Abstract
Background
Despite known benefits to needle biopsy for suspicious breast lesions, variability in the use of this technique has been documented in practice. We sought to study the use of needle biopsy and open surgical biopsy in women with breast cancer, predictors of needle biopsy use, and the effect of biopsy choice on overall number of surgical procedures needed to treat breast cancer.
Methods
We analyzed Surveillance, Epidemiology and End Results (SEER)-Medicare data for 45,542 women diagnosed between 1991–1999 with ductal carcinoma in situ and stage I-II breast cancer. Using diagnosis and procedure codes from three months prior to six months after the SEER diagnosis, we classified the initial biopsy as needle or surgical. Using multivariable logistic regression, we identified patient and tumor characteristics associated with needle biopsy use, and estimated the association between initial biopsy type and likelihood for multiple breast surgeries.
Results
Needle biopsy was the initial procedure for 11,073 (24.3 %) women. In multivariable analyses, needle biopsy use varied significantly by race, year of diagnosis, and tumor size. After controlling for patient and tumor characteristics, needle biopsy use was associated with a reduced likelihood of multiple breast surgeries (OR 0.35, 95% CI 0.34–0.37).
Conclusions
Use of needle biopsy as the initial breast cancer procedure was more common among black women and those with larger tumors, and increased significantly over time. Providers should consider needle biopsy when clinically feasible as the initial breast procedure, because it may reduce the number of surgeries needed to treat breast cancer.
Keywords: breast, diagnostic techniques and procedures, outcome assessment (health care), health services research, SEER Program, Medicare
Patients with suspicious breast anomalies face several options to ascertain initial pathologic diagnosis. Historically, biopsies have been obtained by surgical excision. This procedure, however, causes women with benign breast disease the inconvenience and discomfort of surgery. Additionally, if the margins of the initial excision are close or positive, patients must undergo additional breast surgical procedures. Needle biopsy techniques - including fine needle aspiration, core biopsy, and vacuum-assisted needle biopsy - are less invasive than surgical biopsies and accurately diagnose suspicious breast lesions.1 Using needle biopsy to evaluate breast abnormalities may eliminate the need for surgery altogether when the lesion proves benign. When the needle biopsy shows cancer, subsequent cancer care may be coordinated resulting in fewer surgical procedures overall. One breast center also reported significant cost savings associated with image-guided core biopsy compared with surgical biopsy.2
Despite these documented benefits, data suggest that initial breast biopsy approach is quite variable within3 and across practice settings (S.B. Edge, unpublished data). The purpose of this study was to evaluate the practice patterns and outcomes associated with needle biopsy and surgical excision in the diagnosis of breast cancer in a population-based cohort of women ≥ 65 years old.
PATIENTS and METHODS
Data Sources
We analyzed the Surveillance, Epidemiology, and End-Results (SEER)-Medicare dataset to draw our analytic sample. The SEER-Medicare dataset is a collaborative project of the National Cancer Institute, the respective SEER registries, and the Centers for Medicare and Medicaid Services.4 SEER-Medicare data link tumor registry information with claims for adults enrolled in Medicare; linkage methods have been described previously.5,6 The data set captures roughly 97 percent of incident cancer cases,7 and is especially rich with patients of African-American, Hispanic, Asian, and Hawaiian/Pacific Islander descent.8 We analyzed tumor registry records and claims from the 11 registries that participated in SEER-Medicare between 1991 and 1999. All data were de-identified and the study protocol was deemed exempt from IRB review. A signed data use agreement was executed by the senior author (CCE).
Study Sample
The total number of breast cancer cases in the SEER-Medicare database between 1991 and 1999 was 154,926. Our sample included women with breast cancer diagnosed January 1, 1991 to December 31, 1999 who had a claim for surgical breast excision within six months of their cancer diagnosis (n=93,468). We restricted the sample to women who were diagnosed with Stage I, II, or ductal carcinoma in situ (DCIS) prior to death, survived at least six months after diagnosis, had valid diagnosis dates, did not receive chemo- or radio- therapy prior to surgery and had no history of cancer within the year preceding their breast-cancer surgery (n = 53,010). We excluded 5,481 women diagnosed over the age of 85, 50 women diagnosed below the age of 65, and 1,937 women who did not have continuous Part A and B Medicare coverage throughout the study period. The final analytic sample included 45,542 women. We used claims data for the time period of three months prior to breast cancer diagnosis, through six months after breast cancer diagnosis, to determine study eligibility.
Dependent Variables
Using International Classification of Diseases, 9th Edition (ICD-9) and Current Procedural Terminology (CPT) codes, we identified all breast biopsies and surgical procedures that occurred during the same 9-month window. The list of diagnosis and procedure codes used in this study was developed in consultation with breast cancer clinical experts and appears in Appendix 1. In nearly a third of cases (14,582), women had claims for more than one biopsy procedure during the study period. When multiple biopsy procedure claims occurred on the same day, we considered this to be only one biopsy. When the same type of biopsy claim occurred on more than one day, we treated this as an additional biopsy procedure. We then measured the number of total biopsies and surgical procedures performed on the breast for each patient during the study period. We classified each patient as having received 1) a surgical biopsy before surgical excision if the surgical biopsy was their first or only biopsy procedure, 2) a needle biopsy before surgical excision if the needle biopsy was their first or only biopsy procedure, or 3) one-step surgical excision if they did not have a needle or surgical biopsy reported. Women who had two or more surgical procedures during the study period were considered to have had multiple breast surgeries, and the initial breast biopsy procedure was not included in this count.
Appendix 1.
| Surgery related | ICD-9 Diagnostic Codes | ICD-9 Procedure Codes | HCPCS | CPT | BETOS | CEN | DRG |
|---|---|---|---|---|---|---|---|
| Needle Biopsy | 85.11 | 19000, 19001, 19100, 88170, 19102, 19103, 76095, 76360, 76393, 76942, 88171 | |||||
| Surgical Biopsy | 85.12, 85.20–85.25 | 19110, 19120, 19160, 19125, 19126, 76096, 76393, 76942 | |||||
| Surgical Breast Procedures | 85.12, 85.20, 85.21, 85.22, 85.23, 85.24, 85.25 | 1912x, 1913x, 1914x, 1915x, 1916x, 1917x, 1918x, 1919x, 1920x, 1921x, 1922x, 1923x, 1924x, 1925x, 19260, 19162 | |||||
| Chemotherapy | E933.1, E930.7, V58.1, V66.2, V76.2 | 99.25 | G0355, G0359, G0360, G0361, G0362, Q0083, Q0084, Q0085, J7150, J85xx, J86xx, J87xx, J8999, J9xxx | 964xx, 965xx | O1D | 0331, 0332, 0335 | 410 |
| Radiation | V58.0, V66.1, V67.1 | 92.20–92.29 | C1164, C1174, C1325, C1350, C1700, C1701, C1702, C1703, C1704, C1705, C1706, C1707, C1708, C1709, C1710, C1711, C1712, C1715, C1716, C1717, C1718, C1719, C1720, C1728, C1790, C1791, C1792, C1793, C1794, C1795, C1796, C1797, C1798, C1799, C1800, C1801, C1802, C1803, C1804, C1805, C1806, C2300, C2616, C2622, C2633, G0173, G0174, G0178, G0242, G0243, G0256, G0261, G0273, G0274, G0338, G0339, G0340, Q3001 | 61793, 77XXX, 79030, 79035, 79100, 79200, 79300, 79400, 79420, 79440, 79900, 79999, | P7A | 0330, 0333, 0339, 0973 | 409 |
ICD-9 = International Classification of Diseases, Ninth Revision; HCPCS = Health Care Procedure Coding System; CPT = Current Procedural Terminology; BETOS = Berenson-Eggers Type of Service; CEN = Revenue Center Code; DRG = Diagnosis-Related Group
Independent Variables
Patient characteristics included age at diagnosis, race (white, black, or other, non-white), Hispanic descent, marital status, and presence of comorbidities, as measured by the Klabunde modification of the Charlson Comorbidity Index.9,10 From the SEER registry data, we measured the following tumor characteristics: stage (DCIS, I, or II), tumor size at diagnosis (<2 cm, 2–5 cm, >5 cm, not recorded), histology (ductal, lobular, other), and grade (1, 2, 3, other/unknown). We also obtained the geographic region, urban or rural residence, prior receipt of state financial assistance, and year of diagnosis from the SEER data. Prior care in a teaching hospital was measured using Medicare inpatient claims files. Teaching hospital care was not necessarily associated with the biopsy or surgical procedures analyzed during the study period.
Statistical Analysis
We examined differences in the frequencies of the initial biopsy procedures used for the different patient groups (as defined by sociodemographic, clinical, geographic, and other characteristics). The frequency of the breast biopsy procedures over time was examined using the Cochran-Armitage test for trend. We estimated the likelihood of needle biopsy as the initial biopsy procedure using a logistic regression model that incorporated patient, tumor, and system characteristics as the independent variables. As an initial examination, we compared the total number of surgical breast procedures performed during the study period based on type of initial biopsy with one-way ANOVA. Post-hoc tests were calculated using the Gabriel statistic11 because of uneven group sizes. Finally, we used logistic regression to estimate the likelihood of multiple breast surgeries during the study period. The initial model for multiple surgeries included only patient and tumor characteristics. After retaining significant variables, we added type of initial breast biopsy as a dichotomous predictor variable.
Sensitivity Analyses
We measured type of initial breast biopsy as both categorical (needle biopsy, surgical biopsy, or no biopsy) and dichotomous (initial needle biopsy or not). No significant change in parameter estimates was observed when we compared an ordered logit model for the categorical measure versus a logistic regression model for the dichotomous measure. We also modified our definition of multiple breast surgeries to include three or more surgical breast procedures during the study period. When we replicated our logistic regression model estimating the likelihood of three or more breast surgeries, our findings did not change appreciably.
SAS version 9.1 was used for all analyses. For chi-square and ANOVA results, P values less than .05 were considered statistically significant. Parameter estimates from logistic regression models were expressed as odds ratios and 95% confidence intervals (CI) were calculated.
RESULTS
Baseline Characteristics
Table 1 shows patient and tumor characteristics for the sample by type of initial breast biopsy. A large number of patients (n=16,455) did not have prior inpatient claims to calculate the modified Charlson comorbidity score, or to measure history of care in a teaching hospital. We retained these patients and added a category to our variable to reflect lack of claims data. The distribution of patients by year ranged from 3,460 (7.6%) in 1991 to 5,504 (12.1%) in 1992 (results not shown). The mean age at diagnosis was 74.2 (SD 5.3). Initial needle biopsy was less common for black women and Hispanic women. The distribution of patients varied significantly by geographic region: the largest number of cases was from the West, with subsequent higher rates of needle biopsy. Initial surgical biopsy was more common for women with Stage II disease, tumors < 2 cm in size, and higher grades. There was no relationship between type of initial biopsy and number of comorbid conditions, history of care in a teaching hospital, or history of state assistance.
Table 1.
Characteristics by Initial Breast Biopsy Technique
| Needle Biopsy (N=11,073) | Non-Needle Biopsy (N=34,469) | P | ||||
|---|---|---|---|---|---|---|
| Patient Characteristics | ||||||
| Age at Diagnosis | Mean (SD) | 74.5 | 5.3 | 74.1 | 5.3 | <.001 |
| N | % | N | % | |||
|
|
||||||
| Race | White | 9,970 | 90.0 | 31,290 | 90.8 | <.001 |
| Black | 544 | 4.9 | 1,802 | 5.2 | ||
| Other, non-white | 559 | 5.1 | 1,377 | 4.0 | ||
| Hispanic | 319 | 2.9 | 1,165 | 3.4 | .01 | |
| Married at Diagnosis | 5,100 | 46.1 | 16,349 | 47.4 | .01 | |
| Comorbidities | 0 | 4,278 | 38.6 | 13,334 | 38.7 | .16 |
| 1 | 2,002 | 17.8 | 6,111 | 17.7 | ||
| 2 | 478 | 4.2 | 1,444 | 4.2 | ||
| ≥ 3 | 383 | 3.2 | 1,057 | 3.1 | ||
| Missing | 3,932 | 36.1 | 12,523 | 36.3 | ||
| Tumor Characteristics | ||||||
| Tumor Stage | DCIS | 1,248 | 11.3 | 5,449 | 15.8 | <.001 |
| Stage 1 | 5,149 | 46.5 | 19,242 | 55.8 | ||
| Stage 2 | 4,676 | 42.2 | 9,778 | 28.4 | ||
| Tumor Size | < 2cm | 6,801 | 61.4 | 25,351 | 73.6 | <.001 |
| 2–5 cm | 3,567 | 32.2 | 6,556 | 19.0 | ||
| > 5 cm | 192 | 1.7 | 400 | 1.2 | ||
| Unknown | 513 | 4.6 | 2,162 | 6.3 | ||
| Histology | Ductal | 7,938 | 71.7 | 24,493 | 71.1 | .01 |
| Lobular | 1,096 | 9.9 | 3,187 | 3.3 | ||
| Other | 2,039 | 18.4 | 6,789 | 19.7 | ||
| Grade | 1 | 1,689 | 15.3 | 5,457 | 15.8 | <.001 |
| 2 | 3,978 | 35.9 | 11,349 | 32.9 | ||
| 3 | 2,836 | 25.6 | 6,790 | 19.7 | ||
| Other/Unknown | 2,570 | 23.2 | 10,873 | 31.5 | ||
| System Characteristics | ||||||
| Geographic Region | Northeast | 1,441 | 13.0 | 5,255 | 15.3 | <.001 |
| South | 733 | 6.6 | 1,954 | 5.7 | ||
| Midwest | 2,911 | 26.3 | 10,749 | 31.2 | ||
| West | 5,988 | 54.1 | 16,511 | 47.9 | ||
| Urban Residence | 10,102 | 91.2 | 31,051 | 90.1 | <.001 | |
| History of State Assistance | 1,523 | 13.8 | 4,653 | 13.5 | .50 | |
| Received care in teaching hospital | No | 1,051 | 9.5 | 3,240 | 9.4 | .29 |
| Yes | 6,090 | 55.0 | 18,706 | 54.3 | ||
| Missing | 3,932 | 35.5 | 12,523 | 36.3 | ||
Non-needle biopsy group includes 1,351 patients with one-step surgery (surgical breast excision without claims for biopsy procedures). Western region includes Hawaii.
Biopsy Trends and Predictors
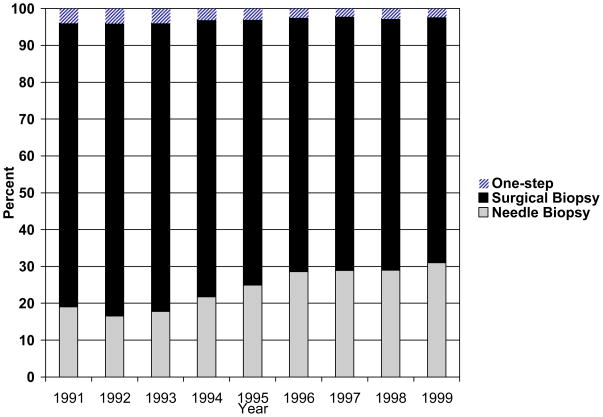
The use of needle biopsy as the initial procedure nearly doubled over the study period from 16.5% in 1992 to 30.9% in 1999, and as expected the use of surgical biopsies decreased over time (Figure 1). Cochran-Armitage tests for trend were significant (z test -24.69, both p < .0001).
Figure 1. Initial Breast Biopsy Technique over Time*.
* One-step surgery defined as surgical excision of breast tissue without a claim for needle or surgical biopsy. Cochran-Armitage test for trend significant (z test -24.69, both p < .0001).
Table 2 shows the results of a multivariable logistic regression model estimating the likelihood of initial needle biopsy. After controlling for other variables, women who were black were less likely to receive needle biopsy then white women (odds ratio = 0.90, 95% CI = 0.81 to 0.99). Compared with white women, women whose race was not black or white were more likely to receive needle biopsy (odds ratio = 1.12, 95% CI = 1.01 to 1.25). The majority of women in the “other” race and ethnicity category are of Asian/Pacific Islander descent; these women are largely concentrated in the West/Pacific region. Hispanic ethnicity is a separate variable from race in the data set. Hispanic women (odds ratio = 0.72, 95% CI = 0.63 to 0.82) were less likely to receive needle biopsy than non-Hispanics. Women with tumors of lower stage, smaller size, and lower grade were less likely to receive initial needle biopsy. Women in the Northeast (odds ratio = 0.74, 95% CI = 0.69 to 0.79) and Midwest (odds ratio = 0.76, 95% CI = 0.72 to 0.80) regions were significantly less likely to receive initial needle biopsy than women in the West. Year of diagnosis remained a significant predictor of initial needle biopsy on multivariable analysis.
Table 2.
Patient, tumor, and system characteristics associated with receipt of needle biopsy as initial technique (N=45,542)
| Odds Ratio | 95% CI | |||
|---|---|---|---|---|
| Patient Characteristics | ||||
| Age at diagnosis | 1.01 | 1.01 | 1.01 | |
| Race | Black | 0.90 | 0.81 | 0.99 |
| Other, non-white | 1.12 | 1.01 | 1.25 | |
| White | - | - | - | |
| Hispanic | 0.72 | 0.63 | 0.82 | |
| Tumor Characteristics | ||||
| Stage | Stage 0 (DCIS) | 0.66 | 0.60 | 0.73 |
| Stage 1 | 0.80 | 0.75 | 0.87 | |
| Stage 2 | - | - | - | |
| Tumor size | < 2 cm | 0.65 | 0.54 | 0.77 |
| 2–5 cm | 1.07 | 0.90 | 1.28 | |
| Missing tumor size | 0.72 | 0.58 | 0.88 | |
| >5 cms | - | - | - | |
| Grade | Other/unknown | 0.77 | 0.72 | 0.82 |
| Grade 1 | 0.84 | 0.79 | 0.91 | |
| Grade 2 | 0.92 | 0.86 | 0.97 | |
| Grade 3 | - | - | - | |
| System Characteristics | ||||
| Region | Northeast | 0.74 | 0.69 | 0.79 |
| South | 1.04 | 0.95 | 1.14 | |
| Midwest | 0.76 | 0.72 | 0.80 | |
| West | - | - | - | |
| Year of diagnosis | 1.11 | 1.10 | 1.12 | |
Likelihood Ratio chi-square 1884.86, 16 df, p < .0001, c-stat: 0.64
Parsimonious model reported: independent variables not significant in univariate or multivariable analyses removed.
Biopsy Technique and Multiple Breast Surgeries
Table 3 shows the mean number of breast surgical procedures based on the type of initial breast biopsy. The average number of breast surgical procedures was 1.75 (SD 0.70, range 1–7 procedures), but significant differences were found by type of initial breast biopsy. Women who had no biopsy and went straight to mastectomy or lumpectomy had the lowest number of surgical procedures (mean 1.24, SD 0.47, range 1–4). Women who had an initial needle biopsy prior to surgical excision had the next lowest number of surgical procedures (mean 1.50, SD 0.65, range 1–6), followed by women who had surgical biopsy before surgical excision (mean 1.86, SD 0.69, range 1–7). The difference in surgical procedures across groups was significant (F = 1030.41, 4 df, p < .01). From post hoc tests performed using a one-way ANOVA model, the differences in the average number of procedures between the groups was also statistically significant (p < .01).
Table 3.
Surgical Breast Procedures by Initial Biopsy Technique*
| Initial Breast Biopsy Technique | N | Mean of Surgical Procedures a | SD | Range |
|---|---|---|---|---|
| One-step surgery | 1,351 | 1.24 | 0.47 | 1–4 |
| Needle | 11,073 | 1.50 | 0.65 | 1–6 |
| Surgical | 33,118 | 1.86 | 0.69 | 1–7 |
| Total | 45,542 | 1.75 | 0.70 | 1–7 |
One-step surgery defined as surgical excision of breast tissue without a claim for needle or surgical biopsy. Differences in means significant in a one-way ANOVA, F = 1577.10, 2 d.f. p < .0001.
Post-Hoc test for Differences across all categories statistically significant at p < .01.
Table 4 shows the results of a logistic regression model to predict multiple breast surgeries, defined as two or more surgical procedures on the breast during the study period (excluding the initial breast biopsy procedure). After controlling for other significant predictors, having an initial needle biopsy was significantly associated with having a decreased likelihood of multiple breast surgeries during the study period (odds ratio = 0.35, 95% CI = 0.34 to 37). Additional characteristics associated with a decreased likelihood of multiple surgeries were age (odds ratio = 0.95, 95% CI = 0.95 to 0.96), having DCIS versus a stage II cancer (odds ratio 0.84, 95% CI 0.77 to 0.91), and having ductal versus other/unspecified histology (odds ratio = 0.85, 95% CI 0.80 to 0.89). When compared with patients with tumors above 5 cms in size, patients with tumors less than 2 cms had increased odds of multiple surgeries (odds ratio = 1.33, 95% CI = 1.12 to 1.59), while patients with tumors between 2 and 5 cms had significantly lower odds of multiple surgeries (odds ratio 0.81, 95% CI 0.68 to 0.96). Compared with patients from the West, women residing in the Northeast and (odds ratio = 0.81, 95% CI = 0.76 to 0.86), Midwest (odds ratio = 1.13, 95% CI = 1.07 to 1.18) regions had significantly different likelihoods for multiple surgeries. Additional significant effects on multiple surgeries were found by year of diagnosis (odds ratio = 1.03, 95% CI = 1.02 to 1.04), and for patients with no prior history of care in a teaching hospital (odds ratio = 0.89, 95% CI = 0.83 to 0.95).
Table 4.
Predictors of Multiple Breast Surgeries (N=45,542)*
| Parameter | OR | 95% CI | ||
|---|---|---|---|---|
| Initial Needle Biopsy | 0.35 | 0.34 | 0.37 | |
| Patient Characteristics | ||||
| Age at diagnosis | 0.95 | 0.95 | 0.96 | |
| Tumor Characteristics | ||||
| Stage | Stage 0 | 0.84 | 0.77 | 0.91 |
| Stage 1 | 1.10 | 1.03 | 1.18 | |
| Stage 2 | - | - | - | |
| Tumor size | < 2 cm | 1.33 | 1.12 | 1.59 |
| 2–5 cms | 0.81 | 0.68 | 0.96 | |
| missing | 1.54 | 1.26 | 1.87 | |
| > 5 cms | - | - | - | |
| Histology | Ductal | 0.85 | 0.80 | 0.89 |
| Lobular | 0.94 | 0.87 | 1.02 | |
| Other | - | - | - | |
| Grade | Other/Unknown | 1.01 | 0.95 | 1.07 |
| Grade 1 | 0.99 | 0.92 | 1.05 | |
| Grade 2 | 0.97 | 0.92 | 1.03 | |
| Grade 3 | - | - | - | |
| System Characteristics | ||||
| Geographic Region | Northeast | 0.81 | 0.76 | 0.86 |
| South | 1.05 | 0.96 | 1.14 | |
| Midwest | 1.13 | 1.07 | 1.18 | |
| West | - | - | ||
| Year of diagnosis | 1.03 | 1.02 | 1.04 | |
| Received care in teaching hospital | No | 0.89 | 0.83 | 0.95 |
| Yes | 0.97 | 0.93 | 1.02 | |
| Missing | - | - | - | |
Likelihood Ratio chi-square 3867.18, 18 d.f., p < .0001, c-statistic 0.66.
Parsimonious model reported: independent variables not significant in univariate or multivariable analyses removed.
DISCUSSION
We found that having an initial needle biopsy was associated with a significant decrease in the likelihood of having multiple surgical procedures on the breast, even after controlling for a number of patient and tumor characteristics. Not surprisingly, needle biopsy use appeared to increase over time. This may have been a reflection of increasing confidence in clinician skill or increasing availability of experienced providers. However, by the end of our study a majority of women (69%) still did not have an initial needle biopsy. Moreover, we found significant variations in the use of needle biopsy by race/ethnicity, geography, and tumor characteristics. Compared with residents of the West, women in the Northeast were significantly less likely to receive both needle biopsy and multiple surgeries, whereas women residing in the South were more likely to have both needle biopsy and multiple surgeries. It is clear that geographic variations are present in breast care, and are worthy of continued study. Specifically, it is interesting to consider why providers in the Midwest are more likely to re-excise while they also perform needle biopsy at a high rate. Black and Hispanic women were less likely to receive needle biopsy, but there were no differences in the likelihood of multiple surgeries by race. It is noteworthy that both tumor size and stage were associated with initial biopsy technique and multiple surgeries, while tumor grade was only associated with surgeries.
There are some limitations inherent in analyses of registry and claims data. For example, only limited data on the characteristics of providers and facilities are available. Additional data might reveal more detailed explanations for the observed variations in initial biopsy type and number of surgeries. For example, availability of experienced providers and tools needed to perform a needle biopsy may have varied significantly from one location to another. Because of our reliance on Medicare data, we are unable to draw any conclusions regarding Medicare enrollees participating in health maintenance organization plans, or the population of women under age 65. We are not able to measure the context of the clinical encounters that may impact the decision to have a needle or surgical biopsy. Correlation of our findings in settings with documentation of clinician and patient deliberations would be helpful. However, the multiple years and geographic regions represented in our data add strength to our findings and conclusions.
These analyses are restricted to studying surgical procedures on the breast, and additional consideration of axillary procedures may be important. Women who receive initial needle biopsy may be more likely to proceed to simultaneous breast and axillary surgery. For patients who receive surgical biopsy and have negative margins, additional axillary surgery is most likely required. As such, we would not expect the findings reported here to change dramatically. Another limitation is our reliance on data through 1999 because this was when the core needle biopsy technique was coming into wider acceptance. Due to delays in availability of SEER-Medicare data, we were not able to assess the proportion of women with cancer diagnosed by needle biopsy in more recent years. Based on individual practice reports, it seems likely that a higher proportion of women have needle biopsy now than what was observed in 1999. For example, about 70% of women with breast cancer treated at National Comprehensive Cancer Network centers from 2002 to 2006, had a needle biopsy performed (S. B. Edge, unpublished data). Regardless, it seems likely that variation in use of initial needle biopsy by geographic and sociodemographic features persists today.
Our study joins a long list of publications that document disparities in breast cancer treatment by race, age, geography, and other patient characteristics. Most recently, a study found that black women, older women, and women living in non-metropolitan areas were less likely to receive radiotherapy after breast conservation surgery.12 Significant differences by geography and race were found in a study comparing breast conversation therapy versus mastectomy.13,14 Black women are also less likely to receive adjuvant radiotherapy for early-stage breast cancer.15 Our findings suggest that disparities in breast care occur even earlier in the diagnostic process.
The National Cancer Policy Board16 and others17 have identified the quality of cancer care – especially breast cancer - as uneven in the United States. The IOM’s Quality Chasm18 report concludes that providers should strive to deliver care that is effective, patient-centered, and equitable. Using needle biopsy rather than surgical biopsy to evaluate suspicious breast abnormalities may lead to a decrease in the need for multiple breast surgeries, and as a result could reduce complications, improve timeliness of care, and most importantly improve patient satisfaction and quality of life. Clinicians should consider whether all patients eligible for needle biopsy in their practice are provided with this option prior to proceeding with further diagnostic or therapeutic interventions. Interventions aimed at increasing the availability and acceptance of this technique and efforts to standardize biopsy techniques may improve quality of care for women with breast cancer.
Acknowledgments
We appreciate the research assistance of Lysa S. Magazu in the development of this manuscript.
Research Support: National Cancer Institute (R25 CA 057711-12, Glorian Sorensen, Principal Investigator).
Footnotes
Presentations: Findings from this paper were presented at the 2007 ASCO Breast Symposium, San Francisco, CA.
Disclaimer: This study used the linked SEER-Medicare database and the authors acknowledge the efforts of the respective agencies in creating the database. The interpretation and reporting of these data are the sole responsibility of the authors. At the time of the study, the corresponding author (CRF) was research fellow, Center for Outcomes and Policy Research, Dana-Farber Cancer Institute, and Department of Society, Human Development, and Health, Harvard School of Public Health
References
- 1.Fajardo LJ, Pisano ET, Caudry DJ, Gastonis CA, Berg WA, Connolly J, et al. Stereotactic and sonographic large-core biopsy of non-palpable breast lesions: Results of the Radiologic Diagnostic Oncology Group V Study. Acad Radiol. 2004;11:293–308. doi: 10.1016/s1076-6332(03)00510-5. [DOI] [PubMed] [Google Scholar]
- 2.Golub RM, Bennett CL, Stinson T, Venta L, Morrow M. Cost minimization study of image-guided core biopsy versus surgical excision biopsy for women with abnormal mammograms. J Clin Oncol. 2004;22:2430–37. doi: 10.1200/JCO.2004.06.154. [DOI] [PubMed] [Google Scholar]
- 3.Hanley C, Kessaram R. Quality of diagnosis and surgical management of breast lesions in a community hospital: Room for improvement? Can J Surg. 2005;49:185–92. [PMC free article] [PubMed] [Google Scholar]
- 4.National Cancer Institute. [Accessed October 21, 2008];Overview of the SEER Program. Available from URL: http://seer.cancer.gov/about/
- 5.Cooper GS, Virnig B, Klabunde CN, Schussler N, Freeman J, Warren JL. Use of SEER-Medicare data for measuring cancer surgery. Medical Care. 2002;40(8 Suppl):IV-43–8. doi: 10.1097/00005650-200208001-00006. [DOI] [PubMed] [Google Scholar]
- 6.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Medical Care. 2002;40(8 Suppl):IV-3–18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 7.Zippin C, Lum D, Hankey BF. Completeness of hospital cancer case reporting from the SEER program of the National Cancer Institute. Cancer. 1995;76:2343–50. doi: 10.1002/1097-0142(19951201)76:11<2343::aid-cncr2820761124>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 8.Ries LAG, Kosary CL, Hankey BF, Miller BA, Harras A, Edwards BK. NIH Publication 97–2789. National Cancer Institute; 1997. SEER cancer statistics review, 1973–1994. [Google Scholar]
- 9.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. Journal of Clinical Epidemiology. 2000;53:1258–67. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 10.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. Journal of Chronic Disease. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 11.Gabriel KR. A simple method of multiple comparisons of means. Journal of the American Statistical Association. 1978;73:364. [Google Scholar]
- 12.Hershman DL, Buono D, McBride RB, Tsai WY, Joseph KA, Grann VR, et al. Surgeon characteristics and receipt of adjuvant radiotherapy in women with breast cancer. J Natl Cancer Inst. 2008;100:199–206. doi: 10.1093/jnci/djm320. [DOI] [PubMed] [Google Scholar]
- 13.Nattinger AB, Gottlieb MS, Veum J, Yahnke D, Goodwin JS. Geographic variation in the use of breast-conserving treatment for breast cancer. N Engl J Med. 1992;326:1102–7. doi: 10.1056/NEJM199204233261702. [DOI] [PubMed] [Google Scholar]
- 14.Lantz PV, Zemencuk JK, Katz SJ. Is mastectomy overused? A call for an expanded research agenda. Health Serv Res. 2002;37:417–31. doi: 10.1111/1475-6773.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joslyn SA. Racial differences in treatment and survival from early-stage breast carcinoma. Cancer. 2002;95:1759–66. doi: 10.1002/cncr.10827. [DOI] [PubMed] [Google Scholar]
- 16.Hewitt M, Simone JV. Ensuring quality cancer care. Washington D.C: National Cancer Policy Board, Institute of Medicine; 1999. [PubMed] [Google Scholar]
- 17.Nattinger AB. Quality of care for breast cancer. Medical Care. 2003;41:341–43. doi: 10.1097/01.MLR.0000060890.54585.08. [DOI] [PubMed] [Google Scholar]
- 18.Institute of Medicine. Crossing the quality chasm : A new health system for the 21st century. Washington, DC: National Academy Press; 2001. [PubMed] [Google Scholar]