Abstract
In the developing nervous system, individual neurons must occupy appropriate positions within circuits. This requires that these neurons recognize and form connections with specific pre- and postsynaptic partners. Cellular recognition is also required for the spacing of cell bodies and the arborization of dendrites, factors that determine the inputs onto a given neuron. These issues are particularly evident in the retina, where different types of neurons are evenly spaced relative to other cells of the same type. This establishes a reiterated columnar circuitry resembling the insect retina. Establishing these mosaic patterns requires that cells of a given type (homotypic cells) be able to sense their neighbors. Therefore, both synaptic specificity and mosaic spacing require cellular identifiers. In synaptic specificity, recognition often occurs between different types of cells in a pre- and postsynaptic pairing. In mosaic spacing, recognition is often occurring between different cells of the same type, or homotypic self-recognition. Dendritic arborization can require recognition of different neurites of the same cell, or isoneuronal self-recognition. The retina is an extremely amenable system for studying the molecular identifiers that drive these various forms of recognition. The different neuronal types in the retina are well defined, and the genetic tools for marking cell types are increasingly available.
In this review we will summarize retinal anatomy and describe cell types in the retina and how they are defined. We will then describe the requirements of a recognition code and discuss newly emerging candidate molecular mechanisms for recognition that may meet these requirements.
Keywords: Dscam, clustered protocadherin, cell adhesion, self-avoidance, synaptic specificity, laminar specificity
Introduction
Establishing cell identity and defining cell types in the developing nervous system is a commonly studied topic, but it is important to consider that cell identity can be defined in different ways, and these differences impact why and how it is studied, and what its purpose may be. The retina provides an excellent system in which to address this, because the final anatomy and circuitry of the adult are quite well established, even in more complicated mammalian systems. Even in a single system such as the retina, several definitions of cell identity and cell-type are available, but researchers are increasingly able to integrate different definitions. Neurons can be categorized functionally; for example, retinal ganglion cells that respond to light (ON cells), to dark (OFF cells), or to motion in a specific directions (direction selective DS cells). Neurons can also be defined anatomically; for example ON bipolar cells or OFF bipolar cells that project to specific synaptic laminae of the inner plexiform layer. Finally, neurons can be identified based on markers. In some cases these markers directly relate to function, such as tyrosine hydroxylase labeling dopaminergic amacrine cells or choline acetyltransferase labeling cholinergic amacrine cells. In other cases the relationship to function is less clear, such as Synaptotagmin2, a synaptic vesicle protein, labeling a specific class of cone bipolar cells. Whether defined functionally, anatomically, or with markers, the identity of a cell represents its terminally differentiated state and therefore its place in the functional circuitry of the retina. In the vertebrate retina, the two main needs for cell identity are establishing the even, mosaic spacing of different cell types within the horizontal plane of the retina and for synaptic specificity, the proper pairing or pre- and postsynaptic cells for functional connectivity, both within the retina and in ganglion cell projections into the brain. The extent to which cell identity contributes to these developmental processes will undoubtedly vary between cell types, depending on factors such as the complexity of the dendritic arbor and axonal trajectory, or the heterogeneity of the surroundings, but it is difficult to imagine a non-random anatomy that does not depend at least in part on a cell's ability to distinguish itself and its homotypic neighbors from other cell types. When identity is finalized during development, what its molecular determinants are, and the extent to which morphology (form) directs function or function determines form is still unclear for most cell types. Encoding cell identity for the purposes of self-recognition during mosaic formation and synaptic specificity requires a molecular code that is sufficiently diverse and specific to allow these processes to proceed in a complicated environment consisting of many cell types. In this review, we will describe the definitions and purposes of cell identity, discuss recent results and emerging tools to help in studies of cell identity, and discuss candidate molecules that may contribute to a cell identity code in the developing nervous system. It is important to remember that there will be many exceptions to the “rules” we are describing, both across species, and between cell types in a single species. This review is intended to speak in generalizations about cell identity, drawing largely on examples from mice and Drosophila, where genetics has provided some insights into mechanisms, and using as examples cell types for which we have the best markers and therefore the most information.
Retinal anatomy and cell types
Simple systems: Drosophila
Simple model organisms such as Drosophila provide a powerful system for studying neurodevelopment, in part because their neuroanatomy is largely “hardwired” and more reproducible than in vertebrate systems (Figure 1A). The Drosophila retina consists of approximately 800 ommatidia, the individual facets that comprise the compound eye. Each ommatidium contains 8 photoreceptors, or R cells, which transduce light into neuronal signals much as vertebrate photoreceptors do. R1 through R6 detect visible light and project axons to the lamina of the fly central nervous system. R7 and R8 detect UV light and project axons through the lamina to the medulla of the fly brain (reviewed in Sanes and Zipursky, 2010). Within each ommatidium, the arrangement of R cells is highly stereotyped, as are the projections of the R cell axons into the central nervous system, and these projections maintain a “Retinotopic” arrangement. The cell fate determination of the R cells is largely a series of inductive events that represents a programmed pattern of differentiation in the eye imaginal disc of the larval fly (Tomlinson, 1990; Rubin, 1989). Therefore, self-recognition is not required to establish cell identities in the fly retina since each ommatidium is isolated from its neighbors and contains only a single R cell of each type. However, heterotypic recognition is required for the synaptic specificity of R cell axonal projections. The R1–6 projections stop in the lamina and form functional connections with specific lamina neurons (L cells), whereas the R7 and R8 projections proceed through the lamina without stopping and form connections in the medulla.
Figure 1. Retinal circuits in Drosophila and vertebrates.
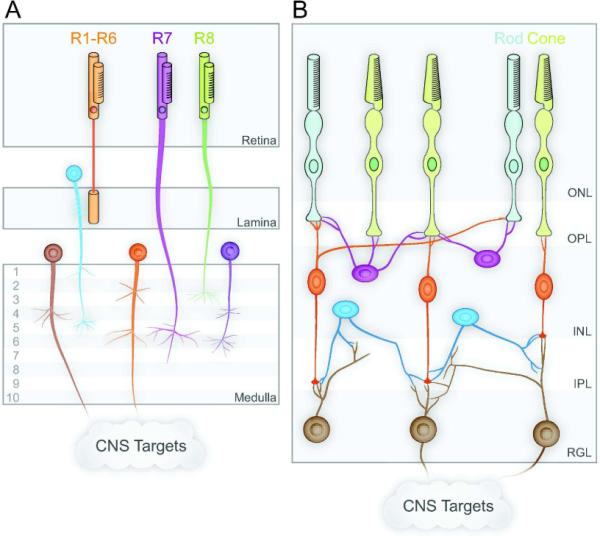
(A) Drosophila visual system in which photoreceptors R1–R6 project to the lamina, R7–R8 as well as lamina neurons project to specific layers of the medulla, and medulla neurons project to central targets. (B) In the vertebrate visual system, rod and cone photoreceptors in the outer nuclear layer (ONL) and bipolar cells (orange) and horizontal cells (purple) in the inner nuclear layer (INL) form synapses in the intervening outer plexiform layer (OPL). Bipolar cells and amacrine cells (blue) synapse with retinal ganglion cells (brown) from the retinal ganglion layer (RGL) in specific laminae of the intervening inner plexiform layer (IPL).
The vertebrate retina
The vertebrate retina has a more complex anatomy than the fly retina (Sanes and Zipursky, 2010). The basic columnar circuitry is largely preserved, but without the distinct anatomical boundaries provided by the ommatidia. The vertebrate retina typically has three cell layers separated by two synaptic plexiform layers. These include an outer layer of photoreceptors (the outer nuclear layer) which synapse onto a middle layer of interneurons (the inner nuclear layer) containing horizontal, bipolar, and amacrine cells, which in turn synapse onto the innermost layer of retinal ganglion cells, which project axons to the brain through the optic nerve (Figure 1B).
However, each of these cell types has multiple subtypes. For example, photoreceptors are comprised of both rods and cones, which differ in their morphology, light sensitivity, and arrangement in the retina. Furthermore, the types and composition of photoreceptor subtypes varies in different regions of the retina and in different species. Therefore, photoreceptors reflect the challenges of discussing cell types, which may have finer distinctions based on anatomy, function, and phylogeny.
Horizontal cells are comparatively uniform; in the mouse retina for example it appears that there is only one sort of horizontal cell, while other species may have more (Fischer et al., 2007). Bipolar cells can be subdivided into those that connect to rods (rod bipolar cells) and those that connect to cones (cone bipolar cells) in the outer plexiform layer (OPL), the synaptic layer separating the outer and inner nuclear layers. Rod bipolar cells are fairly uniform and thus far have defied further subdivision. Cone bipolar cells are more heterogeneous and at least 11 subtypes have been described in mouse (Ghosh et al., 2004; Wassle et al., 2009). Broadly these include “ON” and “OFF” bipolar cells. OFF cells increase their responsiveness when the lights go off and ON cells increase their responsiveness when the lights go on, but more precisely, OFF cells fire when a dark bar of a grating or a dark letter of text falls on the cell's receptive field and ON cells fire when a light bar of a grating or the blank space of a page stimulates the cell's receptive field. Anatomically, OFF cells project to the outer portion of the inner plexiform layer (IPL), nearer to the cell bodies of the inner nuclear layer, where they form synapses with the dendrites of retinal ganglion cells. ON cells project to the inner portion of the inner plexiform layer, nearer the cell bodies of the retinal ganglion cells, where they also form synapses with retinal ganglion cells.
Not surprisingly, there are also several types of retinal ganglion cells. These include those that stratify their dendritic arbors in the OFF laminae of the IPL and therefore receive input from OFF bipolar cells, termed OFF ganglion cells. Others stratify in the ON portion of the IPL and are termed ON ganglion cells. Still others stratify their dendrites in both ON and OFF laminae of the IPL and are therefore termed bistratified ganglion cells, which are often sensitive to motion in a specific direction (direction-selective, bistratified ganglion cells; Barlow et al., 1964; Demb, 2007; Kim et al., 2010; Huberman et al., 2009). Other ganglion cells may be minimally involved in image-forming vision, but function in ambient light detection and circadian entrainment. These are the intrinsically photoresponsive retinal ganglion cells (ipRGCs) that express melanopsin, their own photopigment (Hattar et al., 2002; Provencio et al., 2002). However, it has recently become clear that even melanopsin-expressing ipRGCs have multiple subtypes (Schmidt and Kofuji, 2009; Ecker et al., 2010). While the number of subtypes varies between species and by method used to catalogue them, in total, at least 15 subtypes of retinal ganglion cell have been described in mammals to date (Rockhill et al., 2002; Coombs et al., 2006; Devries and Baylor, 1997).
However, the most diverse cell type in the retina is the amacrine cell, with at least 25 subtypes described to date in rabbit. These subtypes range from abundant AII amacrine cells that function in the rod circuit to rare wide-field amacrine cells, where a single cell has an enormous neurite arbor that spans much of the retina (MacNeil and Masland, 1998).
Defining cell types
In discussing the many types and subtypes of cells in the vertebrate retina, it becomes clear that many different criteria are used to define a cell type. Typically these include functional definitions, anatomical definitions, and histochemical or immunological markers that distinguish different cell types. In cases such as photoreceptors, these definitions are easy to reconcile and integrate. Rods have a different cell morphology than cones (anatomy) and also respond differently to light (saturating in bright light and functioning in low light, whereas cones function in bright light and are not sensitive enough to respond to low light, hence a functional distinction). Furthermore, these functions are dependent on different opsins expressed by each cell type, and these opsins therefore serve as markers of the different cell types and even different subtypes of cones. Therefore, it is easy to correlate functional, anatomical and marker-based definitions of the different photoreceptor types.
This is less easily done for other neuronal types in the retina. For example, melanopsin-expressing ipRGCs initially appeared to be a single type based on the use of melanopsin as a marker of their ability to transduce light signals. However, it is now clear that there are multiple subtypes based on anatomy, including where in the IPL the cells stratify their dendrites and where in the brain they project their axons (Schmidt and Kofuji, 2009; Ecker et al., 2010). Whether all ipRGC subtypes serve a common purpose of detecting ambient light levels and functional differences are determined simply by their CNS targets is under investigation, but the variations in anatomy suggest that such a model will be an over-simplification. Therefore, a single marker, melanopsin, that is probably important for function, intrinsic photoresponsiveness of the ganglion cells, is not demarcating a single anatomical subtype of ganglion cell and probably not a single functional subtype either.
Mosaics and cell types
An additional defining feature of individual cell types in the vertebrate retina is the spacing of the cell somas into a mosaic pattern (Cook, 1998). As described, the functional circuitry of the vertebrate retina depends on an approximation of the columnar circuitry of the insect eye. Achieving this anatomy requires that each “column” contains the appropriate cell types in the appropriate numbers. As a result, neurons of a given type are evenly distributed in their horizontal spacing. Importantly, the dendrites of these neurons may overlap extensively with their homotypic neighbors. This overlap is termed the coverage factor and is the relationship between the area covered by a neuron's neurite arbor and the number of neurons in a given area. The exact rules in establishing mosaic spacing of cell bodies and the coverage factor of dendritic arbors vary with cell type, as some cells are abundant and densely packed whereas others are rare and sparsely distributed (MacNeil and Masland, 1998). Coverage factors also vary with cell type, and even within individuals or mouse strains for a particular cell type, and even when cell density is changed through mutations, a mosaic pattern persists, but with different spacing, reflecting the altered density (Lin et al., 2004; Whitney et al., 2009). Cell body spacing and coverage and shape of the dendritic arbor may be related properties, but they needn't be co-dependent since cell intrinsic mechanisms are probably also involved in establishing dendrite morphology. Cell body spacing of most cell types can be described in terms of an “exclusion zone” around each cell body. This represents a radial distance from a given cell body within which it is very unlikely that the soma of another cell of the same type will be encountered. Statistical tests of this spacing principle include Density Recovery Profiling and nearest neighbor analysis (Rockhill et al., 2000; Rodieck, 1991). Interestingly, some cells have extremely regular and orderly spacing, such as cholinergic starburst amacrine cells, whereas others are nearly random once outside their exclusion zone, such as dopaminergic amacrine cells, if the packing density of the cells allows spacing that exceeds the exclusion zone radius (Galli-Resta, 2002; Keeley and Reese, 2010).
Three mechanisms have been proposed for the formation of mosaic patterns (reviewed in Cook and Chalupa, 2000). Each of these mechanisms is likely to contribute to varying degrees in different cell types and in different species, but conclusive experimental data defining these contributions is lacking and additional mechanisms may also be involved. First, neurons that differentiate early may regulate the cell-fate decisions of later cells, inhibiting the generation of additional homotypic cells and promoting the generation of different cell types. This mechanism is highly analogous to the development of the Drosophila retina (McCabe et al., 1999) and computational modeling in the fish retina indicates it could give rise to an appropriate mosaic pattern (Tyler et al., 2005; Cameron and Carney, 2004). However, it is unclear whether the targets of these inductive signals may be the neuroprogenitors themselves or a population of postmitotic cells that remains plastic, and there is little direct experimental data to support the model. A second mechanism is lateral (tangential) migration of cells to assume their correct position in the mosaic pattern. There is definitive experimental evidence to indicate that retinal neurons do undergo lateral migration; however, the extent to which this migration contributes to mosaic patterning has not been resolved (Reese et al., 1995, 1999; Galli-Resta et al., 1997; Galli-Resta, 2000). The third mechanism is sculpting of mosaic patterns through developmental cell death, in which misplaced or supernumerary neurons are eliminated (Jeyarasasingam et al., 1998; Cusato et al., 2001; Raven et al., 2003). This mechanism may again contribute to mosaic patterning, but even animals such as goldfish or Xenopus have mosaic patterning despite very little ganglion cell death in retinal development (Wilson, 1971, Easter et al., 1981). Newer hypotheses, such as the contribution of mechanical forces to the formation of layers and mosaics in the retina, are attractive but have yet to be extensively examined (Galli-Resta et al., 2008). In sum, the mechanisms by which mosaic patterns in the retina arise are poorly understood.
The production of mosaics by regulation of cell-fate decisions may be less dependent on homotypic recognition; however, the other mechanisms clearly require homotypic recognition, and therefore neurons need a signal capable of telling them of the status of neighboring homotypic cells (Figure 2). Since changes in cell density alter but do not eliminate the mosaic pattern, cells must respond to the uniformity of this signal, and not its absolute intensity (Lin et al., 2004; Keeley and Reese, 2010). Since the dendritic arbors for most amacrine and ganglion cell populations are largely overlapping (coverage factors greater than 1 for most retinal neurons), these signals also presumably function over quite short distances (MacNeil and Masland, 1998; Devries and Baylor, 1997). These signals may be diffusible cues such as ATP, or contact mediated signals such as cell surface proteins (Galli-Resta et al., 2008, 2002), and this again may vary with cell type.
Figure 2. Recognition events requiring cell identity.
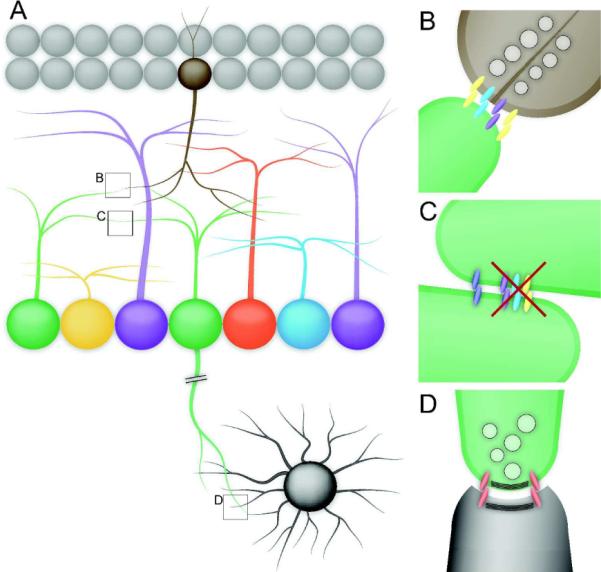
A neuron's identity specifies its proper position in a circuit. Neurons in the retina must recognize appropriate heterotypic cells in order to form functional connections within the retina (B) and in central targets (D). They must also recognize homotypic cells in order to maintain mosaic spacing (C). These recognition events require cell-type specific molecules, and the molecules required for each type of recognition may be different. Therefore, homotypic cells share a repertoire of cell-type specific recognition and adhesion molecules. At interactions between homotypic cells, inappropriate adhesions must masked to prevent excessive fasciculation and to maintain proper dendrite arborization.
Laminar Specificity
In addition to the mosaic spacing of homotypic cell bodies in the horizontal plane of the retina, cells also have a vertical laminar specificity, both for the position of the cell soma and also for the stratification of the processes. For example, dopaminergic amacrine cell bodies are always found at the bottom of the inner nuclear layer, immediately adjacent to the inner plexiform layer. The neurites of these cells arborize horizontally, but are largely constrained vertically and stratify just below the somas in the inner plexiform layer. Some cell types have more tightly defined laminae in which they stratify their processes than others, but this is considered a defining characteristic of cell types. For example, the 11 types of cone bipolar cells can be defined not just by markers, but also anatomically by where their axons terminate in the inner plexiform layer (Haverkamp and Wässle, 2000; Ghosh et al., 2004; Wassle et al., 2009). Processes of heterotypic cells that occupy a single lamina in the inner plexiform layer often form synapses. This is most readily conceptualized for the axons of bipolar cells and the dendrites of ganglion cells; however, the amacrine cells also constitute both pre- and postsynaptic partners in the inner plexiform layer. Therefore, presumably, the heterotypic cell identifiers that prescribe synaptic specificity are also enriched in specific laminae of the inner plexiform layer (Figure 2). However, there are also cell intrinsic signals that help determine dendrite morphology (Moore et al., 2002; Grueber et al., 2003; Sugimura et al., 2004; Li et al., 2004; Kim et al., 2006; Jan and Jan, 2010; Hattori et al., 2007b). It is worth considering whether the same molecular identifiers that drive synaptic specificity are necessarily driving laminar specificity, or if the laminar specificity results from a de facto segregation of processes that are destined to connect and find the appropriate lamina through cell intrinsic pathways that determine their morphology. In zebrafish, ganglion cells stratify their dendrites in appropriate laminae with preexisting amacrine neurites (Mumm et al., 2006). Therefore, at least the amacrine cells must have stratified their processes in the absence of cues from their major synaptic targets. The elimination of supernumerary processes and connections may also refine lamination. The extent to which ganglion cells stratify precisely from the outset versus refine their laminar specificity during development varies between different cell types (Kim et al., 2010). The ability to misdirect lamination through the expression of putative cell surface identifiers would argue that cell surface proteins such as adhesion molecules are indeed instructive and therefore directing lamination (Yamagata and Sanes, 2008). Alternatively, the preservation of functional synaptic connectivity in the absence of these proteins and the preservation of synaptic specificity even when laminar specificity is lost would argue that the two processes can be separated (Fuerst et al., 2009; Matsuoka et al., 2011).
Dendrite arborization
We have discussed the requirement of cell identity and recognition in heterotypic populations of cells for synaptic and laminar specificity and self-recognition between homotypic cells for mosaic formation. However, self-recognition among the neurites of a single cell is also important for dendrite arborization. As most neurons are extending neurites, these processes need to avoid one another to establish an arbor as opposed to an intertwined mass of dendrites. Many points of cell morphology are determined by cell-intrinsic signals, but ordering complex arbors also requires self-recognition and self-avoidance, as demonstrated by recent studies of Drosophila sensory neurons (Soba et al., 2007; Hughes et al., 2007; Matthews et al., 2007).
Adhesion and anti-adhesion
Self-recognition and synaptic specificity are largely examined in terms of cell adhesion signals that positively regulate which cells should connect. However, neurodevelopment cannot occur only through adhesive mechanisms, even with differing affinities, without some means of preventing inappropriate adhesion. If cell-surface-associated adhesion molecules are shared between homotypic cells and between these cells and their heterotypic synaptic partners, then normal synaptic connectivity needs to be promoted whereas aberrant adhesion such as fasciculation needs to be prevented (Figure 2C). Molecular mechanisms of self-avoidance are also emerging alongside candidate mechanisms for adhesion (Millard and Zipursky, 2008; Fuerst and Burgess, 2009).
Ultimately then it would seem that all of the scenarios above may be simultaneously in play. Cell identity is needed for recognition of homotypic neighbors and appropriate mosaic spacing of cell bodies. It is also needed for heterotypic recognition for synaptic specificity and laminar specificity. But are the molecular identifiers the same for all of these processes and are the same mechanisms used? In considering this, it seems likely that synaptic specificity is the most fundamentally important of these processes. Not connecting to the right partner has a deleterious impact on the function of a circuit. The other processes, horizontal mosaic formation and vertical laminar stratification, may simply serve to optimize the connectivity that is driven by the more basic code for synaptic specificity. In order to maximize the likelihood of pre- and postsynaptic partners encountering one another, their processes should costratify in the inner plexiform layer. If they do not, they may still form functional connections when they do encounter one another, but the number of functional connections may be markedly reduced. Similarly, mosaic spacing would serve to optimize a cell connecting with the correct number of appropriate pre- or postsynaptic partners, but the spacing itself may not be essential for the formation of these connections (Figure 3). The test of this will be to examine each parameter, synaptic specificity, laminar specificity, and mosaic spacing, in a system where one is perturbed and the others are largely intact. To date this has been a challenge, particularly for synaptic specificity, where a mutant that selectively disrupts the fidelity of synaptic connectivity has not been identified. As discussed below, mutations are now emerging that disrupt mosaic spacing, or more precisely, fail to maintain mosaic spacing, and these same mutations may also impact laminar specificity. As one might predict, these mutations also reduce the number of synapses formed, but do not appear to dramatically change the specificity of those connections that do form, although this has not been exhaustively analyzed. Therefore the recognition events that allow the optimization of circuit formation through laminar specificity and mosaic spacing may be related, but the basic machinery driving the formation of synapses may be distinct from the mechanisms driving the optimization.
Figure 3. Cellular strategies to optimize synaptic specificity.
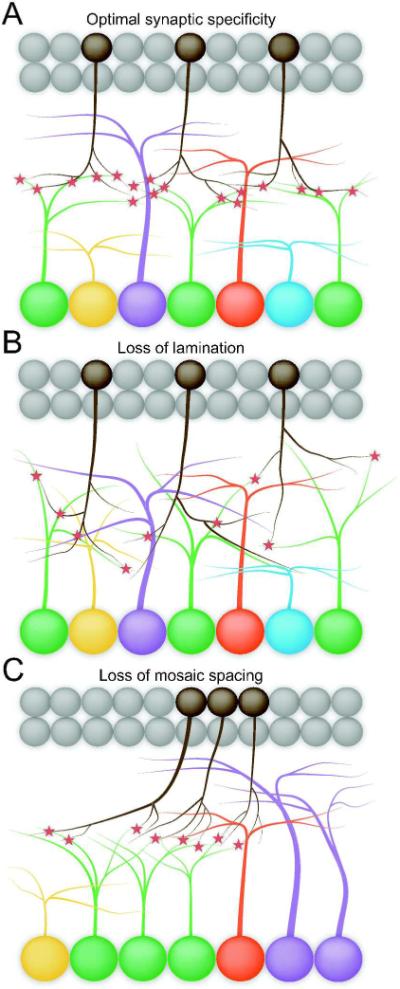
Forming synaptic connections between appropriate partners is fundamental to neural circuit formation and is a key purpose of cell type identity. Two features of retinal organization serve to optimize synaptic specificity. Elaborating processes in specific laminae increases the chances of suitable pre- and postsynaptic partners finding each other, while mosaic spacing optimizes the number of partners available to each neuron (A). Dendritic lamination could be lost without disrupting synaptic specificity per se, although it would decrease the likelihood of partners encountering each other (B). Likewise, specificity could be maintained without mosaic spacing, but individual cells would not be able to partner with the normal number of neurons (C).
Genetic tools for studying cell identity
The ability to easily visualize specific neuronal populations is rapidly advancing, particularly through transgenic labeling techniques in mice. Instead of relying on dye fills or Golgi stains for anatomy, or the serendipitous identification of a cell-type-specific marker, transgenic labeling with easily visualized markers such as GFP or Cre recombinase which can be combined with reporters allows the rapid screening of cell populations (Kim et al., 2008; Huberman et al., 2008, 2009; Siegert et al., 2009). A labeled population is usually considered a distinct cell type if it meets the criteria of having a uniform morphology including dendritic arbor size, stratifcation of the arbor in a specific layer of the retina, and projections to a specific target or targets in the brain in the case of ganglion cells. In addition, the cell bodies should be regularly spaced in a mosaic pattern. The genes identified as having these specific expression patterns can then be tested in other species and the reporters can be used to isolate cells, which can then be further analyzed by expression profiling or transcriptome sequencing. In addition to genetically encoded reporters, genes and mutations that perturb mosaic patterning and laminar specificity are emerging, as described below. It will be interesting to examine the impact of these mutations on synaptic specificity and the function of retinal circuits.
Options for molecular identifiers?
Drosophila
In Drosophila, individual neurons derive identity from stochastic expression of Dscam1 isoforms. Dscam1, a member of the immunoglobulin (Ig) superfamliy of adhesion molecules, promotes neurite repulsion (Zhu et al., 2006; Matthews et al., 2007; Hughes et al., 2007; Soba et al., 2007). What makes Dscam1 particularly well suited to confer cell identity is its extraordinary diversity: The Dscam1 gene contains 4 blocks of alternative exons encoding for 38,016 different proteins with 19,008 distinct extracellular domains (Figure 4A). A given cell expresses 10–50 isoforms, each of which is remarkably precise in its preference for isoform specific homophilic trans interactions (Wojtowicz et al., 2004, 2007; Neves et al., 2004). As a result, each neuron has an individual Dscam1 signature causing repulsion of isoneural processes while allowing interactions between the neurites of separate neurons. The neurites of neurons mutant for Dscam1 fail to diverge from each other to develop the appropriate morphology (reviewed in Zipursky and Sanes, 2010).
Figure 4. Drosophila Dscams and vertebrate Protocadherins provide diverse recognition signals.
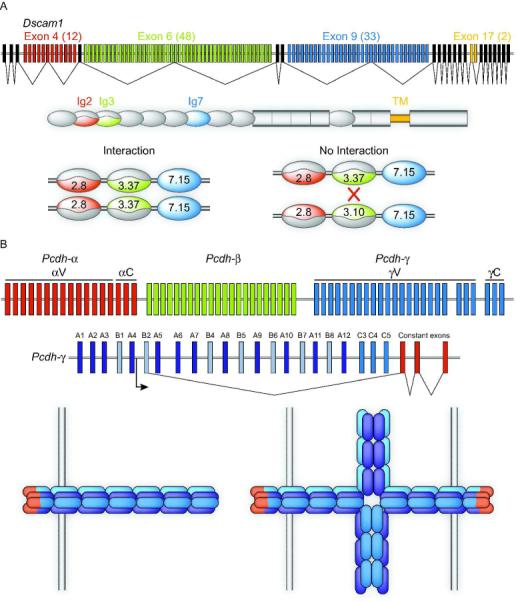
The Drosophila Dscam1 locus contains four blocks of alternatively spliced exons (A). Random variable exon choice for exon 4 (of which there are 12 choices), exon 6 (48 choices) and exon 9 (33 choices) results in 19,008 possible combinations of Ig domains 2, 3, and 7 respectively. These Ig domains determine the specificity of Dscam interaction such that each isoform will only interact with an identical isoform. If even one of the three variable Ig domains is divergent, then interactions will not occur. Individual Drosophila neurons stochastically express fewer than 50 of the 19008 isoforms, resulting in the ability of a cell to recognize its own processes through homophilic interaction while being invisible to its neighboring neurons. The vertebrate clustered Protocadherin (Pcdh) locus is composed of three clusters of exons, termed α, β, and γ, arrayed back to back (B). Each exon encodes an entire extracellular domain, a transmembrane domain, and a cytoplasmic domain. Members of the α and γ subfamilies have a cluster-specific shared cytoplasmic c-terminus encoded by three constant exons. Each variable exon has its own promoter, transcription of which results in long mRNA transcripts from which the intervening introns and variable exons are spliced. Proteins encoded by the γ locus form cis tetramers indiscriminately of isoform. These tetramers comprise the adhesive unit of the γ-Pcdhs, and are strictly homophilic in trans with tetramers of the same composition. Thus, the 22 γ-Pcdhs alone can generate at least 12,560 adhesive units. If the organization of isoforms within tetramers is important (as well as composition) or if α and β isoforms can participate, then the number of specific adhesive units is considerably greater. Therefore, the clustered Pcdhs are capable of encoding recognition signals in vertebrates as diverse as those encoded by Drosophila Dscam1.
Dscam1-dependent dendrite self-avoidance has been illustrated in several neuron types. Olfactory projection neurons and interneurons both failed to appropriately elaborate dendritic arbors in the antennal lobe without Dscam1 (Zhu et al., 2006). Similarly, Dscam1 mutant sensory neurons in the body wall showed significantly more dendrite self-crossings than did wild type neurons (Matthews et al., 2007; Hughes et al., 2007; Soba et al., 2007). The self-crossing phenotype could be rescued by overexpressing a single Dscam1 isoform, but this also induced inappropriate repulsion with the dendrites of neighboring neurons (Matthews et al., 2007; Hughes et al., 2007; Soba et al., 2007). In fact, thousands of Dscam1 isoforms are required for these neurons to robustly distinguish self from non-self (Hattori et al., 2007a, 2009). This level of complexity allows the stochastic expression of Dscam1 isoforms to uniquely label each neuron in a field for isoneuronal self-avoidance, while allowing interaction with other neurons.
Dscam1, along with its paralog Dscam2, also regulates synapse specificity in the visual system, but again does so through a homotypic repulsion mechanism. Tetrad synapses made between photoreceptor terminals and postsynaptic cells in the lamina always involve postsynaptic sites from one L1 neuron and one L2 neuron, along with two variable postsynaptic partners. In neurons lacking both Dscam1 and Dscam2, this specificity was lost, such that many tetrad synapses contained two postsynaptic specializations from a L1 or L2 neuron at the expense of the other (Millard et al., 2010). Thus, Dscam mediated repulsion prevents two postsynaptic processes from the same cell from occupying the same tetrad synapse.
While Dscam1 confers individual cell identity important for isoneural recognition, other cell type specific molecules control proper wiring in the Drosophila visual system. Some molecules act repulsively like Dscam1, and others mediate adhesive signals similar to those proposed by Roger Sperry's chemoaffinity hypothesis (Sperry, 1963). Repulsive mechanisms that underlie axon tiling help facilitate targeting to the lamina and to the medulla. Photoreceptors R1–R6 from a single ommatidium project axons in a fascicle toward the lamina. At the lamina, the axons defasiculate and each one innervates a separate specifically targeted cartridge (Sanes and Zipursky, 2010). In R cells mutant for the protocadherin Flamingo, axons defasciculate, but target diffusely (Lee et al., 2003). Further analyses of R1–R6 projections in which only a single axon was mutant for Flamingo demonstrated that Flamingo mediates repulsive interactions between axons to facilitate proper targeting (Chen and Clandinin, 2008). Axons projecting into the medulla form tiled columns in which processes from different cell types overlap. For this to occur by a repulsive mechanism, each cell type must have distinct cues to maintain spacing from homotypic axons while remaining indifferent to those of other cell types. The molecules that mediate axon tiling in the medulla found thus far are consistent with this hypothesis. Dscam2, which is very similar to Dscam1 but without the vast diversity, mediates repulsive interactions between L1 axons to confine them to specific columns in the medulla (Millard et al., 2007). Millard and colleagues investigated other cell types and found that Dscam2 is not required for tiling of L2, R7, or R8 axons. A different Ig superfamily member, Turtle, was recently found to mediate tiling of R7 axons through homophilic repulsion (Ferguson et al., 2009), and R8 axon tiling depends on Flamingo (Senti et al., 2003).
In addition to tiling into columns, axons innervating the medulla terminate at specific laminae. Thus far, it seems that lamina-specific targets depend on adhesive mechanisms. Shinza-Kameda and colleagues found that targeting R8 axons to lamina M3 required the homophilic adhesion molecule Capricious on axons and targets. Ectopic expression in R7 cells, which do not normally express Capricious, caused their axons to terminate in M3 instead of their normal targets in M6 (Shinza-Kameda et al., 2006). Capricious expression is limited to R8 cells and their medullary targets, as one would expect of a chemoaffinity molecule. However, Nern et al., in 2008 found that temporal regulation of the more ubiquitously expressed N-Cadherin is involved in targeting L3 and L5 to their targets. Thus, the developing nervous system can recycle chemoaffinity markers even within the same structure through precise temporal regulation.
Vertebrate systems
There are two vertebrate genes encoding Dscams: Dscam and DscamL1. Neither gene has been found to generate diverse isoforms through alternative splicing; thus individual cell identity in the vertebrate nervous system cannot depend on Dscams. It has been suggested that the clustered Protocadherins (Pcdhs) could impart individual identity (Zipursky and Sanes, 2010). The ~50 clustered Pcdhs are encoded by three gene clusters (α, β, and γ) ordered sequentially on mouse chromosome 18 (Wu et al., 1999; Figure 4B). The α and γ clusters are composed of variable and constant exons, such that each Pcdh protein has divergent extracellular sequence with a cluster-specific common cytoplasmic c-terminus. The β cluster has no constant exons. Single cell analysis of Purkinje cells revealed that a given neuron expressed around 5 α and γ isoforms (Esumi et al., 2005; Kaneko et al., 2006). A recent study from Schreiner and Weiner suggested that vertebrate Pcdhs can generate an adhesive code with great diversity, similar to Drosophila Dscam1. Using the γ-Pcdhs, they showed that the molecules formed cis tetramers in an isoform indiscriminant manner. These tetramers are the adhesive unit of the Pcdhs, and each tetramer displayed strong homophilic preference for tetramers of the same composition (Schreiner and Weiner, 2010). There are at least 12,560 possible tetrameric combinations of γ-Pcdhs, and if these tetramers could include members of the α and β clusters, then the possible combinations would be far greater. This suggests a model in which both the choice of isoforms and their relative expression levels determine the complement of adhesive units available to a cell. Thus, the clustered Pcdhs have the diversity and expression pattern required to impart individual cell identity in vertebrates.
Drosophila Dscam1 promotes cell identity through a repulsive mechanism. We do not know if the Pcdhs are repulsive or adhesive, but the cellular phenotypes observed in mice mutant for Pcdh clusters are not particularly similar to those of Dscam1 mutant flies. The α-Pcdhs are important for sorting olfactory neurons to specific glomeruli in the olfactory bulb (Hasegawa et al., 2008) and for targeting serotonergic projections from raphe nucleus to central targets (Katori et al., 2009). The γ-Pcdhs are required for synapse formation and interneuron survival in the spinal cord (Wang et al., 2002; Weiner et al., 2005; Prasad et al., 2008). In the retina, loss of the γ-Pcdhs exacerbated normal developmental cell death, but did not disrupt layer specific dendrite arborization or synapse formation (Lefebvre et al., 2008). Furthermore, γ-Pcdhs do not appear to exclusively signal between processes of the same cell. The γ-Pcdhs are expressed by astrocytes where they are localized to perisynaptic processes in vitro and in vivo. Restricted deletion of the cluster in astrocytes significantly delayed synapse development in vivo. In a co-culture system in which either astrocytes or neurons were mutant for the γ-Pcdhs, normal progression of synapse formation required the adhesion molecules in both cell types in a contact dependent manner (Garrett and Weiner, 2009). The simplest explanation for these data is that γ-Pcdhs mediate contacts between astrocytes and neurons during synapse development. Two separate cells would not necessarily need to express the same precise complement of Pcdh isoforms to interact with each other. The promiscuity of cis tetramer formation suggests that all of the possible combinations of the expressed Pcdhs would form, though the ratios would depend on the relative expression levels. This suggests a competitive model in which cells sharing a few isoforms would form weak interactions, while those expressing many shared isoforms would experience stronger interactions.
In vertebrates, the phenotypes that most resemble mutations in Drosophila Dscams are caused not by mutations in the Pcdhs, but by mutations in the mammalian Dscams. In the retinas of mice mutant for Dscam or DscamL1, neuronal subtypes no longer formed evenly spaced intermingled mosaics, rather the dendrites fasciculated with each other, and the cell bodies were pulled into clumps (Fuerst et al., 2008, 2009). Not all subtypes were affected, only those that normally express the now missing gene. However, neither Dscam nor DscamL1 genes can generate the diverse proteins required to confer individual cell identity, and consistent with this, the clumps and fascicles of retinal neurons that form in the absence of Dscam seem to be largely cell-type specific, suggesting cell identity is preserved. This indicates that vertebrate neurons may use a different strategy to define self than those of Drosophila. Compared to Drosophila, vertebrate cell identity may be defined to a greater extent by diverse adhesive interactions. These adhesive interactions are likely to be important for the homotypic recognition required for mosaic formation and lamina specific synaptic partnering, but become undesirable in excess, leading to the fascicles and clumps of homotypic neurons observed in Dscam and DscamL1 mutants. Therefore, mammals may use the Dscams to mask cell type specific adhesion, keeping it in check. This hypothesized adhesive masking mechanism is subtly but significantly different than the repulsive mechanism of Drosophila Dscams. Rather than active repulsion between isoneural and homotypic neurites, adhesive masking allows neurites to be indifferent to each other. Indeed, as discussed above, most cell types in the mammalian retina have a coverage factor greater than one, indicating extensive overlap of homotypic neurites, and that is difficult to explain by a strictly repulsive mechanism.
The cell type specific adhesion molecules masked by the Dscams are still unknown, but they are likely to be the molecules responsible for the diverse homotypic and heterotypic recognition interactions. For the reasons discussed above, the clustered Pcdhs are particularly good candidates, as are the molecules known to regulate lamina specific dendrite and axon termination. Retinal ganglion cells project their dendrites to distinct layers in the IPL, and target their axons to specific layers in structures in the CNS. It is possible that the same molecules could be necessary for lamina specific targeting of the dendrites and axons of the same cell, but thus far individual molecules have only been identified as essential for one or the other.
In targeting to the superior colliculus (SC), RGCs maintain two types of identity: positional identity to preserve retinotopic organization and cell type identity to innervate the appropriate laminae of the structure. Positional identity is encoded by gradients of cell surface molecules in the retina and SC. Gradients of A- and B-type ephrins and their receptors, EphAs and EphBs respectively, are the most well characterized positional cues, but gradients of repulsive guidance molecule (RGM) and its receptor neogenin, as well as members of the Wnt family may also be involved. Positional identity is not the focus of this review, and the gradients that regulate retinotopic maps have been well reviewed in recent years (Flanagan, 2006; Luo and Flanagan, 2007; Clandinin and Feldheim, 2009; Scicolone et al., 2009). A recent study investigated the developmental time course underlying laminar restriction of axons in the SC. They found that RGC axons cover several laminae and form synapses throughout early postnatal time points (<P5) then, through synapse elimination and axon retraction, become restricted to specific laminae (Cheng et al., 2010). For one cell type at least, this developmental laminar restriction did not require cholinergic retinal waves, indicating cell-type specific molecules direct the process (Huberman et al., 2009). Adhesion molecules are also important for laminar targeting in the chick optic tectum, the functional equivalent to the SC in chick. In a classic study using retina and tectum co-cultures, blocking N-cadherin disrupted the laminar targeting of cultured RGC axons. This disruption was observed when RGCs were targeting into PFA-fixed tectum, indicating dependence on actual contact rather than a diffusible secreted cue (Inoue and Sanes, 1997).
The first molecules found to regulate laminar restriction of dendrite arborization in the IPL were the Sidekicks (Yamagata et al., 2002). Sidekick-1 and -2 are members of the Ig superfamily expressed in a subset of retinal ganglion cells that target specific sublaminae in the chick retina. Overexpression of either Sidekick in RGCs that normally do not express the molecule caused aberrant lamination to the layers normally populated by Sidekick expressing neurons (Yamagata et al., 2002). Interestingly, Dscam and DscamL were also found to regulate lamina targeting in the chick IPL (Yamagata and Sanes, 2008, 2010). This finding is seemingly at odds with what has been demonstrated in mouse. In mice mutant for Dscam, DscamL1, or both, synaptic lamination was preserved. Furthermore, in DscamL1 mutants, paired recordings of AII amacrine cells and rod bipolar cells – synaptic partners that both express DscamL1 – synaptic pairing was maintained (Fuerst et al., 2009). This disparity could be explained by species (genetic) differences or by phenotypic variability. Indeed, a new mutant allele of Dscam that displayed greater laminar disorganization in the IPL was recently discovered in a different genetic background (Fuerst et al., 2010). Thus, Dscam may have distinct, competing roles based on genetic context. These data are also consistent with the hypothesis that laminar specificity is not the same phenomenon as synaptic specificity, but that lamination optimizes appropriate pairing. In DscamL1 mutants, pairing is maintained, but the total number of synapses is reduced (Fuerst et al., 2009). This, despite the substantial increase in cell number in these mutants, suggests that aberrant mosaic spacing or lamination may prevent pre- and postsynaptic cells from encountering each other in sufficient number, but does not prevent them from forming functional synapses when they do contact each other. In further support of this hypothesis, it was recently shown that the Semaphorin Sema6A and its receptor PlexinA4 are required for proper laminar targeting of several cell types in the mouse retina (Matsuoka et al., 2011). Interestingly, even though dopaminergic amacrine cell neurites are mistargeted in the IPL in these mutants, they still form synapses with ipRGCs, their appropriate synaptic partners (Matsuoka et al., 2011).
Conclusion
Cell identity is required for neurons to recognize self, homotypic cells, and heterotypic cells. In the vertebrate retina, these diverse recognition events allow the cell to form dendritic arbors in intermingled mosaics with homotypic cells and to target their dendrites and axons to specific laminae to form specific synapses. The Dscams are required to maintain mosaic spacing in the retina and may be important for lamination, but appear to be dispensable for synaptic specificity. This suggests that mosaic spacing and lamination may optimize synaptic pairing, but that these processes may operate through different mechanisms. Indeed, the synaptic phenotypes in the retinas mutant for the Dscams are consistent with a loss of optimization: Fewer total synapses develop despite a significant increase in cell number.
In Drosophila, Dscam1 can account for individual cell identity because of its diversity and directly repulsive properties. Vertebrate Dscams certainly cannot. Most RGCs express the same Dscam, and in its absence cell type identity is retained. However, the Dscam mutants may prove instructive in identifying cell type specific adhesive codes. Together with mouse lines labeling genetically identified mosaic cell types, we may begin to unravel the adhesive cues responsible for cell identity and diverse recognition that become unmasked in Dscam mutants.
Acknowledgements
The authors would like to thanks Drs. Kevin Seburn and Gareth Howell for comments on the manuscript and Mr. Jessie Hammer for assistance with the figures. This work is supported by the NEI (RO1EY018605, RWB), AMG is supported by T32NS051112-04.
References
- Barlow HB, Hill RM, Levick WR. Retinal ganglion cells responding selectively to direction and speed of image motion in the rabbit. The Journal of Physiology. 1964;173:377–407. doi: 10.1113/jphysiol.1964.sp007463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron DA, Carney LH. Cellular patterns in the inner retina of adult zebrafish: quantitative analyses and a computational model of their formation. The Journal of comparative neurology. 2004;471:11–25. doi: 10.1002/cne.11040. [DOI] [PubMed] [Google Scholar]
- Chen P, Clandinin TR. The cadherin Flamingo mediates level-dependent interactions that guide photoreceptor target choice in Drosophila. Neuron. 2008;58:26–33. doi: 10.1016/j.neuron.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng T, Liu X, Faulkner RL, Stephan AH, Barres BA, Huberman AD, Cheng H. Emergence of lamina-specific retinal ganglion cell connectivity by axon arbor retraction and synapse elimination. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2010;30:16376–16382. doi: 10.1523/JNEUROSCI.3455-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clandinin TR, Feldheim DA. Making a visual map: mechanisms and molecules. Current opinion in neurobiology. 2009;19:174–80. doi: 10.1016/j.conb.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JE. Getting to grips with neuronal diversity: What is a neuronal type? In: Chalupa LM, Finlay BL, editors. Development and Organization of the Retina: From Molecules to Function. Plenum Press; New York: 1998. pp. 91–120. [Google Scholar]
- Cook JE, Chalupa LM. Retinal mosaics: new insights into an old concept. Trends Neurosci. 2000;1:26–34. doi: 10.1016/s0166-2236(99)01487-3. [DOI] [PubMed] [Google Scholar]
- Coombs J, van der List D, Wang G, Chalupa LM. Morphological properties of mouse retinal ganglion cells. Neuroscience. 2006;140:123–136. doi: 10.1016/j.neuroscience.2006.02.079. [DOI] [PubMed] [Google Scholar]
- Cusato K, Stagg SB, Reese BE. Two phases of increased cell death in the inner retina following early elimination of the ganglion cell population. J Comp Neurol. 2001;439(4):440–9. doi: 10.1002/cne.1361. [DOI] [PubMed] [Google Scholar]
- Demb JB. Cellular mechanisms for direction selectivity in the retina. Neuron. 2007;55:179–186. doi: 10.1016/j.neuron.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Devries SH, Baylor DA. Mosaic arrangement of ganglion cell receptive fields in rabbit retina. Journal of Neurophysiology. 1997;78:2048–2060. doi: 10.1152/jn.1997.78.4.2048. [DOI] [PubMed] [Google Scholar]
- Easter SS, Rusoff AC, Kish PE. The growth and organization of the optic nerve and tract in juvenile and adult goldfish. J. Neurosci. 1981;I:793–8ll. doi: 10.1523/JNEUROSCI.01-08-00793.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker JL, Dumitrescu ON, Wong KY, Alam NM, Chen S, LeGates T, Renna JM, Prusky GT, Berson DM, Hattar S. Melanopsin-expressing retinal ganglion-cell photoreceptors: cellular diversity and role in pattern vision. Neuron. 2010;67:49–60. doi: 10.1016/j.neuron.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esumi S, Kakazu N, Taguchi Y, Hirayama T, Sasaki A, Hirabayashi T, Koide T, Kitsukawa T, Hamada S, Yagi T. Monoallelic yet combinatorial expression of variable exons of the protocadherin-alpha gene cluster in single neurons. Nat Genet. 2005;37:171. doi: 10.1038/ng1500. [DOI] [PubMed] [Google Scholar]
- Ferguson K, Long H, Cameron S, Chang W, Rao Y. The conserved Ig superfamily member Turtle mediates axonal tiling in Drosophila. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2009;29:14151–14159. doi: 10.1523/JNEUROSCI.2497-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AJ, Stanke JJ, Aloisio G, Hoy H, Stell WK. Heterogeneity of horizontal cells in the chicken retina. The Journal of Comparative Neurology. 2007;500:1154–1171. doi: 10.1002/cne.21236. [DOI] [PubMed] [Google Scholar]
- Flanagan JG. Neural map specification by gradients. Current Opinion in Neurobiology. 2006;16:59–66. doi: 10.1016/j.conb.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Fuerst PG, Bruce F, Tian M, Wei W, Elstrott J, Feller MB, Erskine L, Singer JH, Burgess RW. DSCAM and DSCAML1 function in self-avoidance in multiple cell types in the developing mouse retina. Neuron. 2009;64:484–97. doi: 10.1016/j.neuron.2009.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerst PG, Burgess RW. Adhesion molecules in establishing retinal circuitry. Current opinion in neurobiology. 2009 doi: 10.1016/j.conb.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerst PG, Fuerst PG, Koizumi A, Koizumi A, Masland RH, Masland RH, Burgess RW, Burgess RW. Neurite arborization and mosaic spacing in the mouse retina require DSCAM. Nature. 2008;451:470. doi: 10.1038/nature06514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerst PG, Harris BS, Johnson KR, Burgess RW. A novel null allele of mouse DSCAM survives to adulthood on an inbred C3H background with reduced phenotypic variability. Genesis (New York, N.Y.: 2000) 2010;48:578–584. doi: 10.1002/dvg.20662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli-Resta L. Local, possibly contact-mediated signalling restricted to homotypic neurons controls the regular spacing of cells within the cholinergic arrays in the developing rodent retina. Development. 2000;127(7):1509–16. doi: 10.1242/dev.127.7.1509. [DOI] [PubMed] [Google Scholar]
- Galli-Resta L. Putting neurons in the right places: local interactions in the genesis of retinal architecture. Trends in neurosciences. 2002;25:638–43. doi: 10.1016/s0166-2236(02)02279-8. [DOI] [PubMed] [Google Scholar]
- Galli-Resta L, Leone P, Bottari D, Ensini M, Rigosi E, Novelli E. The genesis of retinal architecture: an emerging role for mechanical interactions? Prog Retin Eye Res. 2008;27:260–83. doi: 10.1016/j.preteyeres.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Galli-Resta L, Novelli E, Viegi A. Dynamic microtubule-dependent interactions position homotypic neurones in regular monolayered arrays during retinal development. Development (Cambridge, England) 2002;129:3803–3814. doi: 10.1242/dev.129.16.3803. [DOI] [PubMed] [Google Scholar]
- Galli-Resta L, Resta G, Tan SS, Reese BE. Mosaics of islet-1-expressing amacrine cells assembled by short-range cellular interactions. J Neurosci. 1997;17(20):7831–8. doi: 10.1523/JNEUROSCI.17-20-07831.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett AM, Weiner JA. Control of CNS Synapse Development by -Protocadherin-Mediated Astrocyte-Neuron Contact. Journal of Neuroscience. 2009;29:11723–11731. doi: 10.1523/JNEUROSCI.2818-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh KK, Bujan S, Haverkamp S, Feigenspan A, Wässle H. Types of bipolar cells in the mouse retina. The Journal of Comparative Neurology. 2004;469:70–82. doi: 10.1002/cne.10985. [DOI] [PubMed] [Google Scholar]
- Grueber WB, Jan LY, Jan YN. Different levels of the homeodomain protein cut regulate distinct dendrite branching patterns of Drosophila multidendritic neurons. Cell. 2003;112:805–18. doi: 10.1016/s0092-8674(03)00160-0. [DOI] [PubMed] [Google Scholar]
- Hasegawa S, Hamada S, Kumode Y, Esumi S, Katori S, Fukuda E, Uchiyama Y, Hirabayashi T, Mombaerts P, Yagi T. The protocadherin-alpha family is involved in axonal coalescence of olfactory sensory neurons into glomeruli of the olfactory bulb in mouse. Molecular and cellular neurosciences. 2008 doi: 10.1016/j.mcn.2008.01.016. [DOI] [PubMed] [Google Scholar]
- Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science (New York, N.Y.) 2002;295:1065–1070. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori D, Chen Y, Matthews BJ, Salwinski L, Sabatti C, Grueber WB, Zipursky SL. Robust discrimination between self and non-self neurites requires thousands of Dscam1 isoforms. Nature. 2009;461:644–648. doi: 10.1038/nature08431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori D, Demir E, Kim HW, Viragh E, Zipursky SL, Dickson BJ. Dscam diversity is essential for neuronal wiring and self-recognition. Nature. 2007a;449:223–227. doi: 10.1038/nature06099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori Y, Sugimura K, Uemura T. Selective expression of Knot/Collier, a transcriptional regulator of the EBF/Olf-1 family, endows the Drosophila sensory system with neuronal class-specific elaborated dendritic patterns. Genes to Cells: Devoted to Molecular & Cellular Mechanisms. 2007b;12:1011–1022. doi: 10.1111/j.1365-2443.2007.01107.x. [DOI] [PubMed] [Google Scholar]
- Haverkamp S, Wässle H. Immunocytochemical analysis of the mouse retina. The Journal of comparative neurology. 2000;424:1–23. [PubMed] [Google Scholar]
- Huberman AD, Manu M, Koch SM, Susman MW, Lutz AB, Ullian EM, Baccus SA, Barres BA. Architecture and activity-mediated refinement of axonal projections from a mosaic of genetically identified retinal ganglion cells. Neuron. 2008;59:425–38. doi: 10.1016/j.neuron.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman AD, Wei W, Elstrott J, Stafford BK, Feller MB, Barres BA. Genetic identification of an On-Off direction-selective retinal ganglion cell subtype reveals a layer-specific subcortical map of posterior motion. Neuron. 2009;62:327–34. doi: 10.1016/j.neuron.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes ME, Bortnick R, Tsubouchi A, Bäumer P, Kondo M, Uemura T, Schmucker D. Homophilic Dscam interactions control complex dendrite morphogenesis. Neuron. 2007;54:417–27. doi: 10.1016/j.neuron.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue A, Sanes J. Lamina-specific connectivity in the brain: regulation by N-cadherin, neurotrophins, and glycoconjugates. Science. 1997;276:1428. doi: 10.1126/science.276.5317.1428. [DOI] [PubMed] [Google Scholar]
- Jan Y, Jan LY. Branching out: mechanisms of dendritic arborization. Nat Rev Neurosci. 2010;11:316–28. doi: 10.1038/nrn2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyarasasingam G, Snider CJ, Ratto GM, Chalupa LM. Activity-regulated cell death contributes to the formation of ON and OFF alpha ganglion cell mosaics. The Journal of Comparative Neurology. 1998;394:335–343. [PubMed] [Google Scholar]
- Kaneko R, Kato H, Kawamura Y, Esumi S, Hirayama T, Hirabayashi T, Yagi T. Allelic gene regulation of Pcdh-alpha and Pcdh-gamma clusters involving both monoallelic and biallelic expression in single Purkinje cells. The Journal of biological chemistry. 2006;281:30551–60. doi: 10.1074/jbc.M605677200. [DOI] [PubMed] [Google Scholar]
- Katori S, Hamada S, Noguchi Y, Fukuda E, Yamamoto T, Yamamoto H, Hasegawa S, Yagi T. Protocadherin-alpha family is required for serotonergic projections to appropriately innervate target brain areas. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:9137–47. doi: 10.1523/JNEUROSCI.5478-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeley PW, Reese BE. Morphology of dopaminergic amacrine cells in the mouse retina: independence from homotypic interactions. The Journal of Comparative Neurology. 2010;518:1220–1231. doi: 10.1002/cne.22270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I, Zhang Y, Meister M, Sanes J. Laminar Restriction of Retinal Ganglion Cell Dendrites and Axons: Subtype-Specific Developmental Patterns Revealed with Transgenic Markers. Journal of Neuroscience. 2010;30:1452. doi: 10.1523/JNEUROSCI.4779-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I, Zhang Y, Yamagata M, Meister M, Sanes JR. Molecular identification of a retinal cell type that responds to upward motion. Nature. 2008;452:478–82. doi: 10.1038/nature06739. [DOI] [PubMed] [Google Scholar]
- Kim MD, Jan LY, Jan YN. The bHLH-PAS protein Spineless is necessary for the diversification of dendrite morphology of Drosophila dendritic arborization neurons. Genes & Development. 2006;20:2806–2819. doi: 10.1101/gad.1459706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R, Clandinin T, Lee C, Chen P, Meinertzhagen I, Zipursky S. The protocadherin Flamingo is required for axon target selection in the Drosophila visual system. Nat Neurosci. 2003;6:557. doi: 10.1038/nn1063. [DOI] [PubMed] [Google Scholar]
- Lefebvre JL, Zhang Y, Meister M, Wang X, Sanes JR. gamma-Protocadherins regulate neuronal survival but are dispensable for circuit formation in retina. Development (Cambridge, England) 2008;135:4141–51. doi: 10.1242/dev.027912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Wang F, Menut L, Gao F. BTB/POZ-zinc finger protein abrupt suppresses dendritic branching in a neuronal subtype-specific and dosage-dependent manner. Neuron. 2004;43:823–34. doi: 10.1016/j.neuron.2004.08.040. [DOI] [PubMed] [Google Scholar]
- Lin B, Wang SW, Masland RH. Retinal ganglion cell type, size, and spacing can be specified independent of homotypic dendritic contacts. Neuron. 2004;43:475–485. doi: 10.1016/j.neuron.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Luo L, Flanagan JG. Development of continuous and discrete neural maps. Neuron. 2007;56:284–300. doi: 10.1016/j.neuron.2007.10.014. [DOI] [PubMed] [Google Scholar]
- MacNeil MA, Masland RH. Extreme Diversity among Amacrine Cells: Implications for Function. Neuron. 1998;20:971–982. doi: 10.1016/s0896-6273(00)80478-x. [DOI] [PubMed] [Google Scholar]
- Matsuoka RL, Nguyen-Ba-Charvet KT, Parray A, Badea TC, Chédotal A, Kolodkin AL. Transmembrane semaphorin signalling controls laminar stratification in the mammalian retina. Nature. 2011;470:259–263. doi: 10.1038/nature09675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews BJ, Kim ME, Flanagan JJ, Hattori D, Clemens JC, Zipursky SL, Grueber WB. Dendrite self-avoidance is controlled by Dscam. Cell. 2007;129:593–604. doi: 10.1016/j.cell.2007.04.013. [DOI] [PubMed] [Google Scholar]
- McCabe KL, Gunther EC, Reh TA. The development of the pattern of retinal ganglion cells in the chick retina: mechanisms that control differentiation. Development (Cambridge, England) 1999;126:5713–5724. doi: 10.1242/dev.126.24.5713. [DOI] [PubMed] [Google Scholar]
- Millard SS, Flanagan JJ, Pappu KS, Wu W, Zipursky SL. Dscam2 mediates axonal tiling in the Drosophila visual system. Nature. 2007;447:720–4. doi: 10.1038/nature05855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millard SS, Lu Z, Zipursky SL, Meinertzhagen IA. Drosophila dscam proteins regulate postsynaptic specificity at multiple-contact synapses. Neuron. 2010;67:761–768. doi: 10.1016/j.neuron.2010.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millard SS, Zipursky SL. Dscam-mediated repulsion controls tiling and self-avoidance. Current Opinion in Neurobiology. 2008;18:84–89. doi: 10.1016/j.conb.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore AW, Jan LY, Jan YN. hamlet, a binary genetic switch between single- and multiple- dendrite neuron morphology. Science. 2002;297:1355–8. doi: 10.1126/science.1072387. [DOI] [PubMed] [Google Scholar]
- Mumm JS, Williams PR, Godinho L, Koerber A, Pittman AJ, Roeser T, Chien C, Baier H, Wong ROL. In vivo imaging reveals dendritic targeting of laminated afferents by zebrafish retinal ganglion cells. Neuron. 2006;52:609–621. doi: 10.1016/j.neuron.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nern A, Zhu Y, Zipursky SL. Local N-cadherin interactions mediate distinct steps in the targeting of lamina neurons. Neuron. 2008;58:34–41. doi: 10.1016/j.neuron.2008.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves G, Zucker J, Daly M, Chess A. Stochastic yet biased expression of multiple Dscam splice variants by individual cells. Nature Genetics. 2004;36:240–246. doi: 10.1038/ng1299. [DOI] [PubMed] [Google Scholar]
- Prasad T, Wang X, Gray PA, Weiner JA. A differential developmental pattern of spinal interneuron apoptosis during synaptogenesis: insights from genetic analyses of the protocadherin-gamma gene cluster. Development (Cambridge, England) 2008;135:4153–64. doi: 10.1242/dev.026807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencio I, Rollag MD, Castrucci AM. Photoreceptive net in the mammalian retina. This mesh of cells may explain how some blind mice can still tell day from night. Nature. 2002;415:493. doi: 10.1038/415493a. [DOI] [PubMed] [Google Scholar]
- Raven MA, Eglen SJ, Ohab JJ, Reese BE. Determinants of the exclusion zone in dopaminergic amacrine cell mosaics. J Comp Neurol. 2003;461(1):123–36. doi: 10.1002/cne.10693. [DOI] [PubMed] [Google Scholar]
- Reese BE, Harvey AR, Tan SS. Radial and tangential dispersion patterns in the mouse retina are cell-class specific. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:2494–2498. doi: 10.1073/pnas.92.7.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese BE, Necessary BD, Tam PP, Faulkner-Jones B, Tan SS. Clonal expansion and cell dispersion in the developing mouse retina. Eur J Neurosci. 1999;8:2965–78. doi: 10.1046/j.1460-9568.1999.00712.x. [DOI] [PubMed] [Google Scholar]
- Rockhill RL, Euler T, Masland RH. Spatial order within but not between types of retinal neurons. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:2303–2307. doi: 10.1073/pnas.030413497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockhill RL, Daly FJ, MacNeil MA, Brown SP, Masland RH. The diversity of ganglion cells in a mammalian retina. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2002;22:3831–3843. doi: 10.1523/JNEUROSCI.22-09-03831.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodieck RW. The density recovery profile: a method for the analysis of points in the plane applicable to retinal studies. Visual Neuroscience. 1991;6:95–111. doi: 10.1017/s095252380001049x. [DOI] [PubMed] [Google Scholar]
- Rubin GM. Development of the Drosophila retina: inductive events studied at single cell resolution. Cell. 1989;57:519–520. doi: 10.1016/0092-8674(89)90120-7. [DOI] [PubMed] [Google Scholar]
- Sanes JR, Zipursky SL. Design principles of insect and vertebrate visual systems. Neuron. 2010;66:15–36. doi: 10.1016/j.neuron.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt TM, Kofuji P. Functional and morphological differences among intrinsically photosensitive retinal ganglion cells. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2009;29:476–482. doi: 10.1523/JNEUROSCI.4117-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner D, Weiner JA. Combinatorial homophilic interaction between gamma-protocadherin multimers greatly expands the molecular diversity of cell adhesion. Proceedings of the National Academy of Sciences of the United States of America. 2010 doi: 10.1073/pnas.1004526107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scicolone G, Ortalli AL, Carri NG. Key roles of Ephs and ephrins in retinotectal topographic map formation. Brain Research Bulletin. 2009;79:227–247. doi: 10.1016/j.brainresbull.2009.03.008. [DOI] [PubMed] [Google Scholar]
- Senti K, Usui T, Boucke K, Greber U, Uemura T, Dickson BJ. Flamingo regulates R8 axon-axon and axon-target interactions in the Drosophila visual system. Current Biology: CB. 2003;13:828–832. doi: 10.1016/s0960-9822(03)00291-4. [DOI] [PubMed] [Google Scholar]
- Shinza-Kameda M, Takasu E, Sakurai K, Hayashi S, Nose A. Regulation of layer-specific targeting by reciprocal expression of a cell adhesion molecule, capricious. Neuron. 2006;49:205–213. doi: 10.1016/j.neuron.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Siegert S, Scherf BG, Punta KD, Didkovsky N, Heintz N, Roska B. Genetic address book for retinal cell types. Nature Neuroscience. 2009;12:1197–204. doi: 10.1038/nn.2370. [DOI] [PubMed] [Google Scholar]
- Soba P, Zhu S, Emoto K, Younger S, Yang S, Yu H, Lee T, Jan LY, Jan Y. Drosophila sensory neurons require Dscam for dendritic self-avoidance and proper dendritic field organization. Neuron. 2007;54:403–16. doi: 10.1016/j.neuron.2007.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperry RW. Chemoaffinity in the orderly growth of nerve fiber patterns and connections. Proceedings of the National Academy of Sciences of the United States of America. 1963;50:703–710. doi: 10.1073/pnas.50.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimura K, Satoh D, Estes P, Crews S, Uemura T. Development of morphological diversity of dendrites in Drosophila by the BTB-zinc finger protein abrupt. Neuron. 2004;43:809–22. doi: 10.1016/j.neuron.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Tomlinson A. The molecular basis of pattern formation in the developing compound eye of Drosophila. Seminars in Cell Biology. 1990;1:229–239. [PubMed] [Google Scholar]
- Tyler MJ, Carney HL, Cameron DA. Control of cellular pattern formation in the vertebrate inner retina by homotypic regulation of cell-fate decisions. Journal of Neuroscience. 2005;25:4565–76. doi: 10.1523/JNEUROSCI.0588-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Weiner J, Levi S, Craig A, Bradley A, Sanes J. Gamma protocadherins are required for survival of spinal interneurons. Neuron. 2002;36:843. doi: 10.1016/s0896-6273(02)01090-5. [DOI] [PubMed] [Google Scholar]
- Wassle H, Puller C, Muller F, Haverkamp S. Cone Contacts, Mosaics, and Territories of Bipolar Cells in the Mouse Retina. J. Neurosci. 2009;29:106–117. doi: 10.1523/JNEUROSCI.4442-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner J, Wang X, Tapia J, Sanes J. Gamma protocadherins are required for synaptic development in the spinal cord. Proc Natl Acad Sci U S A. 2005;102:8. doi: 10.1073/pnas.0407931101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney IE, Raven MA, Ciobanu DC, Williams RW, Reese BE. Multiple genes on chromosome 7 regulate dopaminergic amacrine cell number in the mouse retina. Investigative Ophthalmology & Visual Science. 2009;50:1996–2003. doi: 10.1167/iovs.08-2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA. Optic nerve fibre counts and retinal ganglion cell counts during development of Xenopus laevis (Daudin) Q. J. Exp. Physiol. 1971;56:83–91. doi: 10.1113/expphysiol.1971.sp002110. [DOI] [PubMed] [Google Scholar]
- Wojtowicz WM, Flanagan JJ, Millard SS, Zipursky SL, Clemens JC. Alternative splicing of Drosophila Dscam generates axon guidance receptors that exhibit isoform-specific homophilic binding. Cell. 2004;118:619–33. doi: 10.1016/j.cell.2004.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtowicz WM, Wu W, Andre I, Qian B, Baker D, Zipursky SL. A vast repertoire of Dscam binding specificities arises from modular interactions of variable Ig domains. Cell. 2007;130:1134–45. doi: 10.1016/j.cell.2007.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Maniatis T. A striking organization of a large family of human neural cadherin-like cell adhesion genes. Cell. 1999;97:779. doi: 10.1016/s0092-8674(00)80789-8. [DOI] [PubMed] [Google Scholar]
- Yamagata M, Sanes JR. Synaptic Localization and Function of Sidekick Recognition Molecules Require MAGI Scaffolding Proteins. Journal of Neuroscience. 2010;30:3579–3588. doi: 10.1523/JNEUROSCI.6319-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata M, Weiner J, Sanes J. Sidekicks: synaptic adhesion molecules that promote lamina-specific connectivity in the retina. Cell. 2002;110:649. doi: 10.1016/s0092-8674(02)00910-8. [DOI] [PubMed] [Google Scholar]
- Yamagata M, Sanes JR. Dscam and Sidekick proteins direct lamina-specific synaptic connections in vertebrate retina. Nature. 2008;451:465–9. doi: 10.1038/nature06469. [DOI] [PubMed] [Google Scholar]
- Zhu H, Hummel T, Clemens JC, Berdnik D, Zipursky SL, Luo L. Dendritic patterning by Dscam and synaptic partner matching in the Drosophila antennal lobe. Nature Neuroscience. 2006;9:349–355. doi: 10.1038/nn1652. [DOI] [PubMed] [Google Scholar]
- Zipursky SL, Sanes JR. Chemoaffinity revisited: dscams, protocadherins, and neural circuit assembly. Cell. 2010;143:343–353. doi: 10.1016/j.cell.2010.10.009. [DOI] [PubMed] [Google Scholar]


