Abstract
Background and Purpose
Perihematomal edema formation and consequent cell death contribute to the delayed brain injury evoked by intracerebral hemorrhage (ICH). In this study we aimed to evaluate the effect of α7 nicotinic acetylcholine receptor (α7nAChR) stimulation, on behavior, brain edema and neuronal apoptosis. Furthermore we aimed to determine the role of the pro-apoptotic glycogen synthase kinase-3β (GSK-3β) after experimental ICH.
Methods
Male CD-1 mice (n=109) were subjected to intracerebral infusion of autologous blood (n=88) or sham surgery (n=21). ICH animals received either vehicle administration, 4 or 12 mg/kg of α7nAChR agonist PHA-543613, 12 mg/kg of α7nAChR agonist PNU-282987, 6 mg/kg of α7nAChR antagonist methyllycaconitine (MLA), 15 μg/kg of phosphatidylinositol 3-kinase (PI3K) inhibitor wortmannin or PHA-543613 combined with MLA or wortmannin. Behavioral deficits and brain water content were evaluated at 24 and 72 hours after surgery. Western blotting and immunofluorescence staining were utilized for the quantification and localization of activated Akt (p-Akt), GSK-3β (p-GSK-3β) and cleaved caspase-3 (CC3). Neuronal cell death was quantified via terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL).
Results
α7nAChR stimulation improved neurological outcome and reduced brain edema at 24 and 72 hours after surgery (p<0.05 compared to vehicle). Furthermore, PHA-543613 treatment increased p-Akt and decreased p-GSK-3β and CC3 expressions in the ipsilateral hemisphere (p<0.05, respectively), which was reversed by MLA and wortmannin. P-Akt, p-GSK-3β and CC3 were generally localized in neurons. PHA-543613 reduced neuronal cell death in the perihematomal area (p<0.05).
Conclusion
α7nAChR stimulation improved functional and morphological outcomes after experimental ICH in mice. PHA-543613 reduced the expression of pro-apoptotic GSK-3β via the PI3K-Akt signaling pathway.
Keywords: α7 nicotinic acetylcholine receptor, glycogen synthase kinase-3, intracerebral hemorrhage, apoptosis, caspase-3, PHA-543613, PHA-282987
Introduction
The prognosis of spontaneous intracerebral hemorrhage (ICH) is often less favorable than that of similar-sized ischemic strokes.1 Approximately 15% of all cerebrovascular accidents are hemorrhagic in nature, not including the considerable proportion of ischemic brain lesions (30%) that will eventually undergo hemorrhagic transformation.2, 3 The expanding blood clot as well as the subsequent formation of brain edema, induce cellular and molecular processes, which provoke neuronal apoptosis, thus aggravating the injury after ICH.3-5 Consequently, prospective treatments reducing brain edema or apoptotic cell death may provide a neuroprotective strategy for ICH-patients.
α7 nicotinic acetylcholine receptors (α7nAChR) are expressed in neurons, neuroglia and endothelial cells of the mammalian brain.6-8 In addition to their effects as ligand-gated ion-channels, α7nAChR stimulation attenuates neuronal apoptosis in various disease models, particularly via activation of the _ENREF_6phosphatidylinositol 3-kinase (PI3K) - Akt signaling pathway.7-9
Activated Akt inhibits the glycogen synthase kinase-3β (GSK-3β) in neurons and nonneuronal cells by serine-9 phosphorylation.10 Several pro-apoptotic stimuli, including oxygen-glucose deprivation and growth factor withdrawal, have been reported to increase the activity -as well as the tyrosine-216 phosphorylation - of GSK-3β.10-12 Phosphorylated GSK-3β (p-GSK-3β, Tyr216) induces caspase-3-activation, an early step in the execution phase of cellular apoptosis.13 Furthermore, stimulated GSK-3β has been found to aggravate the brain injury after experimental ischemic stroke11 and subarachnoid hemorrhage;14 however the role of GSK-3β in ICH remains unexplored.
In this present study we aim to investigate two hypotheses: (A) Selective α7nAChR agonists, PHA-543613 and PNU-282987, ameliorate behavioral and morphological outcomes (brain edema) after experimental ICH in mice and (B) α7nAChR stimulation reduces activated GSK-3β via the PI3K-Akt signaling pathway, eventually decreasing neuronal apoptosis.
Materials and Methods
Animals and Intracerebral Blood Infusion
All procedures were conducted following an institutionally approved protocol in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Male CD-1 mice (n=109, weighing 35-45g; Charles River, Wilmington, MA) were housed in a light and temperature controlled environment with unlimited access to food and water.
Hemorrhagic stroke in mice was induced using a modified double infusion model of autologous whole blood (30μl) as previously reported.15, 16 Briefly, mice were anesthetized via intraperitoneal co-injection of ketamine (100mg/kg) and xylazine (10mg/kg) in a 2:1 v/v ratio. Positioned prone onto a stereotactic head frame (Kopf Instruments, Tujunga, CA), a craniotomy was performed and a 27-gauge needle was inserted into the right hemisphere (stereotactic coordinates from bregma: 0.2 mm anterior, 2.0 mm lateral and 3.0 mm in depth). 5 μl of autologous whole blood, collected from the central tail artery, were infused at a rate of 2 μl/min. The needle was then lowered to the target position of 3.7 mm in depth. After a waiting period of 5 minutes, 25 μl of autologous whole blood were infused into the right basal ganglia. The needle was left in place for an additional 10 minutes after the completed infusion, before being withdrawn at a rate of 1mm/min. The craniotomy was sealed with bone wax and the scalp suture closed. All mice were allowed to fully recover under observation. Sham operated animals were subjected to needle insertion only.
Experimental Groups and Pharmacological Interventions
Experimental groups consisted of mice subjected to ICH (n=88) or sham surgery (n=21). After ICH-induction, animals were randomly selected and treated with either 4 or 12 mg/kg of α7nAChR agonist PHA-543613 (PHA-4mg, PHA-12mg) or with 12 mg/kg of α7nAChR agonist PNU-282987 (PNU-12mg). All treatments were administered as an intraperitoneal injection at 1 hour after surgery. Alternatively, ICH-mice received 6 mg/kg of α7nAChR antagonist methyllycaconitine (MLA) intraperitoneally, at 15 minutes - or PI3K inhibitor wortmannin (15μg/kg) intravenously, at 90 minutes prior to PHA-543613 (12mg/kg) injection (PHA+MLA, PHA+Wort). MLA and wortmannin were also administered without consecutive α7nAChR agonist treatment (MLA-6mg, Wort-15μg). Sham and vehicle animals received an equivalent volume of normal saline, as given to animals in the intervention groups (0.2ml). All drugs were purchased from Sigma-Aldrich (St. Louis, MO) and processed according to previously published protocols.8, 17
Assessment of Behavioral Outcome
Behavioral outcomes were assessed in a blinded fashion at 24 and 72 hours after surgery. The sensorimotor Garcia Test 18 has been modified for use in mice after experimental hemorrhagic stroke. This composite assessment consists of seven individual tests evaluating spontaneous activity (I), axial sensation (II), vibrissae proprioception (III), symmetry of limb movement (IV), lateral turning (V), forelimb outstretching (VI) and climbing (VII). Each test received a score between 0 (worst performance) and 3 (best performance) and a total Garcia Score was calculated as the sum of all sub-tests (maximum = 21 points). For the Corner Turn Test19, mice were allowed to advance into a 30° angled corner. To exit the corner, mice could turn either to the right or to the left. The choice of turning side was recorded for each out of 10 to 15 trials and a score was calculated as number of left turns/all turns × 100 (%). Reflexive motor ability of the animal's contralateral (left) forelimb, to respond to a vibrissae-elicited excitation, was assessed via the Forelimb Placing Test.19 The score was expressed as number of successful paw placements out of 10 consecutive vibrissae stimulations. Spatial exploratory and cognitive performances in mice were evaluated with the T-Maze task as previously reported.20 This procedure relies on the rodent's natural behavior to explore novelty and thus alternate between the right and the left arm of a T-Maze. Spontaneous alternations were recorded as a percentage of all trials. All behavioral scores were converted as a percentage of average sham performance (=100%).
Measurement of Brain Water Content
Brain water content was measured at 24 and 72 hours after surgery as previously reported.16 Briefly, mice were decapitated under lethal isoflurane anesthesia and brains were quickly removed. A coronal brain section of 4 mm thickness was separated 2 mm anterior and posterior of the needle tract, and then further divided into ipsilateral and contralateral cortex and basal ganglia. The cerebellum was additionally collected as an internal control. All brain specimens were weighed using an analytical microbalance (APX-60, Denver Instrument, Bohemia, NY) in order to obtain the wet weight. Samples were then dried at 100°C for 24 hours before determining the dry weight. The brain water content (%) was calculated as (wet weight – dry weight)/ wet weight × 100.
Western Blotting
Mice were euthanized at 24 hours after surgery and ipsilateral hemispheres were isolated and processed as previously described.16 Equal amounts of protein (50μg) were separated by SDA-PAGE, then transferred onto nitrocellulose membranes and incubated with the respective primary and secondary antibodies (1:1000). The following primary antibodies were obtained from Cell Signaling Technology: anti-p-Akt (Ser473), anti-Akt, anti-GSK-3β and anti-Cleaved-Caspase-3. Anti-p-GSK-3β (Tyr216), anti-β-Actin and all secondary antibodies were purchased from Santa Cruz Biotechnology. Immunoblots were visualized with the ECL Plus chemiluminescence reagent kit (Amersham Bioscience, Arlington Heights, IL) and densitometrically quantified using the software ImageJ (NIH). Results were expressed as relative density ratio, normalized to the mean value of the sham group.
Immunofluorescence Staining
Mice were euthanized at 24 hours after surgery and brain specimens were processed as previously described with minor modifications.21 Triple immunofluorescence was performed using the neuronal marker anti-NeuN (1:100, Millipore, Temecula, CA) and anti-p-GSK-3β (Tyr216, 1:100) in combination with terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL), (In situ Cell Death Detection Kit, Fluorescein, Roche Inc., Mannheim, Germany). Double immunofluorescence staining was performed using anti-NeuN and anit-Cleaved-Caspase-3 (1:1000). Microphotographs were analyzed using a fluorescent microscope and Magna Fire SP system (Olympus). TUNEL positive neurons were counted at x400 magnification in four perihematomal areas (500μm × 500μm grids) and the data was expressed as cells/mm2.
Statistical Analysis
Data were expressed as mean±S.E.M. and statistically analyzed with One-way ANOVA followed by Tukey post hoc test. All behavior data was expressed as mean±S.E.M. of sham performance and analyzed with Kruskal-Wallis One Way Analysis of Variance on Ranks, followed by the Student-Newman-Keuls Method. A P value of <0.05 was considered statistically significant. All statistical analyses were performed using SigmaPlot version 10.0 for Windows.
Results
PHA-543613-mediated attenuation of behavioral deficits and brain edema at 24 hours after ICH is dependent on the PI3K-Akt signaling pathway
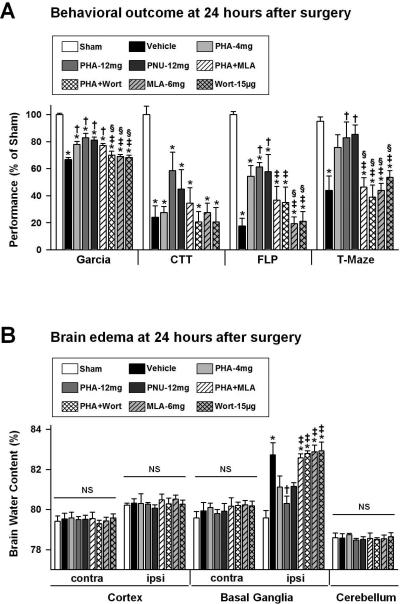
Behavioral deficits were evaluated at 24 hours after surgery (n=6 per group). Mice subjected to ICH presented a significantly worse Garcia Test performance than sham-operated animals (p<0.05, Figure 1A), however treatment with α7nAChR agonists PHA-543613 (PHA-4mg, PHA-12mg) or PNU-282987 (PNU-12mg) improved the outcome significantly (p<0.05, compared to vehicle). Mice within the pharmacological intervention groups additionally received an injection of α7nAChR antagonist MLA (PHA+MLA) or PI3K inhibitor wortmannin (PHA+Wort), prior to PHA-543613 (12mg/kg) administration, in order to assess whether the observed behavioral improvements are dependent on α7nAChR induced activation of the PI3KAkt signaling pathway. Wortmannin, as anticipated, reversed the initial attenuation of behavioral deficits observed with PHA-543613. The two applied inhibitors, MLA (6mg/kg) and wortmannin (15μg/kg), did not worsen the behavioral outcome of the Garcia Test when administered alone (p>0.05, compared to vehicle). After experimental right-sided ICH, mice turned less frequently to the impaired (left) side while performing the Corner Turn Test (p<0.05 compared to sham); however no differences were found between treated and untreated ICH-animals (p>0.05). Furthermore, vehicle animals demonstrated significantly impaired contralateral (left) forelimb function, evaluated via the Forelimb Placing Test, as well as a reduced number of spontaneous alterations during the T-Maze assessment (p<0.05 compared to sham). Treatments of PHA-543613 (PHA-12mg) or PNU-282987 (PNU-12mg) improved the forelimb placing ability and increased the number of spontaneous alterations in ICH-animals (p<0.05 compared to vehicle). In contrast, mice receiving α7nAChR agonist PHA-543613 (12mg/kg) combined with MLA (PHA+MLA) or wortmannin (PHA+Wort) showed no significant differences compared to the respective vehicle group (p>0.05).
Figure 1.
Statistical analysis of behavioral outcome (A) and brain edema (B) at 24 hours after ICH induction or sham surgery. Data are expressed as mean ± SEM. * p<0.05 compared to sham, † p<0.05 compared to vehicle, ‡ p<0.05 compared to PHA-12mg, § p<0.05 compared to PNU-12mg. N=6 in each group. Garcia: Garcia Test, CTT: Corner Turn Test, FLP: Forelimb Placing Test, contra: contralateral, ipsi: ipsilateral, NS: not significant.
Brain edema was evaluated at 24 hours after surgery (n=6 per group). Treated mice (PHA-12mg) showed a significantly reduced brain water content in the ipsilateral basal ganglia (p<0.05, compared to vehicle, Figure 1B), however co-administration of MLA (PHA+MLA) or wortmannin (PHA+Wort) reversed this effect entirely (p<0.05 compared to PHA-12mg). The latter compounds did not increase brain edema compared to the vehicle (p>0.05). No significant differences were evident between all groups, in contra- and ipsilateral cortex, contralateral basal ganglia or in the cerebellum (p>0.05).
PHA-543613 attenuates behavioral deficits and brain edema at 72 hours after ICH
Sham-operated, vehicle and treated (PHA-12mg) animals (n=6 per group) were utilized to evaluate behavioral outcomes and brain edema at 72 hours after surgery (Figure 2). PHA-543613 treatment significantly improved performances of Garcia, Corner Turn, Forelimb Placing and T-Maze Tests as compared to the vehicle group (p<0.05).
Figure 2.
Statistical analysis of behavioral outcome (A) and brain edema (B) at 72 hours after ICH induction or sham surgery. Data are expressed as mean ± SEM. * p<0.05 compared to sham, † p<0.05 compared to vehicle. N=6 in each group. Garcia: Garcia Test, CTT: Corner Turn Test, FLP: Forelimb Placing Test, contra: contralateral, ipsi: ipsilateral.
Brain water content of the ipsilateral basal ganglia was significantly higher in the vehicle group than in sham animals (p<0.05, Figure 2B), and PHA-543613 treatment (PHA-12mg) reduced the amount of brain edema significantly (p<0.05, compared to vehicle). No significant differences were evident between all groups, in contra- and ipsilateral cortex, contralateral basal ganglia or in the cerebellum (p>0.05).
PHA-543613 activates the PI3K-Akt signaling pathway and decreases protein expressions of p-GSK-3β and cleaved caspase-3 at 24 hours after ICH
To further confirm that PHA-543613-mediated neuroprotection is dependent on the anti-apoptotic PI3K-Akt signaling pathway, Western blot analysis was conducted and the changes in protein levels of phosphorylated Akt (p-Akt, Ser473), phosphorylated GSK-3β (p-GSK-3β, Tyr216) and cleaved caspase-3 (CC3) were quantified. The treatment (PHA-12mg) increased phosphorylation of Akt significantly, compared to both the sham and the vehicle group (p<0.05, n=5 per group, Figure 3A). MLA and wortmannin (PHA+MLA, PHA+Wort) significantly decreased the level of p-Akt at 24 hours after ICH (p<0.05, compared to PHA-12mg). GSK-3β phosphorylation was significantly reduced by PHA-543613 (p<0.05, compared to vehicle, Figure 3B); however MLA and wortmannin reversed this effect entirely. Lastly, PHA-12mg brain specimens contained significantly decreased quantities of CC3 (p<0.05, compared to vehicle, Figure 1C), and wortmannin (PHA+Wort) abolished the anti-apoptotic effect of the treatment (p<0.05, compared to PHA-12mg).
Figure 3.
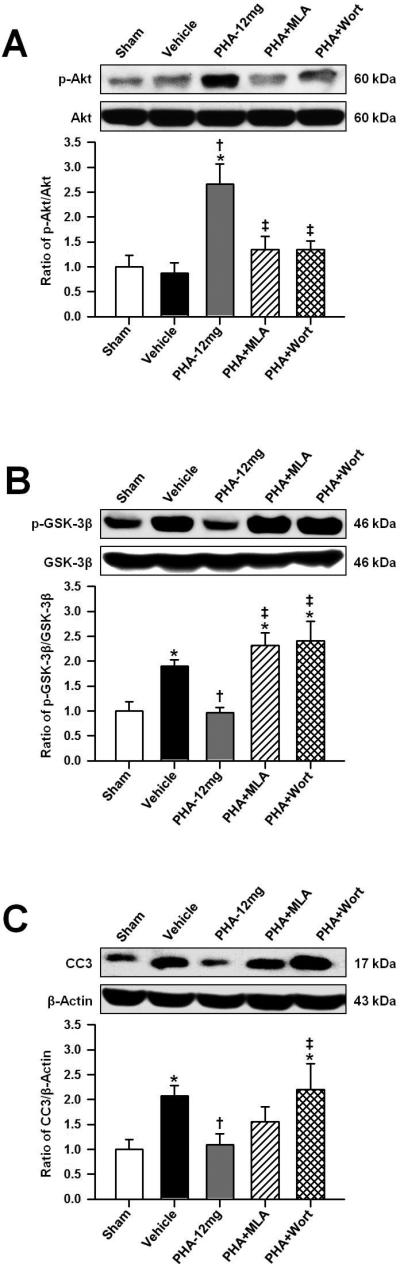
Representative Western blots and densitometric quantification of p-Akt/Akt (A), p-GSK-3β/GSK-3β (B) and CC3/β-Actin (C) at 24 hours after ICH induction or sham surgery. Data in bar graphs are expressed as mean ± SEM. * p<0.05 compared to sham, † p<0.05 compared to vehicle, ‡ p<0.05 compared to PHA-12mg. N=5 in each group.
PHA-543613 reduces neuronal apoptosis at 24 hours after ICH
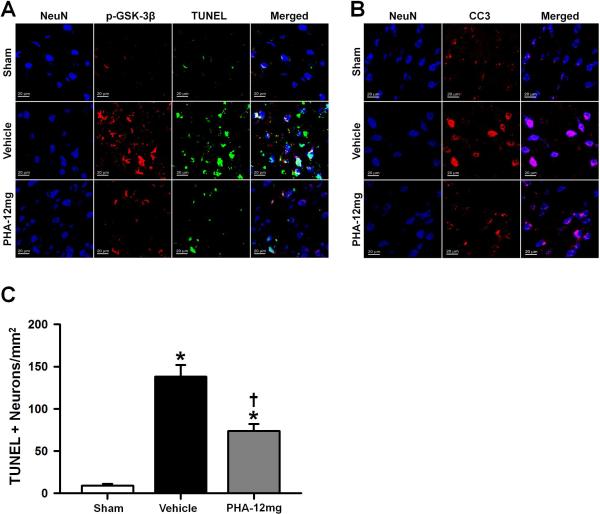
Immunofluorescence staining and neuronal cell death quantification was performed in sham, vehicle and PHA-12mg animals (n=4 per group). All mice were sacrificed at 24 hours after ICH or sham surgery and immunoreactivities of NeuN, p-GSK-3β, TUNEL and CC3 were evaluated in the perihematomal area or near the needle tract in sham animals. Consistent with the Western blot results, the immunofluorescence analysis revealed decreased expressions of p-GSK-3β (Figure 4A) and CC3 (Figure 4B) in the treatment group (PHA-12mg). TUNEL positive cells (Figure 4A) were less apparent after PHA-543613 treatment. Immunoreactivities of p-GSK-3β, TUNEL and CC3 were generally colocalized within NeuN positive cells (neurons).
Figure 4.
(A) Representative microphotographs of immunofluorescence staining showing colocalization of NeuN (DAPI/blue), p-GSK-3β (Texas Red/red) and TUNEL (FITC/green) at 24 hours after surgery. PHA-543613 treatment decreased perihematomal immunoreactivities of p-GSK-3β and TUNEL. (B) Representative microphotographs showing colocalization of NeuN (DAPI/blue) and CC3 (Texas Red/red) at 24 hours after surgery. PHA-543613 treatment decreased perihematomal immunoreactivity of CC3. (C) Neuronal cell death quantification. PHA-543613 treatment decreased the number of TUNEL positive neurons in the perihematomal area at 24 hours after surgery. Data in bar graphs are expressed as mean ± SEM. * p<0.05 compared to sham, † p<0.05 compared to vehicle, ‡ p<0.05 compared to PHA-12mg. N=4 in each group.
The total number of TUNEL and NeuN double-stained cells (TUNEL+ neurons) in the perihematomal area was significantly decreased after PHA-543613 treatment compared to the vehicle group at 24 hours after ICH (p<0.05, Figure 4C).
Discussion
The first aim of this present study was to investigate whether α7nAChR agonists, PHA-543613 and PNU-282987, ameliorate behavioral deficits and brain edema after experimental ICH in mice. Hemispheric hemorrhage, affecting the basal ganglia, has been reported to cause debilitating sensorimotor as well as cognitive deficits in humans.22, 23 Intending to achieve a translational perspective, we included sensorimotor (Garcia, Corner Turn and Forelimb Placing Test) and cognitive (T-Maze) assessments in our experiment. The composite Garcia Test sensitively detects sensorimotor consequences of unilateral hemorrhagic or ischemic brain injury.8, 18 Both the Corner Turn and the Forelimb Placing Test have been widely used to evaluate lateralizing behaviors as well as sensorimotor impairments in preclinical ICH studies.19 During the T-Maze assessment, rodents normally select alternating arms on consecutive trials (spontaneous alterations).20 A decreased number of those spontaneous alterations correlates well with the severity of the cognitive impairment in injured animals. 24 High dose treatment (12mg/kg) of PHA-543613 or PNU-282987 significantly improved the outcome of all conducted behavioral tests, with the exception of the corner turn test performance at 24 hours after surgery (compared to vehicle). Even mild unilateral brain injuries may cause a lateralizing behavior in rodents, and the outcome of the Corner Turn Test after experimental ICH, was previously reported to improve to a lesser extent than the outcome of the Forelimb Placing Test.19 Brain edema, defined as an increase in the water content of brain tissue, is observed in acute and delayed stages after ICH.25 Several studies suggest a close association between the degree of perihematomal brain edema and poor outcome in patients.25, 26 Our results showed significantly reduced brain water content of the ipsilateral basal ganglia in the PHA-12mg group, at 24 and 72 hours after ICH-induction (compared to vehicle). The aforementioned findings support our first hypothesis that α7nAChR agonists PHA-543613 and PNU-282987 ameliorate behavioral and morphological outcomes (brain edema) after ICH in mice. PHA-543613, in a concentration of 12 mg/kg, ameliorated the brain injury to a greater extent than 4 mg/kg of PHA-543613 and 12 mg/kg of PNU-282987 (Figure 1), and was therefore exclusively applied in the following experiments (Figures 2, 3 and 4). We previously reported that short-term activation of the α7nAchR via PNU-282987 did not alter physiological parameters (blood pressure, heart rate) or plasma concentrations of Na+, K+, Cl-, and glucose in rodents.8 The observed amelioration of the brain injury in the treatment groups (PHA-4mg, PHA-12mg and PNU-12mg) were unlikely to be caused by changes of physiological parameters.
Aiming to further examine the dependence between α7nAChR-induced neuroprotection and the PI3K-Akt signaling pathway, we administered either MLA or wortmannin, prior to PHA-543613 (12mg/kg).
MLA is a potent competitor, interfering with the [125I] α-bungarotoxin binding site of the α7nAChR (Ki=1.4nm).27, 28 MLA is quickly redistributed from the vasculature to the brain where it extensively inhibits the receptor activation by PHA-543613 (Ki=8.8nm).17, 27, 28 Wortmannin inhibits PI3K irreversibly, and is commonly used to prevent downstream phosphorylation of Akt.8 Animals receiving these interventions in addition to the treatment did not show functional or morphological improvements at 24 hours after surgery. Furthermore, MLA or wortmannin administered alone did not worsen the injury compared to the vehicle. These results indicate that α7nAChR agonists exert their neuroprotective effects through the PI3K-Akt signaling pathway. Consistent with these findings, earlier studies demonstrated the ability of α7nAChR-agonists to trigger PI3K-activation, possibly through stimulation of the receptor's catalytic intracellular domain; however, the exact mechanism of this process remains yet to be discovered.7, 29 Akt, a serine/threonine kinase, which is directly activated by PI3K-mediated phosphorylation, stimulates several anti-apoptotic mechanisms, among them the inhibition of GSK-3β.10 Belonging also to the serine/threonine kinase family, GSK-3β has been described to activate pro-apoptotic caspase-3,13 thus aggravating neuronal injury after experimental ischemic stroke11 and subarachnoid hemorrhage.14
In this study, we additionally intended to show the protective effect of GSK-3β-inhibition. PHA-543613 treatment significantly increased the protein expression of activated Akt (p-Akt, Ser473) in the ipsilateral hemisphere at 24 hours after surgery, which successively reduced the expression of activated GSK-3β (p-GSK-3β, Tyr216) and cleaved caspase-3 (CC3). MLA or wortmannin, in combination with PHA-543613, showed similar levels of the respective proteins as the vehicle group. These results support the proposed anti-apoptotic effect of α7nAChR agonism through PI3K-Akt activation and resulting GSK-3β-inhibition.
Given the fact that α7nAChR are not only expressed in neurons but also in neuroglia and endothelial cells of the mammalian brain,6 we used immunofluorescence staining to show colocalizations of p-GSK-3β and CC3 with NeuN (neuronal marker). Both target proteins were generally localized in neurons, and PHA-543613 treatment reduced the intensity of p-GSK-3β and CC3 immunofluorescence in the perihematomal area. We furthermore used TUNEL, to detect apoptotic cell death, and observed a significantly reduced density of apoptotic neurons in the treatment group (compared to vehicle). Added together, these findings support our second hypothesis, suggesting that α7nAChR-stimmulation reduces activated GSK-3β via the PI3K-Akt signaling pathway, thus decreasing the incidence of neuronal apoptosis after ICH in mice.
In this study we investigated anti-apoptotic effects of the α7nAChR; however previously reported anti-inflammatory properties of this receptor subtype may also participate in neuroprotection following hemorrhagic stroke.30 Inflammatory mechanisms, mediated by cellular components such as resident immune cells of the brain (microglia), are involved in the progression of the ICH-induced brain injury.31 Activation of α7nAChR in microglial cells inhibits the production of pro-inflammatory cytokines but not the production of anti-inflammatory cytokines, thereby reducing the local inflammatory response.30 These anti-inflammatory effects of the α7nAChR may additionally protect the blood brain barrier integrity after experimental ICH. Inhibition of GSK-3β will also increase the expression of its downstream protein β-catenin, an important component of the blood brain barrier.32, 33 Furthermore, α7nAChR stimulation resulted in enhanced cognition and attention in both animals as well as humans, and α7nAChR agonists have been represented as promising treatments for schizophrenia and Alzheimer's disease.6, 9, 17, 27
In conclusion, this study found that α7nAChR-stimulation improved functional and morphological outcomes after experimental ICH in mice. We demonstrated that PHA-543613 treatment reduced the expression of pro-apoptotic GSK-3β via the PI3K-Akt signaling pathway, and the pharmacological reversal thereof obliterated this effect. Additional pre-clinical studies are needed to investigate potential anti-inflammatory effects of α7nAChR agonists, and it is also essential to elucidate further mechanisms on how α7nAChR-stimulation reduces brain edema and neuronal apoptosis after experimental ICH, as shown in this present study.
Acknowledgments
Sources of Funding
This study was supported by NIH grant RO1NS053407 to J.H. Zhang.
Footnotes
Disclosures
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jorgensen HS, Nakayama H, Raaschou HO, Olsen TS. Intracerebral hemorrhage versus infarction: Stroke severity, risk factors, and prognosis. Annals of neurology. 1995;38:45–50. doi: 10.1002/ana.410380110. [DOI] [PubMed] [Google Scholar]
- 2.Lyden PD, Zivin JA. Hemorrhagic transformation after cerebral ischemia: Mechanisms and incidence. Cerebrovascular and brain metabolism reviews. 1993;5:1–16. [PubMed] [Google Scholar]
- 3.Matsushita K, Meng W, Wang X, Asahi M, Asahi K, Moskowitz MA, et al. Evidence for apoptosis after intercerebral hemorrhage in rat striatum. Journal of cerebral blood flow and metabolism. 2000;20:396–404. doi: 10.1097/00004647-200002000-00022. [DOI] [PubMed] [Google Scholar]
- 4.Aronowski J, Hall CE. New horizons for primary intracerebral hemorrhage treatment: Experience from preclinical studies. Neurol Res. 2005;27:268–279. doi: 10.1179/016164105X25225. [DOI] [PubMed] [Google Scholar]
- 5.Qureshi AI, Suri MF, Ostrow PT, Kim SH, Ali Z, Shatla AA, et al. Apoptosis as a form of cell death in intracerebral hemorrhage. Neurosurgery. 2003;52:1041–1047. discussion 1047-1048. [PubMed] [Google Scholar]
- 6.Lightfoot AP, Kew JN, Skidmore J. Alpha7 nicotinic acetylcholine receptor agonists and positive allosteric modulators. Prog Med Chem. 2008;46:131–171. doi: 10.1016/S0079-6468(07)00003-3. [DOI] [PubMed] [Google Scholar]
- 7.Takada-Takatori Y, Kume T, Sugimoto M, Katsuki H, Sugimoto H, Akaike A. Acetylcholinesterase inhibitors used in treatment of alzheimer's disease prevent glutamate neurotoxicity via nicotinic acetylcholine receptors and phosphatidylinositol 3-kinase cascade. Neuropharmacology. 2006;51:474–486. doi: 10.1016/j.neuropharm.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 8.Duris K, Manaenko A, Suzuki H, Rolland WB, Krafft PR, Zhang JH. {alpha}7 nicotinic acetylcholine receptor agonist pnu-282987 attenuates early brain injury in a perforation model of subarachnoid hemorrhage in rats. Stroke. 2011 doi: 10.1161/STROKEAHA.111.619965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaw S, Bencherif M, Marrero MB. Janus kinase 2, an early target of alpha 7 nicotinic acetylcholine receptor-mediated neuroprotection against abeta-(1-42) amyloid. J Biol Chem. 2002;277:44920–44924. doi: 10.1074/jbc.M204610200. [DOI] [PubMed] [Google Scholar]
- 10.Grimes CA, Jope RS. The multifaceted roles of glycogen synthase kinase 3beta in cellular signaling. Progress in neurobiology. 2001;65:391–426. doi: 10.1016/s0301-0082(01)00011-9. [DOI] [PubMed] [Google Scholar]
- 11.Valerio A, Bertolotti P, Delbarba A, Perego C, Dossena M, Ragni M, et al. Glycogen synthase kinase-3 inhibition reduces ischemic cerebral damage, restores impaired mitochondrial biogenesis and prevents ros production. J Neurochem. 2011;116:1148–1159. doi: 10.1111/j.1471-4159.2011.07171.x. [DOI] [PubMed] [Google Scholar]
- 12.Bhat RV, Shanley J, Correll MP, Fieles WE, Keith RA, Scott CW, et al. Regulation and localization of tyrosine216 phosphorylation of glycogen synthase kinase-3beta in cellular and animal models of neuronal degeneration. Proc Natl Acad Sci U S A. 2000;97:11074–11079. doi: 10.1073/pnas.190297597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.King TD, Bijur GN, Jope RS. Caspase-3 activation induced by inhibition of mitochondrial complex i is facilitated by glycogen synthase kinase-3beta and attenuated by lithium. Brain research. 2001;919:106–114. doi: 10.1016/s0006-8993(01)03005-0. [DOI] [PubMed] [Google Scholar]
- 14.Endo H, Nito C, Kamada H, Yu F, Chan PH. Akt/gsk3beta survival signaling is involved in acute brain injury after subarachnoid hemorrhage in rats. Stroke. 2006;37:2140–2146. doi: 10.1161/01.STR.0000229888.55078.72. [DOI] [PubMed] [Google Scholar]
- 15.Wang J, Fields J, Dore S. The development of an improved preclinical mouse model of intracerebral hemorrhage using double infusion of autologous whole blood. Brain Res. 2008;1222:214–221. doi: 10.1016/j.brainres.2008.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma Q, Manaenko A, Khatibi NH, Chen W, Zhang JH, Tang J. Vascular adhesion protein-1 inhibition provides antiinflammatory protection after an intracerebral hemorrhagic stroke in mice. J Cereb Blood Flow Metab. 2010;31:881–893. doi: 10.1038/jcbfm.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wishka DG, Walker DP, Yates KM, Reitz SC, Jia S, Myers JK, et al. Discovery of n-[(3r)-1-azabicyclo[2.2.2]oct-3-yl]furo[2,3-c]pyridine-5-carboxamide, an agonist of the alpha7 nicotinic acetylcholine receptor, for the potential treatment of cognitive deficits in schizophrenia: Synthesis and structure--activity relationship. J Med Chem. 2006;49:4425–4436. doi: 10.1021/jm0602413. [DOI] [PubMed] [Google Scholar]
- 18.Garcia JH, Wagner S, Liu KF, Hu XJ. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke. 1995;26:627–634. doi: 10.1161/01.str.26.4.627. [DOI] [PubMed] [Google Scholar]
- 19.Hua Y, Schallert T, Keep RF, Wu J, Hoff JT, Xi G. Behavioral tests after intracerebral hemorrhage in the rat. Stroke. 2002;33:2478–2484. doi: 10.1161/01.str.0000032302.91894.0f. [DOI] [PubMed] [Google Scholar]
- 20.MacLellan CL, Langdon KD, Churchill KP, Granter-Button S, Corbett D. Assessing cognitive function after intracerebral hemorrhage in rats. Behavioural brain research. 2009;198:321–328. doi: 10.1016/j.bbr.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Ostrowski RP, Colohan AR, Zhang JH. Mechanisms of hyperbaric oxygen-induced neuroprotection in a rat model of subarachnoid hemorrhage. Journal of cerebral blood flow and metabolism. 2005;25:554–571. doi: 10.1038/sj.jcbfm.9600048. [DOI] [PubMed] [Google Scholar]
- 22.Gebel JM, Broderick JP. Intracerebral hemorrhage. Neurol Clin. 2000;18:419–438. doi: 10.1016/s0733-8619(05)70200-0. [DOI] [PubMed] [Google Scholar]
- 23.Nys GM, van Zandvoort MJ, de Kort PL, Jansen BP, de Haan EH, Kappelle LJ. Cognitive disorders in acute stroke: Prevalence and clinical determinants. Cerebrovasc Dis. 2007;23:408–416. doi: 10.1159/000101464. [DOI] [PubMed] [Google Scholar]
- 24.Deacon RM, Rawlins JN. T-maze alternation in the rodent. Nat Protoc. 2006;1:7–12. doi: 10.1038/nprot.2006.2. [DOI] [PubMed] [Google Scholar]
- 25.Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet neurology. 2006;5:53–63. doi: 10.1016/S1474-4422(05)70283-0. [DOI] [PubMed] [Google Scholar]
- 26.Zazulia AR, Diringer MN, Derdeyn CP, Powers WJ. Progression of mass effect after intracerebral hemorrhage. Stroke. 1999;30:1167–1173. doi: 10.1161/01.str.30.6.1167. [DOI] [PubMed] [Google Scholar]
- 27.Toyohara J, Hashimoto K. Alpha7 nicotinic receptor agonists: Potential therapeutic drugs for treatment of cognitive impairments in schizophrenia and alzheimer's disease. Open Med Chem J. 2010;4:37–56. doi: 10.2174/1874104501004010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ward JM, Cockcroft VB, Lunt GG, Smillie FS, Wonnacott S. Methyllycaconitine: A selective probe for neuronal alpha-bungarotoxin binding sites. FEBS Lett. 1990;270:45–48. doi: 10.1016/0014-5793(90)81231-c. [DOI] [PubMed] [Google Scholar]
- 29.de Jonge WJ, Ulloa L. The alpha7 nicotinic acetylcholine receptor as a pharmacological target for inflammation. Br J Pharmacol. 2007;151:915–929. doi: 10.1038/sj.bjp.0707264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marrero MB, Bencherif M, Lippiello PM, Lucas R. Application of alpha7 nicotinic acetylcholine receptor agonists in inflammatory diseases: An overview. Pharm Res. 2011;28:413–416. doi: 10.1007/s11095-010-0283-7. [DOI] [PubMed] [Google Scholar]
- 31.Wang J. Preclinical and clinical research on inflammation after intracerebral hemorrhage. Prog Neurobiol. 2010;92:463–477. doi: 10.1016/j.pneurobio.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nelson WJ, Nusse R. Convergence of wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. Beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 1997;16:3797–3804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]