Abstract
The development of Parkinson's disease is accompanied by concurrent activation of caspase-3 and apoptosis of dopaminergic neurons of human patients and rodent models. The role of caspase-3, a final executioner of apoptosis, in the pathogenesis of Parkinson's disease, however, remains to be determined. Here, we show that gene disruption of caspase-3 protects mice from 1-methyle-4-phenyl-1,2,3,6-tetrahmydropyridine (MPTP)-induced Parkinsonian syndrome, as reflected by reversal of MPTP-induced bradykinesia and decreased tyrosine hydroxylase expression in the nigra-striatum. MPTP treatment resulted in increased caspase-3 activation and apoptosis in the substantia nigra of wild-type mice at 24 h after the inception of MPTP treatment, as compared with vehicle-treated control animals. Gene disruption of caspase-3 prevented MPTP-induced apoptosis in the substantia nigra. At 7 days after MPTP treatment, tyrosine hydroxylase expression was suppressed and infiltration of activated microglia and astrocytes was markedly increased in the nigra-striatum of wild-type mice. All of these alterations following MPTP treatment were blocked by disruption of caspase-3 in mice. These results clearly indicate that caspase-3 activation is required for the development of MPTP-induced Parkinson's disease in mice. These findings suggest that activation of caspase-3-mediated apoptosis of dopaminergic neurons in the early stage may play an important role in the pathogenesis of Parkinson's disease.
Keywords: Parkinson's disease, MPTP, caspase-3, apoptosis, substantia nigra, striatum
Introduction
Parkinson's disease (PD) is the second most common neurodegenerative disorder after Alzheimer's disease, affecting approximately 1–2 % of the population over the age of 65 [1]. PD is characterized by a progressive loss of dopaminergic neurons in the substantia nigra pars compacta. The loss of dopaminergic afferents from the substantia nigra to the striatum and putamen results in extra pyramidal motor dysfunction, including tremor, rigidity, and bradykinesia. Another hallmark feature of PD is gliosis, accumulatuion of activated microglia and astrocytes in the substantia nigra and striatum [2], although it remains to be clarified how gliosis is initiated and sustained in PD. The symptoms of PD can be ameliorated by medications, such as precursors of dopamines, but these remedies cannot prevent or retard the progression of neurodegeneration. To develop new preventive and/or therapeutic strategies against PD, therefore, it is important to enhance our understanding of the molecular pathogenesis of the disease.
Activation of caspases and apoptosis of dopaminergic neurons have been implicated in the pathogenesis of PD. Caspase-3 is the downstream executioner of apoptosis in the cascades of caspases. Activated caspase-3 has been shown in the substantia nigra of patients with PD [3,4], although controversial results were also reported [5]. Likewise, numerous studies have demonstrated increased apoptosis of dopamine neurons in the substantia nigra of PD patients by in situ labeling methods, TdT-mediated dUTP-biotin nick end labeling (TUNEL) staining, and electron microscopy [6,7,8,9], while no apoptic neurons were observed in other studies [5,10]. It is important to note that the rate of neuronal death in PD patients is very low, and hence very sensitive methods are necessary for detection of apoptosis in PD. This has been argued as a potential contributor to the controversial results in previous literature.
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) causes a severe and irreversible Parkinsonian syndrome in humans, non-human primates, and rodents, which fully replicates the clinical and pathological hallmarks of idiopathic PD [11,12]. These major pathological features of PD include selective degeneration and loss of dopaminergic neurons in the substantia nigra, as reflected by suppressed expression of tyrosine hydroxylase (TH), the rate-limiting enzyme of dopamine biosynthesis, and gliosis. Thus, the MPTP model has been the most commonly used and best characterized rodent model of PD.
Administration of MPTP induces activation of caspases, including caspase-3, 8, 9, and 11 [13,14,15], and poptotic DNA fragmentation in the substantia nigra of rodents [16,17,18,19,20,21]. Apoptosis in the substantia nigra after MPTP-treatment is, therefore, associated with activation of caspases, including caspase-3, in mice. A previous study has shown that gene disruption of caspase-11 protects mice from MPTP-induced PD [22]. Treatment with a broad-spectrum caspase inhibitor partially prevented MPTP-induced loss of dopaminergic neurons in mice [23]. It is important to note, however, that activities of caspases-1, 4, and 11 are essential for maturation of proinflammatory cytokines, including interleukin-1 (IL-1), as well as execution of apoptosis. For example, caspase-11 mediates the activation of caspase-1 (also known as interleukin-1 converting enzyme), the primary activator of pro-IL-1, leading to enhanced inflammation, although caspase-11 can also promote caspase-3 cleavage (activation). It is possible, therefore, caspase-11 ablation and broad-spectrum caspase inhibitior might exert the protective effects by blocking proinflammatory cytokine maturation rather than direct inhibition of apoptosis.
Moreover, the controversial results have been reported about the role of caspases in 1-methyl-4-phenylpyridinium iodide (MPP+, MPTP's toxic metabolite)-induced cell death in cultured neuronal cells. Some studies have shown that inhibitors of caspases, including caspase-3, protect neuronal cells from MPP+-induced apoptosis [24,25,26]. In other studies, however, caspase inhibitors failed to decrease MPP+-induced neuronal cell death [27,28,29], although 6-hydroxydopamine-induced death was blocked by caspase inhibition in these studies [27,28]. The role of caspase-3 in PD remains to be determined and has not yet been investigated in caspase-3 knockout mice in vivo. In the present study, therefore, we studied the effects of gene disruption of caspase-3 on MPTP-induced PD in mice.
Materials and methods
Animals and MPTP administration
All experiments were carried out in accordance with institutional guidelines and the study protocol was approved by the Massachusetts General Hospital Subcommittee on Research Animal Care. The animal care facility is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. We used male wild-type C57BL/6 mice (Jackson Laboratory, Bar Harbor, ME) and caspase-3-deficient mice on C57BL/6 background, which were kindly provided by Dr. T.W. Mak [30], at 8 weeks of age. Body weight did not differ between wild-type and caspase-3 knockout mice at 8 weeks of age (BW [g]: WT: 23.9 ± 0.3 [mean ± SEM]; KO: 23.8 ± 0.8). The mice were housed in a pathogen-free animal facility maintained at 25ºC and illuminated by 12:12-h light-dark cycles. The mice were provided with standard rodent chow and water ad libitum. Mice received four intraperitoneal injections of MPTP (20 mg/kg BW) or saline with an interval of 2 h over a 6-h period as previously described [31].
Immunohistochemistry
Immunohistochemical analysis was performed as previously described [32]. Briefly, mice were anesthetized with pentobarbital sodium (50 mg/kg BW, IP) and then perfused transcardially with phosphate-buffered saline (PBS). The brains were removed and immersed in 4% paraformaldehyde for 1 day. Then, the samples were serially immersed in phosphate buffer containing 10% and 20% sucrose (w/v) for 24 h each and frozen in O.C.T. compound. Serial 10-μm coronal sections were prepared using a cryostat (Jencons Scientific, Bridgevill, PA). After inactivation of endogenous peroxidase activity by hydrogen peroxide, the sections were incubated in blocking solution (10% normal goat serum in PBS) for 1 h, followed by incubation with a primary antibody (TH [1:1,000, Millipore, Bedford, MA], Iba1 [1:500, Wako Chemicals USA, Richmond, VA], GFAP [1:500, Dako, Carpinteria, CA], or cleaved caspase-3 [1:200, Cell Signaling, Danvers, MA]) at 4ºC. The sections were washed three times with PBS and incubated with biotinylated goat anti-secondary antibody (1:200, Vector Laboratories, Burlingame, CA). They were further soaked with horseradish peroxidase (HRP)-labeled streptoavidin-biotin complex (Vectastain Elite ABC kit; Vector, Burlingame, CA) and visualized by diaminobenzidine (DAB). To evaluate the numbers of Iba1- and GFAP-positive cells, the 10-μm thick coronal sections with an interval of 50 μm in the striatum (0.26–0.56 mm anterior from the bregma) were analyzed as previously described [33,34].
Double labeling for DNA fragmentation and tyrosine hydroxylase
Sections were processed for fluorescence TUNEL staining by using the ApopTag Fluorescein In Situ Apoptosis Detection Kit (Promega, Madison, WI) according to the manufacturer's instructions. After the sections were incubated in blocking solution for 1 h, they were incubated with rabbit anti-TH antibody overnight at 4ºC, followed by incubation with Rhodamine RedTX-conjugated goat anti-rabbit antibody (1:200, Jackson Immunoreasearch Laboratories, West Grove, PA) for 1 h. The fluorescence images were captured using appropriate filters under a microscope (Nikon ECLIPSE TE-2000-S).
Immunoblotting
For the evaluation of TH protein expression, substantia nigra and striatum were dissected and frozen at 24 h or 7 days after the MPTP injections [35], and homogenized in lysis buffer (50mM Tris-HCl pH 7.4, 150 mM NaCl, 1% Triton X-100, 1 mM EDTA, 1 mM DTT, 1 mM phenylmethylsulfonyl fluoride, 2 mM sodium vanadate, protease inhibitor cocktail [Sigma]) as previously described [36]. Protein samples (15 μg/lane) were subjected to a standard SDS-polyacrylamide gel electrophoresis with a Mini-Protean System (Bio-Rad, Hercules, CA) and were electrophoretically transferred to a polyvinylidene difluoride membrane (Bio-Rad). The membranes were soaked in blocking buffer (GE Healthcare, Waukesha, WI) for 1 h and incubated overnight at 4°C with anti-TH (1:50,000), anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody (1:75,000, Trevigen, Gaithersburg, MD), or cleaved caspase-3 [1:5,000], followed by incubation with HRP-conjugated anti-rabbit IgG antibody (1:75,000, GE Healthcare) for 1 h at room temperateure. Immunoreactive bands were detected with Amersham ECL Advance Western Blotting Detection Kit (GE Healthcare). Densitometric analysis of the results was carried out with NIH Image software (ver. 1.62).
Tail suspension test
Tail suspension tests were performed in a sound-proof room as described previously [37] with minor modifications. Briefly, mice were suspended by the tails (45-cm high). The duration of immobility was measured for 5 min just after the inception of suspension.
Statistical analysis
Data are shown as mean ± SEM. Statistical analysis was performed with one-way analysis of variance, followed by Bonferroni's Multiple Comparison Test or Student t test. A value of p<0.05 was considered statistically significant.
Results
MPTP-induced apoptosis in the substantia nigra was prevented by caspase-3 deficiency in mice
As shown previously [15,20], immunohistochemical and immunoblot analyses revealed that cleaved (activated) caspase-3 was increased in the substantia nigra at 24 h after the inception of MPTP injections in wild-type mice, as compared with saline-treated mice (Fig. 1). The protein expression of GAPDH was not affected by MPTP administration. Consistent with activation of caspase-3, MPTP treatment resulted in induction of apoptosis in the substantia nigra in wild-type mice, as indicated by TUNEL staining. In contrast, caspase-3 deficiency blocked MPTP-induced apoptosis in the substantia nigra (Fig. 2). When treated with saline alone, no difference was found in apoptosis in the substantia nigra between wild-type and caspase-3 knockout mice.
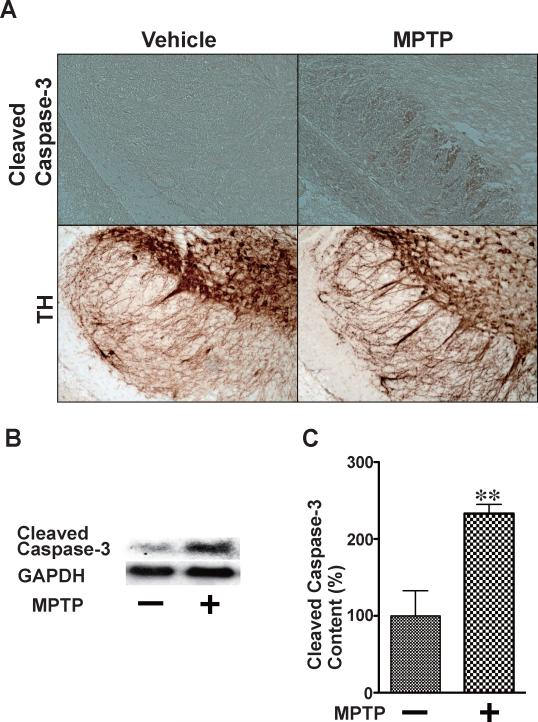
Fig. 1.
MPTP-induced activation of caspase-3 in the substantia nigra. Immunohistochemistry (A) and immunoblotting (B, C) revealed that MPTP administration resulted in increased cleavage (activation) of caspase-3 in the substantia nigra of wild-type mice at 24 h after the inception of MPTP injections, as compared with saline (vehicle) alone. The protein expression of GAPDH was not affected by MPTP treatment. n=3–4 per group. **p<0.01 vs. vehicle.
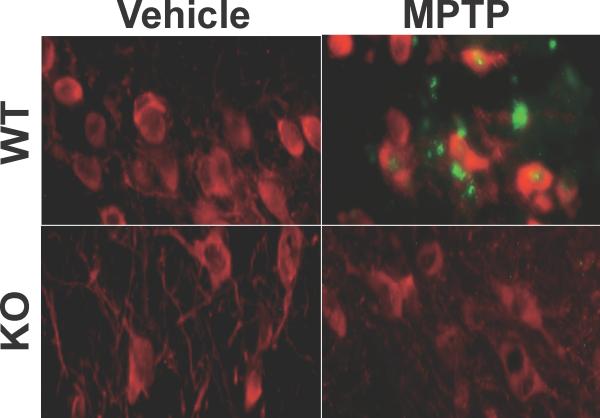
Fig. 2.
Caspase-3 deficiency inhibited MPTP-induced apoptosis in the substantia nigra in mice. MPTP administration induced apoptosis in the substantia nigra of wild-type (WT) mice, as judged by double staining for TUNEL (green) and tyrosine hydroxylase (TH, red), at 24 h after the inception of MPTP injections. In caspase-3 knockout (KO) mice, however, MPTP failed to induce apoptosis in the substantia nigra.
MPTP-induced bradykinesia and decreased expression of tyrosine hydroxylase were reverted by caspase-3 deficiency in mice
Next, we examined the effects of gene disruption of caspase-3 on MPTP-induced PD in mice. At 7 days after MPTP injections, wild-type mice exhibited a marked bradykinesia, as judged by the duration of immobilization in the tail suspension test, as compared with vehicle-treated control animals. In stark contrast, administration of MPTP failed to prolong immobilization time during the tail suspension test in caspase-3 knockout mice (Fig. 3A).
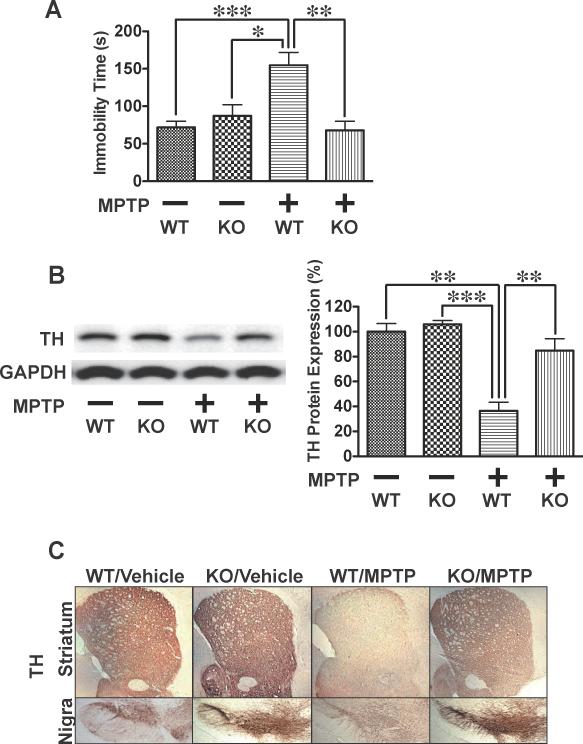
Fig. 3.
Caspase-3 deficiency prevented MPTP-induced bradykinesia and reduction in tyrosine hydroxylase expression in mice. At 7 days after MPTP administration, the tail suspension test showed prolonged immobility time in wild- type (WT) mice compared to vehicle alone (A). In contrast, MPTP did not affect duration of immobility in caspase-3 knockout (KO) mice. Immunoblot (B) and immunohistochemical (C) analyses revealed that the protein expression of tyrosine hydroxylase (TH) was markedly decreased in the nigra-striatum of wild-type, but not caspase-3 knockout, mice at 7 days after MPTP administration compared with vehicle alone. The protein expression of GAPDH was not altered by MPTP or caspase-3 deficiency. n=4 per group. *p<0.05, **p<0.01, ***p<0.001.
Immunoblot and immunohistochemical analyses revealed that the protein expression of tyrosine hydroxylase (TH) was markedly suppressed in the substantia nigra and striatum of MPTP-treated wild-type mice relative to sham animals (Figs. 3B,C). Caspase-3 deficiency completely blocked MPTP-induced decrease in TH expression in the nigra-striatum. Neither MPTP nor caspase-3 deficiency altered GAPDH expression. When treated with saline alone, the results of the tail suspension test and TH expression in the nigra-striatum did not differ between wild-type and caspase-3 knockout mice. Saline injections did not affect the results of the tail suspension test and TH expression in the nigra-striatum in wild-type mice, as compared with naïve mice without injections (data not shown).
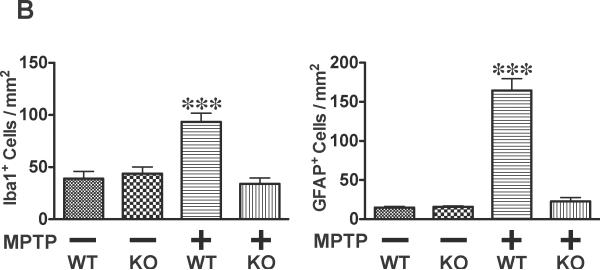
At 7 days after MPTP injections, the decreased TH expression in MPTP-treated wild-type mice was accompanied by increased numbers of ionized calcium binding adaptor molecule 1 (Iba1)-positive microglia and glial fibrillary acidic protein (GFAP)-positive astrocytes in the substantia nigra and striatum, relative to sham animals (Fig. 4). In contrast, neither Iba1- nor GFAP- positive cells were increased by MPTP treatment in the nigra-striatum of caspase-3 knockout mice. When treated with saline alone, no difference was found in Iba1- and GFAP-positive cells in the nigra-striatum between wild-type and caspase-3 knockout mice.
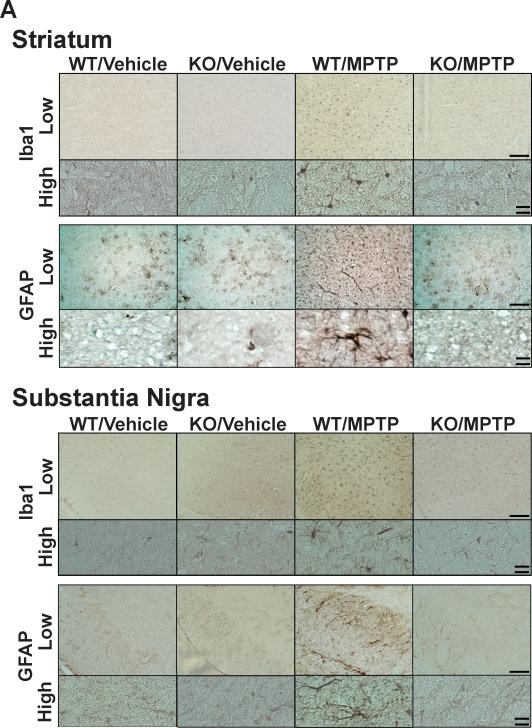
Fig. 4.
Caspase-3 deficiency blocked MPTP-induced infiltration of activated microglia and astrocytes in the nigra-striatum in mice. Immunohistochemistry showed that accumulation of Iba1-positive microglia and GFAP-positive astrocytes in the striatum and substantia nigra of wild-type (WT) mice at 7 days after MPTP administration, as compared with vehicle alone. In caspase-3 knockout (KO) mice, however, MPTP failed to increase Iba1- and GFAP-positive cells in the nigra-striatum (A). The numbers of GFAP- and Iba1-positive cells in the striatum was increased in wild-type, but not caspase-3 knockout, mice at 7 days after MPTP administration relative to sham animals (B). n=5–6 per group. ***p<0.001 vs. WT with vehicle, and KO with vehicle and MPTP. Single bar (low magnification)=200 μm; Double bar (high magnification)=20 μm.
Discussion
Our data clearly demonstrate that gene disruption of caspase-3 completely blocked MPTP-induced PD in mice, as judge by the tail suspension test, TH expression, and accumulation of microglia and astrocytes in the nigra-striatum (Figs. 3,4). Moreover, MPTP-induced apoptosis in dopaminergic neurons in the substantia nigra was prevented by caspase-3 ablation (Figs. 1,2). These findings indicate that caspase-3 activation is required for MPTP-induced PD in mice.
Our results are in line with a previous study that caspase-11 deficiency prevents MPTP-induced PD in mice [22]. In their study, they did not examine apoptosis following MPTP treatment. Under normal condition few neuronal cells express caspase-11, while caspase-11 is abundantly expressed in microglia. The authors discussed that caspase-11 activation-mediated inflammation in microglia plays an important role in MPTP neurotoxicity [22]. In contrast, caspase-3 is ubiquitously expressed, including neurons. Our findings suggest that caspase-3-dependent apoptosis in neurons in the substantia nigra may play a privotal role in MPTP-induced PD in mice.
Previous studies have shown that caspase-3 activation and apoptosis occur in the early stage of the development of PD following MPTP administration in the substantia nigra in wild-type mice [15,20]. As opposed to transient activation of caspase-3 and apoptosis in the early stage, activation and accumulation of microglia and astrocytes persist in the nigra-striatum in MPTP-induced PD [38,39], contributing to sustained inflammation in the lesion. These findings suggest that caspase-3 activation and subsequent apoptosis of dopamine neurons in the substantia nigra may be the initial step essential for the development of PD, including progressive suppression of TH expression and gliosis. Our data seem to support the postulated scenario in which insult by MPTP injections results in activation of caspase-3-mediated apoptosis of dopamine neurons, which, in turn, leads to persistent loss of TH in the nigra-striatum along with sustained activation and infiltration of microglia and astrocytes.
In addition, this raises the possibility that the controversial results on apoptosis of dopaminergic neurons in PD patients [3,4,5] might be partly explained by the presumption that apoptosis of dopamine neurons is the early pathological event rather than consequence of the disease development. In accord with previous studies [15,20], we found significant increase in TUNEL-positive cells at 1 day, but not 7 days, after MPTP treatment in wild-type mice, as compared with sham animals (Fig. 2 and data not shown). One can speculate, therefore, that apoptotic changes in dopamine neurons may or may not be present at the end of disease stage when postmortem samples are collected.
At 24 h after the inception of MPTP treatment, TUNEL-positive cells were found in both TH-positive and -negative cells in the substantia nigra of wild-type mice (Fig. 2). A previous study has shown that in the substantia nigra neurons with a strong signal for cleaved (activated) caspase-3 show reduction in TH immunoreactivity following MPTP administration, whereas neurons with a weak cleaved caspase-3 signal show strong TH positivity [40]. The protein expression of TH was slightly decreased at 1 day after MPTP treatment relative to sham mice, although the decrease in TH expression progressed and culminated at 7 days post-MPTP treatment (data not shown). It is possible, therefore, that some of the TUNEL-positive cells in the substantia nigra might show reduced or undetectable TH immunoreactivity due to loss of TH expression during the process of apoptosis, although those cells used to express TH prior to MPTP treatment. Further studies are required to clarify this point.
In conclusion, our results demonstrate that activation of caspase-3 is required for MPTP-induced apoptosis in the substantia nigra and development of PD. These findings indicate a critical role of caspase-3 in MPTP-induced PD in mice. Taken together with previous studies [15, 20], these findings suggest that caspase-3-dependent apoptosis of dopaminergic neurons might be the essential initial event that triggers subsequent progressive loss of TH, sustained inflammation, and gliosis in MPTP-induced PD in mice.
Acknowledgements
We are grateful to Dr. T.W. Mak for providing caspase-3 knockout mice. We thank Dr. C. Waeber for helpful technical assistance. This work was partly supported by research grants to M.K. (R01 DK05827), F.I. (R01 HL101930), and the Microscopy and Image Analysis Core of Massachusetts General Hospital (P30 NS045776) from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Alves G, Forsaa EB, Pedersen KF, Dreetz Gjerstad M, Larsen JP. Epidemiology of Parkinson's disease. J Neurol. 2008;255(Suppl 5):18–32. doi: 10.1007/s00415-008-5004-3. [DOI] [PubMed] [Google Scholar]
- [2].Teismann P, Tieu K, Cohen O, Choi DK, Wu DC, Marks D, Vila M, Jackson-Lewis V, Przedborski S. Pathogenic role of glial cells in Parkinson's disease. Mov Disord. 2003;18:121–9. doi: 10.1002/mds.10332. [DOI] [PubMed] [Google Scholar]
- [3].Hartmann A, Hunot S, Michel PP, Muriel MP, Vyas S, Faucheux BA, Mouatt-Prigent A, Turmel H, Srinivasan A, Ruberg M, Evan GI, Agid Y, Hirsch EC. Caspase-3: A vulnerability factor and final effector in apoptotic death of dopaminergic neurons in Parkinson's disease. Proc Natl Acad Sci U S A. 2000;97:2875–80. doi: 10.1073/pnas.040556597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Tatton NA. Increased caspase 3 and Bax immunoreactivity accompany nuclear GAPDH translocation and neuronal apoptosis in Parkinson's disease. Exp Neurol. 2000;166:29–43. doi: 10.1006/exnr.2000.7489. [DOI] [PubMed] [Google Scholar]
- [5].Jellinger KA. Cell death mechanisms in Parkinson's disease. J Neural Transm. 2000;107:1–29. doi: 10.1007/s007020050001. [DOI] [PubMed] [Google Scholar]
- [6].Mochizuki H, Goto K, Mori H, Mizuno Y. Histochemical detection of apoptosis in Parkinson's disease. J Neurol Sci. 1996;137:120–3. doi: 10.1016/0022-510x(95)00336-z. [DOI] [PubMed] [Google Scholar]
- [7].Tatton NA, Maclean-Fraser A, Tatton WG, Perl DP, Olanow CW. A fluorescent double-labeling method to detect and confirm apoptotic nuclei in Parkinson's disease. Ann Neurol. 1998;44:S142–8. doi: 10.1002/ana.410440721. [DOI] [PubMed] [Google Scholar]
- [8].Mochizuki H, Mori H, Mizuno Y. Apoptosis in neurodegenerative disorders. J Neural Transm. 1997;(Suppl 50):125–40. doi: 10.1007/978-3-7091-6842-4_13. [DOI] [PubMed] [Google Scholar]
- [9].Kingsbury AE, Mardsen CD, Foster OJ. DNA fragmentation in human substantia nigra: apoptosis or perimortem effect? Mov Disord. 1998;13:877–84. doi: 10.1002/mds.870130604. [DOI] [PubMed] [Google Scholar]
- [10].Wullner U, Kornhuber J, Weller M, Schulz JB, Loschmann PA, Riederer P, Klockgether T. Cell death and apoptosis regulating proteins in Parkinson's disease--a cautionary note. Acta Neuropathol. 1999;97:408–12. doi: 10.1007/s004010051005. [DOI] [PubMed] [Google Scholar]
- [11].Burns RS, Chiueh CC, Markey SP, Ebert MH, Jacobowitz DM, Kopin IJ. A primate model of parkinsonism: selective destruction of dopaminergic neurons in the pars compacta of the substantia nigra by N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Proc Natl Acad Sci U S A. 1983;80:4546–50. doi: 10.1073/pnas.80.14.4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Heikkila RE, Hess A, Duvoisin RC. Dopaminergic neurotoxicity of 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine in mice. Science. 1984;224:1451–3. doi: 10.1126/science.6610213. [DOI] [PubMed] [Google Scholar]
- [13].Viswanath V, Wu Y, Boonplueang R, Chen S, Stevenson FF, Yantiri F, Yang L, Beal MF, Andersen JK. Caspase-9 activation results in downstream caspase-8 activation and bid cleavage in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson's disease. J Neurosci. 2001;21:9519–28. doi: 10.1523/JNEUROSCI.21-24-09519.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hartmann A, Troadec JD, Hunot S, Kikly K, Faucheux BA, Mouatt-Prigent A, Ruberg M, Agid Y, Hirsch EC. Caspase-8 is an effector in apoptotic death of dopaminergic neurons in Parkinson's disease, but pathway inhibition results in neuronal necrosis. J Neurosci. 2001;21:2247–55. doi: 10.1523/JNEUROSCI.21-07-02247.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Turmel H, Hartmann A, Parain K, Douhou A, Srinivasan A, Agid Y, Hirsch EC. Caspase-3 activation in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated mice. Mov Disord. 2001;16:185–9. doi: 10.1002/mds.1037. [DOI] [PubMed] [Google Scholar]
- [16].Hassouna I, Wickert H, Zimmermann M, Gillardon F. Increase in bax expression in substantia nigra following 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) treatment of mice. Neurosci Lett. 1996;204:85–8. doi: 10.1016/0304-3940(96)12323-5. [DOI] [PubMed] [Google Scholar]
- [17].Serra PA, Sciola L, Delogu MR, Spano A, Monaco G, Miele E, Rocchitta G, Miele M, Migheli R, Desole MS. The neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine induces apoptosis in mouse nigrostriatal glia. Relevance to nigral neuronal death and striatal neurochemical changes. J Biol Chem. 2002;277:34451–61. doi: 10.1074/jbc.M202099200. [DOI] [PubMed] [Google Scholar]
- [18].Tatton NA, Kish SJ. In situ detection of apoptotic nuclei in the substantia nigra compacta of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated mice using terminal deoxynucleotidyl transferase labelling and acridine orange staining. Neuroscience. 1997;77:1037–48. doi: 10.1016/s0306-4522(96)00545-3. [DOI] [PubMed] [Google Scholar]
- [19].Vila M, Jackson-Lewis V, Vukosavic S, Djaldetti R, Liberatore G, Offen D, Korsmeyer SJ, Przedborski S. Bax ablation prevents dopaminergic neurodegeneration in the 1-methyl- 4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson's disease. Proc Natl Acad Sci U S A. 2001;98:2837–42. doi: 10.1073/pnas.051633998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Novikova L, Garris BL, Garris DR, Lau YS. Early signs of neuronal apoptosis in the substantia nigra pars compacta of the progressive neurodegenerative mouse 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine/probenecid model of Parkinson's disease. Neuroscience. 2006;140:67–76. doi: 10.1016/j.neuroscience.2006.02.007. [DOI] [PubMed] [Google Scholar]
- [21].Spooren WP, Gentsch C, Wiessner C. TUNEL-positive cells in the substantia nigra of C57BL/6 mice after a single bolus of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Neuroscience. 1998;85:649–51. doi: 10.1016/s0306-4522(97)00640-4. discussion 653. [DOI] [PubMed] [Google Scholar]
- [22].Furuya T, Hayakawa H, Yamada M, Yoshimi K, Hisahara S, Miura M, Mizuno Y, Mochizuki H. Caspase-11 mediates inflammatory dopaminergic cell death in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson's disease. J Neurosci. 2004;24:1865–72. doi: 10.1523/JNEUROSCI.3309-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Yang L, Sugama S, Mischak RP, Kiaei M, Bizat N, Brouillet E, Joh TH, Beal MF. A novel systemically active caspase inhibitor attenuates the toxicities of MPTP, malonate, and 3NP in vivo. Neurobiol Dis. 2004;17:250–9. doi: 10.1016/j.nbd.2004.07.021. [DOI] [PubMed] [Google Scholar]
- [24].Dodel RC, Du Y, Bales KR, Ling ZD, Carvey PM, Paul SM. Peptide inhibitors of caspase-3-like proteases attenuate 1-methyl-4-phenylpyridinum-induced toxicity of cultured fetal rat mesencephalic dopamine neurons. Neuroscience. 1998;86:701–7. doi: 10.1016/s0306-4522(98)00154-7. [DOI] [PubMed] [Google Scholar]
- [25].Kaul S, Kanthasamy A, Kitazawa M, Anantharam V, Kanthasamy AG. Caspase-3 dependent proteolytic activation of protein kinase C delta mediates and regulates 1-methyl-4-phenylpyridinium (MPP+)-induced apoptotic cell death in dopaminergic cells: relevance to oxidative stress in dopaminergic degeneration. Eur J Neurosci. 2003;18:1387–401. doi: 10.1046/j.1460-9568.2003.02864.x. [DOI] [PubMed] [Google Scholar]
- [26].Gomez C, Reiriz J, Pique M, Gil J, Ferrer I, Ambrosio S. Low concentrations of 1-methyl-4-phenylpyridinium ion induce caspase-mediated apoptosis in human SH-SY5Y neuroblastoma cells. J Neurosci Res. 2001;63:421–8. doi: 10.1002/1097-4547(20010301)63:5<421::AID-JNR1037>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- [27].Choi WS, Yoon SY, Oh TH, Choi EJ, O'Malley KL, Oh YJ. Two distinct mechanisms are involved in 6-hydroxydopamine- and MPP+-induced dopaminergic neuronal cell death: role of caspases, ROS, and JNK. J Neurosci Res. 1999;57:86–94. doi: 10.1002/(SICI)1097-4547(19990701)57:1<86::AID-JNR9>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- [28].Han BS, Hong HS, Choi WS, Markelonis GJ, Oh TH, Oh YJ. Caspase-dependent and -independent cell death pathways in primary cultures of mesencephalic dopaminergic neurons after neurotoxin treatment. J Neurosci. 2003;23:5069–78. doi: 10.1523/JNEUROSCI.23-12-05069.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chu CT, Zhu JH, Cao G, Signore A, Wang S, Chen J. Apoptosis inducing factor mediates caspase-independent 1-methyl-4-phenylpyridinium toxicity in dopaminergic cells. J Neurochem. 2005;94:1685–95. doi: 10.1111/j.1471-4159.2005.03329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Woo M, Hakem R, Soengas MS, Duncan GS, Shahinian A, Kagi D, Hakem A, McCurrach M, Khoo W, Kaufman SA, Senaldi G, Howard T, Lowe SW, Mak TW. Essential contribution of caspase 3/CPP32 to apoptosis and its associated nuclear changes. Genes Dev. 1998;12:806–19. doi: 10.1101/gad.12.6.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Jackson-Lewis V, Przedborski S. Protocol for the MPTP mouse model of Parkinson's disease. Nat Protoc. 2007;2:141–51. doi: 10.1038/nprot.2006.342. [DOI] [PubMed] [Google Scholar]
- [32].Chiba T, Yamada M, Sasabe J, Terashita K, Shimoda M, Matsuoka M, Aiso S. Amyloid-beta causes memory impairment by disturbing the JAK2/STAT3 axis in hippocampal neurons. Mol Psychiatry. 2009;14:206–22. doi: 10.1038/mp.2008.105. [DOI] [PubMed] [Google Scholar]
- [33].Oki C, Watanabe Y, Yokoyama H, Shimoda T, Kato H, Araki T. Delayed treatment with arundic acid reduces the MPTP-induced neurotoxicity in mice. Cell Mol Neurobiol. 2008;28:417–30. doi: 10.1007/s10571-007-9241-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Frau L, Borsini F, Wardas J, Khairnar AS, Schintu N, Morelli M. Neuroprotective and anti-inflammatory effects of the adenosine A(2A) receptor antagonist ST1535 in a MPTP mouse model of Parkinson's disease. Synapse. 2011;65:181–8. doi: 10.1002/syn.20833. [DOI] [PubMed] [Google Scholar]
- [35].Liberatore GT, Jackson-Lewis V, Vukosavic S, Mandir AS, Vila M, McAuliffe WG, Dawson VL, Dawson TM, Przedborski S. Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat Med. 1999;5:1403–9. doi: 10.1038/70978. [DOI] [PubMed] [Google Scholar]
- [36].Shinozaki S, Inoue Y, Yang W, Fukaya M, Carter EA, Ming-Yu Y, Fischman A, Tompkins R, Kaneki M. Farnesyltransferase inhibitor improved survival following endotoxin challenge in mice. Biochem Biophys Res Commun. 2010;391:1459–64. doi: 10.1016/j.bbrc.2009.12.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Mori A, Ohashi S, Nakai M, Moriizumi T, Mitsumoto Y. Neural mechanisms underlying motor dysfunction as detected by the tail suspension test in MPTP-treated C57BL/6 mice. Neurosci Res. 2005;51:265–74. doi: 10.1016/j.neures.2004.11.008. [DOI] [PubMed] [Google Scholar]
- [38].McGeer PL, McGeer EG. Glial reactions in Parkinson's disease. Mov Disord. 2008;23:474–83. doi: 10.1002/mds.21751. [DOI] [PubMed] [Google Scholar]
- [39].Teismann P, Schulz JB. Cellular pathology of Parkinson's disease: astrocytes, microglia and inflammation. Cell Tissue Res. 2004;318:149–61. doi: 10.1007/s00441-004-0944-0. [DOI] [PubMed] [Google Scholar]
- [40].Eberhardt O, Coelln RV, Kugler S, Lindenau J, Rathke-Hartlieb S, Gerhardt E, Haid S, Isenmann S, Gravel C, Srinivasan A, Bahr M, Weller M, Dichgans J, Schulz JB. Protection by synergistic effects of adenovirus-mediated X-chromosome-linked inhibitor of apoptosis and glial cell line-derived neurotrophic factor gene transfer in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson's disease. J Neurosci. 2000;20:9126–34. doi: 10.1523/JNEUROSCI.20-24-09126.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]