Abstract
Elapid snakes throughout the world are considered very lethal containing neurotoxic venoms that affect the nervous system. When humans are envenomated it is considered a serious medical emergency, and antivenom is the main form of treatment considered, in spite of the fact that some patients may only survive under intensive therapy treatment such as respiratory support. Coral snakes are part of the family Elapidae and envenomations by these snakes are very low (< 2% of total snakebites) in most countries from southeastern United States to Argentina. In the United States there are only two species of coral snakes of medical importance which belong to the Micrurus genera: Micrurus fulvius fulvius (Eastern coral snake) and M. tener tener (Texas coral snake). In 2006, Wyeth pharmaceutical notified customers that the production of the North American Coral Snake Antivenin (NACSA) in the U.S. was discontinued and adequate supplies were available to meet historical needs through the end of October 2008; and therefore, it is of utmost important to consider other antivenoms as alternatives for the treatment of coral snake envenoming. One logical alternative is the coral snake antivenom, Coralmyn, produced by the Mexican company, Bioclon. In order to compare neutralization between NACSA and Coralmyn antivenoms with the North American coral snake venoms, the venom lethal doses (LD50) and antivenom effective doses (ED50) were determined in 18–20 g, female, BALB/c mice. Additionally, venom comparisons were determined through a non reduced SDS-PAGE for M. f. fulvius, M. t. tener and the Mexican coral snake venom, M. nigrocinctus nigrocinctus. Coralmyn antivenom was able to effectively neutralize 3 LD50 doses of all venom from both M. t. tener and M. f. fulvius, while Wyeth antivenom only neutralized M. f. fulvius venom and was not effective in neutralizing 3 LD50 doses of M. t. tener venom. Coralmyn is effective in the neutralization of both clinically important coral snake venoms in the U.S.
Keywords: Coral snakes; Micrurus fulvius fulvius; Micrurus tener tener; venom, antivenom; neutralization, North American Coral Snake Antivenin; Coralmyn
Introduction
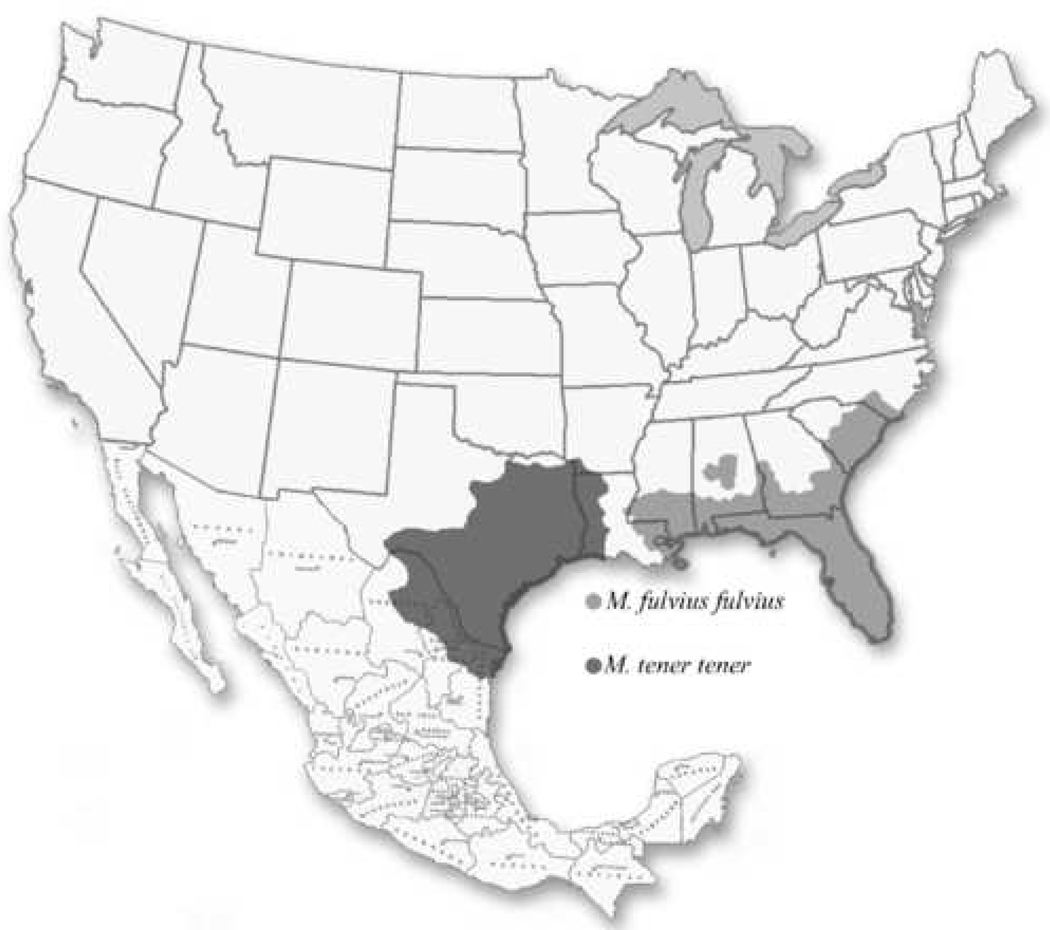
The coral snakes are the only Elapids that exist in the Americas and are represented by three genera: Leptomicrurus, Micruroides and Micrurus (Sandner-Montilla, 1985; Pifano et al., 1986; de Brandao Prieto da Silva, 2001). In the United States there exist three coral snakes representing two genera, which are Micruroides euryxanthus, Micrurus tener tener, and Micrurus fulvius fulvius. The latter two are clinically more significant only because the former displays a shy, elusive behavior that rarely comes into contact with humans (Shaw, 1971). Although the venom of the Micruroides is less toxic than Micrurus, they all present neurotoxic symptoms (McColloguh and Gennaro, 1971; Wingert and Wainschel, 1975; Aird, 1991; Dalo, 1989; Parrish, 1967) . The Micrurus species can be found from south, eastern Florida westward to east and south Texas; M. f. fulvius are found east of the Mississippi river and M. t. tener are found west of the Mississippi river (Campbell and Lamar, 2004; Fig. 1). Envenomation by these snakes can cause death due to the alpha neurotoxins that cause muscle paralysis and respiratory arrest due to postsynaptic, nondepolarization blockage at the neuromuscular junction by binding competitively to the acetylcholine receptor (Lee, 1970 and 1972; Pettigrew and Glass, 1985; Vital-Brazil, 1987; Alape-Giron et al, 1996; Rosso et al, 1996; Francis et al., 1997 ). The current NACSA will no longer be available after October 2008; and therefore, it is important to determine the ability of other coral snake antivenoms to neutralize the toxic effects of the North American coral snake venoms. The Sonoran coral snake (M. euryxanthus) was not included in this study since it is a relatively harmless snake and no deaths have ever been attributed to its bite (McCollough and Gennaro, 1963; Parrish and Khan, 1967).
Figure 1.
Geographical distribution of Micrurus fulvius fulvius and M. tener tener.
Since coral snake antivenom became available in 1967 (Kitchens and Van Mierop, 1987), no deaths have been reported in the U.S.; however, before coral snake antivenom, the estimated case fatality rate was 10% (Parrish and Khan, 1967; Norris and Dart, 1989; German et al., 2005), and the cause of death was respiratory or cardiovascular failure. The best and most acceptable form of treatment after coral snake envenoming is with the use of antivenom. This study compared the efficacy of the Mexican antivenom, Coralmyn®, and U.S. antivenom, Wyeth’s North American Coral Snake Antivenin to neutralize the toxicity effects of M. t. tener (Texas coral snake) and M. f. fulvius (Eastern coral snake) venom. Ultimately, the purpose of this study is to determine if Coralmyn antivenom is as effective in neutralizing the U.S. coral snake venoms. It is crucial to find a safe and effective antivenom that will replace the current U.S. one, which will no longer be available after October 2008 (Peterson, 2006).
Methods
2.1. Venoms
Two pools of venom from M. f. fulvius (Eastern coral snake) were purchased from Biotoxins Incorporated in St. Cloud, FL (Pool I-Lot#: MF/05A; Pool II-Lot#: MF/038.). Each pool contained venom from approximately 60 specimens found in south central Florida. Venom from M. t. tener was extracted from coral snakes found in the Houston area (Pool I) and South Texas area (Pool II) at the Natural Toxins Research Center (NTRC) on the average of every two weeks. Each pool contained venom from approximately 10 specimens. The snakes were allowed to bite into a para-film over a 15 mL test tube. The venoms were centrifuged for 5 min at 23°C at 12,800 × g to remove cellular debris. The venom supernatant was then transferred to vials with the proper labels and stored at −90°C until lyophilized.
2.2. Antivenoms
Coralmyn® is a polyclonal antivenom (Fab)2 fragment with an equine origin, and produced by Instituto Bioclon in Mexico using venom from Micrurus nigrocinctus nigrocinctus (Black banded coral snake). The North American Coral Snake Antivenin is a polyclonal IgG with an equine origin, and produced by Wyeth® located in the United States using venom from Micrurus fulvius fulvius (Eastern coral snake).
2.3. Lethal Dose (LD50)
Five groups of eight mice for each venom were housed in cages and observed throughout the quarantine period and experiments. The endpoint of lethality of the mice was determined after 48 hr. The LD50 of the venoms listed in Tables 1 were determined in BALB/c mice. Venoms were dissolved in 0.85% saline at the highest test dose per mouse. Serial dilutions of 1.5 using saline were made to obtain four additional concentrations. All solutions during the experiment were stored at 0° C and warmed to 37°C just before being injected into mice. The lethal toxicity was determined by injecting 0.2 mL of venom (containing dosages ranging between 2.5 to 13.5 µg/mouse for M. f. fulvius venoms and dosages ranging from 4.5 to 22.5 µg/mouse for M. t. tener venoms) into a tail vein of 18–20 g female BALB/c mice. The injections were administered using a 1-mL syringe fitted with a 30-gauge, 0.5-inch needle. Saline controls were used. The LD50 was calculated by the Spearman-Karber method for each pool of venom (n=3 ±SD).
Table 1.
LD50 of North American Coral Snake Venoms and ED50 of Wyeth and Bioclon Antivenom
| Species | Pool | LD50 (i.v.) (mg/kg ± SD)c |
Mean LD50 for species |
NACSA ED50 (mL/mg±SD)d |
NACSA Mean ED50 (mL/mg) |
NACSA ED50 (mL/mg±SD)e |
Coralmyn ED50 (mL/mg ± SD)d |
Coralmyn ED50 (mL/mg ± SD)e |
Coralmyn Mean ED50 (mL/mg) |
|---|---|---|---|---|---|---|---|---|---|
| M. f. f. | 0.279 | 10/2.39 | 10/5.5 | ||||||
| Ia | 0.268±0.029 | 10/2.17± 0.4 | 1/0.217 ± 0.04 | 10/4.86 ± 0.7 | 1/0.486 ± 0.071 | ||||
| M. f. f. | |||||||||
| IIa | 0.291±0.055 | 10/2.61± 0.3 | <10/0.76 | 1/0.261 ± 0.03 | 10/6.11 ± 1.2 | 1/0.611 ± 0.124 | 10/11.17 | ||
| M. t. t. | Ib | 0.822±0.118 | 0.779 | <10/±0.76 | < 1/ 0.076 | 10/10.11 ± 1.7 | 1/1.011 ± 0.172 | ||
| M. t. t. | IIb | 0.736±0.133 | <10/ 0.76 | < 1/ 0.076 | 10/12.23 ± 3.9 | 1/1.223 ± 0.395 | |||
Pooled venom obtained from Biotoxins Inc., St. Cloud, FL.
Pooled venom obtained from the NTRC, Kingsville, TX.
The LD50 is the concentration of venom (mg/kg body weight) required to kill 50% of the BALB/c mice injected iv with 0.2 or 0.5 mL of the various snake venoms. LD50 was calculated using the Spearman-Karber method.
Expressed as mL of antivenom/mg of venom neutralized; ED50 values were determined against 3 LD50 of venoms. n=3
Expressed as the amount of protein (mg) neutralized by 1 mL of antivenom.
2.4. Antivenom efficacy dose (ED50)
Five groups of eight mice were challenged with a mixture of antivenom containing 3 LD50 of venom. Five doses of antivenom were used. Coralmyn and NACSA vials were reconstituted with 5 or 10 mL, respectively with WFI (water for injection) and all subsequent dilutions were made with sterile 0.85% saline. Stock venom solutions were freshly prepared at 0°C before being used. For each group of mice, 3 venom LD50 were mixed with antivenom and incubated at 37°C for 30 min. Each mouse was injected with 0.2 mL (NACSA) or 0.5 mL (Coralmyn) of venom/antivenom freshly mixed and into the tail vein of mice. The mice were observed for 48 hr and the percent survival and ED50 was calculated by the Spearman-Karber method for both antivenoms (n=3 ±SD).
2.5. Non-reduced SDS PAGE of Micrurus venoms
A total of 6 µg of each venom (M. t. tener pools I –II, M. f. fulvius pools I – II and M. n. nigrocinctus) were run non-reduced on a 10–20%Tris-Glycine SDS PAGE (Invitrogen™). A XCell SureLock™ system with Tris-Glycine SDS running buffer (10X) diluted to 1X at a voltage of 130 for 90 min using a Bio-Rad PowerPac™ Basic was used. SeeBlue Plus2 markers ranging from 3–250 kDa were used as controls.
Results
Venom lethality and antivenom efficacy
The LD50 of two North American coral snakes venoms and ED50 of two commercial antivenoms against the North American coral snakes were determined by intravenous route (Table 1 and Table 2). Micrurus fulvius fulvius venom was 3.4 times more toxic than M. t. tener venom 0.23 and 0.8 mg/kg, respectively (Table 1). Coralmyn antivenom was able to neutralize both North American coral snake venoms effectively with a mean ED50 of 507.5 and 319 LD50/5 mL vial for M. f. fulvius and M. t. tener, respectively (Table 2). The NACSA was effective in neutralizing M. f. fulvius venom with a mean ED50 of 449 LD50/10 mL vial, but was not effective in neutralizing at least 44 LD50 of M. t. tener venom.
Table 2.
LD50 per mouse and ED50 per vials
| Species | Pool | LD50 mg/mouse |
NACSA ED50 (mL vial/LD50)a |
NACSA Mean ED50 (mL vial/LD50) |
NACSA ED50 (mL/LD50)b |
Coralmyn ED50 (mL vial/LD50)c |
Coralmyn Mean ED50 (mL vial/LD50) |
Coralmyn ED50 (mL /LD50)b |
|---|---|---|---|---|---|---|---|---|
| 10/449 | 1/42.6 | 5/507.5 | 1/95.4 | |||||
| M. f. f. | I | 0.00509 | 10/426 | 5/477 | ||||
| 1/47.2 | 1/107.6 | |||||||
| M. f. f. | II | 0.00552 | 10/472 | 5/538 | ||||
| <10/44 | 1/<0.44 | 5/319 | 1/64.6 | |||||
| M. t. t. | I | 0.01561 | 10/< 44 | 5/323 | ||||
| 1/<0.44 | 1/63 | |||||||
| M. t. t. | II | 0.01398 | 10/< 44 | 5/315 | ||||
Wyeth’s insert states that one 10 mL vial neutralizes 250 LD50 of M. fulvius venom.
Expressed as the number of LD50 neutralized by 1 mL of antivenom.
Bioclon’s insert states that one 5 mL vial neutralizes 450 LD50 of Micrurus ssp venom.
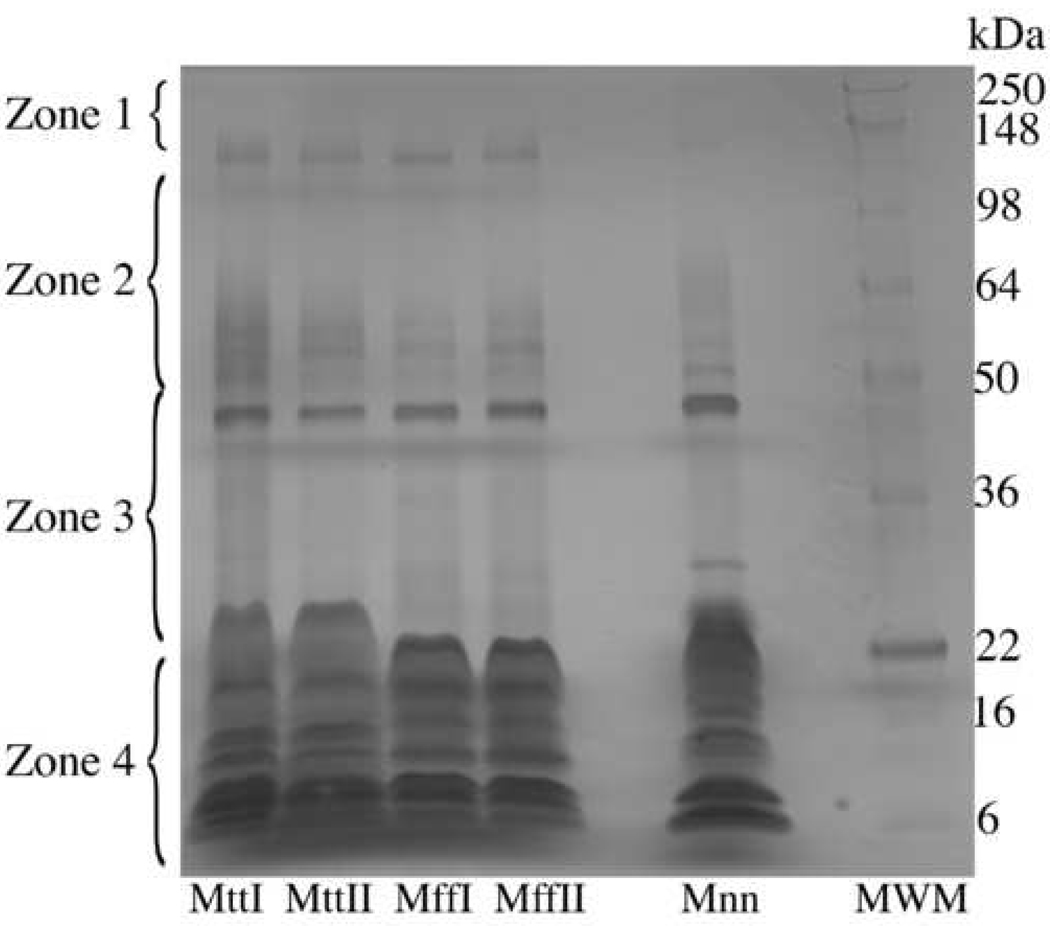
Non-reduced SDS PAGE of Micrurus venoms
Micrurus tener tener showed approximately 19 bands, M. f. fulvius showed 15 bands, and M. n. nigrocinctus showed approximately 17 bands (Fig. 2). All protein bands ranged between 150 to 5 kDa. In general, these three Micrurus venoms share some common and some different characteristics.
Figure 2.
Non-reduced SDS PAGE of Micrurus venoms. A 10–20% Tris-Glycine gel was used to run 6 µg of M. t. tener venoms pools I and II, M. f. fulvius venoms pools I and II and M. n. nigrocinctus venom. An XCell SureLock™ electrophoresis system with Tris-Glycine SDS running buffer was used. The gel was run at 130V, 90 min on a Bio-Rad PowerPac™ Basic. The markers used were SeeBlue Plus2 (Invitrogen). Mtt: M. t. tener, Mff: M. f. fulvius, and Mnn: M. n. nigrocinctus.
Discussion
Parrish and Khan (1967) reported that bites of the Eastern coral snakes are believed to be more severe than that of the Texas coral snakes, and the lethality doses in our study confirms their findings since the LD50 for M. f. fulvius and M. t. tener averaged at 0.279 and 0.779 mg/kg, respectively (Table 1). The majority of the symptoms of coral snake envenomation in the United States have been reported as a result of Eastern coral snakes (McCollough and Gennaro, 1963; Kitchens and Van Mierop, 1987; German et al., 2005). Many of these symptoms include local swelling, respiratory arrest, seizures, paresthesias, vomiting, nausea, dizziness, lethargy, ptosis, among others (Ramsey and Klickstein, 1962; Mosely, 1966; McCollough and Gennaro, 1963; Pifano et al., 1986). Morgan et al. (2007) reported that these symptoms maybe isolated to mainly the Eastern coral snake with the exception of paresthesias. These same investigators reported that 37 patients bitten by the Texas coral snake did not develop neurologic symptoms, and many had more local pain and swelling than that reported for the Eastern coral snake envenomations (Morgan et al, 2007). Nonetheless, Eastern coral snake envenomations will still remain an issue in treatment when the NACSA is no longer available. Eastern coral snake venom does produce neurological symptoms that warrant treatment with antivenom.
The manufacturers of the NACSA state that their antivenom, one vial equaling 10 mL, is able to neutralize 250 LD50, which is equivalent to 2 mg of M. f. fulvius venom. On the other hand, the manufacturers of Coralymn state that their antivenom, one vial equaling 5 mL, is able to neutralize 450 LD50, which is 5 mg of M. n. nigrocinctus venom. In this study using BALB/c mice, NACSA was able to neutralize an average of 449 LD50 of M. f. fulvius venom per vial and less than 44 LD50 for M. t. tener venom. Coralmyn antivenom was able to neutralize an average of 507.5 LD50 of M. f. fulvius venom and 319 LD50 of M. t. tener venom (Table 2).
In this study, the two North American coral snake venoms showed differences in lethal potency. Micrurus fulvius fulvius had an LD50 of 0.268 to 0.291 mg/kg, which is similar to the LD50 of M. fulvius venom in other studies (Arce et al., 2003; de Roodt et al., 2004). On the other hand, M. t. tener had an LD50 of 0.736 to 0.822 mg/kg, which is similar to the LD50 of the Mexican coral snake venom, M. n. nigrocinctus (de Roodt et al., 2004).
Both antivenoms showed different neutralizing efficacy against both species of coral snakes (Table 1 and Table 2). The NACSA was effective against M. f. fulvius venom (ED50=10 mL of antivenom neutralizing a mean of 449 LD50 of venom); however the antivenom was not effective in neutralizing 3 LD50 doses of M. t. tener venom. At 10 mL, the NACSA was still not capable of neutralizing 44 LD50 of venom (Table 2). On a milligram basis, the NACSA neutralized M. f. fulvius effectively (10 mL neutralizing a mean of 2.4 mg and less than 0.76 mg for M. t. tener venom). Coralmyn antivenom was effective in neutralizing both M. f. fulvius and M. t. tener venoms. Neutralization was more effective against M. t. tener venom on a milligram basis (ED50= 10 mL of antivenom neutralizing a little over 10 mg of venom), while neutralization of M. f. fulvius venom was only half as effective (ED50=10 mL of antivenom neutralizing an average of 5.5 mg of venom) (Table 1). However, on an LD50 basis, Coralmyn was more effective in neutralizing M. f. fulvius venom (10 mL neutralizing a mean of 507.5 LD50 and 319 LD50 for M. t. tener venom) (Table 2). As in previous studies (Consroe et al., 1995; Arce et al., 2003; Sánchez et al., 2003), the more toxic venoms on an LD50 basis are easier to neutralize because less venom is required to administer an LD50; and thus a more logical way to test antivenom efficacy is first protect the mouse with antivenom and then determine the LD50 as previously suggested (Sánchez et al., 2003). The ability of Coralmyn to neutralize the Texas coral snake venom more effectively than the NACSA could be due to the fact that the Texas coral snake and the Mexican black-banded coral snake are more geographically related (Campbell and Lamar, 2004). According to the SDS-PAGE, M. t. tener and M. n. nigrocinctus venom shared more common bands than M. n. nigrocinctus and M. f. fulvius venom (Fig. 2). The similarity of M. n. nigrocinctus and M. t. tener venom profiles is consistent with the ability of Coralymn to neutralize these venoms effectively.
Until recently, most of the published article pertaining to coral snake neutralization in the USA has been limited to that of the M. f. fulvius (Cohen et al., 1968 and 1971; Amuy et al., 1997; de Brandão da Silva et al., 2001; Wisniewski et al., 2003; de Roodt et al., 2004). This present study reveals a lower effectiveness of the NACSA to neutralize the venom of the Texas coral snake, and recent assessments of Texas coral snake bites have questioned the necessity of antivenom administration when bitten by these Texas snakes (Standford et al., 2005; Borys et al., 2005; Morgan et al., 2007). Further studies are needed to completely rule out the need for antivenom in the cases of Texas coral snake bites. Nonetheless, our study shows that the Mexican antivenom, Coralmyn, is effective in neutralizing the Texas coral snake venom in a mouse model; and thus, could be an alternative in treating patients with both Eastern and Texas coral snake envenomations. Unlike the NACSA, Coralmyn is an F(ab’)2 antibody and it less likely to cause adverse reactions such as anaphylaxis in patients (Sutherland, 1977; Dart and McNally, 2001); furthermore, (Fab’)2 antibodies are distributed faster into the tissue of Coralmyn (Chippaux and Goyffon, 1998) and the reconstitution is immediate as opposed to 45 min for NACSA (Table 3) (Kanavage et al., 2006).
Table 3.
Comparison of NACSA and Coralymn
| Characteristics | NACSA | Coralmyn |
|---|---|---|
| Produced | United States of America | Mexico |
| Origin | Equine | Equine |
| Antibody Type | IgG | (Fab’)2 |
| Molecular Weight | 110 kDa | 50 kDa |
| Reconstitution (min) | 45a | Immediateb |
| Distribution (h) | >3c | 3 c |
| Elimination (h) | >100 c | 60 c |
| Tissue Affinity | High c | Moderate-High c |
| Elimination Route | Immune Tissue c | Immune Tissue c |
| Binds Complement | Yes c | No c |
| ED50 d | 1 vial (10 mL) neutralizes 250 LD50 | 1 vial (5 mL) neutralizes 450 LD50 |
Current study
Manufacture’s antivenom insert information
The goal of this study was to determine if Coralmyn antivenom was capable in neutralizing the North American coral snake venoms so that the U.S. may have an effective alternative in treating these envenomations when the NACSA will no longer be available. The results not only show that Coralmyn is effective in neutralizing both coral snake venoms tested, but it is more effective in neutralizing M. t. tener venom, than the NACSA. Furthermore, Coralmyn is able to neutralize perhaps the most clinically important coral snake venom, M. f. fulvius, with a mean of 507.5 LD50 per vial versus 449 LD50 per vial of the North American approved product.
Acknowledgments
This research was supported by Wyeth Laboratory (Grant #460505), Instituto Bioclon (Grant #460506), NTRC at Texas A&M University-Kingsville: NIH/NCRR #1 P40 RR018300-01, NIH/RIMI #5 PMD000216-02, and NIH/SCORE #5 S06 GM008107-29; and grants from FONACIT (G-2005000400), Caracas,Venezuela. We are grateful to Nora Diaz De Leon, NTRC administrative officer for technical assistance, Mark Hockmuller and Lucy Arispe.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aird SD, da Silva NJ. Comparative enzymatic composition of Brazilian coral snake (Micrurus) venoms. Comp. Biochem. Physiol. Part B. 1991;99:287–294. doi: 10.1016/0305-0491(91)90043-d. [DOI] [PubMed] [Google Scholar]
- Alape-Giron A, Stiles B, Schmidt J, Giron-Cortés M, Thelestam M, Jornvall H, Bergman T. Characterization of multiple nicotinic acetylcholine receptor-binding protiens and phospholipases A2 from the venom of the coral snake Micrurus nigrocinctus. FEBS Lett. 1996;380:29–32. doi: 10.1016/0014-5793(95)01543-4. [DOI] [PubMed] [Google Scholar]
- Amuy E, Alape-Girón A, Lomonte B, Thelestam M, Gutiérrez JM. Development of immunoassays for determination of circulating venom antigens during envenomations by coral snakes (Micrurus species) Toxicon. 1997;35:1605–1616. doi: 10.1016/s0041-0101(97)00045-7. [DOI] [PubMed] [Google Scholar]
- Arce V, Rojas E, Ownby CL, Rojas G, Gutiérrez JM. Preclinical assessment of the ability of polyvalent (Crotalinae) and anticoral (Elapidae) antivenoms produced in Costa Rica to neutralize the venoms of North American snakes. Toxicon. 2003;41:851–860. doi: 10.1016/s0041-0101(03)00043-6. [DOI] [PubMed] [Google Scholar]
- Borys DJ, Tobleman WR, Standford RD, Morgan DL. Is antivenin required for all Texas coral snake (Micrurus fulvius tenere) envenomation? Abstract: Clinical Toxicology. 2005;43:709. [Google Scholar]
- Campbell JA, Lamar WW. The Venomous Reptiles of the Western Hemisphere. New York: Cornell University Press; 2004. [Google Scholar]
- Chippaux JP, Goyffon M. Venoms, antivenoms and immunotherapy. Toxicon. 1998;36:823–846. doi: 10.1016/s0041-0101(97)00160-8. [DOI] [PubMed] [Google Scholar]
- Cohen P, Dawson JH, Seligmann EB. Cross-neutralization of Micrurus fulvius fulvius (Coral snake) venom by anti-micrurus Carinicauda dumerilii serum. Am. J. Trop. Med. Hyg. 1968;17:308–310. doi: 10.4269/ajtmh.1968.17.308. [DOI] [PubMed] [Google Scholar]
- Cohen P, Berkeley WH, Seligmann EB. Coral Snake Venoms: In vitro relation of neutralizing and precipitating antibodies. Am. J. Trop. Med. Hyg. 1971;20:646–649. [PubMed] [Google Scholar]
- Consroe P, Egen N, Russell FE, Gerrish K, Smith DC, Sidki A, Landon JT. Comparison of a new ovine antigen binding fragment (Fab) antivenin for United States Crotalidae with the commercial antivenin for protection against venom-induced lethality in mice. Am. J. Trop. Med. Hyg. 1995;53:507–510. doi: 10.4269/ajtmh.1995.53.507. [DOI] [PubMed] [Google Scholar]
- Dalo N, Perales J, Munoz R, Martinez B, Moussatche H. Neuromuscular blocking activity of a fraction isolated from the coral snake venom. Toxicon. 1989;27:40. [Google Scholar]
- Dart RC, McNally J. Efficacy, safety, and use of snake antivenoms in the United States. Ann. Emerg. Med. 2001;37:181–188. doi: 10.1067/mem.2001.113372. [DOI] [PubMed] [Google Scholar]
- de Brandao Prieto da Silva AR, Yamagushi IK, Morais JF, Higashi HG, Raw I, Ho PL, de Oliveira JS. Cross reactivity of different specific Micrurus antivenom sera with homologous and heterologous snake venoms. Toxicon. 2001;39:949–953. doi: 10.1016/s0041-0101(00)00233-6. [DOI] [PubMed] [Google Scholar]
- de Roodt AR, Paniagua-Solis JF, Dolab JA, Estévez-Ramiréz J, Ramos-Cerrillo B, Litwin S, Dokmetjian JC, Alagón A. Effectiveness of two common antivenoms for North, Central, and South American Micrurus envenomations. J Toxicology. 2004;42:171–178. doi: 10.1081/clt-120030943. [DOI] [PubMed] [Google Scholar]
- Francis BR, da Silva Junior NJ, Seebart C, Casaise Silva LL, Schmidt JJ, Kaiser II. Toxins isolated from the venom of the Brazilian coral snake (Micrurus frontalis frontalis) include hemorrhagic type phospholipases A2 and postsynaptic neurotoxins. Toxicon. 1997;35:1193–1203. doi: 10.1016/s0041-0101(97)00031-7. [DOI] [PubMed] [Google Scholar]
- German BT, Hack JB, Brewer K, Meggs WJ. Pressure-immobilization of bandages delay toxicity in a porcine model of Eastern coral snake (Micrurus fulvius fulvius) envenomation. Ann. Emerg. Med. 2005;45:603–608. doi: 10.1016/j.annemergmed.2004.11.025. [DOI] [PubMed] [Google Scholar]
- Kanavage AD, Boyer LV, McNally J, Osterhout JJ. Resistance of antivenom proteins to foaming-induced denaturation. Toxicon. 2006;47:445–452. doi: 10.1016/j.toxicon.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Kitchens CS, Van Mierop LH. Envenomation by the Eastern coral snake (Micrurus fulvius fulvius) JAMA. 1987;258:1615–1618. [PubMed] [Google Scholar]
- Lee CY. Elapid neurotoxins and their mode of action. Clin. Toxicol. 1970;3:457–452. doi: 10.3109/15563657008990119. [DOI] [PubMed] [Google Scholar]
- Lee CY. Chemistry and pharmacology of polypeptide toxins in snake venoms. Ann. Rev. Pharmacol. 1972;12:265–286. doi: 10.1146/annurev.pa.12.040172.001405. [DOI] [PubMed] [Google Scholar]
- McCollough MC, Gennaro JF. Treatment of venomous snakebite in the United States. In: Minton SA, editor. Snake Venoms and Envenomation. New York: Marcel Dekker; 1971. pp. 137–154. [Google Scholar]
- Morgan DL, Borys DJ, Stanford R, Kjar D, Tobleman W. Texas coral snake (Micrurus tener) bites. Southern Med. Assoc. 2007;100:152–156. doi: 10.1097/01.smj.0000253596.39121.19. [DOI] [PubMed] [Google Scholar]
- Norris RL, Dart RC. Apparent coral snake envenomation in a patient without visible fang marks. Am. J. Emerg. Med. 1989;7:402–405. doi: 10.1016/0735-6757(89)90047-8. [DOI] [PubMed] [Google Scholar]
- Parrish HM, Khan MS. Bites of coral snakes: report of 11 representative cases. Am. J. Med. Sci. 1967;253:561–568. [PubMed] [Google Scholar]
- Peterson ME. Snake bite: Coral snakes. Clin. Tech. Small Anim. Pract. 2006;21:183–186. doi: 10.1053/j.ctsap.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Pífano F, Trujillo M, Rodríguez-Acosta A. Sobre el emponzoñamiento producido por las corales ponzoñozas del trópico americano, especialmente en Venezuela. Med. Crit. Ven. 1986;1:96–101. [Google Scholar]
- Pttigrew LC, Glass JP. Neurologic complications of a coral snake bite. Neurology. 1985;35:589–592. doi: 10.1212/wnl.35.4.589. [DOI] [PubMed] [Google Scholar]
- Rosso JP, Vargas-Rosso O, Gutiérrez JM, Rochat H, Bougis PE. Characterization of alpha-neurotoxin and phospholipase A2 activities from Micrurus venoms. Determination of the amino acid sequence and receptor-binding abilit of the major alpha-neurotoxin from Micrurus nigrocinctus nigrocinctus. Eur. J. Biochem. 1996;238:231–239. doi: 10.1111/j.1432-1033.1996.0231q.x. [DOI] [PubMed] [Google Scholar]
- Sánchez EE, Galán JA, Perez JC, Rodríguez-Acosta A, Chase PB, Pérez JC. The efficacy of two antivenoms against the venom of North American snakes. Toxicon. 2003;41:357–365. doi: 10.1016/s0041-0101(02)00330-6. [DOI] [PubMed] [Google Scholar]
- Sandner-Montilla F. La creación de la familia Micruridae para las corales de América de la superfamilia Elapidoea. Mem. Cient. Ofidiol. Inst. Ven. Ofidiol. 1985;8:14–21. [Google Scholar]
- Shaw CE. The coral snakes, genera Micrurus and Micruroides, of the United States and northern Mexico. In: Bucherl W, Buckley EE, editors. Venomous Animals and Their Venoms. San Diego: Academic; 1971. pp. 157–172. [Google Scholar]
- Standford RD, Borys DJ, Morgan DL, Tobleman WR. Red on yellow kill a fellow, but not in Texas: Five-years of Texas coral snake (Micrurus fulvius tenere) envenomations. Abstract: Clinical Toxicology. 2005;43:707. [Google Scholar]
- Sutherland SK. Serum Reactions. An analysis of commericial antivenoms and the possible role of anticomplementary activity in denovo reactions to antivenoms and antitoxins. Med. J. Aust. 1977;1:613–615. [PubMed] [Google Scholar]
- Vital-Brazil OV. Coral snake venoms: mode of action and pathophysiology of experimental envenomation. Rev. Inst. Med. Trop. Sao Paulo. 1987;29:119–126. doi: 10.1590/s0036-46651987000300001. [DOI] [PubMed] [Google Scholar]
- Wingert WA, Wainschel J. Diagnosis and management of envenomation by poisonous snakes. South. Med. J. 1975;68:1015–1026. doi: 10.1097/00007611-197508000-00019. [DOI] [PubMed] [Google Scholar]
- Wisniewski MS, Hill RE, Havey JM, Bogdan GM, Dart RC. Australian tiger snake (Notechis scutatus) and Mexican coral snake (Micrurus species) antivenoms prevent death from United States coral snake (Micrurus fulvius fulvius) venom in a mouse model. 2003;41:7–10. doi: 10.1081/clt-120018264. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Progress in the characterization of venoms and standardization of antivenom. Geneva: World Health Organization; WHO Offset Publication. 1981;58 [PubMed]