Abstract
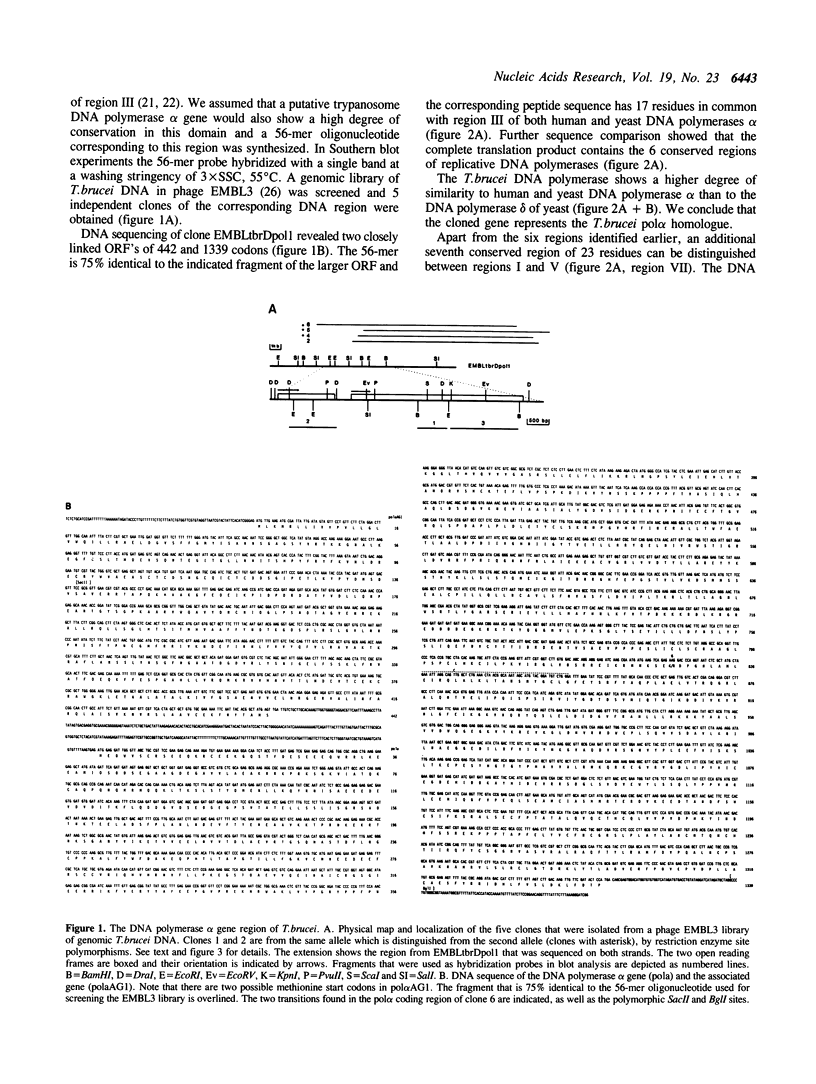
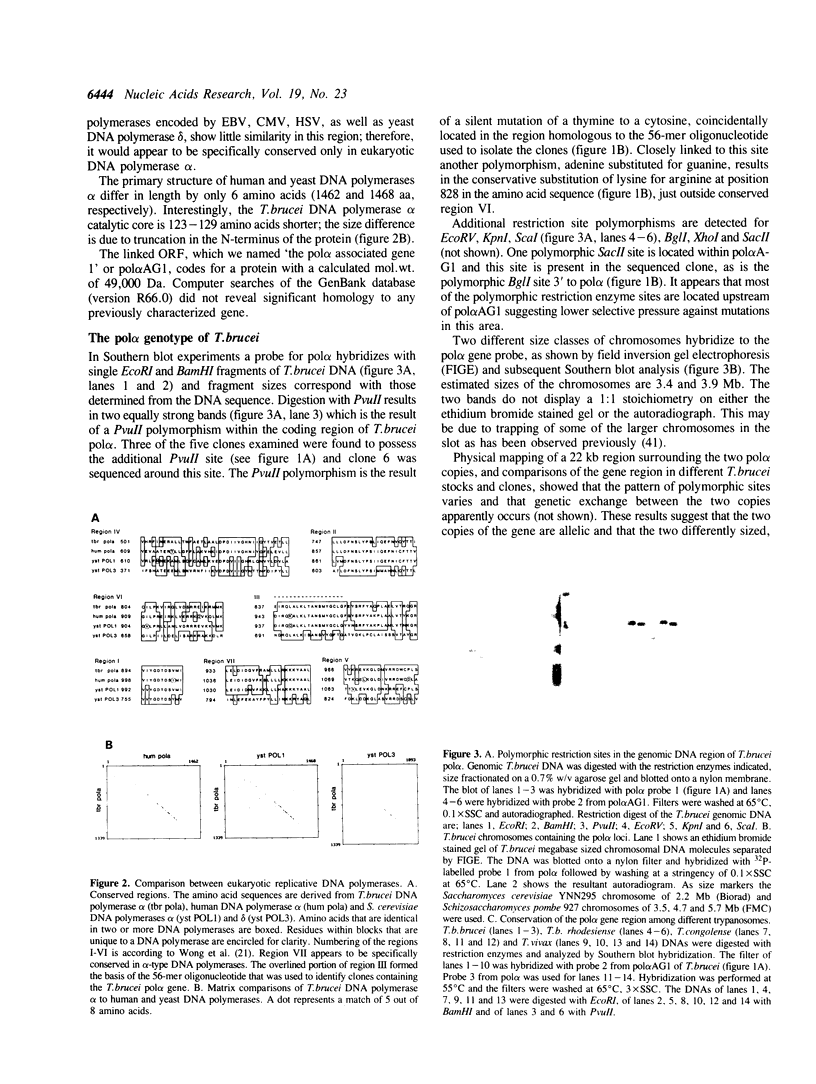
As an initial step towards the characterization of replicative DNA polymerases of trypanosomes, we have cloned, sequenced and examined the expression of the Trypanosoma (Trypanozoon) brucei brucei gene that encodes the DNA polymerase alpha catalytic core (pol alpha). The protein sequence contains the six conserved regions that have been recognized previously in eukaryotic and viral replicative DNA polymerases. In addition, we have identified a seventh region which appears to be conserved primarily in alpha-type DNA polymerases. The T.brucei DNA pol alpha core N-terminus is 123 and 129 amino acids smaller than that of the human and yeast homologue, respectively. The gene is separated by 386 bp from an upstream open reading frame (ORF) of 442 codons. Stable transcripts of the upstream sequence are detected in both dividing and non-dividing forms, while pol alpha transcripts are detected principally in dividing forms. Allelic copies of the T.brucei pol alpha region exhibit restriction site polymorphisms; one such sequence polymorphism affects the amino acid sequence of the T.brucei DNA pol alpha core. The T.brucei pol alpha region cross-hybridizes weakly with that of T.(Nannomonas) congolense and T.(Duttonella) vivax.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aboagye-Kwarteng T., ole-MoiYoi O. K., Lonsdale-Eccles J. D. Phosphorylation differences among proteins of bloodstream developmental stages of Trypanosoma brucei brucei. Biochem J. 1991 Apr 1;275(Pt 1):7–14. doi: 10.1042/bj2750007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer G. A., Heller H. M., Burgers P. M. DNA polymerase III from Saccharomyces cerevisiae. I. Purification and characterization. J Biol Chem. 1988 Jan 15;263(2):917–924. [PubMed] [Google Scholar]
- Borst P. Discontinuous transcription and antigenic variation in trypanosomes. Annu Rev Biochem. 1986;55:701–732. doi: 10.1146/annurev.bi.55.070186.003413. [DOI] [PubMed] [Google Scholar]
- Boulet A., Simon M., Faye G., Bauer G. A., Burgers P. M. Structure and function of the Saccharomyces cerevisiae CDC2 gene encoding the large subunit of DNA polymerase III. EMBO J. 1989 Jun;8(6):1849–1854. doi: 10.1002/j.1460-2075.1989.tb03580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun R., Schönenberger Cultivation and in vitro cloning or procyclic culture forms of Trypanosoma brucei in a semi-defined medium. Short communication. Acta Trop. 1979 Sep;36(3):289–292. [PubMed] [Google Scholar]
- Burgers P. M., Bambara R. A., Campbell J. L., Chang L. M., Downey K. M., Hübscher U., Lee M. Y., Linn S. M., So A. G., Spadari S. Revised nomenclature for eukaryotic DNA polymerases. Eur J Biochem. 1990 Aug 17;191(3):617–618. doi: 10.1111/j.1432-1033.1990.tb19165.x. [DOI] [PubMed] [Google Scholar]
- Burgers P. M. Eukaryotic DNA polymerases alpha and delta: conserved properties and interactions, from yeast to mammalian cells. Prog Nucleic Acid Res Mol Biol. 1989;37:235–280. doi: 10.1016/s0079-6603(08)60700-x. [DOI] [PubMed] [Google Scholar]
- Burgers P. M. Mammalian cyclin/PCNA (DNA polymerase delta auxiliary protein) stimulates processive DNA synthesis by yeast DNA polymerase III. Nucleic Acids Res. 1988 Jul 25;16(14A):6297–6307. doi: 10.1093/nar/16.14.6297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes J. J., Downey K. M., Black V. L., So A. G. A new mammalian DNA polymerase with 3' to 5' exonuclease activity: DNA polymerase delta. Biochemistry. 1976 Jun 29;15(13):2817–2823. doi: 10.1021/bi00658a018. [DOI] [PubMed] [Google Scholar]
- Campbell J. L. Eukaryotic DNA replication. Annu Rev Biochem. 1986;55:733–771. doi: 10.1146/annurev.bi.55.070186.003505. [DOI] [PubMed] [Google Scholar]
- Campbell J. Eukaryotic DNA replication: yeast bares its ARSs. Trends Biochem Sci. 1988 Jun;13(6):212–217. doi: 10.1016/0968-0004(88)90086-2. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cross G. A. Cellular and genetic aspects of antigenic variation in trypanosomes. Annu Rev Immunol. 1990;8:83–110. doi: 10.1146/annurev.iy.08.040190.000503. [DOI] [PubMed] [Google Scholar]
- De Lange T., Liu A. Y., Van der Ploeg L. H., Borst P., Tromp M. C., Van Boom J. H. Tandem repetition of the 5' mini-exon of variant surface glycoprotein genes: a multiple promoter for VSG gene transcription? Cell. 1983 Oct;34(3):891–900. doi: 10.1016/0092-8674(83)90546-9. [DOI] [PubMed] [Google Scholar]
- Evans R. M., Hollenberg S. M. Zinc fingers: gilt by association. Cell. 1988 Jan 15;52(1):1–3. doi: 10.1016/0092-8674(88)90522-3. [DOI] [PubMed] [Google Scholar]
- Evers R., Hammer A., Köck J., Jess W., Borst P., Mémet S., Cornelissen A. W. Trypanosoma brucei contains two RNA polymerase II largest subunit genes with an altered C-terminal domain. Cell. 1989 Feb 24;56(4):585–597. doi: 10.1016/0092-8674(89)90581-3. [DOI] [PubMed] [Google Scholar]
- Gibbs J. S., Chiou H. C., Bastow K. F., Cheng Y. C., Coen D. M. Identification of amino acids in herpes simplex virus DNA polymerase involved in substrate and drug recognition. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6672–6676. doi: 10.1073/pnas.85.18.6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson W. C., Borst P. Size-fractionation of the small chromosomes of Trypanozoon and Nannomonas trypanosomes by pulsed field gradient gel electrophoresis. Mol Biochem Parasitol. 1986 Feb;18(2):127–140. doi: 10.1016/0166-6851(86)90033-2. [DOI] [PubMed] [Google Scholar]
- Gibson W. C., Miles M. A. The karyotype and ploidy of Trypanosoma cruzi. EMBO J. 1986 Jun;5(6):1299–1305. doi: 10.1002/j.1460-2075.1986.tb04359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaguni L. S., Lehman I. R. Eukaryotic DNA polymerase-primase: structure, mechanism and function. Biochim Biophys Acta. 1988 Jul 13;950(2):87–101. doi: 10.1016/0167-4781(88)90001-2. [DOI] [PubMed] [Google Scholar]
- Knopf C. W. The herpes simplex virus type 1 DNA polymerase gene: site of phosphonoacetic acid resistance mutation in strain Angelotti is highly conserved. J Gen Virol. 1987 May;68(Pt 5):1429–1433. doi: 10.1099/0022-1317-68-5-1429. [DOI] [PubMed] [Google Scholar]
- Kouzarides T., Bankier A. T., Satchwell S. C., Weston K., Tomlinson P., Barrell B. G. Sequence and transcription analysis of the human cytomegalovirus DNA polymerase gene. J Virol. 1987 Jan;61(1):125–133. doi: 10.1128/jvi.61.1.125-133.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanham S. M., Godfrey D. G. Isolation of salivarian trypanosomes from man and other mammals using DEAE-cellulose. Exp Parasitol. 1970 Dec;28(3):521–534. doi: 10.1016/0014-4894(70)90120-7. [DOI] [PubMed] [Google Scholar]
- Larder B. A., Kemp S. D., Darby G. Related functional domains in virus DNA polymerases. EMBO J. 1987 Jan;6(1):169–175. doi: 10.1002/j.1460-2075.1987.tb04735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathe R. Synthetic oligonucleotide probes deduced from amino acid sequence data. Theoretical and practical considerations. J Mol Biol. 1985 May 5;183(1):1–12. doi: 10.1016/0022-2836(85)90276-1. [DOI] [PubMed] [Google Scholar]
- Lee M. Y., Tan C. K., Downey K. M., So A. G. Further studies on calf thymus DNA polymerase delta purified to homogeneity by a new procedure. Biochemistry. 1984 Apr 24;23(9):1906–1913. doi: 10.1021/bi00304a003. [DOI] [PubMed] [Google Scholar]
- Lucchini G., Mazza C., Scacheri E., Plevani P. Genetic mapping of the Saccharomyces cerevisiae DNA polymerase I gene and characterization of a pol1 temperature-sensitive mutant altered in DNA primase-polymerase complex stability. Mol Gen Genet. 1988 Jun;212(3):459–465. doi: 10.1007/BF00330850. [DOI] [PubMed] [Google Scholar]
- Lumsden W. H., Herbert W. J. Pedigrees of the Edinburgh Trypanosoma (Trypanozoon) antigenic types (ETat). Trans R Soc Trop Med Hyg. 1975;69(2):205–208. doi: 10.1016/0035-9203(75)90156-x. [DOI] [PubMed] [Google Scholar]
- Majiwa P. A., Matthyssens G., Williams R. O., Hamers R. Cloning and analysis of Trypanosoma (Nannomonas) congolense ILNat 2.1 VSG gene. Mol Biochem Parasitol. 1985 Jun;16(1):97–108. doi: 10.1016/0166-6851(85)90052-0. [DOI] [PubMed] [Google Scholar]
- Matthews J. T., Carroll R. D., Stevens J. T., Haffey M. L. In vitro mutagenesis of the herpes simplex virus type 1 DNA polymerase gene results in altered drug sensitivity of the enzyme. J Virol. 1989 Nov;63(11):4913–4918. doi: 10.1128/jvi.63.11.4913-4918.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E. N., Turner M. J. Analysis of antigenic types appearing in first relapse populations of clones of Trypanosoma brucei. Parasitology. 1981 Feb;82(1):63–80. doi: 10.1017/s0031182000041871. [DOI] [PubMed] [Google Scholar]
- Nantulya V. M., Musoke A. J., Rurangirwa F. R., Saigar N., Minja S. H. Monoclonal antibodies that distinguish Trypanosoma congolense, T. vivax and T. brucei. Parasite Immunol. 1987 Jul;9(4):421–431. doi: 10.1111/j.1365-3024.1987.tb00520.x. [DOI] [PubMed] [Google Scholar]
- Pays E., Steinert M. Control of antigen gene expression in African trypanosomes. Annu Rev Genet. 1988;22:107–126. doi: 10.1146/annurev.ge.22.120188.000543. [DOI] [PubMed] [Google Scholar]
- Pizzagalli A., Valsasnini P., Plevani P., Lucchini G. DNA polymerase I gene of Saccharomyces cerevisiae: nucleotide sequence, mapping of a temperature-sensitive mutation, and protein homology with other DNA polymerases. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3772–3776. doi: 10.1073/pnas.85.11.3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prelich G., Tan C. K., Kostura M., Mathews M. B., So A. G., Downey K. M., Stillman B. Functional identity of proliferating cell nuclear antigen and a DNA polymerase-delta auxiliary protein. Nature. 1987 Apr 2;326(6112):517–520. doi: 10.1038/326517a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro S. Z., Kimmel B. E. Differential protein synthesis during the life cycle of the protozoan parasite Trypanosoma brucei. J Protozool. 1987 Feb;34(1):58–62. doi: 10.1111/j.1550-7408.1987.tb03132.x. [DOI] [PubMed] [Google Scholar]
- Shapiro S. Z., Naessens J., Liesegang B., Moloo S. K., Magondu J. Analysis by flow cytometry of DNA synthesis during the life cycle of African trypanosomes. Acta Trop. 1984 Dec;41(4):313–323. [PubMed] [Google Scholar]
- So A. G., Downey K. M. Mammalian DNA polymerases alpha and delta: current status in DNA replication. Biochemistry. 1988 Jun 28;27(13):4591–4595. doi: 10.1021/bi00413a001. [DOI] [PubMed] [Google Scholar]
- Stillman B. Initiation of eukaryotic DNA replication in vitro. Annu Rev Cell Biol. 1989;5:197–245. doi: 10.1146/annurev.cb.05.110189.001213. [DOI] [PubMed] [Google Scholar]
- Tan C. K., Castillo C., So A. G., Downey K. M. An auxiliary protein for DNA polymerase-delta from fetal calf thymus. J Biol Chem. 1986 Sep 15;261(26):12310–12316. [PubMed] [Google Scholar]
- Tsurimoto T., Melendy T., Stillman B. Sequential initiation of lagging and leading strand synthesis by two different polymerase complexes at the SV40 DNA replication origin. Nature. 1990 Aug 9;346(6284):534–539. doi: 10.1038/346534a0. [DOI] [PubMed] [Google Scholar]
- Tsurumi T., Maeno K., Nishiyama Y. A single-base change within the DNA polymerase locus of herpes simplex virus type 2 can confer resistance to aphidicolin. J Virol. 1987 Feb;61(2):388–394. doi: 10.1128/jvi.61.2.388-394.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Ploeg L. H., Schwartz D. C., Cantor C. R., Borst P. Antigenic variation in Trypanosoma brucei analyzed by electrophoretic separation of chromosome-sized DNA molecules. Cell. 1984 May;37(1):77–84. doi: 10.1016/0092-8674(84)90302-7. [DOI] [PubMed] [Google Scholar]
- Vickerman K. Developmental cycles and biology of pathogenic trypanosomes. Br Med Bull. 1985 Apr;41(2):105–114. doi: 10.1093/oxfordjournals.bmb.a072036. [DOI] [PubMed] [Google Scholar]
- Wang T. S., Wong S. W., Korn D. Human DNA polymerase alpha: predicted functional domains and relationships with viral DNA polymerases. FASEB J. 1989 Jan;3(1):14–21. doi: 10.1096/fasebj.3.1.2642867. [DOI] [PubMed] [Google Scholar]
- Wong S. W., Wahl A. F., Yuan P. M., Arai N., Pearson B. E., Arai K., Korn D., Hunkapiller M. W., Wang T. S. Human DNA polymerase alpha gene expression is cell proliferation dependent and its primary structure is similar to both prokaryotic and eukaryotic replicative DNA polymerases. EMBO J. 1988 Jan;7(1):37–47. doi: 10.1002/j.1460-2075.1988.tb02781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]