Abstract
In patients with multiple sclerosis (MS), a diffuse axonal degeneration occurring throughout the white matter of the central nervous system causes progressive neurologic disability. The underlying mechanism is unclear. This review describes a number of pathways by which dysfunctional astrocytes in MS might lead to axonal degeneration. White-matter astrocytes in MS show a reduced metabolism of adenosine triphosphate-generating phosphocreatine, which may impair the astrocytic sodium potassium pump and lead to a reduced sodium-dependent glutamate uptake. Astrocytes in MS white matter appear to be deficient in β2 adrenergic receptors, which are involved in stimulating glycogenolysis and suppressing inducible nitric oxide synthase (NOS2). Glutamate toxicity, reduced astrocytic glycogenolysis leading to reduced lactate and glutamine production, and enhanced nitric oxide (NO) levels may all impair axonal mitochondrial metabolism, leading to axonal degeneration. In addition, glutamate-mediated oligodendrocyte damage and impaired myelination caused by a decreased production of N-acetylaspartate by axonal mitochondria might also contribute to axonal loss. White-matter astrocytes may be considered as a potential target for neuroprotective MS therapies.
Keywords: astrocytes, axonal degeneration, energy metabolism, multiple sclerosis
The majority of patients with multiple sclerosis (MS) begin with a relapsing-remitting course, which often after several years of disease duration converts into a progressive disease (secondary progressive MS). In a minority of patients, progressive neurologic deterioration without remission occurs from the disease onset (primary progressive MS). Multiple sclerosis is traditionally viewed as a T cell-driven autoimmune disease against myelin of the central nervous system (Compston and Coles, 2008). Substantial evidence indicates that inflammation has a key role in the development of focal demyelinating lesions that constitute the pathological substrate for relapses. However, the progressive phase of MS reflects a poorly understood insidious axonal degeneration that is age related and independent of relapses (Confavreux and Vukusic, 2006; Koch et al, 2007a). Pathological studies have shown that axonal degeneration occurs diffusely throughout the normal appearing white matter (Evangelou et al, 2000). This neurodegenerative component is associated with inflammation (Frischer et al, 2009), but there is growing awareness that inflammatory mechanisms alone cannot explain this degenerative process. Immunomodulatory drugs, which reduce the development of focal lesions and relapses, are not effective in progressive MS (Wilkins and Scolding, 2008), and pathological studies show ongoing demyelination and axonal degeneration despite pronounced immunosuppression (Metz et al, 2007).
Axonal degeneration might be caused by Wallerian degeneration secondary to axonal injury in focal demyelinating lesions (Ferguson et al, 1997; Trapp et al, 1998). However, both magnetic resonance imaging and neuropathological studies found a lack of correlation between focal lesion load and axonal loss in the spinal cord (Bergers et al, 2002; DeLuca et al, 2006; Kutzelnigg et al, 2005), indicating that mechanisms other than Wallerian degeneration contribute to this progressive axonal degeneration.
A current hypothesis suggests that axonal mitochondrial energy failure may lead to axonal degeneration in MS (Su et al, 2009; Trapp and Stys, 2009). There is an increasing awareness that astrocytes have an important role in a variety of neurodegenerative diseases (De Keyser et al, 2008; Kimelberg and Nedergaard, 2010; Ransom et al, 2003), but their possible role in the pathogenesis of MS has received little attention. This review describes a number of pathways by which dysfunctional white-matter astrocytes in MS might lead to axonal mitochondrial energy failure and axonal degeneration.
Phosphocreatine metabolism
Phosphorus magnetic resonance spectroscopy of the brain produces multiple peaks representing high-energy phosphorus compounds, including phosphocreatine (PCr) and adenosine triphosphate (ATP). Minderhoud et al (1992) found that compared with healthy controls, MS patients had increased PCr/β-ATP ratios in the centrum semiovale, and this correlated with clinical measures of MS severity. The β-ATP peak does not contain contributions from other components and appears to be constant under different metabolic conditions (van der Knaap and Pouwels, 2005), suggesting that PCr levels were elevated. Husted et al (1994) found significantly increased PCr/total 31P ratio values in the normal appearing white matter of the centrum semiovale, but not in focal MS lesions of MS patients versus healthy controls. Steen et al (2010) corroborated these findings by detecting significantly increased PCr/β-ATP and PCr/total 31P ratios in the normal appearing white matter of the centrum semiovale of MS patients, compared with healthy controls.
The results from these three independent studies indicate that PCr levels in the normal appearing white matter of MS patients are increased, suggesting that this source of energy generated by mitochondrial creatine kinase (CK) is not properly used.
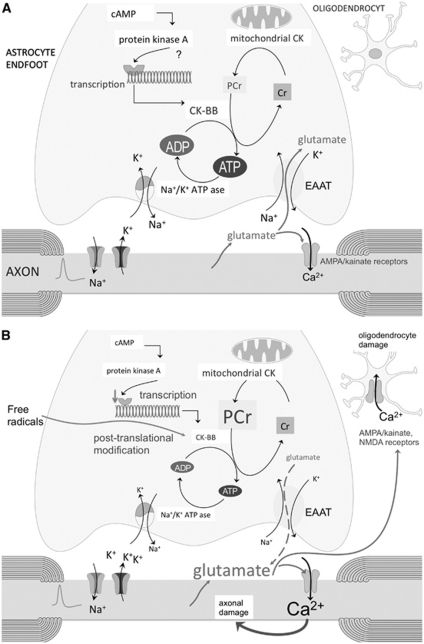
Phosphocreatine acts as a metabolic buffer that is transported from mitochondria to high-energy consuming areas in the cytosol (Figure 1A). Cytosolic CK catalyzes the reversible transfer of the phosphor group from PCr to ADP, generating ATP at a much faster rate than glycolysis and oxidative phosphorylation (Brosnan and Brosnan, 2007). The brain cytosolic CK isoform is CK-BB, which in white matter appears to be exclusively present in astrocytes in both rat and human brain (Tachikawa et al, 2004; Thompson et al, 1980).
Figure 1.
(A) Schematic representation of the astrocyte phosphocreatine (PCr)–creatine (Cr) cycle. Mitochondrial creatine kinase (CK) ensures that adenosine triphosphate (ATP), produced by oxidative phosphorylation, is converted into PCr. Phosphocreatine diffuses to the thin astrocytic processes where it is metabolized to Cr by CK-BB to generate ATP. The expression of CK-BB is stimulated by cAMP (this has been showed in a human astrocytoma cell line and needs confirmation in astrocytes). The propagation of action potentials along axons leads not only to the expulsion of K+ but also to rapid vesicular release into the extracellular fluid of glutamate. The most ATP consuming activity in the astrocytic end feet is the Na+/K+-ATP pump. It takes up K+ released by axons after each depolarization, and it establishes the Na+ gradient necessary for glutamate uptake by the astrocytic Na+-dependent glutamate transporters. (B) In multiple sclerosis (MS), CK-BB levels and activity are reduced by free radicals and/or decreased transcription due to reduced cAMP formation secondary to a loss of astrocytic β2 adrenergic receptors. As a consequence, PCr is not properly metabolized, leading to failure of the Na+/K+-ATP pump and reversal of glutamate uptake by the glutamate transporters. Enhanced glutamate levels in the extracellular space surrounding axons may overactivate axonal α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionic acid (AMPA)/kainate receptors and lead to glutamate-mediated axonal degeneration. Oligodendrocytes, which express AMPA and N-methyl--aspartate (NMDA) receptors, are also sensitive to excitotoxic damage. EAAT, Excitatory Amino Acid Transporter.
N-acetylaspartate (NAA) is an amino acid that is primarily localized to neurons and their axons, where it is synthesized by mitochondria (Bates et al, 1996; Moffett et al, 2007; Patel and Clark, 1979). There was no correlation between the decreased NAA/Cr ratio and the increased PCr/β-ATP and PCr/total 31P ratios, suggesting that the reduced astrocytic PCr metabolism in the normal appearing white matter of MS subjects is not secondary to a decreased axonal energy metabolism or axonal loss (Steen et al, 2010). Compared with controls without central nervous system disease, significantly reduced levels and activity of CK-BB were found in postmortem normal appearing white-matter brain samples in patients with progressive MS, which might provide an explanation for the elevated PCr levels (Steen et al, 2010; Figure 1B).
Mechanisms that may lead to a decreased CK-BB concentration in cerebral white matter are a reduced transcription or posttranslational modification of the enzyme. Decreased transcription might be caused by a deficiency of astrocytic β2 adrenergic receptors in MS white matter (De Keyser et al, 1999; Zeinstra et al, 2000). Activation of these receptors by norepinephrine increases the levels of intracellular cAMP. Norepinephrine, activating these β2 adrenergic receptors, is probably released from axonal varicosities that are present along noradrenergic axons throughout the white matter (Chiti and Teschemacher, 2007). Reduction of intracellular cAMP levels impairs the transcription of CK-BB in cultured human U87-MG glioblastoma cells (Kuzhikandathil and Molloy, 1994, 1999).
The mechanism underlying a loss of β2 adrenergic receptors on astrocytes in MS is unclear. The same finding has been made in dogs following an encephalomyelitis caused by the canine distemper morbillivirus. This condition leads to a chronic demyelinating disease that is very similar to MS, including an axonal degeneration throughout the normal appearing white matter (Seehusen and Baumgartner, 2010; Vandevelde and Zurbriggen, 2005). Although an infectious component has long been suspected, no specific transmissible agent has so far been linked convincingly to MS. Searches for the presence of a morbillivirus in postmortem MS brain tissue have been inconclusive (Geeraedts et al, 2004; Lassmann et al, 2003).
Posttranslational modification of CK-BB might be caused by free radicals, especially by the oxidation of thiol groups of its structure (Wolosker et al, 1996). There are indications that posttranslational oxidative modification of the enzyme may contribute to decreased CK-BB activity in the cerebral cortex in a number of neurodegenerative disorders, including Alzheimer's disease (Aksenov et al, 2000; Aksenov et al, 1999). In MS, there is increased production of reactive oxygen species, not only in focal inflammatory white-matter lesions (Langemann et al, 1992), but also throughout the normal appearing white matter (Graumann et al, 2003). Further research is needed to find out which mechanism is primarily involved.
Possible consequences of an impaired phosphocreatine metabolism
The most ATP consuming activity in astrocytic end feet during axonal electrogenesis is the Na+/K+-ATP pump. It takes up K+ released by axons in the extracellular space after each depolarization, and it establishes the Na+ gradient necessary for glutamate uptake by the astrocytic Na+-dependent glutamate transporters (Figure 1A; Anderson and Swanson, 2000; Danbolt, 2001). Axonal release of glutamate is thought to represent a widespread mechanism for activity-dependent signaling at the axon–glia interface throughout the white matter. Glutamate in white-matter axons is stored in vesicles, and the propagation of action potentials along these axons leads to rapid vesicular release of glutamate into the extracellular fluid by exocytosis (Kukley et al, 2007; Ziskin et al, 2007). White-matter axons contain voltage-gated Ca2+ channels and the vesicle fusion machinery that are necessary for this type of activity-mediated exocytosis (Alix and Domingues, 2011). The functional significance of this vesicular glutamate release is unclear. After stimulation of white-matter axons, synaptic-like potentials mediated by α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionic acid (AMPA) receptors have been recorded in patch-clamped NG2+ glia (Kukley et al, 2007).
In human white matter, the Excitatory Amino Acid Transporter 1 is expressed in oligodendrocytes and astrocytes, whereas Excitatory Amino Acid Transporter 2, which has the largest role in regulating extracellular glutamate concentration, is essentially located throughout the processes of astrocytes (Domercq and Matute, 1999; Vallejo-Illarramendi et al, 2006). These transporters move glutamate into astrocytes and oligodendrocytes against a steep concentration gradient by coupling glutamate translocation to the transmembrane Na+, K+ gradients. These gradients are maintained by the membrane Na+/K+-ATP pump, such that glutamate uptake is ultimately ATP dependent.
Reduced activity of the astrocytic Na+/K+-ATP pump will lead to high extracellular K+ concentrations and reversal of glutamate uptake by glutamate transporters (Figure 1B; Rose et al, 2009). Peripheral astrocytic processes containing mitochondria (Lovatt et al, 2007) terminate as fine astrocytic endings that contact the axonal nodes (Raine, 1984). There is evidence from in-vitro studies that PCr, which is transported from the mitochondria, contributes as an energy source in the distal astrocytic processes, which contain glutamate transporters (Reichenbach et al, 2010). Inhibition of the Na+/K+ pump in astrocyte cultures by ouabain decreases energy expenditure, and led to a raise in PCr levels (Silver and Erecinska, 1997). However, a glutamate challenge to cultured astrocytes, requiring enhanced glutamate uptake, was associated with enhanced PCr consumption (Fonseca et al, 2005; Sonnewald et al, 1997). The addition of the glutamine synthetase inhibitor methionine sulfoximine had no effect on the PCr concentrations, suggesting that Na+/K+ pump activity was involved (Sonnewald et al, 1997).
Compared with healthy controls, subjects with MS have increased glutamate levels throughout the normal appearing white matter (Srinivasan et al, 2005), and in the cerebrospinal fluid (Sarchielli et al, 2003). Central myelinated axons express functional kainate and Ca2+ permeable AMPA receptors, which on overstimulation by glutamate may lead to excitotoxic damage of axons, caused by an increased influx of Ca2+(Ouardouz et al, 2009a, 2009b).
Oligodendrocytes, expressing AMPA/kainate and N-methyl--aspartatereceptors, are also sensitive to glutamate toxicity, which is associated with caspase-3 activation, DNA fragmentation, apoptotic cell death, and demyelination (Domercq et al, 2005; Karadottir and Attwell, 2007; Salter and Fern, 2005; Xu et al, 2008). Damage of the myelin sheath itself may contribute to axonal degeneration by reducing trophic support to axons and impairing axonal transport (Nave, 2010). Irvine and Blakemore (2008) elegantly showed that myelin loss contributes to the axon loss, and that remyelination can protect against axonal loss. They inhibited remyelination in the cuprizone model by exposing the brain to X-irradiation before cuprizone intoxication. This resulted in a significant increase in the extent of axonal degeneration and loss compared with nonirradiated cuprizone-fed mice. Restoring the remyelinating capacity in these X-irradiated mice, by transplanting embryo-derived neural progenitors, resulted in a significant increase in axon survival compared with nontransplanted X-irradiated cuprizone-intoxicated mice.
Axonal energy metabolism
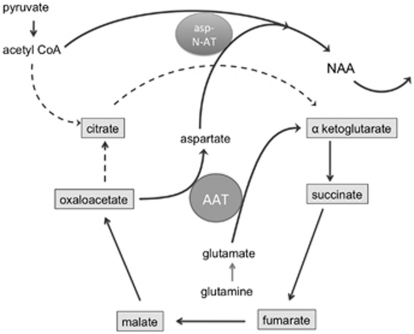
Axonal mitochondrial ATP metabolism and the synthesis of NAA are indirectly linked (Figure 2). Aspartate aminotransferase facilitates the conversion of glutamate to α-ketoglutarate and oxaloacetate to aspartate. Glutamate in neurons and their axons is mainly derived from glutamine that is shuttled from astrocytes to neurons, because pyruvate carboxylase is a glia-specific enzyme (Hertz et al, 2007). Pyruvate kinase converts pyruvate to oxaloacetate. After condensation of oxaloacetate with acetyl CoA, the resulting citrate molecule can be converted to α-ketoglutarate and subsequently to glutamine. There are some indications that carboxylation of pyruvate, supporting the formation of glutamate, may also occur in neurons, but the mechanism is not clear (Fan et al, 2010; Hassel, 2001). Acetylation of aspartate by the neuronal enzyme aspartate N-acetyltransferase results in the formation of NAA that is exported from mitochondria. The formation of NAA thereby favors the conversion of glutamate to α-ketoglutarate, which is a mechanism in neurons to bypass the slower citrate synthase reaction in the tricarboxylic acid cycle (Yudkoff et al, 1994). The levels of NAA in normal appearing white matter, as measured by 1H magnetic resonance spectroscopy, are thus considered to be both a marker of axonal mitochondrial function and axonal integrity.
Figure 2.
Schematic representation of the possible mechanism underlying N-acetylaspartate (NAA) synthesis by axonal mitochondria. The dashed lines indicate the part of the tricarboxylic acid cycle in neurons that can be bypassed by the conversion of glutamate and oxaloacetate to α-ketoglutarate and aspartate via the enzyme aspartate aminotransferase (AAT). Glutamine is synthetized in astrocytes, released in the extracellular space and taken up by the axons. Acetylation of aspartate by aspartate N-acetyltransferase (asp-N-AT) leads to the formation of NAA, which is removed from neuronal mitochondria, thereby favoring the conversion of glutamate to α-ketoglutarate, which can enter the truncated tricarboxylic acid cycle for adenosine triphosphate (ATP) production.
Several 1H magnetic resonance spectroscopy studies of the normal appearing white matter in MS subjects showed decreased levels of NAA compared with healthy controls (Aboul-Enein et al, 2010; Chard et al, 2002; De Stefano et al, 1998, 2001, 2002; Fu et al, 1998; Leary et al, 1999; Lee et al, 2000). Decreased NAA levels in normal appearing white matter are already present at the early stages of the disease and progresses over time (De Stefano et al, 2001). Cader et al (2007) investigated the relationship between callosal size, diffusion magnetic resonance imaging parameters, and NAA concentrations in the corpus callosum in MS patients. Relative changes in NAA were not directly related to diffusion-derived parameters of axonal loss. Another study in MS patients estimated the structural contributions to NAA, as assessed by axial diffusivity derived from diffusion tensor imaging and cross-sectional volumetric imaging in the spinal cord (Ciccarelli et al, 2010b). Lower residual variance in NAA, reflecting information specific to axonal mitochondrial metabolism, was associated with greater clinical disability independent of structural damage. These findings support the idea that metabolic mitochondrial dysfunction in axons, and not just axonal loss, is an important determinant of reduced NAA concentrations in the normal appearing white matter of MS patients.
Reductions of NAA in normal appearing white matter of drug naive MS subjects were partially reversible in a 2-year longitudinal assessment in the early stages of relapsing-remitting MS (Tiberio et al, 2006). Only 30% of the patients in this study started with interferon β during the evaluation period. Spontaneous recovery of NAA concentrations in focal lesion has also been documented in brain and spinal cord of MS patients (Ciccarelli et al, 2010a; Davie et al, 1994). These spontaneous improvements may reflect either restored mitochondrial activity or a compensatory increase in the number of mitochondria. Histopathological studies found increased numbers of mitochondria and an upregulation of mitochondrial cytochrome C oxidase (complex IV) in axons in both chronic lesions and normal appearing white matter of MS subjects (Mahad et al, 2009; Witte et al, 2009).
An increase in NAA levels in normal appearing white matter of relapsing-remitting MS subjects has been reported after 2 years of treatment with glatiramer acetate (Khan et al, 2005), and after 1 year of treatment with β-interferon (Narayanan et al, 2001). However, numbers of patients in these studies were low, and spontaneous improvement as observed in early onset MS could not be excluded. Another study found no effect of 1-year treatment with β-interferon on white-matter NAA levels in relapsing-remitting MS (Parry et al, 2003). More targeted therapies to improve axonal mitochondrial function should be able to show energy recovering effects within days or weeks. The oral administration of fluoxetine, which is believed to stimulate glycogenolysis in astrocytes, significantly increased the NAA/Cr ratio in cerebral white matter of MS patients within 2 weeks (Mostert et al, 2006).
Possible causes of reduced axonal mitochondrial function
A number of mechanisms may lead to a reduced axonal mitochondrial energy metabolism in the normal appearing white matter of subjects with MS (Figures 3 and 4). A first mechanism may be related to nitric oxide (NO). Nitric oxide synthase (NOS2) is increased in both active focal lesions and throughout the normal appearing white matter (Broholm et al, 2004). On immunostaining, NOS2-positive cells appear to be predominantly astrocytes, and norepinephrine or other agents that elevate cAMP inhibit the expression of NOS2 in astrocytes (Feinstein, 1998; Feinstein et al, 1993). A loss of astrocytic β2 adrenergic receptors in MS might explain why astrocytes in MS plaques and normal appearing white matter express high levels of NOS2 (De Keyser et al, 2004). Enhanced levels of NO can compete with oxygen for the binding domain on cytochrome C oxidase (complex IV in the mitochondrial respiratory chain). This may reduce the electron flow and subsequently ATP synthesis. The increased expression and activity of cytochrome C oxidase, observed in chronic lesions and normal appearing white matter of MS subjects, might represent a compensation mechanism to overcome NO occupancy of the enzyme (Mahad et al, 2009; Witte et al, 2009).
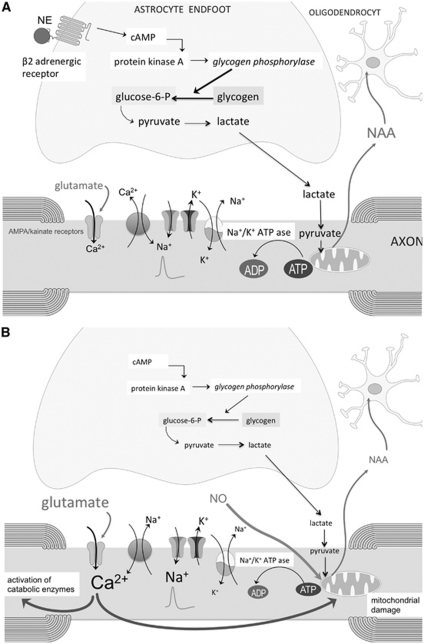
Figure 3.
(A) Schematic representation of the astrocyte-axonal lactate shuttle. Glycogenolysis is mediated by glycogen phosphorylase, which is activated by cAMP-activated protein kinase A in response to neurochemical signals, such as norepinephrine (NE). Glycolysis leads to the formation of lactate, which is taken up by axons via a monocarboxylic acid transporter, and believed to be converted into pyruvate as energy substrate for mitochondrial oxidative metabolism. N-acetylaspartate (NAA) produced by axonal mitochondria is released in the extracellular space and taken up by oligodendrocytes. (B) In multiple sclerosis (MS), white-matter astrocytes are deficient in β2 adrenergic receptors, resulting in decreased formation of cAMP and subsequently a decrease in glycogenolysis, leading to a decreased production of lactate. This causes a reduction in axonal mitochondrial metabolism as evidenced by a decreased formation of NAA. Reduction in adenosine triphosphate (ATP) supply by mitochondria will lead to failure of the axonal Na+/K+ pump, resulting in the accumulation of Na+ in the axoplasma and stimulation of the Na+/Ca2+ exchanger to operate in the reverse Ca2+ import mode. Together with an overactivation of α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionic acid (AMPA)/kainate receptors, due to enhanced extracellular glutamate levels, this will increase intraaxonal levels of Ca2+, which in turn will lead to an overstimulation of various Ca2+-dependent catabolic enzymes and mitochondrial damage. Enhanced levels of nitric oxide (NO) may contribute to mitochondrial dysfunction.
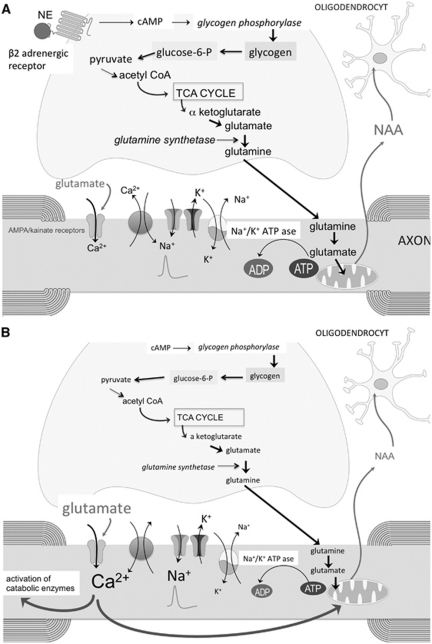
Figure 4.
(A) Schematic representation of the astrocyte-axonal glutamine shuttle. Glycogenolysis is mediated by glycogen phosphorylase, which is activated by cAMP-activated protein kinase A in response to neurochemical signals, such as norepinephrine (NE). Glycogen can be degraded via glucose-6-phosphate into pyruvate, which is introduced in the tricarboxylic acid (TCA) cycle. α-Ketoglutarate can leave the TCA cycle to form glutamate that is converted to glutamine by glutamine synthetase. Glutamine is released in the extracellular space, and taken up by the axons to be used in the axonal TCA cycle (see Figure 2). N-acetylaspartate (NAA) produced by axonal mitochondria is released in the extracellular space and taken up by oligodendrocytes. (B) In multiple sclerosis (MS), white-matter astrocytes are deficient in β2 adrenergic receptors, resulting in decreased glycogenolysis and production of glutamine. This may impair axonal mitochondrial metabolism as evidenced by a decreased formation of NAA, and lead to axonal damage by mechanisms described in Figure 3. AMPA, α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionic acid.
A second mechanism is toxicity caused by increased glutamate levels, which have been detected throughout the normal appearing white matter (Srinivasan et al, 2005). Enhanced extracellular glutamate levels might result from a reduced astrocyte energy metabolism and failure of glutamate uptake (see above). An increased influx of Ca2+ into axons by overstimulation of AMPA/kainate receptors (Ouardouz et al, 2009a, 2009b) may damage mitochondria by promoting Ca2+ entry into the matrix, opening of the permeability transition pore, and release of cytochrome C into the cytosol (Gunter et al, 2004).
A third possible mechanism is an impaired glycogenolysis caused by a deficiency of β2 adrenergic receptors in white-matter astrocytes (De Keyser et al, 1999; Zeinstra et al, 2000). Astrocytes are the main glycogen reservoir in the central nervous system. In-vitro studies on mouse central white matter suggest that activation of astrocytic β adrenergic receptors by norepinephrine stimulates glycogenolysis to produce lactate that is released by astrocytes and is taken up by axons, where it may serve as energy source (Figure 3A; Brown et al, 2004; Brown and Ransom, 2007; Tekkok et al, 2005; Wender et al, 2000). In axons, lactate is converted to pyruvate, which is a source of acetyl CoA required for the synthesis of NAA. In isolated rat brain mitochondria, the efflux of NAA was no longer detectable in the absence of pyruvate (Patel and Clark, 1979). In MS, decreased astrocytic glycogenolysis and lactate formation may compromise axonal mitochondrial metabolism and the synthesis of NAA (Figure 3B). A contribution of astrocyte glycogenolysis in axonal mitochondrial energy metabolism in MS is supported by the finding that a short course with fluoxetine, which stimulates glycogenolysis in primary cultures of mice astrocytes (Allaman et al, 2011; Zhang et al, 1993) increased NAA levels in the centrum semiovale of MS subjects (Mostert et al, 2006).
A fourth mechanism to be considered is a reduced astrocytic oxidative metabolism and decreased astrocytic synthesis of glutamine. This would impair the glutamine shuttle as energy source for axons (Figures 2, 4A, and 4B; Hertz and Gibbs, 2009). Compared with controls, several nuclear-encoded mitochondrial genes and the functional activities of mitochondrial respiratory chain complexes I and III were decreased in motor cortex from MS patients (Dutta et al, 2006). The authors claimed that the reduced mitochondrial gene expression was specific for neurons, but this is difficult to prove since protoplasmic astrocytes in motor cortex also exhibit robust oxidative metabolism (Lovatt et al, 2007). Whether a reduced oxidative metabolism occurs in white-matter astrocytes in MS has not been studied. Compared with myelinated hippocampus and demyelinated motor cortex, demyelinated hippocampus dissected from postmortem MS brains showed a downregulation of glutamine synthetase (Dutta et al, 2011). A deficiency in white-matter astrocytic β2 adrenergic receptors and reduced glycogenolysis in MS might impair astrocytic oxidative metabolism and glutamine synthetase activity. Support for this hypothesis comes from studies showing that the addition of dibutyryl-cAMP, which is a cell permeable cAMP analog, enhanced glutamine synthetase activity in astrocyte cultures (Brookes, 1992; Stanimirovic et al, 1999).
Enhanced consumption of ATP leads to an increase in oxypurines (uric acid, hypoxanthine, and xanthine) and purine nucleosides (inosine, adenosine, and guanosine), which are ATP breakdown products. Higher cerebrospinal fluid and serum concentrations of these end products were found in MS subjects compared with controls (Amorini et al, 2009; Lazzarino et al, 2010). In a follow-up study, higher baseline ATP metabolites were associated with a more severe progression of disability and brain atrophy 3 years later, suggesting that an increased energy demand precedes the axonal degeneration in MS. Neuron-specific enolase is a critical enzyme in neuro-axonal glycolysis, where it converts 2-phospho-D glycerate to phosphoenolpyruvate. Multiple sclerosis patients with a clinically relevant progression of disability after 5 years of follow-up had lower baseline plasma neuron-specific enolase levels than those who remained clinically stable (Koch et al, 2007b), supporting the hypothesis that reduced axonal metabolic activity may precede axonal degeneration and progression of disability in MS.
Consequences of axonal energy failure
A decreased ATP production by mitochondria reduces the activity of the axolemmal Na+/K+ pump, leading to intraaxonal accumulation of Na+ and relative axonal depolarization. This may open voltage-gated Ca2+ channels and induce reversal of the Na+/K+ exchanger, leading to intraaxonal accumulation of Ca2+ (Figure 3). The intraaxonal Ca2+ overload will damage mitochondria (see above) and inappropriately stimulate a variety of Ca2+-dependent catabolic enzyme systems, including proteases, phospholipases, and calpains, ultimately leading to axonal degeneration (Stys, 2005). In addition, reduced formation of NAA may impair myelin membrane turnover and lead to loss of myelin. The maintenance of myelin requires a continuous and dynamic process of myelin component catabolism and recycling (Ando et al, 2003). N-acetylaspartate is transported from axons to the cytoplasm of oligodendrocytes, where aspartoacylase cleaves the acetate moiety for use in fatty acid and steroid synthesis. The fatty acids and steroids produced then go on to be used as building blocks for myelin lipid synthesis (Moffett et al, 2007). As mentioned above, axons losing their myelin sheath are prone to degeneration (Irvine and Blakemore, 2008).
Benign multiple sclerosis
It has long been recognized that a subgroup of individuals with MS shows little or no progression in severity of the disease over time. This so-called “benign MS” can be arbitrarily defined by minimal or no disability after at least 10 years of observation (Ramsaransing and De Keyser, 2006). A less axonal degenerative process appears to be present in this subset of MS patients (Gauthier et al, 2009). Compared with controls, patients with benign MS only showed a nonsignificant trend for elevated PCr ratios in the normal appearing white matter (Steen et al, 2010), and NAA levels in the normal appearing white matter were also relatively preserved (Benedetti et al, 2009; Davie et al, 1997; Steen et al, 2010). Patients with a relatively benign course of MS therefore represent an interesting subgroup to better understand compensatory mechanisms of astrocytic and axonal energy metabolism. It is not known whether patients with benign MS have less elevated glutamate levels in the normal appearing white matter. Genetic association studies may give worthwhile clues. A recent genome-wide association analysis in patients with MS found a significant association between genes with high relevance to glutamate biology and both brain glutamate levels and the degree of neurodegeneration over 1 year of follow-up (Baranzini et al, 2010). This finding needs to be confirmed, and the role of the gene products clarified.
Conclusion
Evidence is evolving that a defective axonal energy metabolism has a role in the diffuse axonal degeneration in MS. A number of findings suggest that at least part of this defective axonal metabolism might be secondary to astrocyte dysfunction. A deficiency in astrocytic β2 adrenergic receptors may be responsible for a reduced glycogenolysis, resulting in a decreased formation of lactate and glutamine, which are energy sources for axons, and for increased levels of NO. An impaired PCr metabolism in astrocytes may lead to increased levels of glutamate in the extracellular space surrounding axons. All these mechanisms can converge to an intraaxonal Ca2+ overload that further impairs mitochondrial function and stimulates catabolic enzymes, leading to axonal degeneration. In addition, decreased NAA levels and excitotoxic damage of oligodendrocytes may impair axonal myelination, and contribute to axonal degeneration. A better insight into these different processes and the protective factors that underlie a relatively benign disease course might ultimately lead to new therapies that slow down the progression of disability in patients with MS.
Acknowledgments
Our research on the role of astrocytes in MS is supported by FWO Belgium (http://www.fwo.be) and the Belgian Charcot Foundation (http://www.fondation-charcot.org).
The authors declare no conflict of interest.
References
- Aboul-Enein F, Krssak M, Hoftberger R, Prayer D, Kristoferitsch W. Reduced NAA-levels in the NAWM of patients with MS is a feature of progression. A study with quantitative magnetic resonance spectroscopy at 3 Tesla. PLoS One. 2010;5:e11625. doi: 10.1371/journal.pone.0011625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksenov M, Aksenova M, Butterfield DA, Markesbery WR. Oxidative modification of creatine kinase BB in Alzheimer's disease brain. J Neurochem. 2000;74:2520–2527. doi: 10.1046/j.1471-4159.2000.0742520.x. [DOI] [PubMed] [Google Scholar]
- Aksenova MV, Aksenov MY, Payne RM, Trojanowski JQ, Schmidt ML, Carney JM, Butterfield DA, Markesbery WR. Oxidation of cytosolic proteins and expression of creatine kinase BB in frontal lobe in different neurodegenerative disorders. Dement Geriatr Cogn Disord. 1999;10:158–165. doi: 10.1159/000017098. [DOI] [PubMed] [Google Scholar]
- Alix JJ, Domingues AM. White matter synapses: form, function, and dysfunction. Neurology. 2011;76:397–404. doi: 10.1212/WNL.0b013e3182088273. [DOI] [PubMed] [Google Scholar]
- Allaman I, Fiumelli H, Magistretti PJ, Martin JL. Fluoxetine regulates the expression of neurotrophic/growth factors and glucose metabolism in astrocytes. Psychopharmacology. 2011;216:75–84. doi: 10.1007/s00213-011-2190-y. [DOI] [PubMed] [Google Scholar]
- Amorini AM, Petzold A, Tavazzi B, Eikelenboom J, Keir G, Belli A, Giovannoni G, Di Pietro V, Polman C, D'Urso S, Vagnozzi R, Uitdehaag B, Lazzarino G. Increase of uric acid and purine compounds in biological fluids of multiple sclerosis patients. Clin Biochem. 2009;42:1001–1006. doi: 10.1016/j.clinbiochem.2009.03.020. [DOI] [PubMed] [Google Scholar]
- Anderson CM, Swanson RA. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia. 2000;32:1–14. [PubMed] [Google Scholar]
- Ando S, Tanaka Y, Toyoda Y, Kon K. Turnover of myelin lipids in aging brain. Neurochem Res. 2003;28:5–13. doi: 10.1023/a:1021635826032. [DOI] [PubMed] [Google Scholar]
- Baranzini SE, Srinivasan R, Khankhanian P, Okuda DT, Nelson SJ, Matthews PM, Hauser SL, Oksenberg JR, Pelletier D. Genetic variation influences glutamate concentrations in brains of patients with multiple sclerosis. Brain. 2010;133:2603–2611. doi: 10.1093/brain/awq192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates TE, Strangward M, Keelan J, Davey GP, Munro PM, Clark JB. Inhibition of N-acetylaspartate production: implications for 1H MRS studies in vivo. Neuroreport. 1996;7:1397–1400. [PubMed] [Google Scholar]
- Benedetti B, Rovaris M, Rocca MA, Caputo D, Zaffaroni M, Capra R, Bertolotto A, Martinelli V, Comi G, Filippi M. In-vivo evidence for stable neuroaxonal damage in the brain of patients with benign multiple sclerosis. Mult Scler. 2009;15:789–794. doi: 10.1177/1352458509103714. [DOI] [PubMed] [Google Scholar]
- Bergers E, Bot JC, De Groot CJ, Polman CH, Lycklama a Nijeholt GJ, Castelijns JA, van der Valk P, Barkhof F. Axonal damage in the spinal cord of MS patients occurs largely independent of T2 MRI lesions. Neurology. 2002;59:1766–1771. doi: 10.1212/01.wnl.0000036566.00866.26. [DOI] [PubMed] [Google Scholar]
- Broholm H, Andersen B, Wanscher B, Frederiksen JL, Rubin I, Pakkenberg B, Larsson HB, Lauritzen M. Nitric oxide synthase expression and enzymatic activity in multiple sclerosis. Acta Neurol Scand. 2004;109:261–269. doi: 10.1111/j.1600-0404.2004.00207.x. [DOI] [PubMed] [Google Scholar]
- Brookes N. Regulation of the glutamine content of astrocytes by cAMP and hydrocortisone: effect of pH. Neurosci Lett. 1992;147:139–142. doi: 10.1016/0304-3940(92)90579-v. [DOI] [PubMed] [Google Scholar]
- Brosnan JT, Brosnan ME. Creatine: endogenous metabolite, dietary, and therapeutic supplement. Annu Rev Nutr. 2007;27:241–261. doi: 10.1146/annurev.nutr.27.061406.093621. [DOI] [PubMed] [Google Scholar]
- Brown AM, Baltan Tekkok S, Ransom BR. Energy transfer from astrocytes to axons: the role of CNS glycogen. Neurochem Int. 2004;45:529–536. doi: 10.1016/j.neuint.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Brown AM, Ransom BR. Astrocyte glycogen and brain energy metabolism. Glia. 2007;55:1263–1271. doi: 10.1002/glia.20557. [DOI] [PubMed] [Google Scholar]
- Cader S, Johansen-Berg H, Wylezinska M, Palace J, Behrens TE, Smith S, Matthews PM. Discordant white matter N-acetylasparate and diffusion MRI measures suggest that chronic metabolic dysfunction contributes to axonal pathology in multiple sclerosis. Neuroimage. 2007;36:19–27. doi: 10.1016/j.neuroimage.2007.02.036. [DOI] [PubMed] [Google Scholar]
- Chard DT, Griffin CM, McLean MA, Kapeller P, Kapoor R, Thompson AJ, Miller DH. Brain metabolite changes in cortical grey and normal-appearing white matter in clinically early relapsing-remitting multiple sclerosis. Brain. 2002;125:2342–2352. doi: 10.1093/brain/awf240. [DOI] [PubMed] [Google Scholar]
- Chiti Z, Teschemacher AG. Exocytosis of norepinephrine at axon varicosities and neuronal cell bodies in the rat brain. FASEB J. 2007;21:2540–2550. doi: 10.1096/fj.06-7342com. [DOI] [PubMed] [Google Scholar]
- Ciccarelli O, Altmann DR, McLean MA, Wheeler-Kingshott CA, Wimpey K, Miller DH, Thompson AJ. Spinal cord repair in MS: does mitochondrial metabolism play a role. Neurology. 2010a;74:721–727. doi: 10.1212/WNL.0b013e3181d26968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccarelli O, Toosy AT, De Stefano N, Wheeler-Kingshott CA, Miller DH, Thompson AJ. Assessing neuronal metabolism in vivo by modeling imaging measures. J Neurosci. 2010b;30:15030–15033. doi: 10.1523/JNEUROSCI.3330-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:1502–1517. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- Confavreux C, Vukusic S. Natural history of multiple sclerosis: a unifying concept. Brain. 2006;129:606–616. doi: 10.1093/brain/awl007. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Davie CA, Barker GJ, Thompson AJ, Tofts PS, McDonald WI, Miller DH. 1H magnetic resonance spectroscopy of chronic cerebral white matter lesions and normal appearing white matter in multiple sclerosis. J Neurol Neurosurg Psychiatry. 1997;63:736–742. doi: 10.1136/jnnp.63.6.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davie CA, Hawkins CP, Barker GJ, Brennan A, Tofts PS, Miller DH, McDonald WI. Serial proton magnetic resonance spectroscopy in acute multiple sclerosis lesions. Brain. 1994;117 (Part 1:49–58. doi: 10.1093/brain/117.1.49. [DOI] [PubMed] [Google Scholar]
- De Keyser J, Mostert JP, Koch MW. Dysfunctional astrocytes as key players in the pathogenesis of central nervous system disorders. J Neurol Sci. 2008;267:3–16. doi: 10.1016/j.jns.2007.08.044. [DOI] [PubMed] [Google Scholar]
- De Keyser J, Wilczak N, Leta R, Streetland C. Astrocytes in multiple sclerosis lack beta-2 adrenergic receptors. Neurology. 1999;53:1628–1633. doi: 10.1212/wnl.53.8.1628. [DOI] [PubMed] [Google Scholar]
- De Keyser J, Zeinstra E, Wilczak N. Astrocytic beta2-adrenergic receptors and multiple sclerosis. Neurobiol Dis. 2004;15:331–339. doi: 10.1016/j.nbd.2003.10.012. [DOI] [PubMed] [Google Scholar]
- De Stefano N, Matthews PM, Fu L, Narayanan S, Stanley J, Francis GS, Antel JP, Arnold DL. Axonal damage correlates with disability in patients with relapsing-remitting multiple sclerosis. Results of a longitudinal magnetic resonance spectroscopy study. Brain. 1998;121 (Part 8:1469–1477. doi: 10.1093/brain/121.8.1469. [DOI] [PubMed] [Google Scholar]
- De Stefano N, Narayanan S, Francis GS, Arnaoutelis R, Tartaglia MC, Antel JP, Matthews PM, Arnold DL. Evidence of axonal damage in the early stages of multiple sclerosis and its relevance to disability. Arch Neurol. 2001;58:65–70. doi: 10.1001/archneur.58.1.65. [DOI] [PubMed] [Google Scholar]
- De Stefano N, Narayanan S, Francis SJ, Smith S, Mortilla M, Tartaglia MC, Bartolozzi ML, Guidi L, Federico A, Arnold DL. Diffuse axonal and tissue injury in patients with multiple sclerosis with low cerebral lesion load and no disability. Arch Neurol. 2002;59:1565–1571. doi: 10.1001/archneur.59.10.1565. [DOI] [PubMed] [Google Scholar]
- DeLuca GC, Williams K, Evangelou N, Ebers GC, Esiri MM. The contribution of demyelination to axonal loss in multiple sclerosis. Brain. 2006;129:1507–1516. doi: 10.1093/brain/awl074. [DOI] [PubMed] [Google Scholar]
- Domercq M, Etxebarria E, Perez-Samartin A, Matute C. Excitotoxic oligodendrocyte death and axonal damage induced by glutamate transporter inhibition. Glia. 2005;52:36–46. doi: 10.1002/glia.20221. [DOI] [PubMed] [Google Scholar]
- Domercq M, Matute C. Expression of glutamate transporters in the adult bovine corpus callosum. Brain Res Mol Brain Res. 1999;67:296–302. doi: 10.1016/s0169-328x(99)00072-8. [DOI] [PubMed] [Google Scholar]
- Dutta R, Chang A, Doud MK, Kidd GJ, Ribaudo MV, Young EA, Fox RJ, Staugaitis SM, Trapp BD. Demyelination causes synaptic alterations in hippocampi from multiple sclerosis patients. Ann Neurol. 2011;69:445–454. doi: 10.1002/ana.22337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta R, McDonough J, Yin X, Peterson J, Chang A, Torres T, Gudz T, Macklin WB, Lewis DA, Fox RJ, Rudick R, Mirnics K, Trapp BD. Mitochondrial dysfunction as a cause of axonal degeneration in multiple sclerosis patients. Ann Neurol. 2006;59:478–489. doi: 10.1002/ana.20736. [DOI] [PubMed] [Google Scholar]
- Evangelou N, Esiri MM, Smith S, Palace J, Matthews PM. Quantitative pathological evidence for axonal loss in normal appearing white matter in multiple sclerosis. Ann Neurol. 2000;47:391–395. [PubMed] [Google Scholar]
- Fan TW, Yuan P, Lane AN, Higashi RM, Wang Y, Hamidi AB, Zhou R, Guitart X, Chen G, Manji HK, Kaddurah-Daouk R. Stable isotope-resolved metabolomic analysis of lithium effects on glial-neuronal metabolism and interactions. Metabolomics. 2010;6:165–179. doi: 10.1007/s11306-010-0208-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein DL. Suppression of astroglial nitric oxide synthase expression by norepinephrine results from decreased NOS-2 promoter activity. J Neurochem. 1998;70:1484–1496. doi: 10.1046/j.1471-4159.1998.70041484.x. [DOI] [PubMed] [Google Scholar]
- Feinstein DL, Galea E, Reis DJ. Norepinephrine suppresses inducible nitric oxide synthase activity in rat astroglial cultures. J Neurochem. 1993;60:1945–1948. doi: 10.1111/j.1471-4159.1993.tb13425.x. [DOI] [PubMed] [Google Scholar]
- Ferguson B, Matyszak MK, Esiri MM, Perry VH. Axonal damage in acute multiple sclerosis lesions. Brain. 1997;120 (Part 3:393–399. doi: 10.1093/brain/120.3.393. [DOI] [PubMed] [Google Scholar]
- Fonseca LL, Monteiro MA, Alves PM, Carrondo MJ, Santos H. Cultures of rat astrocytes challenged with a steady supply of glutamate: new model to study flux distribution in the glutamate-glutamine cycle. Glia. 2005;51:286–296. doi: 10.1002/glia.20209. [DOI] [PubMed] [Google Scholar]
- Frischer JM, Bramow S, Dal-Bianco A, Lucchinetti CF, Rauschka H, Schmidbauer M, Laursen H, Sorensen PS, Lassmann H. The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain. 2009;132:1175–1189. doi: 10.1093/brain/awp070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu L, Matthews PM, De Stefano N, Worsley KJ, Narayanan S, Francis GS, Antel JP, Wolfson C, Arnold DL. Imaging axonal damage of normal-appearing white matter in multiple sclerosis. Brain. 1998;121 (Part 1:103–113. doi: 10.1093/brain/121.1.103. [DOI] [PubMed] [Google Scholar]
- Gauthier SA, Berger AM, Liptak Z, Duan Y, Egorova S, Buckle GJ, Glanz BI, Khoury SJ, Bakshi R, Weiner HL, Guttmann CR. Rate of brain atrophy in benign versus early multiple sclerosis. Arch Neurol. 2009;66:234–237. doi: 10.1001/archneurol.2008.567. [DOI] [PubMed] [Google Scholar]
- Geeraedts F, Wilczak N, van Binnendijk R, De Keyser J. Search for morbillivirus proteins in multiple sclerosis brain tissue. Neuroreport. 2004;15:27–32. doi: 10.1097/00001756-200401190-00007. [DOI] [PubMed] [Google Scholar]
- Graumann U, Reynolds R, Steck AJ, Schaeren-Wiemers N. Molecular changes in normal appearing white matter in multiple sclerosis are characteristic of neuroprotective mechanisms against hypoxic insult. Brain Pathol. 2003;13:554–573. doi: 10.1111/j.1750-3639.2003.tb00485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunter TE, Yule DI, Gunter KK, Eliseev RA, Salter JD. Calcium and mitochondria. FEBS Lett. 2004;567:96–102. doi: 10.1016/j.febslet.2004.03.071. [DOI] [PubMed] [Google Scholar]
- Hassel B. Pyruvate carboxylation in neurons. J Neurosci Res. 2001;66:755–762. doi: 10.1002/jnr.10044. [DOI] [PubMed] [Google Scholar]
- Hertz L, Gibbs ME. What learning in day-old chickens can teach a neurochemist: focus on astrocyte metabolism. J Neurochem. 2009;109 (Suppl 1:10–16. doi: 10.1111/j.1471-4159.2009.05939.x. [DOI] [PubMed] [Google Scholar]
- Hertz L, Peng L, Dienel GA. Energy metabolism in astrocytes: high rate of oxidative metabolism and spatiotemporal dependence on glycolysis/glycogenolysis. J Cereb Blood Flow Metab. 2007;27:219–249. doi: 10.1038/sj.jcbfm.9600343. [DOI] [PubMed] [Google Scholar]
- Husted CA, Goodin DS, Hugg JW, Maudsley AA, Tsuruda JS, de Bie SH, Fein G, Matson GB, Weiner MW. Biochemical alterations in multiple sclerosis lesions and normal-appearing white matter detected by in vivo 31P and 1H spectroscopic imaging. Ann Neurol. 1994;36:157–165. doi: 10.1002/ana.410360207. [DOI] [PubMed] [Google Scholar]
- Irvine KA, Blakemore WF. Remyelination protects axons from demyelination-associated axon degeneration. Brain. 2008;131:1464–1477. doi: 10.1093/brain/awn080. [DOI] [PubMed] [Google Scholar]
- Karadottir R, Attwell D. Neurotransmitter receptors in the life and death of oligodendrocytes. Neuroscience. 2007;145:1426–1438. doi: 10.1016/j.neuroscience.2006.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan O, Shen Y, Caon C, Bao F, Ching W, Reznar M, Buccheister A, Hu J, Latif Z, Tselis A, Lisak R. Axonal metabolic recovery and potential neuroprotective effect of glatiramer acetate in relapsing-remitting multiple sclerosis. Mult Scler. 2005;11:646–651. doi: 10.1191/1352458505ms1234oa. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK, Nedergaard M. Functions of astrocytes and their potential as therapeutic targets. Neurotherapeutics. 2010;7:338–353. doi: 10.1016/j.nurt.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M, Mostert J, Heersema D, De Keyser J. Progression in multiple sclerosis: further evidence of an age dependent process. J Neurol Sci. 2007a;255:35–41. doi: 10.1016/j.jns.2007.01.067. [DOI] [PubMed] [Google Scholar]
- Koch M, Mostert J, Heersema D, Teelken A, De Keyser J. Plasma S100beta and NSE levels and progression in multiple sclerosis. J Neurol Sci. 2007b;252:154–158. doi: 10.1016/j.jns.2006.11.012. [DOI] [PubMed] [Google Scholar]
- Kukley M, Capetillo-Zarate E, Dietrich D. Vesicular glutamate release from axons in white matter. Nat Neurosci. 2007;10:311–320. doi: 10.1038/nn1850. [DOI] [PubMed] [Google Scholar]
- Kutzelnigg A, Lucchinetti CF, Stadelmann C, Bruck W, Rauschka H, Bergmann M, Schmidbauer M, Parisi JE, Lassmann H. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain. 2005;128:2705–2712. doi: 10.1093/brain/awh641. [DOI] [PubMed] [Google Scholar]
- Kuzhikandathil EV, Molloy GR. Transcription of the brain creatine kinase gene in glial cells is modulated by cyclic AMP-dependent protein kinase. J Neurosci Res. 1994;39:70–82. doi: 10.1002/jnr.490390110. [DOI] [PubMed] [Google Scholar]
- Kuzhikandathil EV, Molloy GR. Proximal promoter of the rat brain creatine kinase gene lacks a consensus CRE element but is essential for the cAMP-mediated increased transcription in glioblastoma cells. J Neurosci Res. 1999;56:371–385. doi: 10.1002/(SICI)1097-4547(19990515)56:4<371::AID-JNR5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Langemann H, Kabiersch A, Newcombe J. Measurement of low-molecular-weight antioxidants, uric acid, tyrosine and tryptophan in plaques and white matter from patients with multiple sclerosis. Eur Neurol. 1992;32:248–252. doi: 10.1159/000116835. [DOI] [PubMed] [Google Scholar]
- Lassmann H, Reindl M, Rauschka H, Berger J, Aboul-Enein F, Berger T, Zurbriggen A, Lutterotti A, Bruck W, Weber JR, Ullrich R, Schmidbauer M, Jellinger K, Vandevelde M. A new paraclinical CSF marker for hypoxia-like tissue damage in multiple sclerosis lesions. Brain. 2003;126:1347–1357. doi: 10.1093/brain/awg127. [DOI] [PubMed] [Google Scholar]
- Lazzarino G, Amorini AM, Eikelenboom MJ, Killestein J, Belli A, Di Pietro V, Tavazzi B, Barkhof F, Polman CH, Uitdehaag BM, Petzold A. Cerebrospinal fluid ATP metabolites in multiple sclerosis. Mult Scler. 2010;16:549–554. doi: 10.1177/1352458510364196. [DOI] [PubMed] [Google Scholar]
- Leary SM, Davie CA, Parker GJ, Stevenson VL, Wang L, Barker GJ, Miller DH, Thompson AJ. 1H magnetic resonance spectroscopy of normal appearing white matter in primary progressive multiple sclerosis. J Neurol. 1999;246:1023–1026. doi: 10.1007/s004150050507. [DOI] [PubMed] [Google Scholar]
- Lee MA, Blamire AM, Pendlebury S, Ho KH, Mills KR, Styles P, Palace J, Matthews PM. Axonal injury or loss in the internal capsule and motor impairment in multiple sclerosis. Arch Neurol. 2000;57:65–70. doi: 10.1001/archneur.57.1.65. [DOI] [PubMed] [Google Scholar]
- Lovatt D, Sonnewald U, Waagepetersen HS, Schousboe A, He W, Lin JH, Han X, Takano T, Wang S, Sim FJ, Goldman SA, Nedergaard M. The transcriptome and metabolic gene signature of protoplasmic astrocytes in the adult murine cortex. J Neurosci. 2007;27:12255–12266. doi: 10.1523/JNEUROSCI.3404-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahad DJ, Ziabreva I, Campbell G, Lax N, White K, Hanson PS, Lassmann H, Turnbull DM. Mitochondrial changes within axons in multiple sclerosis. Brain. 2009;132:1161–1174. doi: 10.1093/brain/awp046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz I, Lucchinetti CF, Openshaw H, Garcia-Merino A, Lassmann H, Freedman MS, Atkins HL, Azzarelli B, Kolar OJ, Bruck W. Autologous haematopoietic stem cell transplantation fails to stop demyelination and neurodegeneration in multiple sclerosis. Brain. 2007;130:1254–1262. doi: 10.1093/brain/awl370. [DOI] [PubMed] [Google Scholar]
- Minderhoud JM, Mooyaart EL, Kamman RL, Teelken AW, Hoogstraten MC, Vencken LM, Gravenmade EJ, van den Burg W. In vivo phosphorus magnetic resonance spectroscopy in multiple sclerosis. Arch Neurol. 1992;49:161–165. doi: 10.1001/archneur.1992.00530260063021. [DOI] [PubMed] [Google Scholar]
- Moffett JR, Ross B, Arun P, Madhavarao CN, Namboodiri AM. N-Acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Prog Neurobiol. 2007;81:89–131. doi: 10.1016/j.pneurobio.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostert JP, Sijens PE, Oudkerk M, De Keyser J. Fluoxetine increases cerebral white matter NAA/Cr ratio in patients with multiple sclerosis. Neurosci Lett. 2006;402:22–24. doi: 10.1016/j.neulet.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Narayanan S, De Stefano N, Francis GS, Arnaoutelis R, Caramanos Z, Collins DL, Pelletier D, Arnason BGW, Antel JP, Arnold DL. Axonal metabolic recovery in multiple sclerosis patients treated with interferon beta-1b. J Neurol. 2001;248:979–986. doi: 10.1007/s004150170052. [DOI] [PubMed] [Google Scholar]
- Nave KA. Myelination and the trophic support of long axons. Nat Rev Neurosci. 2010;11:275–283. doi: 10.1038/nrn2797. [DOI] [PubMed] [Google Scholar]
- Ouardouz M, Coderre E, Basak A, Chen A, Zamponi GW, Hameed S, Rehak R, Yin X, Trapp BD, Stys PK. Glutamate receptors on myelinated spinal cord axons: I. GluR6 kainate receptors. Ann Neurol. 2009a;65:151–159. doi: 10.1002/ana.21533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouardouz M, Coderre E, Zamponi GW, Hameed S, Yin X, Trapp BD, Stys PK. Glutamate receptors on myelinated spinal cord axons: II. AMPA and GluR5 receptors. Ann Neurol. 2009b;65:160–166. doi: 10.1002/ana.21539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry A, Corkill R, Blamire AM, Palace J, Narayanan S, Arnold D, Styles P, Matthews PM. Beta-Interferon treatment does not always slow the progression of axonal injury in multiple sclerosis. J Neurol. 2003;250:171–178. doi: 10.1007/s00415-003-0965-8. [DOI] [PubMed] [Google Scholar]
- Patel TB, Clark JB. Synthesis of N-acetyl--aspartate by rat brain mitochondria and its involvement in mitochondrial/cytosolic carbon transport. Biochem J. 1979;184:539–546. doi: 10.1042/bj1840539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine CS. On the association between perinodal astrocytic processes and the node of Ranvier in the C.N.S. J Neurocytol. 1984;13:21–27. doi: 10.1007/BF01148316. [DOI] [PubMed] [Google Scholar]
- Ramsaransing GS, De Keyser J. Benign course in multiple sclerosis: a review. Acta Neurol Scand. 2006;113:359–369. doi: 10.1111/j.1600-0404.2006.00637.x. [DOI] [PubMed] [Google Scholar]
- Ransom B, Behar T, Nedergaard M. New roles for astrocytes (stars at last) Trends Neurosci. 2003;26:520–522. doi: 10.1016/j.tins.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Reichenbach A, Derouiche A, Kirchhoff F. Morphology and dynamics of perisynaptic glia. Brain Res Rev. 2010;63:11–25. doi: 10.1016/j.brainresrev.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Rose EM, Koo JC, Antflick JE, Ahmed SM, Angers S, Hampson DR. Glutamate transporter coupling to Na,K-ATPase. J Neurosci. 2009;29:8143–8155. doi: 10.1523/JNEUROSCI.1081-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter MG, Fern R. NMDA receptors are expressed in developing oligodendrocyte processes and mediate injury. Nature. 2005;438:1167–1171. doi: 10.1038/nature04301. [DOI] [PubMed] [Google Scholar]
- Sarchielli P, Greco L, Floridi A, Gallai V. Excitatory amino acids and multiple sclerosis: evidence from cerebrospinal fluid. Arch Neurol. 2003;60:1082–1088. doi: 10.1001/archneur.60.8.1082. [DOI] [PubMed] [Google Scholar]
- Seehusen F, Baumgartner W. Axonal pathology and loss precede demyelination and accompany chronic lesions in a spontaneously occurring animal model of multiple sclerosis. Brain Pathol. 2010;20:551–559. doi: 10.1111/j.1750-3639.2009.00332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver IA, Erecinska M. Energetic demands of the Na+/K+ ATPase in mammalian astrocytes. Glia. 1997;21:35–45. doi: 10.1002/(sici)1098-1136(199709)21:1<35::aid-glia4>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Sonnewald U, Westergaard N, Schousboe A. Glutamate transport and metabolism in astrocytes. Glia. 1997;21:56–63. doi: 10.1002/(sici)1098-1136(199709)21:1<56::aid-glia6>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Srinivasan R, Sailasuta N, Hurd R, Nelson S, Pelletier D. Evidence of elevated glutamate in multiple sclerosis using magnetic resonance spectroscopy at 3 T. Brain. 2005;128:1016–1025. doi: 10.1093/brain/awh467. [DOI] [PubMed] [Google Scholar]
- Stanimirovic DB, Ball R, Small DL, Muruganandam A. Developmental regulation of glutamate transporters and glutamine synthetase activity in astrocyte cultures differentiated in vitro. Int J Dev Neurosci. 1999;17:173–184. doi: 10.1016/s0736-5748(99)00028-3. [DOI] [PubMed] [Google Scholar]
- Steen C, Wilczak N, Hoogduin JM, Koch M, De Keyser J. Reduced creatine kinase B activity in multiple sclerosis normal appearing white matter. PLoS One. 2010;5:e10811. doi: 10.1371/journal.pone.0010811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stys PK. General mechanisms of axonal damage and its prevention. J Neurol Sci. 2005;233:3–13. doi: 10.1016/j.jns.2005.03.031. [DOI] [PubMed] [Google Scholar]
- Su KG, Banker G, Bourdette D, Forte M. Axonal degeneration in multiple sclerosis: the mitochondrial hypothesis. Curr Neurol Neurosci Rep. 2009;9:411–417. doi: 10.1007/s11910-009-0060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachikawa M, Fukaya M, Terasaki T, Ohtsuki S, Watanabe M. Distinct cellular expressions of creatine synthetic enzyme GAMT and creatine kinases uCK-Mi and CK-B suggest a novel neuron-glial relationship for brain energy homeostasis. Eur J Neurosci. 2004;20:144–160. doi: 10.1111/j.1460-9568.2004.03478.x. [DOI] [PubMed] [Google Scholar]
- Tekkok SB, Brown AM, Westenbroek R, Pellerin L, Ransom BR. Transfer of glycogen-derived lactate from astrocytes to axons via specific monocarboxylate transporters supports mouse optic nerve activity. J Neurosci Res. 2005;81:644–652. doi: 10.1002/jnr.20573. [DOI] [PubMed] [Google Scholar]
- Thompson RJ, Kynoch PA, Sarjant J. Immunohistochemical localization of creatine kinase-BB isoenzyme to astrocytes in human brain. Brain Res. 1980;201:423–426. doi: 10.1016/0006-8993(80)91046-x. [DOI] [PubMed] [Google Scholar]
- Tiberio M, Chard DT, Altmann DR, Davies G, Griffin CM, McLean MA, Rashid W, Sastre-Garriga J, Thompson AJ, Miller DH. Metabolite changes in early relapsing-remitting multiple sclerosis. A two year follow-up study. J Neurol. 2006;253:224–230. doi: 10.1007/s00415-005-0964-z. [DOI] [PubMed] [Google Scholar]
- Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mork S, Bo L. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998;338:278–285. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- Trapp BD, Stys PK. Virtual hypoxia and chronic necrosis of demyelinated axons in multiple sclerosis. Lancet Neurol. 2009;8:280–291. doi: 10.1016/S1474-4422(09)70043-2. [DOI] [PubMed] [Google Scholar]
- Vallejo-Illarramendi A, Domercq M, Perez-Cerda F, Ravid R, Matute C. Increased expression and function of glutamate transporters in multiple sclerosis. Neurobiol Dis. 2006;21:154–164. doi: 10.1016/j.nbd.2005.06.017. [DOI] [PubMed] [Google Scholar]
- van der Knaap MS, Pouwels PJW.2005Magnetic resonance spectroscopy: basic principles and application in white matter disorders Magnetic resonance of myelination and myelin disorders(van der Knaap MS, Valk J, eds).Berlin: Springer; 859–880. [Google Scholar]
- Vandevelde M, Zurbriggen A. Demyelination in canine distemper virus infection: a review. Acta Neuropathol. 2005;109:56–68. doi: 10.1007/s00401-004-0958-4. [DOI] [PubMed] [Google Scholar]
- Wender R, Brown AM, Fern R, Swanson RA, Farrell K, Ransom BR. Astrocytic glycogen influences axon function and survival during glucose deprivation in central white matter. J Neurosci. 2000;20:6804–6810. doi: 10.1523/JNEUROSCI.20-18-06804.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins A, Scolding N. Protecting axons in multiple sclerosis. Mult Scler. 2008;14:1013–1025. doi: 10.1177/1352458508091370. [DOI] [PubMed] [Google Scholar]
- Witte ME, Bo L, Rodenburg RJ, Belien JA, Musters R, Hazes T, Wintjes LT, Smeitink JA, Geurts JJ, De Vries HE, van der Valk P, van Horssen J. Enhanced number and activity of mitochondria in multiple sclerosis lesions. J Pathol. 2009;219:193–204. doi: 10.1002/path.2582. [DOI] [PubMed] [Google Scholar]
- Wolosker H, Panizzutti R, Engelender S. Inhibition of creatine kinase by S-nitrosoglutathione. FEBS Lett. 1996;392:274–276. doi: 10.1016/0014-5793(96)00829-0. [DOI] [PubMed] [Google Scholar]
- Xu GY, Liu S, Hughes MG, McAdoo DJ. Glutamate-induced losses of oligodendrocytes and neurons and activation of caspase-3 in the rat spinal cord. Neuroscience. 2008;153:1034–1047. doi: 10.1016/j.neuroscience.2008.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudkoff M, Nelson D, Daikhin Y, Erecinska M. Tricarboxylic acid cycle in rat brain synaptosomes. Fluxes and interactions with aspartate aminotransferase and malate/aspartate shuttle. J Biol Chem. 1994;269:27414–27420. [PubMed] [Google Scholar]
- Zeinstra E, Wilczak N, De Keyser J. [3H]dihydroalprenolol binding to beta adrenergic receptors in multiple sclerosis brain. Neurosci Lett. 2000;289:75–77. doi: 10.1016/s0304-3940(00)01254-4. [DOI] [PubMed] [Google Scholar]
- Zhang X, Peng L, Chen Y, Hertz L. Stimulation of glycogenolysis in astrocytes by fluoxetine, an antidepressant acting like 5-HT. Neuroreport. 1993;4:1235–1238. doi: 10.1097/00001756-199309000-00006. [DOI] [PubMed] [Google Scholar]
- Ziskin JL, Nishiyama A, Rubio M, Fukaya M, Bergles DE. Vesicular release of glutamate from unmyelinated axons in white matter. Nat Neurosci. 2007;10:321–330. doi: 10.1038/nn1854. [DOI] [PMC free article] [PubMed] [Google Scholar]