Abstract
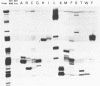
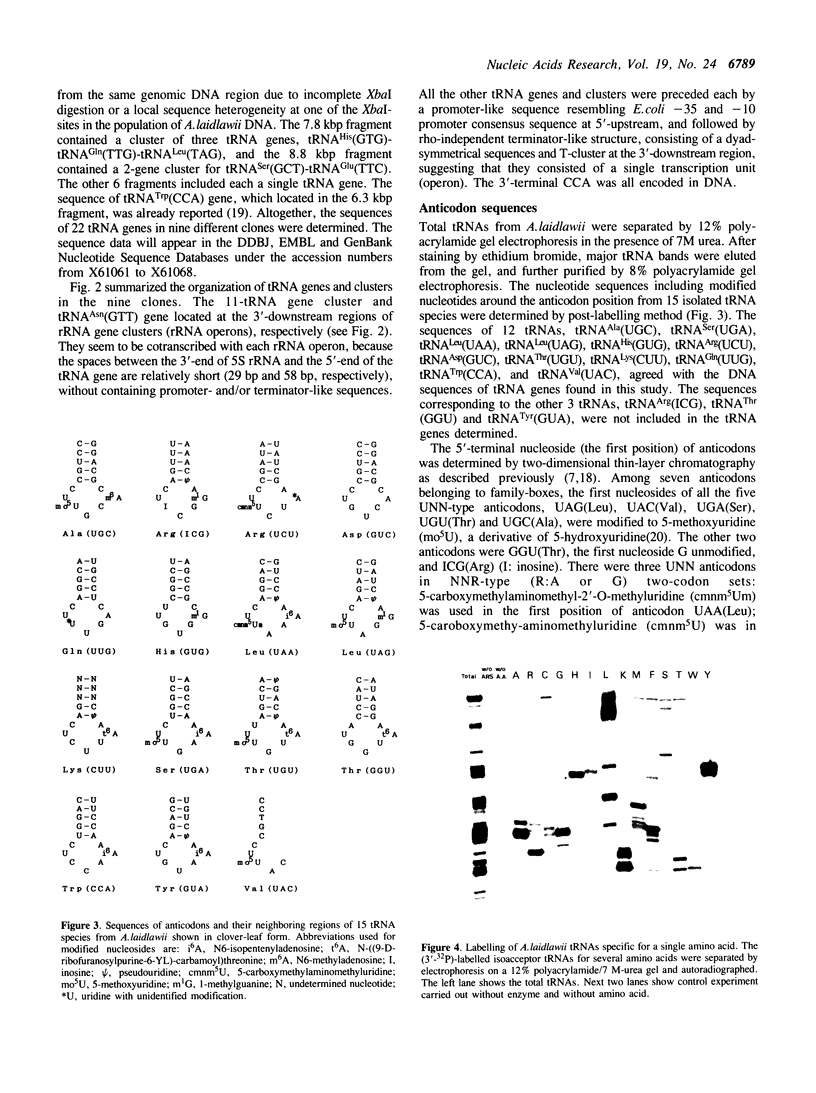
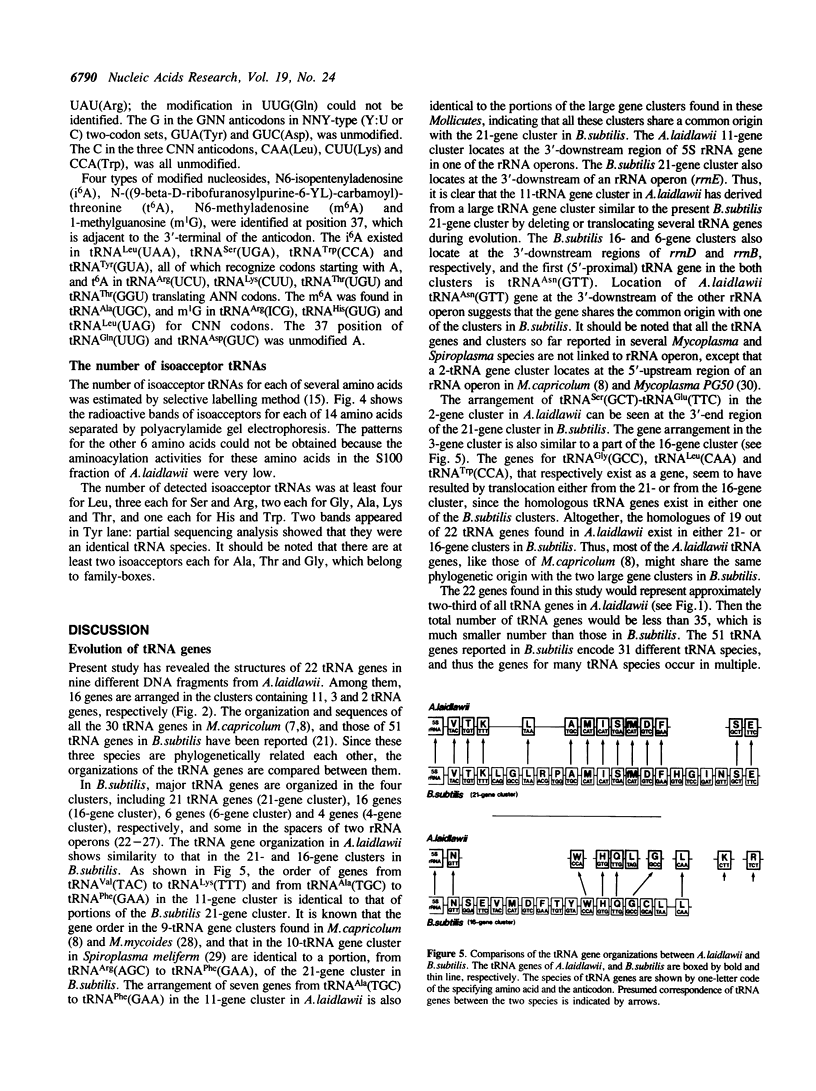
The genes for 22 tRNA species from Acholeplasma laidawii, belonging to the class Mollicutes (Mycoplasmas), have been cloned and sequenced. Sixteen genes are organized in 3 clusters consisting of eleven, three and two tRNA genes, respectively, and the other 6 genes exist as a single gene. The arrangement of tRNA genes in the 11-gene, the 3-gene and the 2-gene clusters reveals extensive similarity to several parts of the 21-tRNA or 16-tRNA gene cluster in Bacillus subtilis. The 11-gene cluster is also similar to the tRNA gene clusters found in other mycoplasma species, the 9-tRNA gene cluster in M.capricolum and in M.mycoides, and the 10-tRNA gene cluster in Spiroplasma meliferm. The results suggest that the tRNA genes in mycoplasmas have evolved from large tRNA gene clusters in the ancestral Gram-positive bacterial genome common to mycoplasmas and B.subtilis. The anticodon sequences including base modifications of 15 tRNA species from A.laidlawii were determined. The anticodon composition and codon-recognition patterns of A.laidlawii resemble those of Bacillus subtilis rather than those of other mycoplasma species.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andachi Y., Yamao F., Iwami M., Muto A., Osawa S. Occurrence of unmodified adenine and uracil at the first position of anticodon in threonine tRNAs in Mycoplasma capricolum. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7398–7402. doi: 10.1073/pnas.84.21.7398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andachi Y., Yamao F., Muto A., Osawa S. Codon recognition patterns as deduced from sequences of the complete set of transfer RNA species in Mycoplasma capricolum. Resemblance to mitochondria. J Mol Biol. 1989 Sep 5;209(1):37–54. doi: 10.1016/0022-2836(89)90168-x. [DOI] [PubMed] [Google Scholar]
- Blanchard A. Ureaplasma urealyticum urease genes; use of a UGA tryptophan codon. Mol Microbiol. 1990 Apr;4(4):669–676. doi: 10.1111/j.1365-2958.1990.tb00636.x. [DOI] [PubMed] [Google Scholar]
- Chevalier C., Saillard C., Bové J. M. Organization and nucleotide sequences of the Spiroplasma citri genes for ribosomal protein S2, elongation factor Ts, spiralin, phosphofructokinase, pyruvate kinase, and an unidentified protein. J Bacteriol. 1990 May;172(5):2693–2703. doi: 10.1128/jb.172.5.2693-2703.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudler R., Schmidhauser C., Parish R. W., Wettenhall R. E., Schmidt T. A mycoplasma high-affinity transport system and the in vitro invasiveness of mouse sarcoma cells. EMBO J. 1988 Dec 1;7(12):3963–3970. doi: 10.1002/j.1460-2075.1988.tb03283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green C. J., Vold B. S. Sequence analysis of a cluster of twenty-one tRNA genes in Bacillus subtilis. Nucleic Acids Res. 1983 Aug 25;11(16):5763–5774. doi: 10.1093/nar/11.16.5763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori H., Osawa S. Origin and evolution of organisms as deduced from 5S ribosomal RNA sequences. Mol Biol Evol. 1987 Sep;4(5):445–472. doi: 10.1093/oxfordjournals.molbev.a040455. [DOI] [PubMed] [Google Scholar]
- Hori H., Sawada M., Osawa S., Murao K., Ishikura H. The nucleotide sequence of 5S rRNA from Mycoplasma capricolum. Nucleic Acids Res. 1981 Oct 24;9(20):5407–5410. doi: 10.1093/nar/9.20.5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inamine J. M., Ho K. C., Loechel S., Hu P. C. Evidence that UGA is read as a tryptophan codon rather than as a stop codon by Mycoplasma pneumoniae, Mycoplasma genitalium, and Mycoplasma gallisepticum. J Bacteriol. 1990 Jan;172(1):504–506. doi: 10.1128/jb.172.1.504-506.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano A., Andachi Y., Ohama T., Osawa S. Novel anticodon composition of transfer RNAs in Micrococcus luteus, a bacterium with a high genomic G + C content. Correlation with codon usage. J Mol Biol. 1991 Sep 20;221(2):387–401. doi: 10.1016/0022-2836(91)80061-x. [DOI] [PubMed] [Google Scholar]
- Kilpatrick M. W., Walker R. T. The nucleotide sequence of glycine tRNA from Mycoplasma mycoides sp. capri. Nucleic Acids Res. 1980 Jun 25;8(12):2783–2786. doi: 10.1093/nar/8.12.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo K., Sone H., Yoshida H., Toida T., Kanatani K., Hong Y. M., Nishino N., Tanaka J. Cloning and sequence analysis of the arginine deiminase gene from Mycoplasma arginini. Mol Gen Genet. 1990 Mar;221(1):81–86. doi: 10.1007/BF00280371. [DOI] [PubMed] [Google Scholar]
- Kuchino Y., Hanyu N., Nishimura S. Analysis of modified nucleosides and nucleotide sequence of tRNA. Methods Enzymol. 1987;155:379–396. doi: 10.1016/0076-6879(87)55026-1. [DOI] [PubMed] [Google Scholar]
- Loughney K., Lund E., Dahlberg J. E. tRNA genes are found between 16S and 23S rRNA genes in Bacillus subtilis. Nucleic Acids Res. 1982 Mar 11;10(5):1607–1624. doi: 10.1093/nar/10.5.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menichi B., Arnold H. H., Heyman T., Dirheimer G., Keith G. Primary structure of Bacillus subtilis tRNAsTyr. Biochem Biophys Res Commun. 1980 Jul 16;95(1):461–467. doi: 10.1016/0006-291x(80)90760-3. [DOI] [PubMed] [Google Scholar]
- Muramatsu T., Yokoyama S., Horie N., Matsuda A., Ueda T., Yamaizumi Z., Kuchino Y., Nishimura S., Miyazawa T. A novel lysine-substituted nucleoside in the first position of the anticodon of minor isoleucine tRNA from Escherichia coli. J Biol Chem. 1988 Jul 5;263(19):9261–9267. doi: 10.1351/pac198961030573. [DOI] [PubMed] [Google Scholar]
- Murao K., Hasegawa T., Ishikura H. 5-methoxyuridine: a new minor constituent located in the first position of the anticodon of tRNAAla, tRNAThr, and tRNAVal from Bacillus subtilis. Nucleic Acids Res. 1976 Oct;3(10):2851–2860. doi: 10.1093/nar/3.10.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murao K., Hasegawa T., Ishikura H. Nucleotide sequence of valine tRNA mo5UAC from bacillus subtilis. Nucleic Acids Res. 1982 Jan 22;10(2):715–718. doi: 10.1093/nar/10.2.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto A., Andachi Y., Yuzawa H., Yamao F., Osawa S. The organization and evolution of transfer RNA genes in Mycoplasma capricolum. Nucleic Acids Res. 1990 Sep 11;18(17):5037–5043. doi: 10.1093/nar/18.17.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oba T., Andachi Y., Muto A., Osawa S. CGG: an unassigned or nonsense codon in Mycoplasma capricolum. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):921–925. doi: 10.1073/pnas.88.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogasawara N., Moriya S., Yoshikawa H. Structure and organization of rRNA operons in the region of the replication origin of the Bacillus subtilis chromosome. Nucleic Acids Res. 1983 Sep 24;11(18):6301–6318. doi: 10.1093/nar/11.18.6301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin S. Molecular biology and genetics of mycoplasmas (Mollicutes). Microbiol Rev. 1985 Dec;49(4):419–455. doi: 10.1128/mr.49.4.419-455.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renbaum P., Abrahamove D., Fainsod A., Wilson G. G., Rottem S., Razin A. Cloning, characterization, and expression in Escherichia coli of the gene coding for the CpG DNA methylase from Spiroplasma sp. strain MQ1(M.SssI). Nucleic Acids Res. 1990 Mar 11;18(5):1145–1152. doi: 10.1093/nar/18.5.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers M. J., Simmons J., Walker R. T., Weisburg W. G., Woese C. R., Tanner R. S., Robinson I. M., Stahl D. A., Olsen G., Leach R. H. Construction of the mycoplasma evolutionary tree from 5S rRNA sequence data. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1160–1164. doi: 10.1073/pnas.82.4.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers M. J., Steinmetz A. A., Walker R. T. The nucleotide sequence of a tRNA gene cluster from Spiroplasma meliferum. Nucleic Acids Res. 1986 Apr 11;14(7):3145–3145. doi: 10.1093/nar/14.7.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson T., Elias P., Lustig F., Guindy Y. S. Cloning and nucleotide sequence analysis of transfer RNA genes from Mycoplasma mycoides. Biochem J. 1985 Nov 15;232(1):223–228. doi: 10.1042/bj2320223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson T., Guindy Y. S., Lustig F., Borén T., Lagerkvist U. Apparent lack of discrimination in the reading of certain codons in Mycoplasma mycoides. Proc Natl Acad Sci U S A. 1987 May;84(10):3166–3170. doi: 10.1073/pnas.84.10.3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka R., Muto A., Osawa S. Nucleotide sequence of tryptophan tRNA gene in Acholeplasma laidlawii. Nucleic Acids Res. 1989 Jul 25;17(14):5842–5842. doi: 10.1093/nar/17.14.5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traboni C., Cortese R., Salvatore F. Selective 32P-labelling of individual species in a total tRNA population. Nucleic Acids Res. 1980 Nov 25;8(22):5223–5232. doi: 10.1093/nar/8.22.5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker R. T., RajBhandary U. L. The nucleotide sequence of formylmethionine tRNA from Mycoplasma mycoides sp. capri. Nucleic Acids Res. 1978 Jan;5(1):57–70. doi: 10.1093/nar/5.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wawrousek E. F., Hansen J. N. Structure and organization of a cluster of sic tRNA genes in the space between tandem ribosomal RNA gene sets in Bacillus subtilis. J Biol Chem. 1983 Jan 10;258(1):291–298. [PubMed] [Google Scholar]
- Wawrousek E. F., Narasimhan N., Hansen J. N. Two large clusters with thirty-seven transfer RNA genes adjacent to ribosomal RNA gene sets in Bacillus subtilis. Sequence and organization of trrnD and trrnE gene clusters. J Biol Chem. 1984 Mar 25;259(6):3694–3702. [PubMed] [Google Scholar]
- Weisburg W. G., Tully J. G., Rose D. L., Petzel J. P., Oyaizu H., Yang D., Mandelco L., Sechrest J., Lawrence T. G., Van Etten J. A phylogenetic analysis of the mycoplasmas: basis for their classification. J Bacteriol. 1989 Dec;171(12):6455–6467. doi: 10.1128/jb.171.12.6455-6467.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y., Ohki M., Ishikura H. The nucleotide sequence of Bacillus subtilis tRNA genes. Nucleic Acids Res. 1983 May 25;11(10):3037–3045. doi: 10.1093/nar/11.10.3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamao F., Muto A., Kawauchi Y., Iwami M., Iwagami S., Azumi Y., Osawa S. UGA is read as tryptophan in Mycoplasma capricolum. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2306–2309. doi: 10.1073/pnas.82.8.2306. [DOI] [PMC free article] [PubMed] [Google Scholar]