Abstract
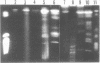
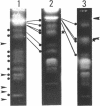
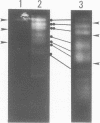
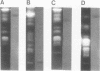
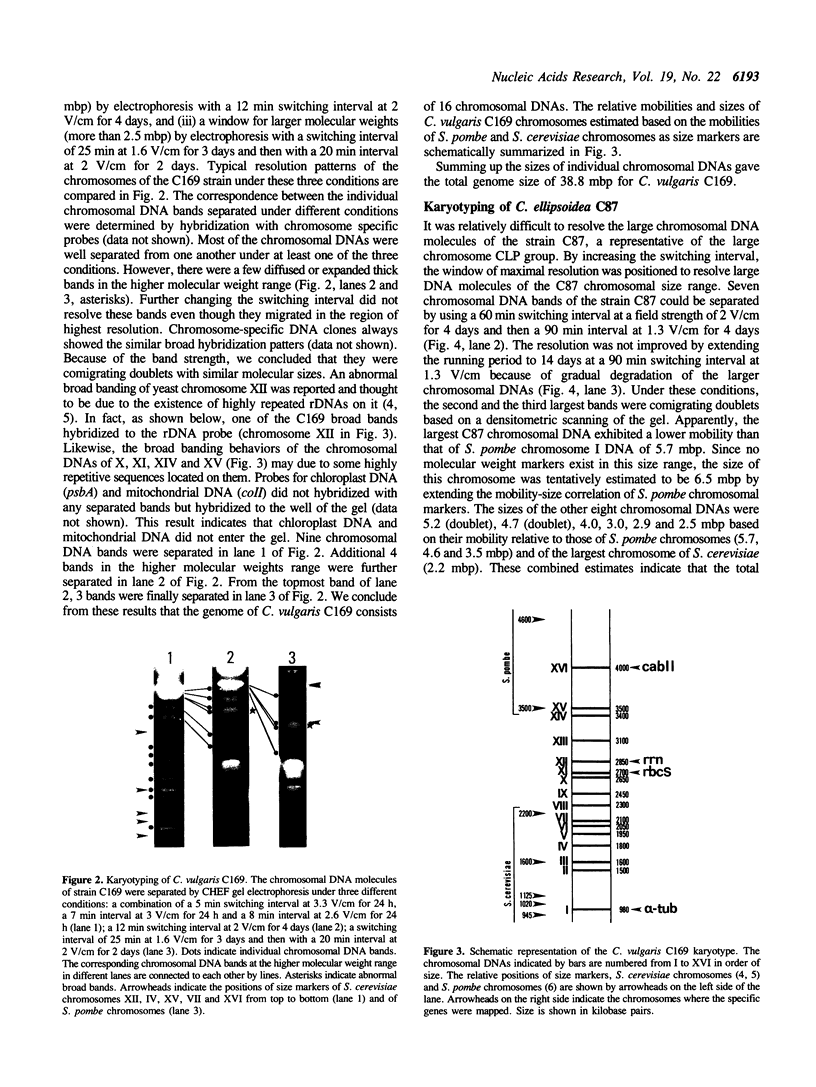
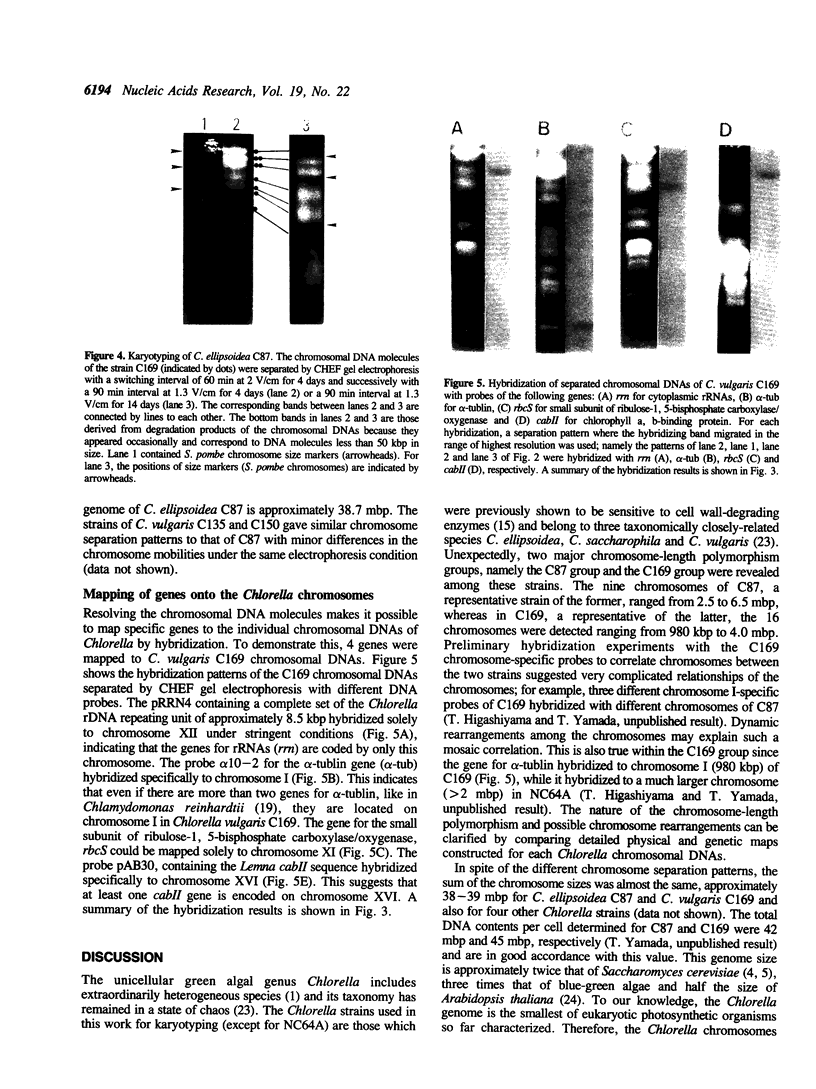
Molecular karyotypes for six strains of four Chlorella species were obtained by using an alternating-field gel electrophoresis system which employs contour-clamped homogenous electric fields (CHEF). The number and migration pattern of the chromosomal DNA molecules varied greatly from strain to strain: for example, nine separated chromosomes of C. ellipsoidea C87 ranged from 2.5 to 6.5 megabase pairs (mbp) in size, whereas 16 chromosomes of C. vulgaris C169 were from 980 kilobase pairs (kbp) to 4.0 mbp. Depending on the chromosome migration patterns, the six strains were classified into two major chromosome-length polymorphism groups. Using hybridization techniques, the genes for alpha-tublin, chlorophyll-a, b-binding proteins, ribosomal RNAs, and the small subunit of ribulose-1, 5-bisphosphate carboxylase/oxygenase (RuBisCO) were mapped on the separated chromosomes of C. vulgaris C169. Since Chlorella chromosomes are small enough to separate and isolate individually by CHEF gel electrophoresis under ordinary conditions, they should serve as excellent materials to study the fundamental molecular structure of plant-type chromosomes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brody H., Carbon J. Electrophoretic karyotype of Aspergillus nidulans. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6260–6263. doi: 10.1073/pnas.86.16.6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu G., Vollrath D., Davis R. W. Separation of large DNA molecules by contour-clamped homogeneous electric fields. Science. 1986 Dec 19;234(4783):1582–1585. doi: 10.1126/science.3538420. [DOI] [PubMed] [Google Scholar]
- Fan J. B., Chikashige Y., Smith C. L., Niwa O., Yanagida M., Cantor C. R. Construction of a Not I restriction map of the fission yeast Schizosaccharomyces pombe genome. Nucleic Acids Res. 1989 Apr 11;17(7):2801–2818. doi: 10.1093/nar/17.7.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J. B., Korman S. H., Cantor C. R., Smith C. L. Giardia lamblia: haploid genome size determined by pulsed field gel electrophoresis is less than 12 Mb. Nucleic Acids Res. 1991 Apr 25;19(8):1905–1908. doi: 10.1093/nar/19.8.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt-Clermont M., Rahire M. Sequence, evolution and differential expression of the two genes encoding variant small subunits of ribulose bisphosphate carboxylase/oxygenase in Chlamydomonas reinhardtii. J Mol Biol. 1986 Oct 5;191(3):421–432. doi: 10.1016/0022-2836(86)90137-3. [DOI] [PubMed] [Google Scholar]
- Johnston J. R., Contopoulou C. R., Mortimer R. K. Karyotyping of yeast strains of several genera by field inversion gel electrophoresis. Yeast. 1988 Sep;4(3):191–198. doi: 10.1002/yea.320040304. [DOI] [PubMed] [Google Scholar]
- Kemp D. J., Corcoran L. M., Coppel R. L., Stahl H. D., Bianco A. E., Brown G. V., Anders R. F. Size variation in chromosomes from independent cultured isolates of Plasmodium falciparum. Nature. 1985 May 23;315(6017):347–350. doi: 10.1038/315347a0. [DOI] [PubMed] [Google Scholar]
- Kohorn B. D., Harel E., Chitnis P. R., Thornber J. P., Tobin E. M. Functional and mutational analysis of the light-harvesting chlorophyll a/b protein of thylakoid membranes. J Cell Biol. 1986 Mar;102(3):972–981. doi: 10.1083/jcb.102.3.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orbach M. J., Vollrath D., Davis R. W., Yanofsky C. An electrophoretic karyotype of Neurospora crassa. Mol Cell Biol. 1988 Apr;8(4):1469–1473. doi: 10.1128/mcb.8.4.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz D. C., Cantor C. R. Separation of yeast chromosome-sized DNAs by pulsed field gradient gel electrophoresis. Cell. 1984 May;37(1):67–75. doi: 10.1016/0092-8674(84)90301-5. [DOI] [PubMed] [Google Scholar]
- Silflow C. D., Chisholm R. L., Conner T. W., Ranum L. P. The two alpha-tubulin genes of Chlamydomonas reinhardi code for slightly different proteins. Mol Cell Biol. 1985 Sep;5(9):2389–2398. doi: 10.1128/mcb.5.9.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellwagen N. C. Orientation of DNA molecules in agarose gels by pulsed electric fields. J Biomol Struct Dyn. 1985 Oct;3(2):299–314. doi: 10.1080/07391102.1985.10508418. [DOI] [PubMed] [Google Scholar]
- VAN Etten J. L., Burbank D. E., Kuczmarski D., Meints R. H. Virus infection of culturable chlorella-like algae and dlevelopment of a plaque assay. Science. 1983 Feb 25;219(4587):994–996. doi: 10.1126/science.219.4587.994. [DOI] [PubMed] [Google Scholar]
- Van der Ploeg L. H., Schwartz D. C., Cantor C. R., Borst P. Antigenic variation in Trypanosoma brucei analyzed by electrophoretic separation of chromosome-sized DNA molecules. Cell. 1984 May;37(1):77–84. doi: 10.1016/0092-8674(84)90302-7. [DOI] [PubMed] [Google Scholar]
- Vollrath D., Davis R. W. Resolution of DNA molecules greater than 5 megabases by contour-clamped homogeneous electric fields. Nucleic Acids Res. 1987 Oct 12;15(19):7865–7876. doi: 10.1093/nar/15.19.7865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T. Repetitive sequence-mediated rearrangements in Chlorella ellipsoidea chloroplast DNA: completion of nucleotide sequence of the large inverted repeat. Curr Genet. 1991 Feb;19(2):139–147. doi: 10.1007/BF00326295. [DOI] [PubMed] [Google Scholar]