Abstract
Purpose
The objective of this study was to determine the role of β-catenin in normal postnatal articular cartilage growth and degeneration.
Methods
We investigated β-catenin gene and protein expression in hip cartilage cells of normal Wistar rats at two, four, six and eight weeks of age by using reverse transcriptase polymerase chain reaction (RT-PCR) and immunohistochemistry. Primary articular chondrocytes from eight week old rats were cultured and treated with LiCl for activation of β-catenin. Collagen X and matrix metalloproteinase 13 (MMP-13) were detected by quantitative RT-PCR and immunofluorescence. A disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS)-4 and 5 were detected by quantitative RT-PCR, and terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) was used for detecting cell apoptosis.
Results
The highest levels of β-catenin expressions were detected in two week old rats, after which a steady decline was observed over the remaining period of observation (p < 0.05). When primary articular chondrocytes from eight week old rats were treated with LiCl, β-catenin mRNA and protein were induced (p < 0.05). Moreover, LiCl-activated β-catenin in chondrocytes was associated with significant concomitant increases in mRNA expression of collagen X and the MMP-13 encoding collagenase 3. Significantly increased mRNA expression of ADAMTS-5 was also seen in primary chondrocytes from eight week old rats after LiCl treatment (p < 0.05). The effect was specific to ADAMTS-5 since ADAMTS-4, which has similar proteolytic activity but different aggrecanase activity, was unaffected. Finally, TUNEL staining revealed that LiCl-activated β-catenin signalling led to increased cell apoptotic events in chondrocytes (p < 0.05).
Conclusions
Our findings suggest that normal spatiotemporal patterns and degrees of Wnt/β-catenin signalling are needed to maintain postnatal articular cartilage growth and function. In the early stages of cartilage development, activation of β-catenin signalling is necessary for articular cartilage growth, while in adult cartilage it leads to degeneration and osteoarthritic-like chondrocytes.
Introduction
Articular cartilage is an essential component of the synovial joints, and loss of this tissue, usually by osteoarthritis (OA), leads to severe incapacitation of joint function [1, 2]. OA manifests as dysplasia and general degeneration of the cartilage [3, 4]; however, the molecular mechanisms underlying cartilage development and degeneration remain to be fully elucidated. Recent studies have shown that β-catenin plays a critical role throughout chondrogenesis and chondrocyte maturation [5–7]. When researchers established ectopic expression of β-catenin in cells of the chondrogenic lineage, chondrocyte differentiation was significantly inhibited but chondrocyte maturation was stimulated, as was ossification during embryonic development [8, 9]. In early mesenchymal precursors, conditional deletion of the β-catenin gene led to enhancement of chondrocyte differentiation, indicating that β-catenin is capable of inhibiting early mesenchymal precursor differentiation into chondrocytes [6, 10]. Surprisingly, however, murine transgenic studies showed that β-catenin promoted the maturation of growth plate chondrocytes after cartilage formation [8, 11]. Another transgenic study that inhibited the β-catenin signalling pathway in mice found that lack of this signal led to defects in postnatal cartilage development [12]. Collectively, these findings indicate the crucial role that β-catenin signalling plays in postnatal cartilage development.
Yet, activation of β-catenin signalling has also been associated with cartilage degeneration and OA progression. High expression levels of β-catenin have been detected in cartilage of human OA patients [13, 14]. Genome-based studies to determine the gene expression profile of OA found that several factors associated with the Wnt/β-catenin pathway, including Wnt proteins and Frizzled receptors, are up-regulated in arthritic cartilage tissues. The low-density lipoprotein receptor-related protein five (LRP5), a Wnt co-receptor, has particularly high expression in human and animal OA cartilage; recently, the catabolic role of LRP5 during human OA was defined as being mediated by the Wnt/β-catenin pathway [14]. The Wnt1-inducible signalling pathway protein one (WISP-1) was strongly increased in the synovium and cartilage of mice with experimental OA, and increased WISP-1 expression was also found in human OA cartilage [15]. In addition, functional deletion of the Frizzled-related protein FrzB in a murine model of OA resulted in disease exacerbation [16, 17]. Likewise, conditional activation of β-catenin signalling in adult mouse articular chondrocytes led to an OA-like phenotype [13]. These findings strongly suggest that the Wnt/β-catenin signalling pathway is involved in the aetiology or progression of OA.
The Wnt signalling pathway has been extensively studied, and its functional contributions to a broad variety of developmental processes, including organogenesis, cell differentiation, morphogenesis and tissue remodelling, are well known [18]. β-catenin has been defined as a key factor of the canonical Wnt signalling pathway. Stability of β-catenin is regulated by functional interaction with a degradation protein complex that is composed of three tumour suppressors: adenomatous polyposis coli (APC), axin and glycogen synthase kinase-3β (GSK-3β). This multiprotein complex induces phosphorylation of serine and threonine residues in the N terminus of β-catenin. Phosphorylated β-catenin is then recognised by the β-transducin repeat-containing protein (β-TrCP), a component of a dedicated E3 ubiquitin ligase complex, whereupon it is ubiquitinated for rapid degradation by proteasomes. The Wnt proteins, on the other hand, act to inhibit the function of the degradation complex, thus stabilising β-catenin in the cytoplasm. Accumulated β-catenin is ultimately transported into the nucleus, where it combines with T-cell factor/lymphoid-enhancing factor (TCF/LEF) to initiate transcription of target genes [18].
Although Wnt/β-catenin signalling is known to be involved in cartilage development and degeneration, the precise role of β-catenin in these processes remains largely uncharacterised. We hypothesised that β-catenin may be highly expressed in early stages of cartilage growth, wherein it may promote cartilage development. In adult cartilage, however, β-catenin would then be expected to be down-regulated, or inactivated, and aberrant activation may result in a detrimental phenotype. In order to confirm these hypotheses, we investigated the expression of β-catenin in articular cartilage from Wistar rats at ages corresponding to human developmental stages of toddler (two weeks old), teenager (four weeks old), young adult (six weeks old) and adult (eight weeks old). Lithium chloride (LiCl), a well-known activator of the Wnt/β-catenin signalling pathway [19–21], was applied to the primary chondrocytes to facilitate investigations into β-catenin effects and functional interactions at different developmental stages.
Materials and methods
Animals and tissue collection
Wistar rats were bred in-house for use. Sacrifice was performed at two, four, six and eight weeks of age (n = ten each) by overdose of general anaesthesia. Total hip joints were excised immediately and divided for processing according to the requirements for subsequent procedures [immunohistochemistry, RNA extraction and quantitative (q) reverse transcriptase polymerase chain reaction (RT-PCR), primary cell culture]. All experiments were carried out according to the protocol approved by the Institutional Ethics Committee.
Immunohistochemistry
Isolated hip joints were fixed in 4% paraformaldehyde, decalcified to complete demineralisation in 10% ethylenediaminetetraacetic acid (EDTA) and then embedded in paraffin. Ten representative sections (5 μm) from various depths of each joint were mounted on slides. The sections were then deparaffinised in xylene, rehydrated and washed three times with phosphate-buffered saline (PBS) for five minutes at room temperature. Endogenous peroxidase activity was blocked by soaking in 3℅ hydrogen peroxide (H2O2) for ten minutes, and antigen retrieval was carried out by microwaving to near boiling (95°C, ten min) in 10 mM of sodium citrate (pH 6.0). Sections were incubated overnight at 4°C with rabbit anti-β-catenin monoclonal antibody (1:50, Abcam, Cambridge, MA, USA). For the negative control reaction, the final incubation was carried out with primary antibody omitted. Thereafter, sections were detected by using the EnVision Detection Kit (Dako, Glostrup, Denmark), according to the manufacturer’s instructions, and by counterstaining with Mayer’s haematoxylin. The total number of chondrocytes per unit area was determined by manually counting the cells present in five randomly chosen regions of each section under ×20 magnification.
Primary rat hip chondrocyte culture and treatment
Hip joints isolated from eight week old rats were washed in D-Hank’s solution immediately upon excision. The femoral head and acetabular articular cartilage were harvested, rewashed with D-Hank’s solution and completely digested by soaking in 0.25% trypsin (Gibco, Invitrogen, Carlsbad, CA, USA) for 15 min in a 37°C shaking water bath. Afterwards, a solution of 0.2% collagenase II [in 10% fetal bovine serum (FBS) and Dulbecco’s modified Eagle’s medium (DMEM), Sigma-Aldrich, St. Louis, MO, USA] was added and the solution was further incubated with shaking for three hours. A 2-ml aliquot of the digested product was collected every hour. The digested product was passed though a 100-μm Swinnex filter to remove any residual fragments, and the filtrate was centrifuged. The pelleted cells were resuspended in complete medium (DMEM with 10% FBS, 1% penicillin/streptomycin, 100 mM L-glutamine). Cells were then counted and plated at the appropriate density in 25-cm culture flasks for incubation in a 5% CO2-air mixture at 37°C. The media were refreshed every three to four days.
In order to stimulate the Wnt signalling pathway, primary articular chondrocytes from eight week old rats plated in six-well plates were treated with 10 mM LiCl (Sigma) for 48 hours. Negative controls were treated exactly the same, except with LiCl omitted from the final incubation step.
Immunofluorescence microscopy
Articular chondrocytes cultured in six-well plates with or without LiCl were fixed with 4% paraformaldehyde for ten minutes and blocked with 10% goat serum in PBS supplemented with 0.2% Triton X-100 (PBST, Sigma). Primary antibody incubation was performed overnight at 4°C for antibodies (all from Abcam and used at 1:100) against β-catenin, collagen II, collagen X and matrix metalloproteinase 13 (MMP-13). After washing, secondary antibody incubation was carried out for one hour at room temperature with anti-rabbit or anti-mouse Alexa Fluor 488 antibody (1:50, Invitrogen). Cells were imaged by fluorescent microscope (Leica Microsystems, Buffalo Grove, IL, USA).
Terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) staining
TUNEL staining was carried out by using a peroxidase-based assay kit (In Situ Cell Death Detection Kit, POD, Roche Applied Science, Mannheim, Germany). Briefly, cells grown in six-well plates were air-dried and fixed in 4% paraformaldehyde in PBS for one hour. After rinsing in PBS, the fixed cells were blocked for ten minutes in 3% H2O2 in methanol, rinsed with PBS and permeabilised by incubation in 0.1% PBST for two minutes on ice. An additional PBS wash was performed and the TUNEL reaction mixture was added for one hour incubation at 37°C in dark, followed by another PBS wash and a 30-min incubation with 4′,6-diamidino-2-phenylindole (DAPI) nuclear stain. Apoptotic cells were observed by fluorescent microscope. The apoptotic cell rates were determined by manually counting the number of TUNEL staining-positive cells and dividing by the number of DAPI-positive cells.
RNA extraction and real-time qRT-PCR analysis
Total tissue and cellular RNA were extracted by using the TRIzol reagent (Invitrogen), according to the manufacturer’s protocol. The purity and concentration of extracted RNA were determined by spectrophotometric measurement of the OD260/280 ratio. Preservation of 28S and 18S rRNA species was used to assess RNA integrity. One microgram total RNA was used to synthesise cDNA with the ReverTra Ace qPCR RT kit (Toyobo, Osaka, Japan) and the following gene-specific PCR primers: collagen X Fw: 5′-GATCATGGAG CTCACGGAAAA-3′, Rev: 5′-CCGTTCGATTCCGCATTG-3′; MMP-13 Fw: 5′-TACGAGCATCCATCCCGA GACC-3′, Rev: 5′-AACCGCAGCACTGAGCCTTTTC-3′; ADAMTS-4 (a disintegrin and metalloproteinase with thrombospondin motifs) Fw: 5′-CTACAACCACCGAACCGAC-3′, Rev: 5′-TGCCAGCCACCAGAACTT-3′; ADAMTS-5 Fw: 5′-GGCTGTGGTGTGCTGTG-3′, Rev: 5′-CTGGTCTTT GGCTTTGAAC-3′; β-catenin Fw: 5′-GCAATCAGGA A AGCAAGC TC-3′, Rev: 5′-TCAGCACTCTGCTTGTGGTC-3′; β-actin Fw: 5′-GGAGATTACTG CCCTGGCTCCTA-3′, Rev: 5′-GACTCATCGTACTCCTGCTTGCTG-3′. qRT-PCR was performed using the SYBR Green® Realtime PCR Master Mix Kit (Toyobo).
Statistical analysis
Descriptive statistical analysis was performed using mean values and standard deviation. Data were analysed with the commercial statistical software SPSS 16.0 and two-tailed Student’s t tests were used. A p value < 0.05 was considered statistically significant.
Results
Expression patterns of β-catenin in postnatal articular cartilage are distinct at different developmental stages
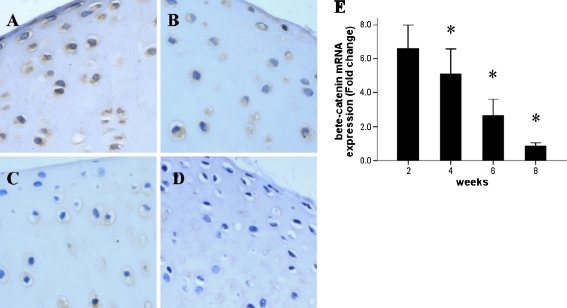
To investigate the role of β-catenin signalling in postnatal articular cartilage, we first determined the temporal expression pattern of β-catenin in articular chondrocytes. Immunohistological analysis revealed that β-catenin was most highly expressed at the earliest stage examined (2 weeks; Fig. 1a). Thereafter, the expression level of β-catenin steadily decreased in an age-dependent manner (Fig. 1a–d). By eight weeks of age, the expression of β-catenin in cartilage had dropped to a level that was below the threshold of immunohistological detection (Fig. 1d). Parallel analysis of the β-catenin mRNA expression, by qPCR, indicated that gene transcription followed the same decreasing expression pattern as was observed for the protein (Fig. 1e).
Fig. 1.
Expression of β-catenin in normal postnatal rat articular cartilage at various postnatal ages: a two weeks, b four weeks, c six weeks and d eight weeks. Expression of β-catenin mRNA (e, detected by qRT-PCR) decreased with age
These results demonstrated that β-catenin signalling is present, and presumably active, during early postnatal articular cartilage growth but disappears and does not contribute to processes in adult cartilage.
LiCl treatment activates β-catenin signalling in adult chondrocytes
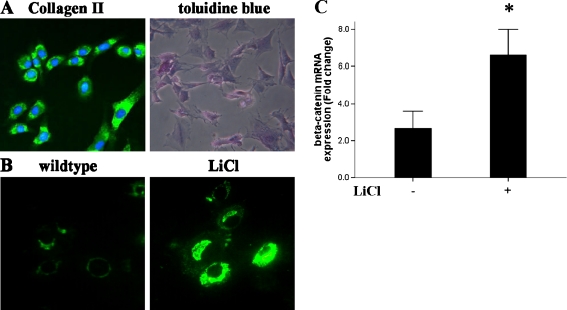
The marked loss of endogenous β-catenin expression in rat articular cartilage at eight weeks of age led us to investigate whether exogenous LiCl could activate β-catenin signalling in isolated tissues from this mature stage. When primary cultured articular chondrocytes from hips of eight week old rats were exposed to LiCl in culture, β-catenin expression was significantly increased (Fig. 2b). Cultures were tested by the expression of collagen II and toluidine blue staining (Fig. 2a). In addition, we examined the effect of LiCl exposure on β-catenin RNA levels and found that β-catenin gene expression was also robustly up-regulated in the LiCl-treated eight week old chondrocytes (Fig. 2c). Thus, these results indicated that LiCl treatment is able to activate the canonical Wnt signalling pathway in mature chondrocytes.
Fig. 2.
β-Catenin signalling was activated in eight week old rat chondrocytes by LiCl treatment. Primary articular chondrocytes were identified by the presence of collagen II and toluidine blue staining (a, ×200). Upon LiCl treatment, higher expressions of β-catenin protein (b, ×400) and mRNA (c) were observed in LiCl-treated adult chondrocytes, as compared to non-treated chondrocytes
LiCl treatment leads to accelerated adult maturation of articular chondrocytes
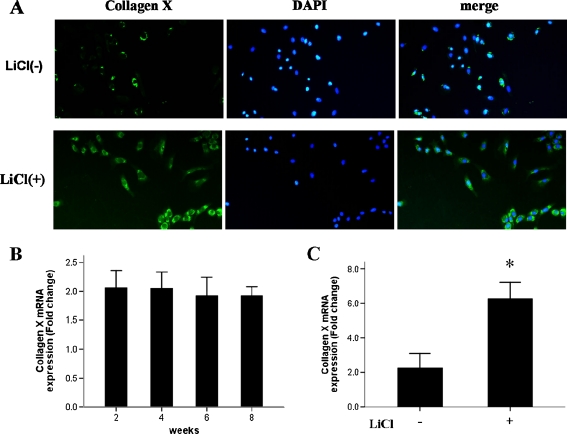
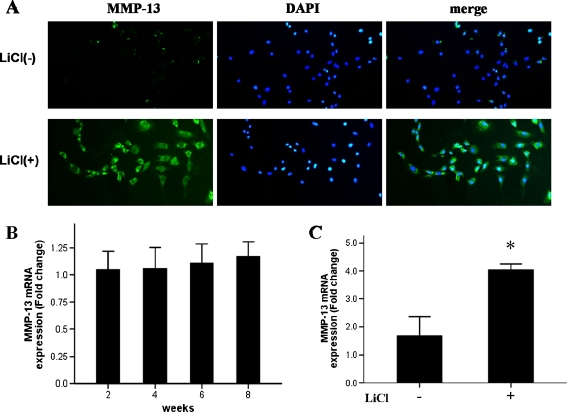
Considering that endogenous β-catenin appeared to be highest at the earliest stages examined (two and four weeks of age in rats), we aimed to determine the effect of β-catenin on chondrocyte maturation. To this end, we isolated articular cartilage from the hips of rats at different stages of cartilage development (two, four, six and eight weeks of age). Total RNA was extracted from these tissues, and the expression of the articular chondrocyte maturation gene markers collagen X and MMP-13 was examined. Surprisingly, neither gene’s expression was significantly changed from among the different ages examined (Figs. 3b and 4b), indicating that β-catenin does not promote articular cartilage maturation during the early stage of postnatal cartilage development.
Fig. 3.
LiCl treatment in chondrocytes leads to high expression of collagen X. Collagen X protein (a, detected by immunofluorescent microscopy, ×200) and mRNA (c) were significantly higher in LiCl-treated chondrocytes, as compared to non-treated chondrocytes in eight week old rats. Collagen X mRNA expression levels in normal cartilage were not significantly different at different ages (b)
Fig. 4.
LiCl treatment in chondrocytes results in high expression of MMP-13. MMP-13 protein (a, detected by immunofluorescent microscopy, ×200) and mRNA (c) were significantly higher in LiCl-treated chondrocytes in eight week old rats, as compared to non-treated chondrocytes. MMP-13 mRNA expression levels in normal cartilage were not significantly different at different ages (b)
We next investigated changes in the maturation status of articular chondrocytes from eight week old rats in response to LiCl treatment. Immunofluorescence microscopy showed that collagen X and MMP-13 protein levels were significantly increased in chondrocytes after exposure to LiCl (Figs. 3a and 4a). To further evaluate the responses of collagen X and MMP-13, total RNA was extracted from LiCl-treated and untreated chondrocytes for analysis by qPCR. Similar to the LiCl-induced effects on the protein expression of these two important chondrocyte maturation markers, LiCl caused significant up-regulation of collagen X and MMP-13 gene expressions (Figs. 3c and 4c). These results suggested that activation of β-catenin may be capable of stimulating chondrocyte maturation in eight week old rats.
LiCl treatment was associated with manifestation of an OA-like phenotype in chondrocytes
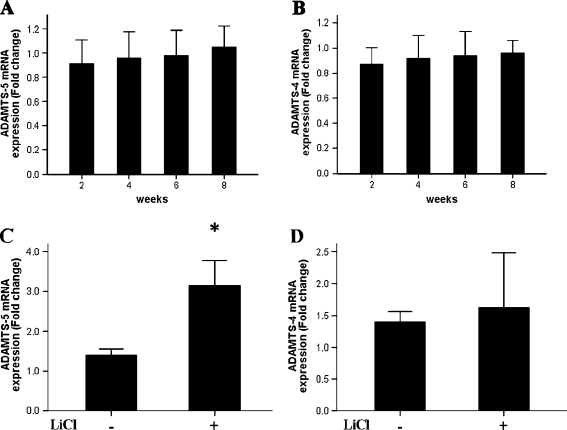
To gain further insight into the OA-like phenotype that was induced in chondrocytes upon β-catenin activation, we evaluated the expression of two known OA-related marker genes: ADAMTS-4 and ADAMTS-5. In normal (untreated) cartilage, the mRNA levels for both OA-related markers were similar at each age examined (observed differences were not statistically significant; Fig. 5a, b). However, upon LiCl treatment ADAMTS-5 expression was significantly enhanced (Fig. 5c). The observed difference in ADAMTS-4 was not statistically different from that in the non-treated cells (Fig. 5d). Therefore, the OA-like phenotype induced by activation of β-catenin likely involves the signalling pathway related specifically to ADAMTS-5 and not the similarly functioning family member ADAMTS-4.
Fig. 5.
Activation of β-catenin signalling leads to high expression of ADAMTS-5, but does not affect ADAMTS-4 expression. ADAMTS-5 (a) and ADAMTS-4 (b) expression in normal cartilage. LiCl-treated chondrocytes in eight week old rats expressed high levels of ADAMTS-5 (c) and normal levels of ADAMTS-4 (d)
LiCl treatment induced apoptosis in mature chondrocytes
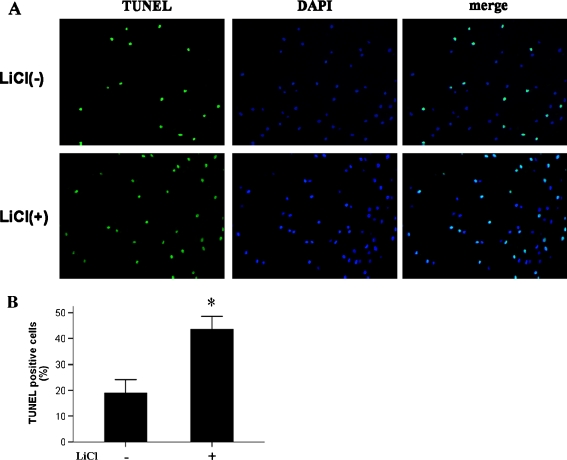
OA is characterised by extensive apoptosis of chondrocytes [22]. To further elucidate the molecular mechanisms underlying the OA-like phenotype induced in chondrocytes by LiCl treatment, cells were examined by TUNEL staining. We found that the number of chondrocytes undergoing apoptosis was significantly higher (by 45%) in samples treated with LiCl than in untreated samples (Fig. 6). Thus, it appeared that activation of β-catenin in chondrocytes of adult rats led to cellular apoptosis.
Fig. 6.
LiCl-induced β-catenin led to apoptosis of chondrocytes from eight week old rats. TUNEL staining-positive chondrocytes (a, ×400) were more prevalent (b) in LiCl-treated samples
Discussion
Articular cartilage is a permanent tissue that undergoes minimal turnover of its cells and matrix as the body ages normally. The zonal articular chondrocytes that compose articular cartilage, along with a unique extracellular matrix architecture, facilitate frictionless movement of joints throughout life [23]. Chondrocyte differentiation processes that occur during endochondral ossification and mature functions are tightly regulated by several signalling pathways [24], including the Wnt/β-catenin pathway [13, 21]. β-Catenin has been shown to act as a critical modulator of multiple steps during chondrocyte formation, maturation and degeneration. Furthermore, β-catenin has been implicated in the pathogenesis of OA disease in humans, as evidenced by genomic-based studies [25, 26]. Animal studies using β-catenin conditional deletion or activation have provided direct evidence about the role of β-catenin in cartilage formation and degeneration [27]. Unfortunately, the data obtained from these animal models were limited by early lethality from gene deletion and multiple organ changes resulting from transgene activation [13]. Thus, in this study we used normal rats at different postnatal ages to detect the dynamic changes in endogenous β-catenin expression corresponding to articular chondrocyte maturation. Rats were examined from two weeks after birth (when chondrocytes are developing) to eight weeks of age (when rats reach sexual maturity).
The function of β-catenin signalling in normal bone formation and postnatal cartilage development are well established [10, 13, 18]. Chen et al. have reported that inhibition of β-catenin signalling causes defects in postnatal cartilage development [12]. In our study, we found higher expression levels of β-catenin at early stages of cartilage development, which steadily decreased as the rats matured into adulthood. These data suggest that the β-catenin signalling pathway is available and presumably necessary and functioning during normal cartilage development. Since β-catenin expression is down-regulated as development concludes, this signalling pathway is not necessary, at least to the same extent, for maintaining the characteristics of articular cartilage after joint maturation. Further investigations into the regulatory mechanisms of β-catenin in early cartilage development will provide insights into the interacting factors and influences that are critical for proper development.
Given the detrimental and severely confounding effects associated with β-catenin deletion and exogenous gene activation, we used LiCl to activate the β-catenin signalling pathway in primary rat chondrocytes. By this approach, we found that collagen X and MMP-13 expressions were significantly increased following LiCl-induced β-catenin activation. It is well known that articular chondrocytes undergo maturation during early OA pathogenesis, after which the characteristic maturational marker genes collagen X [28–30] and MMP-13 [31, 32] are expressed. MMP-13 is a potent enzyme that preferentially targets type II collagen in the cartilage matrix for degradation; interestingly, MMP-13 expression has been found to be increased in human OA joints [33]. The mouse OA model expressing constitutively active MMP-13 presents with an OA-like phenotype [34, 35]. Other OA-associated factors found to be up-regulated in human cartilage are the ADAMTS family members ADAMTS-4 and ADAMTS-5 [36]. We observed increased mRNA expression of ADAMTS-5, but not ADAMTS-4, in LiCl-treated chondrocytes. It has been reported that ablation of ADAMTS-5 protects cartilage from degeneration in animal models of OA [37–39]. Collectively, our data demonstrate that activation of β-catenin (via LiCl) leads to articular chondrocyte maturation and the OA phenotype in eight week old rats. Some studies have also shown that the OA phenotype can be associated with perturbations in other Wnt-related genes, such as Frzb mutation or LRP5 activation [14, 16, 27]. All of these findings considered along with the fact that human OA cartilage expresses high levels of β-catenin [7, 13, 22] support the idea that activation of β-catenin signalling in normal adult chondrocytes leads to OA. However, in our study we did not find significant differences in the temporal mRNA expressions of MMP-13, collagen X or ADAMTS-5 in normal rat articular cartilage tissue. The high levels of β-catenin expression detected at two weeks of age revealed that activation of β-catenin did not result in an OA chondrocyte phenotype during the stage of early articular cartilage development. We speculate that β-catenin signalling is necessary in early articular cartilage development.
The precise molecular mechanisms that activate β-catenin signalling in adult chondrocytes and lead to the OA phenotype remain unknown. To this end, our study revealed a close relationship between the OA phenotype and high expression levels of MMP-13 and ADAMTS-5. It has been reported that apoptosis represents a principal mechanism of chondrocyte degeneration during OA [40–42]. Likewise, we found that LiCl-induced β-catenin signalling in adult chondrocytes led to increased apoptosis. In future studies, we plan to investigate the regulatory mechanisms that interconnect β-catenin, MMP-13, ADAMTS-5 and the apoptosis event in articular chondrocytes at different maturation stages of articular cartilage development.
In summary, in this study we showed for the fist time that β-catenin signalling is crucial for early stage postnatal articular cartilage development but detrimental in adult articular cartilage, where it leads to an OA-like phenotype. Our studies provide new insights into the roles of β-catenin signalling in normal articular chondrocyte function and OA pathogenesis.
References
- 1.Martel-Pelletier J, Boileau C, Pelletier J, Roughley P. Cartilage in normal and osteoarthritis conditions. Best Pract Res Clin Rheumatol. 2008;22(2):351–384. doi: 10.1016/j.berh.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Goldring MB, Goldring SR. Osteoarthritis. J Cell Physiol. 2007;213(3):626–634. doi: 10.1002/jcp.21258. [DOI] [PubMed] [Google Scholar]
- 3.Silva MA, Yamada N, Clarke NM, Roach HI. Cellular and epigenetic features of a young healthy and a young osteoarthritic cartilage compared with aged control and OA cartilage. J Orthop Res. 2009;27(5):593–601. doi: 10.1002/jor.20799. [DOI] [PubMed] [Google Scholar]
- 4.Brandt KD, Dieppe P, Radin EL. Etiopathogenesis of osteoarthritis. Rheum Dis Clin North Am. 2008;34(3):531–559. doi: 10.1016/j.rdc.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 5.Yano F, Kugimiya F, Ohba S, Ikeda T, Chikuda H, Ogasawara T, Ogata N, Takato T, Nakamura K, Kawaguchi H. The canonical Wnt signaling pathway promotes chondrocyte differentiation in a Sox9-dependent manner. Biochem Biophys Res Commun. 2005;333(4):1300–1308. doi: 10.1016/j.bbrc.2005.06.041. [DOI] [PubMed] [Google Scholar]
- 6.Hill TP, Spater D, Taketo MM, Birchmeier W, Hartmann C. Canonical Wnt/beta-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev Cell. 2005;8(5):727–738. doi: 10.1016/j.devcel.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 7.Yuasa T, Otani T, Koike T, Iwamoto M, Enomoto-Iwamoto M. Wnt/β-catenin signaling stimulates matrix catabolic genes and activity in articular chondrocytes: its possible role in joint degeneration. Lab Invest. 2008;88(3):264–274. doi: 10.1038/labinvest.3700747. [DOI] [PubMed] [Google Scholar]
- 8.Tamamura Y, Otani T, Kanatani N, Koyama E, Kitagaki J, Komori T, Yamada Y, Costantini F, Wakisaka S, Pacifici M, Iwamoto M, Enomoto-Iwamoto M. Developmental regulation of Wnt/beta-catenin signals is required for growth plate assembly, cartilage integrity, and endochondral ossification. J Biol Chem. 2005;280(19):19185–19195. doi: 10.1074/jbc.M414275200. [DOI] [PubMed] [Google Scholar]
- 9.Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423(6937):332–336. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- 10.Day TF, Guo X, Garrett-Beal L, Yang Y. Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell. 2005;8(5):739–750. doi: 10.1016/j.devcel.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 11.Akiyama H, Lyons JP, Mori-Akiyama Y, Yang X, Zhang R, Zhang Z, Deng JM, Taketo MM, Nakamura T, Behringer RR, McCrea PD, Crombrugghe B. Interactions between Sox9 and beta-catenin control chondrocyte differentiation. Genes Dev. 2004;18(9):1072–1087. doi: 10.1101/gad.1171104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen M, Zhu M, Awad H, Li TF, Sheu TJ, Boyce BF, Chen D, O’Keefe RJ. Inhibition of beta-catenin signaling causes defects in postnatal cartilage development. J Cell Sci. 2008;121(Pt 9):1455–1465. doi: 10.1242/jcs.020362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu M, Tang D, Wu Q, Hao S, Chen M, Xie C, Rosier RN, O’Keefe RJ, Zuscik M, Chen D. Activation of beta-catenin signaling in articular chondrocytes leads to osteoarthritis-like phenotype in adult beta-catenin conditional activation mice. J Bone Miner Res. 2009;24(1):12–21. doi: 10.1359/jbmr.080901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Papathanasiou I, Malizos KN, Tsezou A. Low-density lipoprotein receptor-related protein 5 (LRP5) expression in human osteoarthritic chondrocytes. J Orthop Res. 2010;28(3):348–353. doi: 10.1002/jor.20993. [DOI] [PubMed] [Google Scholar]
- 15.Blom AB, Brockbank SM, Lent PL, Beuningen HM, Geurts J, Takahashi N, Kraan PM, Loo FA, Schreurs BW, Clements K, Newham P, Berg WB. Involvement of the Wnt signaling pathway in experimental and human osteoarthritis: prominent role of Wnt-induced signaling protein 1. Arthritis Rheum. 2009;60(2):501–512. doi: 10.1002/art.24247. [DOI] [PubMed] [Google Scholar]
- 16.Jones SE, Jomary C. Secreted Frizzled-related proteins: searching for relationships and patterns. Bioessays. 2002;24(9):811–820. doi: 10.1002/bies.10136. [DOI] [PubMed] [Google Scholar]
- 17.Lories RJ, Peeters J, Bakker A, Tylzanowski P, Derese I, Schrooten J, Thomas JT, Luyten FP. Articular cartilage and biomechanical properties of the long bones in Frzb-knockout mice. Arthritis Rheum. 2007;56(12):4095–4103. doi: 10.1002/art.23137. [DOI] [PubMed] [Google Scholar]
- 18.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127(3):469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 19.Li J, Khavandgar Z, Lin SH, Murshed M. Lithium chloride attenuates BMP-2 signaling and inhibits osteogenic differentiation through a novel WNT/GSK3- independent mechanism. Bone. 2011;48(2):321–331. doi: 10.1016/j.bone.2010.09.033. [DOI] [PubMed] [Google Scholar]
- 20.Hiyama A, Sakai D, Risbud MV, Tanaka M, Arai F, Abe K, Mochida J. Enhancement of intervertebral disc cell senescence by WNT/beta-catenin signaling-induced matrix metalloproteinase expression. Arthritis Rheum. 2010;62(10):3036–3047. doi: 10.1002/art.27599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yano F, Kugimiya F, Ohba S, Ikeda T, Chikuda H, Ogasawara T, Ogata N, Takato T, Nakamura K, Kawaguchi H, Chung UI. The canonical Wnt signaling pathway promotes chondrocyte differentiation in a Sox9-dependent manner. Biochem Biophys Res Commun. 2005;333(4):1300–1308. doi: 10.1016/j.bbrc.2005.06.041. [DOI] [PubMed] [Google Scholar]
- 22.Wu Q, Zhu M, Rosier RN, Zuscik MJ, O’Keefe RJ, Chen D. β-catenin, cartilage, and osteoarthritis. Ann N Y Acad Sci. 2010;1192(1):344–350. doi: 10.1111/j.1749-6632.2009.05212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu Q, Huang JH, Sampson ER, Kim K-O, Zuscik MJ, O’Keefe RJ, Chen D, Rosier RN. Smurf2 induces degradation of GSK-3β and upregulates β-catenin in chondrocytes: a potential mechanism for Smurf2-induced degeneration of articular cartilage. Exp Cell Res. 2009;315(14):2386–2398. doi: 10.1016/j.yexcr.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borovecki F, Pecina-Slaus N, Vukicevic S. Biological mechanisms of bone and cartilage remodelling–genomic perspective. Int Orthop. 2007;31(6):799–805. doi: 10.1007/s00264-007-0408-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loughlin J. Genome studies and linkage in primary osteoarthritis. Rheum Dis Clin North Am. 2002;28(1):95–109. doi: 10.1016/S0889-857X(03)00071-1. [DOI] [PubMed] [Google Scholar]
- 26.Spector TD, MacGregor AJ (2004) Risk factors for osteoarthritis: genetics. Osteoarthritis Cartilage 12 Suppl A:S39–S44 [DOI] [PubMed]
- 27.Yuasa T, Kondo N, Yasuhara R, Shimono K, Mackem S, Pacifici M, Iwamoto M, Enomoto-Iwamoto M. Transient activation of Wnt/{beta}-catenin signaling induces abnormal growth plate closure and articular cartilage thickening in postnatal mice. Am J Pathol. 2009;175(5):1993–2003. doi: 10.2353/ajpath.2009.081173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dao DY, Yang X, Flick LM, Chen D, Hilton MJ, O’Keefe RJ. Axin2 regulates chondrocyte maturation and axial skeletal development. J Orthop Res. 2010;28(1):89–95. doi: 10.1002/jor.20954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walker GD, Fischer M, Gannon J, Thompson RC, Jr, Oegema TR., Jr Expression of type-X collagen in osteoarthritis. J Orthop Res. 1995;13(1):4–12. doi: 10.1002/jor.1100130104. [DOI] [PubMed] [Google Scholar]
- 30.Cancel M, Grimard G, Thuillard-Crisinel D, Moldovan F, Villemure I. Effects of in vivo static compressive loading on aggrecan and type II and X collagens in the rat growth plate extracellular matrix. Bone. 2009;44(2):306–315. doi: 10.1016/j.bone.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 31.Meyer RA, Jr, Meyer MH, Ashraf N, Frick S. Changes in mRNA gene expression during growth in the femoral head of the young rat. Bone. 2007;40(6):1554–1564. doi: 10.1016/j.bone.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 32.Kirsch T, Swoboda B, Nah H. Activation of annexin II and V expression, terminal differentiation, mineralization and apoptosis in human osteoarthritic cartilage. Osteoarthritis Cartilage. 2000;8(4):294–302. doi: 10.1053/joca.1999.0304. [DOI] [PubMed] [Google Scholar]
- 33.Orlandi A, Oliva F, Taurisano G, Candi E, Lascio A, Melino G, Spagnoli LG, Tarantino U. Transglutaminase-2 differently regulates cartilage destruction and osteophyte formation in a surgical model of osteoarthritis. Amino Acids. 2008;36(4):755–763. doi: 10.1007/s00726-008-0129-3. [DOI] [PubMed] [Google Scholar]
- 34.Moldovan F, Pelletier JP, Hambor J, Cloutier JM, Martel-Pelletier J. Collagenase-3 (matrix metalloprotease 13) is preferentially localized in the deep layer of human arthritic cartilage in situ: in vitro mimicking effect by transforming growth factor beta. Arthritis Rheum. 1997;40(9):1653–1661. doi: 10.1002/art.1780400915. [DOI] [PubMed] [Google Scholar]
- 35.Wang X, Manner PA, Horner A, Shum L, Tuan RS, Nuckolls GH. Regulation of MMP-13 expression by RUNX2 and FGF2 in osteoarthritic cartilage. Osteoarthritis Cartilage. 2004;12(12):963–973. doi: 10.1016/j.joca.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 36.Plaas A, Osborn B, Yoshihara Y, Bai Y, Bloom T, Nelson F, Mikecz K, Sandy JD. Aggrecanolysis in human osteoarthritis: confocal localization and biochemical characterization of ADAMTS5-hyaluronan complexes in articular cartilages. Osteoarthritis Cartilage. 2007;15(7):719–734. doi: 10.1016/j.joca.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 37.Stanton H, Rogerson FM, East CJ, Golub SB, Lawlor KE, Meeker CT, Little CB, Last K, Farmer PJ, Campbell IK, Fourie AM, Fosang AJ. ADAMTS5 is the major aggrecanase in mouse cartilage in vivo and in vitro. Nature. 2005;434(7033):648–652. doi: 10.1038/nature03417. [DOI] [PubMed] [Google Scholar]
- 38.Malfait AM, Ritchie J, Gil AS, Austin JS, Hartke J, Qin W, Tortorella MD, Mogil JS. ADAMTS-5 deficient mice do not develop mechanical allodynia associated with osteoarthritis following medial meniscal destabilization. Osteoarthritis Cartilage. 2010;18(4):572–580. doi: 10.1016/j.joca.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 39.Glasson SS, Askew R, Sheppard B, Carito B, Blanchet T, Ma HL, Flannery CR, Peluso D, Kanki K, Yang Z, Majumdar MK, Morris EA. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature. 2005;434(7033):644–648. doi: 10.1038/nature03369. [DOI] [PubMed] [Google Scholar]
- 40.Zhong M, Carney DH, Jo H, Boyan BD, Schwartz Z. Inorganic phosphate induces mammalian growth plate chondrocyte apoptosis in a mitochondrial pathway involving nitric oxide and JNK MAP kinase. Calcif Tissue Int. 2011;88(2):96–108. doi: 10.1007/s00223-010-9433-5. [DOI] [PubMed] [Google Scholar]
- 41.Zhu M, Chen M, Zuscik M, Wu Q, Wang YJ, Rosier RN, O’Keefe RJ, Chen D. Inhibition of beta-catenin signaling in articular chondrocytes results in articular cartilage destruction. Arthritis Rheum. 2008;58(7):2053–2064. doi: 10.1002/art.23614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo H, Luo Q, Zhang J, Lin H, Xia L, He C (2011) Comparing different physical factors on serum TNF-alpha levels, chondrocyte apoptosis, caspase-3 and caspase-8 expression in osteoarthritis of the knee in rabbits. Joint Bone Spine. Epub ahead of print [DOI] [PubMed]