Background: BRCA1 inhibits centrosome duplication as part of the DNA damage checkpoint.
Results: Binding partners CRM1, BARD1, and Aurora A have distinct roles in targeting BRCA1 to the centrosome and regulating its activity.
Conclusion: BRCA1 nuclear export stimulates its regulation of centrosome duplication.
Significance: CRM1 mediates shuttling of BRCA1 between nucleus and centrosome and may coordinate its DNA damage response.
Keywords: BRCA1, Cell Biology, Centrosome, Confocal Microscopy, Protein Targeting, BARD1, CRM1, FRAP Assay, Centrosome Amplification, Aurora A Kinase
Abstract
BRCA1 is a DNA damage response protein and functions in the nucleus to stimulate DNA repair and at the centrosome to inhibit centrosome overduplication in response to DNA damage. The loss or mutation of BRCA1 causes centrosome amplification and abnormal mitotic spindle assembly in breast cancer cells. The BRCA1-BARD1 heterodimer binds and ubiquitinates γ-tubulin to inhibit centrosome amplification and promote microtubule nucleation; however regulation of BRCA1 targeting and function at the centrosome is poorly understood. Here we show that both N and C termini of BRCA1 are required for its centrosomal localization and that BRCA1 moves to the centrosome independently of BARD1 and γ-tubulin. Mutations in the C-terminal phosphoprotein-binding BRCT domain of BRCA1 prevented localization to centrosomes. Photobleaching experiments identified dynamic (60%) and immobilized (40%) pools of ectopic BRCA1 at the centrosome, and these are regulated by the nuclear export receptor CRM1 (chromosome region maintenance 1) and BARD1. CRM1 mediates nuclear export of BRCA1, and mutation of the export sequence blocked BRCA1 regulation of centrosome amplification in irradiated cells. CRM1 binds to undimerized BRCA1 and is displaced by BARD1. Photobleaching assays implicate CRM1 in driving undimerized BRCA1 to the centrosome and revealed that when BRCA1 subsequently binds to BARD1, it is less well retained at centrosomes, suggesting a mechanism to accelerate BRCA1 release after formation of the active heterodimer. Moreover, Aurora A binding and phosphorylation of BRCA1 enhanced its centrosomal retention and regulation of centrosome amplification. Thus, CRM1, BARD1 and Aurora A promote the targeting and function of BRCA1 at centrosomes.
Introduction
The breast and ovarian cancer susceptibility protein 1 (BRCA1) is a tumor suppressor protein with multiple functions, which is encoded by a gene whose mutational inactivation contributes to hereditary breast and ovarian cancers (1–4). The BRCA1 protein is a key regulator of the cellular DNA damage response and functions in the nucleus as a component of large multiprotein complexes to maintain genomic integrity through homologous recombination and non-homologous end-joining-based DNA repair (5). BRCA1 interacts with many proteins through an N-terminal RING domain and C-terminal tandem BRCT2 repeats commonly mutated in cancers. The RING domain is a zinc finger motif typical of those found in many ubiquitin ligases and forms part of the BRCA1 sequence that interacts with BARD1 (BRCA1-associated RING domain protein 1) to form a stable BRCA1-BARD1 dimer, with E3 ubiquitin ligase activity (6) that functions in DNA repair (7) and centrosomal duplication (8). Cancer mutations of the BRCT domains, highly folded protein interaction sequences that mediate binding to phosphoserine peptides (9), are known to impair BRCA1 transcription activity (10), DNA repair function (11), and its ability to suppress tumor formation in mice (12).
Centrosomes are non-membranous organelles that nucleate microtubules in interphase and mitosis (13). The centrosome duplicates once during a cell cycle, and the two centrosomes move to opposite poles to form the bipolar mitotic spindle. BRCA1 and BARD1 localize to the centrosome throughout the cell cycle (14, 15) and ensure that centrosome duplication occurs only once during a cell cycle (16), which prevents formation of multipolar spindles, unequal chromosome segregation, and aneuploidy. The loss of BRCA1 by genetic mutation, silencing, or peptide competition results in centrosome amplification in human breast cells, thus contributing to cell transformation and ultimately to aneuploidy as detected in breast tumors (16–19). The BRCA1-BARD1 heterodimer ubiquitinates γ-tubulin, a centrosome component of the γ-tubulin ring complex, and this stimulates initial nucleation of microtubules (14). Further studies from the Parvin laboratory (16, 20) show that BRCA1 ubiquitin ligase activity is essential for the negative regulation of centrosome overduplication (generally referred to as amplification in this study) and also for the centrosomal localization of γ-tubulin. BRCA1 regulates the G2/M cell cycle checkpoint, a mechanism that stalls cell cycle progression after DNA damage to ensure timely repair of the DNA but can inadvertently give time for centrosomes to duplicate more than once in one cell cycle (21). Cell cycle checkpoints are often regulated by kinases, phosphatases, and their substrates. Aurora A kinase is overexpressed in many cancers, including breast cancers (22, 23), and localizes to the centrosome during mitosis (24, 25). Aurora A binds to BRCA1 and moderates its activity at the centrosome by phosphorylation to signal the G2/M-phase transition (26) and microtubule nucleation inhibition via hindering BRCA1 ubiquitin ligase activity (15).
Our laboratory has studied intracellular transport of BRCA1 since 2000, when we first discovered that BRCA1 contains an N-terminal nuclear export sequence (NES) (27). The BRCA1 NES mediates binding to the major nuclear export receptor, CRM1 (chromosome region maintenance protein 1)/exportin-1, and facilitates the nuclear-cytoplasmic shuttling of BRCA1. The precise role that CRM1 plays in regulation of BRCA1 transport or dynamics in the cell is not well understood. It is also striking to note that despite a decade of research by others into the role of BRCA1 at the centrosome, there is currently no understanding of how BRCA1 moves to the centrosome, what sequences are involved, or which binding partners regulate its recruitment or dynamics. This study was therefore aimed at defining the key sequences that localize BRCA1 to the centrosome and to test the hypothesis that CRM1 contributes to this recruitment process and possibly to the BRCA1 regulation of centrosome amplification.
Previously, we identified N-terminal CRM1-binding NESs in both BRCA1 and BARD1 (27, 28). In addition to its nuclear export function, CRM1 has been shown to bind and regulate (i.e. enhance or reduce) the action of several cellular and viral proteins to maintain normal centrosome duplication (29). Forgues et al. (30) observed that CRM1 localized to the centrosome and that CRM1 inhibition by use of the drug leptomycin B (LMB) or CRM1 sequestration by a hepatitis B viral protein, HBx, resulted in formation of supernumerary centrosomes. CRM1 localization at the centrosome was later shown to at least partly involve interaction of its N terminus with Ran-GTP (31). Ran-GTP localizes to the centrosome through its association with the centriole-binding structural protein AKAP450 (32). Thus, CRM1 could potentially provide a docking site for NES-containing regulators of the centrosome. Some evidence for this was shown for nucleophosmin (NPM1), a nucleolar protein whose localization at the centrosome was dependent on binding to CRM1 during mitosis and required to prevent more than one round of centrosome duplication during a cell cycle (33, 34). BRCA2 was also recently implicated in preventing inappropriate centrosome amplification and was found to localize to the centrosome during S and early M phase in a manner partially influenced by CRM1 (35, 36). More recently, we analyzed BARD1 recruitment to centrosomes and showed that BARD1 displays a very fast turnover and BRCA1-independent localization to the centrosome (37). The mutation of the BARD1 NES reduced its centrosomal localization (37). In this study, we have performed the first systematic mapping of sequences critical for centrosomal localization of BRCA1 and describe evidence supporting distinct roles for the N-terminal binding partners CRM1 and BARD1 and C-terminal binding partner Aurora A in regulating BRCA1 localization, dynamics, and function at the centrosome.
EXPERIMENTAL PROCEDURES
Cell Culture, Transfection, and Treatments
Human breast cancer cell line MCF-7 and human osteosarcoma cancer cell line U2OS were cultured in Dulbecco's modified Eagle's medium (DMEM) as described in (38). Human breast cancer cell line HCC1937 (BRCA1 5832InsC mutation) were grown in supplemented RPMI 1640 medium as described (37). At 16 h after seeding, cells were transfected at 50–60% confluence with 2 μg of plasmid DNA (per well in a 6-well plate) using Fugene HD reagent (Promega) according to the manufacturer's instructions. For siRNA transfections, cells were transfected at 40–50% confluence with 3 μg of siRNA (per well in a 6-well plate) using Lipofectamine 2000 reagent (Invitrogen). At 6 h post-transfection, the transfection mix was removed and replaced with medium containing FBS, as described above. Cells were fixed and processed 24–30 h post-transfection for fluorescence microscopy or Western blotting. For LMB treatment, cells were treated with 5 ng/ml (Sigma) for 12 h before fixation and immunostaining. For nocodazole treatment, cells were treated with 10 μm nocodazole 1 h before fluorescence recovery after photobleaching (FRAP) or fixation and immunostaining.
Cell Cycle Analysis by Flow Cytometry
HCC1937 cells were transfected with various yellow fluorescent protein (YFP)-tagged BRCA1 plasmid constructs expressing different forms of BRCA1. 48 h post-transfection, cells were harvested by trypsinization and resuspended 400 μl of PBS, added dropwise to ice-cold 85% ethanol, and incubated for at least 1 h. Prior to flow cytometry, cells were centrifuged at 1500 rpm and resuspended in 600 μl of PBS containing RNase A (1 mg/ml) and propidium iodide (2 mg/ml). Cell cycle/apoptosis profiles were determined using a BD Biosciences FACSCalibur flow cytometer (excitation wavelength 485 nm, emission wavelength 508 nm). The percentage of apoptotic cells was determined by quantifying the sub-G1 DNA content population using CellQuest software.
Plasmids
Many of the plasmids used in this study have been described previously (27, 28, 39–43). Other YFP-labeled BRCA1 constructs were produced by inserting the YFP coding sequence into the NotI site upstream of the BRCA1 coding sequence in FLAG-tagged constructs described previously (27, 39). YFP-BRCA1 297–773 was made using a two-step cloning process. First the NotI/EcoRI site of pF-BRCA1 wt was excised and replaced by a linker made using primers, 5′-G GCC GCA ATG AAT GTA GAA AAG GCT G-3′ and 3′-CGT TAC TTA CAT CTT TTC CGA CTT AA-5′ to create pF-BRCA1 297–1863. The C-terminal sequence of pF-BRCA1 297–1863 from residue 773 was excised with KpnI and religated. YFP was then inserted as above. Specific details of individual cloning techniques and primer sequences are available on request. RFP-pericentrin C241 was supplied as a generous gift by Dr. Sean Munro (44).
Immunofluorescence Microscopy
Centrosomal protein immunostaining was performed as described previously (38), fixing cells with acetone/methanol and staining cells with the following primary antibodies: rabbit polyclonal BRCA1 Ab-P (1:1000; gift from Prof. Jeffrey Parvin), mouse monoclonal γ-tubulin (1:1000; Abcam), mouse monoclonal α-tubulin (1:1000; Sigma), and rabbit polyclonal anti-FLAG (1:2000; Sigma). Bound antibodies were detected with either AlexaFluor 488 (1:500)- or AlexaFluor 594 (1:1000)-conjugated secondary antibodies. Cells were observed and imaged using an Olympus BX-51 fluorescence microscope at ×60 magnification and a Spot RT slider camera 23.1. For each experiment, an average score was obtained from two or three individual experiments, and at least 100 cells were scored for each treatment per experiment. For live imaging, an Olympus FV1000 confocal laser-scanning microscope with a ×60 water objective was used to take z-stack images of co-transfected YFP-CRM1 and RFP-pericentrin C241 at 0.5-μm intervals through the centrosome. The fluorescence intensity of endogenous BRCA1 levels (relative to the centrosome marker, γ-tubulin) in untreated and LMB-treated cells was measured using the Olympus FluoView version 1.6a software and graphed using Microsoft Excel. For each treatment, at least 200 cells over two experiments were quantified.
Fluorescence Recovery after Photobleaching (FRAP)
FRAP was performed on MCF-7 cells co-expressing similar and moderate levels of YFP-BRCA1 constructs and RFP-pericentrin C241 at 30–48 h post-transfection in a humidified CO2 chamber at 37 °C. RFP-pericentrin C241 was co-transfected to mark the centrosome in live cells. The analysis was performed on an Olympus FV1000 confocal laser-scanning microscope with a ×60 water objective. For YFP-tagged construct FRAP, a cell was scanned with laser power (10–13%), and the region of interest was then photobleached at 100% laser power. For the RFP-tagged pericentrin C241 construct, 20% laser power was used for scanning, and 100% laser power was used for bleaching. Data were analyzed with Olympus FluoView version 1.6a software. Each FRAP experiment started with two prebleach image scans followed by bleaching 90–100% of the centrosome for 4 s. Images were collected every 4 s for a total of 60 s, and the size of the scan region and digital zoom (×3.2) was kept constant during each experiment. Each type of FRAP analysis was based on 10–15 cells from at least two independent experiments. Average intensities in all regions of interest, including the background signal and whole cell fluorescence bleaching, were calculated using Olympus Fluoview version 1.6a software. In Microsoft Excel (2007), bleaching from imaging and background fluorescence were deducted for each cell, and an average recovery curve was generated. These data were then entered into GraphPad Prism 5 and determined to best fit a two-phase association curve. t½ and plateau values were determined and used to compare initial speed of recovery and the recovery percentage at the centrosome. The fraction of protein that contributes to the recovery is called the “mobile” fraction, and the protein that does not is called the “immobile” fraction. Spot bleaching of the cytoplasm was carried out for each construct and showed that fluorescence recovery due to cytoplasmic diffusion was <10% for both BRCA1 and pericentrin proteins (data not shown).
CSK Retention Assay
To compare centrosomal retention of endogenous BRCA1 and γ-tubulin, a detergent extraction assay was used to remove soluble proteins from cells prior to fixation and immunofluorescence staining. Cells were grown on poly-l-lysine (0.1 mg/ml; Sigma)-coated coverslips and then incubated in CSK extraction buffer (10 mm Pipes, pH 6.8, 300 mm sucrose, 5 mm MgCl2, 100 mm NaCl, 0.5% Triton X-100) for 8 and 40 min at 32 °C or fixed directly. Cells were fixed with cold acetone/methanol for 3 min at room temperature and then blocked in 3% BSA, PBS and probed with previously described primary and secondary antibodies. Cells were scored for centrosomal localization and imaged using an Olympus BX-51 fluorescence microscope at ×60 magnification and a Spot RT slider camera 23.1. For each experiment, an average score was obtained from two or three individual experiments, and at least 100 cells were scored for each treatment.
Centrosome Amplification Assay
To analyze the ability of different forms of BRCA1 to regulate centrosome amplification, cancer cell line HCC1937 (5382InsC BRCA1 mutant) was transfected with various YFP-tagged BRCA1 constructs. At 24 h after transfection, cells were treated with 10 Gy of ionizing radiation (IR) (300 kV, 10 mA) using an X-RAD 320 Biological Irradiator (Precision X-Ray Inc.) and then allowed to recover at 37 °C with 5% CO2 for 48 h prior to immunostaining and analysis of centrosome number by microscopy.
RESULTS
Cancer Mutations in BRCT Domains Abolish Centrosomal Localization of BRCA1
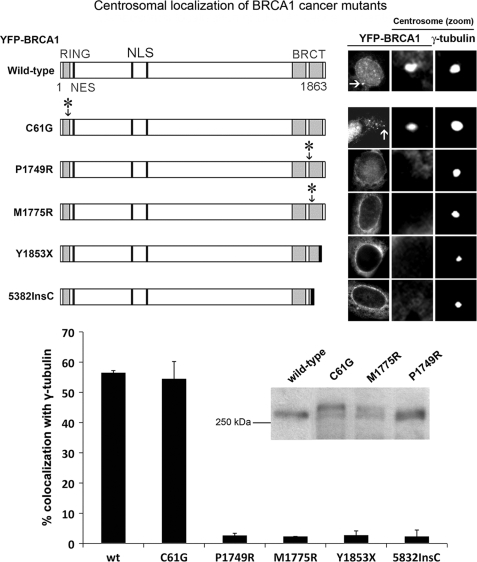
Because centrosomal localization is integral to BRCA1-dependent regulation of centrosome amplification and tumor suppressor function, we compared the effect of known cancer-associated mutations on the localization of BRCA1 to the centrosome. YFP-tagged forms of BRCA1 were transiently expressed in MCF-7 breast cancer cells and analyzed by fluorescence microscopy for co-localization with γ-tubulin, used to mark the centrosome. Wild-type BRCA1 was detected at the centrosome in most (57%) cells (Fig. 1). The cancer-associated N-terminal RING mutation, C61G, which abolishes ubiquitin ligase activity, did not affect centrosomal targeting. On the other hand, each of the BRCT domain point mutations tested (P1749R, M1775R, Y1853X, 5832InsC) completely abolished centrosomal localization (see Fig. 1). The correct size of all point mutants was confirmed by Western blot (Fig. 1, inset; also see Ref. 40). HCC1937 breast cancer cells harbor an endogenous 5382InsC mutation of BRCA1, and by co-staining with antibodies against BRCA1 (Ab-P) and γ-tubulin, we confirmed the ectopic 5382InsC localization pattern, showing that in HCC1937 cells, less than 5% of endogenous mutant BRCA1 stained at the centrosome (supplemental Fig. S1). Therefore, mutations in the BRCT domain (but not the RING domain) abolish BRCA1 targeting to the centrosome. This could be due to the fact that such point mutations disrupt folding of the BRCT domain region (45) and thereby prevent binding of specific proteins required for recruitment of BRCA1 to the centrosome.
FIGURE 1.
Cancer-associated BRCT mutations prevent BRCA1 centrosome localization. Shown is a schematic diagram showing the organization of BRCA1 protein domains (the RING and BRCT domains, the NLS, and the NES) as well as cancer-associated mutations. YFP-tagged BRCA1 cancer-associated mutants were transfected into MCF-7 breast cancer cells and analyzed for co-localization with the centrosome-component γ-tubulin by immunofluorescence microscopy. Representative cell images of YFP-BRCA1 localization are shown in the right-hand panel, in addition to close-up images of the centrosomes, with staining of BRCA1 and γ-tubulin. Cells expressing YFP-tagged BRCA1 were scored for co-localization with the centrosome. Scoring results were obtained from at least three independent experiments, each with at least 100 cells scored (mean ± S.D. (error bars)). Integrity of the BRCA1 point mutants was validated by Western blot (see inset).
The Combination of N Terminus and C-terminal BRCT Domains Targets BRCA1 to Centrosome Independent of BARD1 and γ-Tubulin
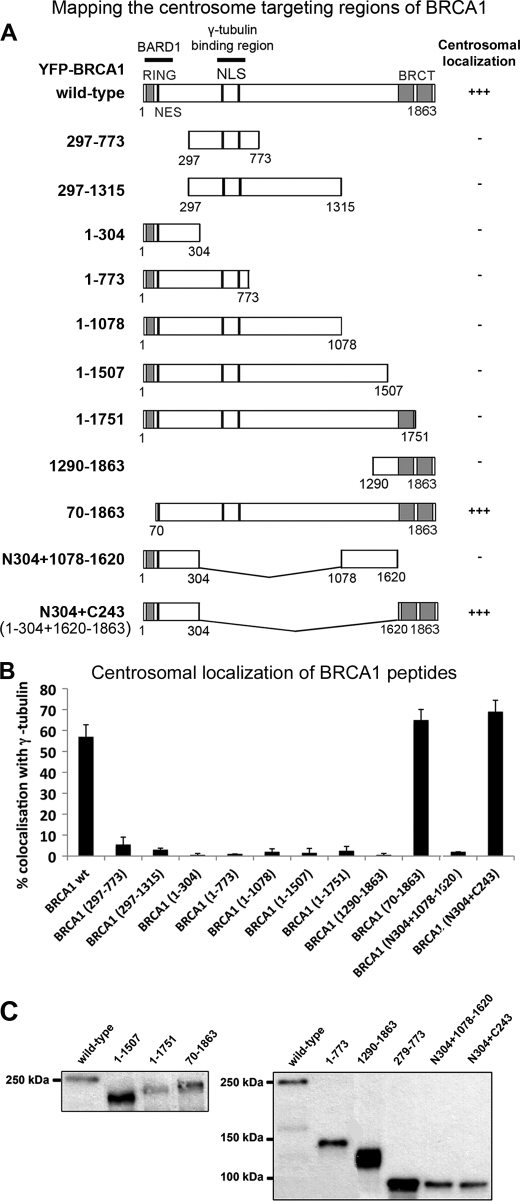
BRCA1 localizes to the centrosome throughout the cell cycle; however, the precise targeting sequence(s) remain unknown. A centrosomal localization sequence has been reported in cyclin E (46), cyclin A2 (47), and BRCA2 (36); however, ClustalW protein sequence alignment revealed no similar targeting element in BRCA1. BRCA1 also lacks a PACT domain, required for targeting pericentrin and AKAP450 to centrosomes (44). Consequently, we used an extensive deletion analysis to map the sequences critical for BRCA1 localization to the centrosome. We transfected YFP-tagged BRCA1 peptides into MCF-7 breast cancer cells and analyzed them by fluorescence microscopy for co-localization with γ-tubulin. Cells expressing wild-type YFP-BRCA1 showed centrosomal accumulation characterized by co-localization with γ-tubulin in asynchronous MCF-7 cells (Fig. 1, A and B). The region spanning amino acids 510–622 of BRCA1 has been reported to bind γ-tubulin and seemed a likely candidate for a centrosomal anchor of BRCA1 (48). Two YFP-tagged protein fragments 297–773 and 297–1312 that encompass the γ-tubulin binding region were transiently expressed in MCF-7 cells and analyzed for co-localization with γ-tubulin. These BRCA1 sequences are detectable in both the nucleus and cytoplasm but did not accumulate at the centrosome (Fig. 2). Thus, γ-tubulin binding is essential for BRCA1 centrosomal functions but not for its localization.
FIGURE 2.
Mapping of BRCA1 centrosome targeting domains. A, diagram showing the position of BRCA1 protein domains: the RING and BRCT domains, NLS, NES, and the binding regions for BARD1 and γ-tubulin. A range of YFP-tagged BRCA1 deletions and fusions were transiently expressed in MCF-7 cells and then fixed with acetone/methanol and analyzed for co-localization of BRCA1 with γ-tubulin by immunofluorescence microscopy (as detailed in the legend to Fig. 1). The relative degree of centrosome-positive BRCA1 staining is indicated in the right-hand panel. B, pYFP-BRCA1-transfected cells were scored for centrosomal localization of ectopic BRCA1 sequences by fluorescence microscopy, and the data were graphed as shown. Scoring data are from three independent experiments, scoring at least 100 cells/experiment (mean ± S.D. (error bars)). C, BRCA1 plasmids not already characterized previously (see “Experimental Procedures”) were confirmed for correct size of the YFP-fusion by Western blot, analyzing total lysates from transfected cells. Note that despite variations in band intensity from total extracts, only cells with similar levels of moderate YFP-BRCA1 expression were analyzed in single cell microscopy assays.
Careful scoring of a series of C-terminal deletions revealed that loss of just 112 amino acids from the C terminus (equivalent to BRCA1 peptide with amino acids 1–1751) completely abolished BRCA1 localization at the centrosome (Fig. 2). We therefore tested the BRCT domains alone (residues 1290–1863) and found that they were not sufficient for BRCA1 centrosomal targeting.
Conversely, it is noteworthy that an N-terminal deletion of the BARD1-interacting RING domain (residues 70–1863), which we previously showed abolishes BARD1 interaction (39) (see also supplemental Fig. S2), did not reduce centrosomal targeting, indicating that although BARD1 co-locates with BRCA1, it is not required for its recruitment to the centrosome. This is unexpected, given that BARD1 is the major known binding partner of BRCA1, and the two proteins form an active heterodimer that is important for the role of BRCA1 in preventing centrosome amplification (14). We found that neither the N terminus alone nor the BRCT domains were sufficient to target BRCA1 to the centrosome; however, a fusion peptide comprising both the N terminus (N304; amino acids 1–304) and C terminus (C243; amino acids 1620–1863) localized to the centrosome equally as efficiently, if not slightly better, than wild-type BRCA1 (N304+C243, 69%; Fig. 2B). To underscore the specificity of this unexpected finding, a different fusion construct that combined the N terminus (N304) and amino acids 1078–1312 was also tested but was negative for centrosomal localization. Similar findings were observed in HCC1937 and U2OS cells (data not shown). The integrity and correct size of each construct expressed in cells was verified by Western blot (Fig. 2C) (27, 28, 39, 40, 43). This finding indicates that cooperation of the N terminus and C-terminal BRCT domains is sufficient to mediate effective BRCA1 targeting to the centrosome, in the absence of binding to BARD1 or γ-tubulin.
Minimal Centrosome Targeting Sequence of BRCA1 (N304+C243) Cannot Regulate DNA Damage-induced Centrosome Amplification in HCC1937 Cells
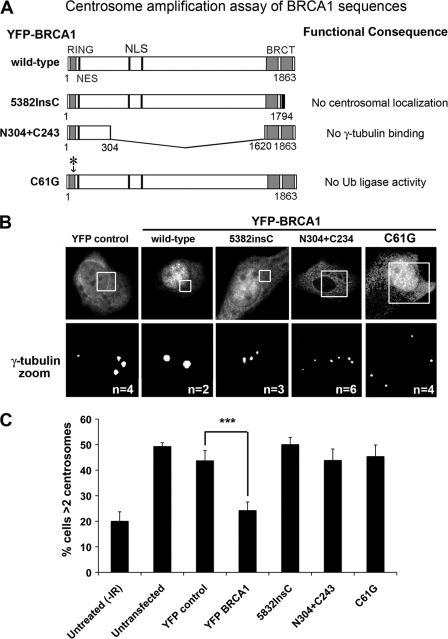
Next we compared the function of specific BRCA1 sequences (Fig. 3A), employing a single cell assay to compare the different sequences for their ability to inhibit centrosome amplification after DNA damage (Fig. 3, B and C). The method utilizes HCC1937 breast cancer cells that express a mutated form of BRCA1 (5282InsC) and mutated p53, resulting in a defective G2/M checkpoint and continuation of the cell cycle after DNA damage, leading to centrosome amplification (50). We observed an increased number of cells (from 20 to 49%) with more than two centrosomes at 48 h after exposure of HCC1937 cells to 10 Gy of IR (Fig. 3), comparable with that previously reported. Centrosome amplification was also observed following overexpression of YFP alone (44%) or YFP-BRCA1 5832InsC mutant (50%), which did not localize to centrosomes. The transient expression of wild-type YFP-BRCA1 inhibited centrosome amplification by 50% (p = 0.0001), as described elsewhere (50). In contrast, the minimal BRCA1 targeting fusion construct N304+C243, which resembles the exon 11 deletion mutant previously shown to disrupt BRCA1 regulation of centrosome number (19), was unable to reduce centrosome amplification (which remained high at 44%). Moreover, the RING mutant YFP-BRCA1 C61G, which can still bind to BARD1 (39) (see also supplemental Fig. S2) but is deficient in ubiquitin ligase activity, was equally as ineffective at inhibiting centrosome amplification in DNA damaged cells (45%). The differences observed were not due to any changes in cell cycle profile, as confirmed by flow cytometry analysis of the cell cycle in transfected HCC1937 cells (supplemental Fig. S3). This is clear evidence that the domains of BRCA1 responsible for centrosome localization are not sufficient for the regulation of centrosome amplification.
FIGURE 3.
Centrosome targeting domains of BRCA1 are insufficient for the regulation of centrosome amplification. A, diagram of different BRCA1 peptides tested for their ability to regulate centrosome amplification (including the minimal centrosomal targeting sequence) and their main functional defects. B, YFP-tagged BRCA1 proteins were transiently expressed in HCC1937 breast cancer cells, which harbor the endogenous BRCA1 mutation 5382InsC and have a defective DNA damage checkpoint. Cells were treated with 10 Gy of IR and left to recover for 48 h. After fixation with acetone/methanol, cells were immunostained with anti-γ-tubulin antibody and scored by microscopy for the number of cells displaying centrosome amplification (>2 centrosomes/cell). Representative cell images are shown for each BRCA1 peptide, showing similar expression levels of transiently expressed proteins and close up images of centrosomes. The number of centrosomes in each imaged cell is noted in white. C, IR-induced centrosome amplification was reduced by overexpression of WT BRCA1 but not by various mutated forms of BRCA1, including the minimal targeting sequence (N304+C243). Scoring results were obtained from three independent experiments with at least 100 cells scored (mean ± S.D. (error bars)). Student's t test was used to determine that only YFP-BRCA1 wild type was statistically significant in regulating centrosome amplification in comparison with YFP control. ***, p < 0.001.
Photobleaching Experiments in Live Cells Identify Dynamic and Immobile Pools of YFP-BRCA1 at Centrosome
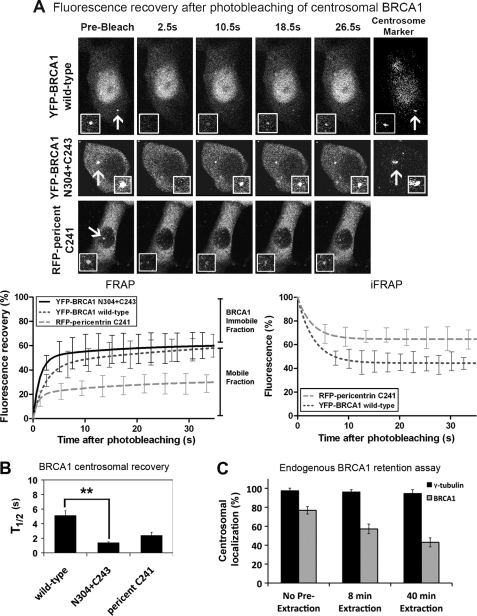
To determine whether the central region (including the binding site for γ-tubulin) of BRCA1 contributes to BRCA1 centrosomal dynamics or retention, we employed FRAP analysis to compare mobility of YFP-BRCA1 wild-type and N304+C243 proteins. First, we determined the minimal bleaching time to completely eliminate centrosome fluorescence using fixed MCF-7 cells (4 s, 100% laser intensity). FRAP assays were conducted on live cells co-expressing the fluorescence-tagged C terminus of pericentriolar matrix component pericentrin (RFP-pericentrin-C241) using an Olympus FV1000 confocal microscope (Fig. 4A). The fluorescent pericentrin provided an effective live cell marker of the centrosome. After a high intensity laser spot bleaching of centrosome fluorescence, recovery was measured every 4 s for a period of at least 60 s. YFP-BRCA1 fluorescence (n = 12) recovered at the bleached centrosome with a t½ of 5.1 s, and YFP-BRCA1 (N304+C243) (n = 10) recovered much faster with a t½ of 1.4 s (p < 0.01; Fig. 4B). Thus, the centrosomal recovery rate of YFP-BRCA1 (N304+C243), which cannot bind γ-tubulin, is faster than wild type in the initial stages of centrosomal association (see also supplemental Table S1). All cells analyzed showed ample cytoplasmic BRCA1 and pericentrin C241 available for recovery to the centrosome. The total extent of recovery of the BRCA1 proteins after 25 s was similar and relatively high compared with RFP-pericentrin, and analysis of the 60-s recovery period (data not shown) suggests that ∼60% of BRCA1 at the centrosome is mobile and dynamic, in contrast to pericentrin, which is much more stable (∼30% mobile; Fig. 4). On the other hand, we identified a significant immobile pool of BRCA1 (∼40% of centrosomal BRCA1) that is relatively stable at the centrosome, independent of γ-tubulin interaction. This was confirmed by a complementary inverse FRAP approach, which involved bleaching of extracentrosomal fluorescence and quantification of the rate of loss from the centrosome (see the graph in the right-hand panel of Fig. 4A). Furthermore, an in vitro assay using mild detergent extraction of soluble proteins revealed centrosome retention of ∼50% of the total endogenous centrosomal BRCA1 pool, whereas γ-tubulin was highly resistant and well retained at centrosomes (Fig. 4C).
FIGURE 4.
Defining the dynamics of YFP-BRCA1 turnover and retention at the centrosome in live cells. A, FRAP analysis was performed on MCF-7 cells transfected with plasmids encoding RFP-pericentrin C241 and YFP-BRCA1 (wild type or N304+C243 mutant). Centrosomes were targeted for laser photobleaching followed by fluorescence time lapse microscopy. Representative prebleach, first image postbleach, and images for 10.5, 18.5, and 26.5 s after bleach are shown for each construct as well as the marker for the centrosome, RFP-pericentrin C241. The insets show higher magnification views of the centrosome. Corresponding FRAP recovery curves are shown for each protein in comparison with RFP-pericentrin C241 (recently described in Ref. 37), indicating the immobile and mobile fractions (left). YFP-BRCA1 (wild type) and RFP-pericentrin were also analyzed by an inverse FRAP (iFRAP) assay, bleaching cellular fluorescence, and then quantifying the rate of loss from the centrosome (right). B, the t½ (half-time ± S.E. (error bars)) and immobile fraction (percentage ± S.E.) are shown for each protein with an average of 10–15 cells over at least two experiments analyzed for each. Student's t test was used to show a significant difference in t½ between wild-type and N304+C243 peptides. **, p < 0.01. C, in an in vitro assay to measure BRCA1 centrosomal retention, cells were treated with CSK detergent buffer for 0, 8, or 40 min prior to fixation with acetone/methanol to remove soluble protein. Cells were then immunostained with BRCA1 antibody, co-stained for γ-tubulin, and scored by microscopy for the percentage of cells still displaying endogenous BRCA1 at the centrosome.
Interestingly, the BRCA1 kinetics differed from that of BARD1, which is more dynamic with only ∼20% immobile at the centrosome (37). Several proteins have been shown to localize to centrosomes via dynein and microtubules (51). To assess whether BRCA1 localizes to centrosomes in a similar manner, cells were further treated with 10 μm nocodazole for 1 h to depolymerize microtubules and then analyzed by immunofluorescence staining or by FRAP assay. We found that nocodazole had no effect on BRCA1 localization or dynamics (supplemental Fig. S4).
Evidence That CRM1-mediated Nuclear Export Stimulates BRCA1 Accumulation at Centrosome in Vitro and in Live Cells
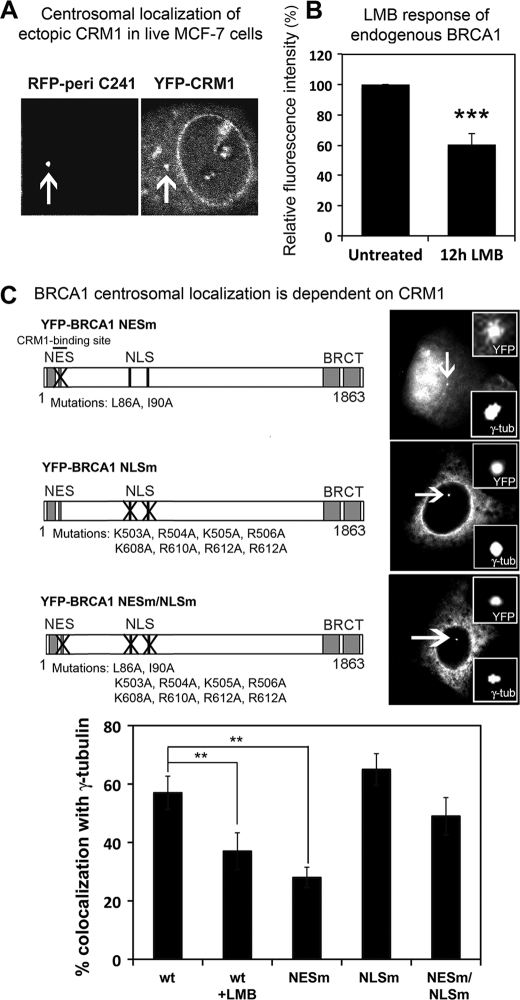
The N and C termini of BRCA1 are critical for localization to the centrosome, although deletion of the N-terminal BARD1 interaction domain did not diminish BRCA1 centrosomal localization (Fig. 2). The N terminus of BRCA1 also contains an NES, a CRM1 binding site that we previously demonstrated facilitates nuclear export of the protein (27). The nuclear export receptor CRM1 is known to localize to the centrosome, and through binding to specific proteins can regulate their impact on centrosome duplication (29, 30). To confirm CRM1 localization at the centrosome, we transfected MCF-7 cells with YFP-CRM1 and RFP-pericentrin C241 and visualized co-localization in live cells using confocal microscopy (Fig. 5A). To test the effect of inhibiting CRM1 on endogenous BRCA1, we treated MCF-7 cells with the CRM1-specific inhibitor, LMB, at 5 ng/ml for 12 h, and co-immunostained with anti-γ-tubulin and BRCA1 (Ab-P) antibodies. LMB inactivates CRM1 through alkylation of cysteine 529, leading to inhibition of NES-dependent nuclear export of proteins (52). Quantification of fluorescence intensity at hundreds of individual centrosomes showed that inhibition of CRM1 by LMB decreased the amount of BRCA1 localized at individual centrosomes by 40% compared with control (Fig. 5B).
FIGURE 5.
CRM1 contributes to BRCA1 centrosomal localization. A, YFP-CRM1 and RFP-pericentrin C241 were transiently co-expressed to show that ectopic CRM1 localizes to the centrosome in live MCF-7 cells. B, MCF-7 cells were treated with 5 ng/ml LMB for 12 h to inhibit CRM1 binding to BRCA1. After fixation with acetone/methanol, cells were stained with anti-γ-tubulin and anti-BRCA1 Ab-P antibodies, and the relative levels of fluorescence intensity at the centrosomes were analyzed by microscopy. >200 cells were analyzed (mean ± S.D. (error bars)). Student's t test was used to show that the loss of BRCA1 staining at the centrosome after LMB was statistically significant. ***, p < 0.001. C, MCF-7 cells were transfected with plasmids encoding wild-type BRCA1 with or without LMB, and nuclear transport mutants of BRCA1 were then scored for co-localization with γ-tubulin. Representative images of each construct are shown with centrosomal localization indicated. Scoring results are from three experiments (mean ± S.D.). Student's t test was used to determine the statistical significance of ectopic BRCA1 loss from the centrosome when CRM1-binding was inhibited by LMB or NES mutation. **, p < 0.01; ***, p < 0.001.
Next, we assessed the centrosomal localization of different BRCA1 nuclear transport mutants (Fig. 5C) in fixed cells to determine how nuclear import or export of BRCA1 influenced its localization at centrosomes. In MCF-7 cells, blocking BRCA1 nuclear export by LMB treatment or mutation of the NES decreased staining of YFP-BRCA1 at the centrosome by 30–50% (Fig. 5C), similar to that seen for endogenous BRCA1. This is consistent with a reduced amount of the BRCA1 NES mutant (NESm) protein in the cytoplasm. On the other hand, when the nuclear localization signal (NLS) of BRCA1 is mutated (NLSm), BRCA1 cannot enter the nucleus and is predominantly localized to the cytoplasm and stained strongly at the centrosome in 65% of cells scored (Fig. 5C). Next, we asked what would happen when both the NLS and NES of BRCA1 are mutated (NESm/NLSm), given that this double transport mutant is stuck in the cytoplasm but also cannot bind to CRM1. Surprisingly, the NESm/NLSm form of BRCA1 displayed a 25% decrease in centrosome staining compared with the NLSm form, and this means that disrupting the binding to CRM1 perturbs centrosome localization of BRCA1 even when it is in the cytoplasm. Therefore, we conclude that CRM1 stimulates BRCA1 localization to the centrosome in two ways, first by exporting it from nucleus to cytoplasm and thereby increasing its cytoplasmic concentration and second by enhancing its localization at the centrosome once in the cytoplasm.
CRM1 Stimulates Dynamic Turnover of BRCA1 at Centrosome
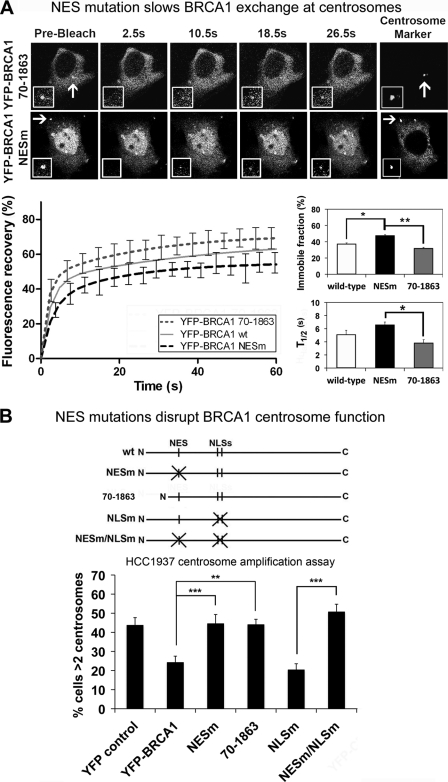
There is surprisingly little published data on the ability of CRM1, the major nuclear export receptor, to regulate protein kinetics in live cells. Therefore we employed FRAP assays on live MCF-7 cells to quantitatively compare two different forms of YFP-BRCA1, one with an unmasked and highly accessible NES (residues 70–1863) (39) and one with a mutated inactive NES (NESm), measuring fluorescence recovery after 4 s of photobleaching (Fig. 6A). Recovery curves showed that YFP-BRCA1 NESm was slower to recover (t½ = 6.62 s, n = 16) than the export-efficient 70–1863 BRCA1 fragment (t½ = 3.86 s, n = 11, p < 0.05) and also slower than wild-type BRCA1 (t½ = 5.14 s). In addition to differences in the recovery rates, the total recovery of fluorescence was slightly reduced for the BRCA1 NES mutant, indicating that it is less mobile than wild-type BRCA1 and considerably less mobile than BRCA1 70–1863 (Fig. 6A). Thus, the NES mutation slows the movement of BRCA1 and causes it to be moderately more retained at centrosomes. The BRCA1 70–1863 sequence does not bind BARD1 and hence is not prone to NES masking by BARD1 (39); this export-active form of BRCA1 displayed high mobility and turnover at the centrosome. By comparison with the above data on BRCA1 dynamics, we found that FRAP analysis of wild-type and NES-mutated forms of YFP-BARD1 revealed little effect of NES mutation on BARD1 exchange dynamics at the centrosome (see supplemental Fig. S5). The results suggest that CRM1 contributes to the recruitment and turnover of BRCA1 at the centrosome, consistent with the role of CRM1 in targeting (Fig. 5).
FIGURE 6.
NES mutation disrupts BRCA1 dynamics and function at the centrosome. A, FRAP analysis was performed on live MCF-7 cells co-transfected with RFP-pericentrin C241 and YFP-BRCA1 mutants, 70–1863 and NESm, deficient in binding to BARD1 and CRM1, respectively. The 70–1863 sequence is highly export-active (39). Centrosome fluorescence was photobleached, and fluorescence recovery was then measured by time lapse microscopy. Representative prebleach, first image postbleach, and images for 10.5, 18.5, and 26.5 s after bleach are shown for each protein. The insets show higher magnification views of the target area. Corresponding recovery curves are shown for the above proteins, as well as wild-type BRCA1. The t½ (half-time ± S.E. (error bars)) and immobile fractions (percentage ± S.E.) are also shown. Statistical significance of changes in immobile fraction and half-time are indicated. *, p < 0.05; ***, p value < 0.001. B, different BRCA1 mutants defective in binding to BARD1 or in nuclear transport were tested for their ability to regulate centrosomal amplification. YFP-tagged BRCA1 proteins were transfected into HCC1937 breast cancer cells, and cells were then treated with 10 Gy of IR and left to recover for 72 h. Cells were fixed and immunostained with anti-γ-tubulin antibody and analyzed for centrosome amplification (>2 centrosomes/cell). Scoring results are from three independent experiments (mean ± S.D.) and Student's t test was used to determine statistical significance. **, p value < 0.01; ***, p value <0.001.
CRM1 Is Critical for BRCA1-mediated Inhibition of Centrosome Amplification
Because CRM1 is implicated in regulation of centrosome number, we tested whether CRM1 affected BRCA1-dependent regulation of centrosome amplification. The HCC1937 cell line was used to assess CRM1-dependent transport mutant forms of BRCA1 (NESm) for their ability to negate centrosome amplification induced by DNA damage (Fig. 6B). Indeed, in contrast to wild-type YFP-BRCA1, we found that export-defective YFP-BRCA1 NESm was unable to prevent centrosome amplification in HCC1937 cells, although many cells showed localization of the NES mutant at the centrosome. Therefore, mutation of the NES, which was previously shown to block regulation of BRCA1 by CRM1 (27), abolished BRCA1 regulation of centrosome number. As in previous experiments (Fig. 5), the YFP-BRCA1 NES/NLSm double mutant was used to rule out indirect effects caused by increased nuclear localization of the NES mutation. YFP-BRCA1 NES/NLSm lacks both nuclear import and export activity and thus locates entirely in the cytoplasm, and this mutant was also unable to reduce the number of cells exhibiting centrosome amplification (49%; see Fig. 6B). In contrast, the NLS mutant form of BRCA1 was equally as effective as wild-type BRCA1 (24%). The export-efficient 70–1862 mutant was unable to regulate centrosome duplication (44%), most likely due to an inability to bind BARD1 and form the ubiquitin ligase heterodimer. The disruption of BRCA1 functionality by the NES mutation was not attributable to changes in cell cycle arrest (see supplemental Fig. S2) or to changes in binding of BARD1 (39). These data show that the CRM1 recognition site is essential for BRCA1 regulation of centrosome number.
Unexpected Differences in Dynamics and Retention of Monomeric BRCA1 and Dimeric BARD1-bound BRCA1 at Centrosome; Association of Dimer Is Less Stable
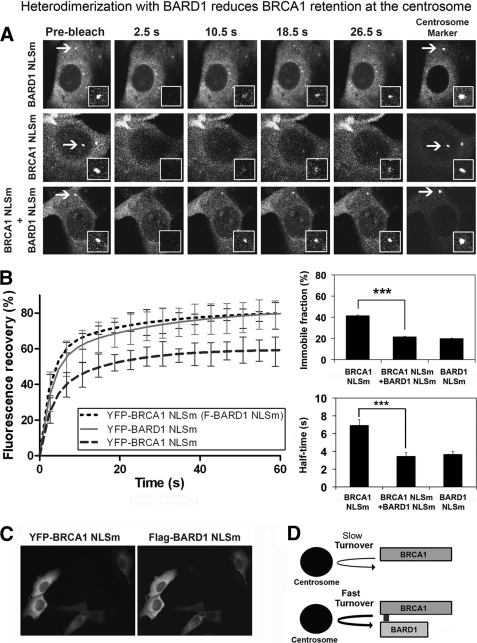
BRCA1 and BARD1 are nuclear-cytoplasmic shuttling proteins that exist predominantly in the nucleus as a heterodimer, due to reciprocal masking of their respective NESs when they bind one another (53, 54). Although the BRCA1-BARD1 dimer is known to ubiquitinate centrosome proteins γ-tubulin (16) and nucleophosmin (55) and to regulate centrosome amplification (8), our findings revealed that BRCA1 can efficiently move to the centrosome in the absence of BARD1 (Fig. 2). Therefore, we asked whether the dynamics of BRCA1 at centrosomes might differ when BRCA1 was free or bound to BARD1. Normally, this issue would be difficult to test because the co-expression of wild-type BRCA1 and BARD1 results in rapid transport of the heterodimer to the nucleus (28, 39). Therefore, to address this, we employed a combination of NLS-mutated forms of BRCA1 and BARD1, enabling assembly of a BRCA1-BARD1 dimer that remained in the cytoplasm and that localizes to the centrosome (see supplemental Fig. S6) rather than shuttling irreversibly to the nucleus like the wild-type BRCA1-BARD1 complex (39).
To quantify the effect of BARD1 co-expression on the centrosomal dynamics of BRCA1, the nuclear import-deficient BRCA1 (NLSm) was analyzed at the centrosome by FRAP assay with and without co-expression of FLAG-BARD1 NLSm (Fig. 7A). Immunostaining of transfected cells confirmed that the two proteins were co-expressed in 99% of cells (Fig. 7C). First, we noted that after photobleaching, the rate of YFP-BRCA1 NLSm recovery at centrosomes was comparable with that of wild-type BRCA1 (t½ = 6.95 s), and it displayed a similar immobile fraction (42%). Analysis of YFP-BARD1 NLSm showed that it recovered at centrosomes almost twice as fast as BRCA1 (t½ = 3.69 s) but with an immobile fraction similar to that of wild-type BARD1 (20.1% immobile; Fig. 7B) (37). Interestingly, in the co-expression experiment when YFP-BRCA1 NLSm recovery was measured in the presence of FLAG-BARD1 NLSm, the BRCA1 mobility and exchange rate increased to a similar level as that seen for BARD1. More specifically, when co-expressed with BARD1, the recovery of YFP-BRCA1 NLSm was accelerated (t½ = 3.47 s) and recovered to almost 80% of initial fluorescence, which corresponds to a 50% reduction in the immobile retained fraction (from 42 to 22%; Fig. 7B). From these data, we conclude that the co-expression of BARD1, resulting in heterodimer formation, speeds up BRCA1 association with the centrosome and decreases its retention (outlined in Fig. 7D). This might contribute to the biased nuclear localization of the wild-type BRCA1-BARD1 heterodimer normally observed in cells (54).
FIGURE 7.
Dimerization with BARD1 reduces BRCA1 retention at the centrosome. A, FRAP analysis was performed on YFP-tagged BRCA1 NLS mutant, BARD1 NLS mutant, and BRCA1 NLS (+FLAG-BARD1 NLS mutant) in transfected MCF-7 cells. Centrosome was marked by co-transfection with RFP-pericentrin C241. Note that co-expression of BRCA1 and BARD1 normally results in nuclear entrapment, whereas expression of NLS-mutated forms permits live cell analysis of the BRCA1-BARD1 heterodimer in the cytoplasm and at centrosomes. Centrosome fluorescence was photobleached, and fluorescence recovery was measured. Representative pre- and postbleach images are shown for each protein. The insets show higher magnification views of the target area. B, corresponding recovery curves are shown for each of the above proteins. The t½ (half-time ± S.E. (error bars)) and immobile fraction (percentage ± S.E.) are also shown (***, p < 0.001). The data show that BARD1 co-expression increases the rate of BRCA1 dynamics and reduces its retention at centrosomes. C, co-expression of BRCA1 and BARD1 was verified by immunofluorescence microscopy in parallel to FRAP experiments. pYFP-BRCA1 NLSm and pFLAG-BARD1 NLSm were co-transfected into MCF-7 cells, fixed with acetone/methanol and stained with anti-FLAG antibody (Sigma) and Hoechst dye. More than 98% of cells displayed co-expression. D, model showing that the heterodimeric form of BRCA1 (BRCA1-BARD1) has a faster exchange rate than monomeric BRCA1.
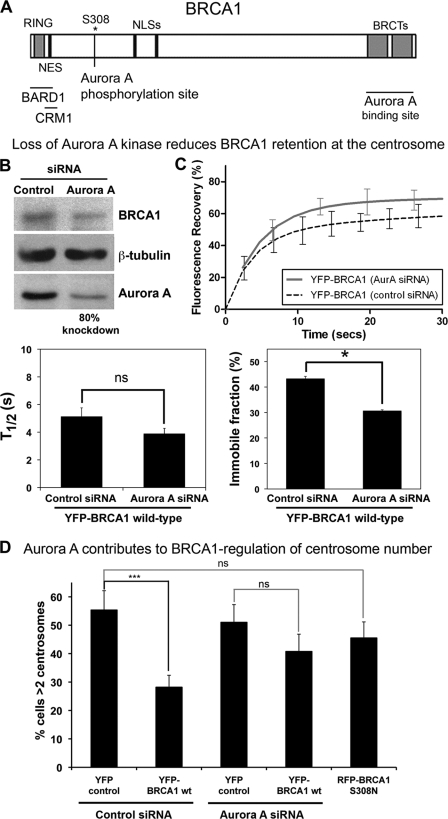
C-terminal Binding Partner, Aurora A Kinase, Contributes to BRCA1 Retention and Function at Centrosome
Thus far, this work has concerned N-terminal partners of BRCA1. In the literature, there are few C-terminal partners that might regulate BRCA1 centrosome dynamics; one of the best candidates is Aurora A kinase, which interacts with BRCA1 at the BRCT domain (Fig. 8A) (26). Aurora A is up-regulated in BRCA1mut breast tumors relative to sporadic breast tumors and has a role in the regulation of BRCA1 function at the centrosome (56). Because the BRCT domains are critical for BRCA1 localization to the centrosome, we used FRAP assays to test the effect of inhibiting Aurora A on the dynamics of YFP-BRCA1 at 72 h after Aurora A knockdown by siRNA (Western blots were performed in parallel and displayed a consistent knockdown of >80%, determined by densitometric analysis; see Fig. 8B). When Aurora A was silenced, YFP-BRCA1 wild-type recovery was moderately slower, with a t½ value of ∼3.9 s compared with control siRNA (∼4.6 s). Although this difference was not statistically significant, it could suggest that inhibition of Aurora A binding or of its kinase activity slows BRCA1 recruitment to the centrosome. Moreover, Aurora A siRNA caused a significant decrease in the immobile pool of BRCA1 (28%) relative to control siRNA (37%), demonstrating that Aurora A contributes to BRCA1 retention at the centrosome (Fig. 8B). The effect of Aurora A on BRCA1 regulation of DNA damage-induced centrosome amplification was also assessed in HCC1937 cells. The cells were transfected with either Aurora A or control siRNA for 16 h and then transfected with YFP-BRCA1 or YFP control plasmid. After 24 h, cells were treated with 10 Gy of IR and fixed and immunostained after a further 48 h. As shown in Fig. 6B, YFP-BRCA1 (28%) was able to substantially reduce the number of cells with >2 centrosomes in comparison with YFP alone (55%) in MCF-7 cells treated with control siRNA. However, in the presence of Aurora A knockdown, YFP-BRCA1 was not able to regulate centrosome amplification after DNA damage (Fig. 8D). Moreover, a mutant version of BRCA1 that cannot be phosphorylated by Aurora A, S308N (26), was unable to regulate centrosome amplification. These data suggest a role for Aurora A in BRCA1 retention at the centrosome as well as a role in the BRCA1-dependent regulation of centrosome number.
FIGURE 8.
Aurora A kinase regulates BRCA1 dynamics and function at the centrosome. A, diagram showing sites where Aurora A kinase binds (BRCT domain) and phosphorylates (Ser-308) BRCA1. B, Western blot analysis to confirm the Aurora A knockdown using siRNA transfection in MCF-7 cells. C, FRAP analysis was performed on live MCF-7 cells transfected with YFP-BRCA1 (and RFP-pericentrin C241 as marker) after transfection with either control or Aurora A siRNA. Corresponding recovery curves are shown, indicating the immobile and mobile fractions and percentage increase in mobility after Aurora A silencing. The fast-phase half-time (t½ ± S.E. (error bars)) and immobile fraction (percentage ± S.E.) are shown for each construct, analyzing 10–15 cells/sample. D, functional assay. pYFP-BRCA1 WT was transfected into HCC1937 breast cancer cells after treatment with Aurora A or control siRNA. pRFP-BRCA1 (S308N) was also transiently expressed in HCC1937 cells. The cells were then irradiated (10 Gy of IR) and left to recover for 48 h. After fixation with acetone/methanol, cells were immunostained with anti-γ-tubulin antibody and scored for cells displaying centrosome amplification. Scoring results were obtained from at least three independent experiments with at least 100 cells scored (mean ± S.D. (error bars)). ***, p < 0.001. ns, not significant.
DISCUSSION
BRCA1 gene mutations lead to centrosome amplification and related mitotic abnormalities in human breast cancers (57) and transgenic mice (18, 19). BRCA1 has been known for some time to locate at the centrosome (58, 59) and, through binding and ubiquitinating γ-tubulin (48), contributes to the regulation of centrosome amplification and microtubule nucleation (14, 16). Despite knowledge of BRCA1 sequences that mediate its ubiquitination activity (amino acids 1–300) (60) and γ-tubulin binding (amino acids 510–622) (48), surprisingly little is known of the sequences or protein interactions that target BRCA1 to the centrosome. Here we show for the first time that BRCA1 is not targeted to the centrosome via its interaction with γ-tubulin but instead by the combination of N- and C-terminal end sequences that flank the central γ-tubulin binding region. The N-terminal nuclear export sequence was important for BRCA1 targeting, turnover, and function at the centrosome, indicating regulation by CRM1. We propose that BRCA1 contributes to the pathway of CRM1-dependent mitotic regulation (model outlined in supplemental Fig. S7).
BRCA1 shuttles between different subcellular compartments, including the nucleus and cytoplasm (54), centrosome (58), and mitochondria (61), to regulate diverse processes. We mapped two separate BRCA1 centrosome-targeting sequences, corresponding to the N terminal (residues 1–304) and C-terminal (residues 1620–1863) BRCT domains, which act cooperatively to localize a GFP marker peptide to the centrosome. Deletion of either sequence abolished BRCA1 localization at the centrosome (Fig. 2). The fusion of N- and C-terminal sequences (named N304+C243) is identical to the core sequence(s) that we previously showed to target BRCA1 to DNA repair foci in cells following exposure to ionizing radiation (40). Although this suggests some overlap in BRCA1 protein complexes at nuclear foci and centrosomes, a critical difference lies in the role of BARD1, which is essential for association of BRCA1 at foci (39) but was dispensable for accumulation at centrosomes (Fig. 2). The binding of BRCA1 to γ-tubulin and BARD1 was not required for its movement to centrosomes (Fig. 2); however, loss of these binding sequences abolished BRCA1 regulation of DNA damage-induced centrosome amplification (Fig. 3). Therefore, the regulation of centrosome amplification and targeting can be distinguished.
Another intriguing aspect is the striking similarity between the minimal centrosome-targeting fusion peptide (N304+C243) and the naturally occurring BRCA1 splice variant Δexon11, which in mouse embryo fibroblasts taken from BRCA1Δexon11/Δexon11 transgenic mice was shown to cause a high proportion of centrosome amplification, multipolar spindles, and aneuploidy (19). The BRCA1(N304-C243) protein retained targeting to centrosomes but lacked the ability to regulate their amplification, which is highly consistent with previous findings and predicts that the Δexon11 splice variants will also display centrosomal localization and could potentially compete with wild-type BRCA1 for binding to specific partners, such as CRM1 or BARD1.
We employed fluorescence photobleaching assays and found that ∼60% of BRCA1 at the centrosome is highly mobile and in continuous exchange between centrosome and surrounding cytoplasm (Fig. 4). Control experiments confirmed that any differences measured in exchange rates of BRCA1 isoforms were not due to variations in expression levels (see supplemental Figs. S8 and S9). It is intriguing to note that the retained centrosomal pool of BRCA1 was twice the amount recently observed for BARD1 (37). The rapid turnover of the mobile BRCA1 pool is reminiscent of previous reports for the centrosomal regulatory proteins Aurora A, Nek2A, CDC-20, and Dynamin-2 (62–65). This contrasts with the relatively long occupancy times of centrosomal structural proteins γ-tubulin and centrin (>5 min) (64, 66). In our assays, ∼40% of YFP-BRCA1 was immobile compared with ∼70% for centrosomal scaffolding protein RFP-pericentrin C241 (Fig. 4). We speculate that the interaction between BRCA1 and γ-tubulin contributes to retention of BRCA1 at the centrosome, and this is supported by our observation that deletion of the γ-tubulin binding sequence modestly increased BRCA1 mobility at this site.
Regulation of BRCA1 by N-terminal Partners CRM1 and BARD1
Recent studies have shown that the major exportin, CRM1, not only mediates nucleocytoplasmic transport but also regulates centrosome duplication to maintain correct assembly of the mitotic spindle (30). It was proposed that centrosomal localization of CRM1 is mediated by its N terminus and binding partner Ran-GTP (31) and that other CRM1 sequences provide a docking site for NES containing proteins. Prior to this study, CRM1 was implicated in the centrosomal localization of nucleophosmin (NPM1) (34), the BRCA1 homologue BRCA2 (35), and BARD1 (37). Of these, NPM1 and BRCA2 were linked to CRM1 control of centrosome duplication (33, 34). NPM1 is a nucleolar protein that binds to CRM1 and localizes at centrosomes during mitosis via interaction with the Ran-CRM1 complex, leading to inhibition of centrosome reduplication. Its functionality and centrosome association is regulated by phosphorylation. NPM1 also binds the N-terminal amino acids 1–122 of BRCA1 and is monoubiquitinated by the BRCA1-BARD1 ubiquitin ligase (55). Therefore, these previous studies indirectly implicated BRCA1-BARD1 in CRM1 regulation of centrosome duplication.
Because NPM1 and BRCA1 are both nuclear shuttling proteins that modulate centrosome duplication, we tested for potential CRM1 regulation of BRCA1. Inactivation of CRM1 by leptomycin B or mutation of the N-terminal NES reduced staining of BRCA1 at the centrosome (Fig. 5). Interestingly, although CRM1 was apparently essential for targeting of NPM1 (33, 34), we observed that CRM1 contributed to, but was not essential for, BRCA1 recruitment to centrosomes. This is explained by our FRAP experiments in living cells, which showed that CRM1 acted not as a retention factor for BRCA1 but more as a driver to stimulate BRCA1 traffic to the centrosome (Fig. 6A). An export-defective BRCA1 mutant displayed a slower recovery and stronger retention at centrosomes than the export-active form, implicating CRM1 in rapid turnover of BRCA1 at the centrosome. Since we first discovered the NES in BRCA1 (27), it has yet to be assigned functional relevance. We found here that in BRCA1-mutated HCC1937 cells exposed to DNA damage, mutation of the BRCA1 NES completely blocked BRCA1 regulation of centrosome amplification (Fig. 6B), suggesting that interaction with CRM1 is essential for BRCA1 function as a centrosome DNA damage checkpoint protein. We propose that CRM1 regulates BRCA1 at the centrosome by (a) increasing centrosomal localization indirectly through nuclear export, (b) increased targeting and turnover, and (c) stimulating its regulation of centrosome amplification.
We speculate that CRM1 helps target BRCA1 to a functionally relevant compartment of the centrosome, such as the centriole, possibly through CRM1-dependent interaction with an enriched Ran-GTP pool anchored by the large centrosomal scaffolding protein AKAP450 (32). It is important to note that CRM1 can only bind to the undimerized form of BRCA1, because the NES (CRM1-binding site) is a core part of the BARD1-binding domain, and both the BRCA1 and BARD1 NESs become masked upon heterodimer formation (39). When the CRM1-bound BRCA1 is brought into proximity with BARD1, the BARD1 will therefore compete with and displace CRM1 (28) to assemble an enzymatically active BRCA1-BARD1 heterodimer, leading to ubiquitination of several substrates required for centrosome amplification control, including γ-tubulin (16), NPM1 (55), and RHAMM (56). Our findings demonstrate that BRCA1 and BARD1 can locate at the centrosome independently of one another and that both proteins are targeted there by CRM1 prior to assembly of the heterodimer (see supplemental Fig. S6) (37). This new concept of post-targeting assembly could explain how the BRCA1-BARD1 dimer is able to form at the centrosome when normally the dimeric complex is rapidly directed into the nucleus and blocked from exiting back to the cytoplasm (54). Finally, our photobleaching assays showed that heterodimer formation significantly reduced the centrosomal retention of BRCA1, hinting at an unanticipated mechanism to accelerate its release after the dimer forms and ubiquitinates its substrates.
Regulation of BRCA1 by C-terminal Partner Aurora A Kinase
BRCA1 checkpoint functions are regulated by phosphorylation (67). A key regulator of BRCA1 mitotic function, Aurora A kinase, binds to the C-terminal BRCT domain of BRCA1 (26) and was therefore a candidate for regulation of BRCA1 dynamics. Indeed, our photobleaching studies revealed that siRNA-mediated knockdown of Aurora A caused a 25% reduction in the immobile pool of BRCA1, indicating that Aurora A contributes to BRCA1 retention at the centrosome. Aurora A is a serine/threonine kinase overexpressed in breast cancers (22, 23) and accumulates at the centrosome in early G2 phase (24, 25, 68). Aurora A phosphorylates BRCA1 at Ser-308 during early M phase, and this was implicated in regulation of the G2 to M transition (26). This kinase has also been implicated in the regulation of BRCA1 inhibition of microtubule nucleation from the centrosome by decreasing ubiquitin ligase activity of the BRCA1-BARD1 heterodimer (49). A further analysis here showed that not only does Aurora A regulate BRCA1 retention at the centrosome in live cells, but its activity (and specifically the phosphorylation of Ser-308) is crucial for BRCA1 regulation of centrosome amplification after DNA damage.
In conclusion, we propose that CRM1 acts as a chaperone to target BRCA1 to a functionally relevant subcompartment(s) of the centrosome. Based on the evidence presented here, we speculate that the ability of CRM1 to drive BRCA1, BARD1, and several of the BRCA1-BARD1 substrates to the centrosome heightens the chance of their proximity, hence expediting BRCA1-BARD1 dimer formation to catalyze ubiquitination of downstream substrates required to regulate centrosome amplification during the DNA damage response. Future experiments to further characterize the spatial and temporal organization of these protein interactions will yield new insights into the G2/M checkpoint function of BRCA1 and its role at the centrosome.
Supplementary Material
Acknowledgments
We are very grateful to Professor Jeffrey Parvin for generously supplying BRCA1 Ab-P antibody and Dr. Sean Munro for pRFP-pericentrin C241, both of which were integral to this study.
This study was supported by grants from the Australian Research Council, the National Health and Medical Research Council (NHMRC) and the Cancer Council of New South Wales.

This article contains supplemental Figs. S1–S9 and Table S1.
- BRCT
- BRCA1 C terminus
- FRAP
- fluorescence recovery after photobleaching
- Gy
- grays
- IR
- ionizing radiation
- NES
- nuclear export sequence
- NESm
- NES mutant
- NLS
- nuclear localization sequence
- NLSm
- NLS mutant
- LMB
- leptomycin B
- RING
- really interesting new gene.
REFERENCES
- 1. Narod S. A., Foulkes W. D. (2004) BRCA1 and BRCA2: 1994 and beyond. Nat. Rev. Cancer 4, 665–676 [DOI] [PubMed] [Google Scholar]
- 2. Antoniou A., Pharoah P. D., Narod S., Risch H. A., Eyfjord J. E., Hopper J. L., Loman N., Olsson H., Johannsson O., Borg A., Pasini B., Radice P., Manoukian S., Eccles D. M., Tang N., Olah E., Anton-Culver H., Warner E., Lubinski J., Gronwald J., Gorski B., Tulinius H., Thorlacius S., Eerola H., Nevanlinna H., Syrjäkoski K., Kallioniemi O. P., Thompson D., Evans C., Peto J., Lalloo F., Evans D. G., Easton D. F. (2003) Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history. A combined analysis of 22 studies. Am. J. Hum. Genet. 72, 1117–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Easton D. F., Ford D., Bishop D. T. (1995) Breast and ovarian cancer incidence in BRCA1 mutation carriers. Breast Cancer Linkage Consortium. Am. J. Hum. Genet. 56, 265–271 [PMC free article] [PubMed] [Google Scholar]
- 4. Levy-Lahad E., Friedman E. (2007) Cancer risks among BRCA1 and BRCA2 mutation carriers. Br. J. Cancer 96, 11–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bekker-Jensen S., Mailand N. (2010) Assembly and function of DNA double-strand break repair foci in mammalian cells. DNA Repair 9, 1219–1228 [DOI] [PubMed] [Google Scholar]
- 6. Xia Y., Pao G. M., Chen H. W., Verma I. M., Hunter T. (2003) Enhancement of BRCA1 E3 ubiquitin ligase activity through direct interaction with the BARD1 protein. J. Biol. Chem. 278, 5255–5263 [DOI] [PubMed] [Google Scholar]
- 7. Thomson T. M., Guerra-Rebollo M. (2010) Ubiquitin and SUMO signaling in DNA repair. Biochem. Soc. Trans. 38, 116–131 [DOI] [PubMed] [Google Scholar]
- 8. Parvin J. D. (2009) The BRCA1-dependent ubiquitin ligase, γ-tubulin, and centrosomes. Environ. Mol. Mutagen. 50, 649–653 [DOI] [PubMed] [Google Scholar]
- 9. Yu X., Chini C. C., He M., Mer G., Chen J. (2003) The BRCT domain is a phospho-protein binding domain. Science 302, 639–642 [DOI] [PubMed] [Google Scholar]
- 10. Krum S. A., Miranda G. A., Lin C., Lane T. F. (2003) BRCA1 associates with processive RNA polymerase II. J. Biol. Chem. 278, 52012–52020 [DOI] [PubMed] [Google Scholar]
- 11. Manke I. A., Lowery D. M., Nguyen A., Yaffe M. B. (2003) BRCT repeats as phosphopeptide-binding modules involved in protein targeting. Science 302, 636–639 [DOI] [PubMed] [Google Scholar]
- 12. Shakya R., Reid L. J., Reczek C. R., Cole F., Egli D., Lin C. S., deRooij D. G., Hirsch S., Ravi K., Hicks J. B., Szabolcs M., Jasin M., Baer R., Ludwig T. (2011) BRCA1 tumor suppression depends on BRCT phosphoprotein binding, but not its E3 ligase activity. Science 334, 525–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moritz M., Braunfeld M. B., Sedat J. W., Alberts B., Agard D. A. (1995) Microtubule nucleation by γ-tubulin-containing rings in the centrosome. Nature 378, 638–640 [DOI] [PubMed] [Google Scholar]
- 14. Sankaran S., Starita L. M., Groen A. C., Ko M. J., Parvin J. D. (2005) Centrosomal microtubule nucleation activity is inhibited by BRCA1-dependent ubiquitination. Mol. Cell. Biol. 25, 8656–8668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sankaran S., Starita L. M., Simons A. M., Parvin J. D. (2006) Identification of domains of BRCA1 critical for the ubiquitin-dependent inhibition of centrosome function. Cancer Res. 66, 4100–4107 [DOI] [PubMed] [Google Scholar]
- 16. Starita L. M., Machida Y., Sankaran S., Elias J. E., Griffin K., Schlegel B. P., Gygi S. P., Parvin J. D. (2004) BRCA1-dependent ubiquitination of γ-tubulin regulates centrosome number. Mol. Cell. Biol. 24, 8457–8466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schlegel B. P., Starita L. M., Parvin J. D. (2003) Overexpression of a protein fragment of RNA helicase A causes inhibition of endogenous BRCA1 function and defects in ploidy and cytokinesis in mammary epithelial cells. Oncogene 22, 983–991 [DOI] [PubMed] [Google Scholar]
- 18. Deng C. X. (2001) Tumorigenesis as a consequence of genetic instability in Brca1 mutant mice. Mutat. Res. 477, 183–189 [DOI] [PubMed] [Google Scholar]
- 19. Xu X., Weaver Z., Linke S. P., Li C., Gotay J., Wang X. W., Harris C. C., Ried T., Deng C. X. (1999) Centrosome amplification and a defective G2-M cell cycle checkpoint induce genetic instability in BRCA1 exon 11 isoform-deficient cells. Mol. Cell 3, 389–395 [DOI] [PubMed] [Google Scholar]
- 20. Sankaran S., Crone D. E., Palazzo R. E., Parvin J. D. (2007) BRCA1 regulates γ-tubulin binding to centrosomes. Cancer Biol. Ther. 6, 1853–1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fukasawa K. (2005) Centrosome amplification, chromosome instability, and cancer development. Cancer Lett. 230, 6–19 [DOI] [PubMed] [Google Scholar]
- 22. Miyoshi Y., Iwao K., Egawa C., Noguchi S. (2001) Association of centrosomal kinase STK15/BTAK mRNA expression with chromosomal instability in human breast cancers. Int. J. Cancer 92, 370–373 [DOI] [PubMed] [Google Scholar]
- 23. Zhou H., Kuang J., Zhong L., Kuo W. L., Gray J. W., Sahin A., Brinkley B. R., Sen S. (1998) Tumor amplified kinase STK15/BTAK induces centrosome amplification, aneuploidy, and transformation. Nat. Genet. 20, 189–193 [DOI] [PubMed] [Google Scholar]
- 24. Kimura M., Kotani S., Hattori T., Sumi N., Yoshioka T., Todokoro K., Okano Y. (1997) Cell cycle-dependent expression and spindle pole localization of a novel human protein kinase, Aik, related to Aurora of Drosophila and yeast Ipl1. J. Biol. Chem. 272, 13766–13771 [DOI] [PubMed] [Google Scholar]
- 25. Gopalan G., Chan C. S., Donovan P. J. (1997) A novel mammalian, mitotic spindle-associated kinase is related to yeast and fly chromosome segregation regulators. J. Cell Biol. 138, 643–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ouchi M., Fujiuchi N., Sasai K., Katayama H., Minamishima Y. A., Ongusaha P. P., Deng C., Sen S., Lee S. W., Ouchi T. (2004) BRCA1 phosphorylation by Aurora-A in the regulation of G2 to M transition. J. Biol. Chem. 279, 19643–19648 [DOI] [PubMed] [Google Scholar]
- 27. Rodríguez J. A., Henderson B. R. (2000) Identification of a functional nuclear export sequence in BRCA1. J. Biol. Chem. 275, 38589–38596 [DOI] [PubMed] [Google Scholar]
- 28. Rodriguez J. A., Schüchner S., Au W. W., Fabbro M., Henderson B. R. (2004) Nuclear-cytoplasmic shuttling of BARD1 contributes to its proapoptotic activity and is regulated by dimerization with BRCA1. Oncogene 23, 1809–1820 [DOI] [PubMed] [Google Scholar]
- 29. Budhu A. S., Wang X. W. (2005) Loading and unloading. Orchestrating centrosome duplication and spindle assembly by Ran/Crm1. Cell Cycle 4, 1510–1514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Forgues M., Difilippantonio M. J., Linke S. P., Ried T., Nagashima K., Feden J., Valerie K., Fukasawa K., Wang X. W. (2003) Involvement of Crm1 in hepatitis B virus X protein-induced aberrant centriole replication and abnormal mitotic spindles. Mol. Cell. Biol. 23, 5282–5292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu Q., Jiang Q., Zhang C. (2009) A fraction of Crm1 locates at centrosomes by its CRIME domain and regulates the centrosomal localization of pericentrin. Biochem. Biophys. Res. Commun. 384, 383–388 [DOI] [PubMed] [Google Scholar]
- 32. Keryer G., Di Fiore B., Celati C., Lechtreck K. F., Mogensen M., Delouvee A., Lavia P., Bornens M., Tassin A. M. (2003) Part of Ran is associated with AKAP450 at the centrosome. Involvement in microtubule-organizing activity. Mol. Biol. Cell 14, 4260–4271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang W., Budhu A., Forgues M., Wang X. W. (2005) Temporal and spatial control of nucleophosmin by the Ran-Crm1 complex in centrosome duplication. Nat. Cell Biol. 7, 823–830 [DOI] [PubMed] [Google Scholar]
- 34. Shinmura K., Tarapore P., Tokuyama Y., George K. R., Fukasawa K. (2005) Characterization of centrosomal association of nucleophosmin/B23 linked to Crm1 activity. FEBS Lett. 579, 6621–6634 [DOI] [PubMed] [Google Scholar]
- 35. Han X., Saito H., Miki Y., Nakanishi A. (2008) A CRM1-mediated nuclear export signal governs cytoplasmic localization of BRCA2 and is essential for centrosomal localization of BRCA2. Oncogene 27, 2969–2977 [DOI] [PubMed] [Google Scholar]
- 36. Nakanishi A., Han X., Saito H., Taguchi K., Ohta Y., Imajoh-Ohmi S., Miki Y. (2007) Interference with BRCA2, which localizes to the centrosome during S and early M phase, leads to abnormal nuclear division. Biochem. Biophys. Res. Commun. 355, 34–40 [DOI] [PubMed] [Google Scholar]
- 37. Brodie K. M., Mok M. T., Henderson B. R. (2012) Characterization of BARD1 targeting and dynamics at the centrosome. The role of CRM1, BRCA1, and the Q564H mutation. Cell. Signal. 24, 451–459 [DOI] [PubMed] [Google Scholar]
- 38. Brodie K. M., Henderson B. R. (2010) Differential modulation of BRCA1 and BARD1 nuclear localization and foci assembly by DNA damage. Cell. Signal. 22, 291–302 [DOI] [PubMed] [Google Scholar]
- 39. Fabbro M., Rodriguez J. A., Baer R., Henderson B. R. (2002) BARD1 induces BRCA1 intranuclear foci formation by increasing RING-dependent BRCA1 nuclear import and inhibiting BRCA1 nuclear export. J. Biol. Chem. 277, 21315–21324 [DOI] [PubMed] [Google Scholar]
- 40. Au W. W., Henderson B. R. (2005) The BRCA1 RING and BRCT domains cooperate in targeting BRCA1 to ionizing radiation-induced nuclear foci. J. Biol. Chem. 280, 6993–7001 [DOI] [PubMed] [Google Scholar]
- 41. Schüchner S., Tembe V., Rodriguez J. A., Henderson B. R. (2005) Nuclear targeting and cell cycle regulatory function of human BARD1. J. Biol. Chem. 280, 8855–8861 [DOI] [PubMed] [Google Scholar]
- 42. Rodriguez J. A., Au W. W., Henderson B. R. (2004) Cytoplasmic mislocalization of BRCA1 caused by cancer-associated mutations in the BRCT domain. Exp. Cell Res. 293, 14–21 [DOI] [PubMed] [Google Scholar]
- 43. Au W. W., Henderson B. R. (2007) Identification of sequences that target BRCA1 to nuclear foci following alkylative DNA damage. Cell. Signal. 19, 1879–1892 [DOI] [PubMed] [Google Scholar]
- 44. Gillingham A. K., Munro S. (2000) The PACT domain, a conserved centrosomal targeting motif in the coiled-coil proteins AKAP450 and pericentrin. EMBO Rep. 1, 524–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Williams R. S., Green R., Glover J. N. (2001) Crystal structure of the BRCT repeat region from the breast cancer-associated protein BRCA1. Nat. Struct. Biol. 8, 838–842 [DOI] [PubMed] [Google Scholar]
- 46. Matsumoto Y., Maller J. L. (2004) A centrosomal localization signal in cyclin E required for Cdk2-independent S phase entry. Science 306, 885–888 [DOI] [PubMed] [Google Scholar]
- 47. Pascreau G., Eckerdt F., Churchill M. E., Maller J. L. (2010) Discovery of a distinct domain in cyclin A sufficient for centrosomal localization independently of Cdk binding. Proc. Natl. Acad. Sci. U.S.A. 107, 2932–2937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hsu L. C., Doan T. P., White R. L. (2001) Identification of a γ-tubulin-binding domain in BRCA1. Cancer Res. 61, 7713–7718 [PubMed] [Google Scholar]
- 49. Sankaran S., Crone D. E., Palazzo R. E., Parvin J. D. (2007) Aurora A kinase regulates breast cancer-associated gene 1 inhibition of centrosome-dependent microtubule nucleation. Cancer Res. 67, 11186–11194 [DOI] [PubMed] [Google Scholar]
- 50. Saladino C., Bourke E., Conroy P. C., Morrison C. G. (2009) Centriole separation in DNA damage-induced centrosome amplification. Environ. Mol. Mutagen. 50, 725–732 [DOI] [PubMed] [Google Scholar]
- 51. Maxwell C. A., Keats J. J., Crainie M., Sun X., Yen T., Shibuya E., Hendzel M., Chan G., Pilarski L. M. (2003) RHAMM is a centrosomal protein that interacts with dynein and maintains spindle pole stability. Mol. Biol. Cell 14, 2262–2276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kudo N., Matsumori N., Taoka H., Fujiwara D., Schreiner E. P., Wolff B., Yoshida M., Horinouchi S. (1999) Leptomycin B inactivates CRM1/exportin 1 by covalent modification at a cysteine residue in the central conserved region. Proc. Natl. Acad. Sci. U.S.A. 96, 9112–9117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Baer R., Ludwig T. (2002) The BRCA1/BARD1 heterodimer, a tumor suppressor complex with ubiquitin E3 ligase activity. Curr. Opin. Genet. Dev. 12, 86–91 [DOI] [PubMed] [Google Scholar]
- 54. Henderson B. R. (2005) Regulation of BRCA1, BRCA2, and BARD1 intracellular trafficking. BioEssays 27, 884–893 [DOI] [PubMed] [Google Scholar]
- 55. Sato K., Hayami R., Wu W., Nishikawa T., Nishikawa H., Okuda Y., Ogata H., Fukuda M., Ohta T. (2004) Nucleophosmin/B23 is a candidate substrate for the BRCA1-BARD1 ubiquitin ligase. J. Biol. Chem. 279, 30919–30922 [DOI] [PubMed] [Google Scholar]
- 56. Pujana M. A., Han J. D., Starita L. M., Stevens K. N., Tewari M., Ahn J. S., Rennert G., Moreno V., Kirchhoff T., Gold B., Assmann V., Elshamy W. M., Rual J. F., Levine D., Rozek L. S., Gelman R. S., Gunsalus K. C., Greenberg R. A., Sobhian B., Bertin N., Venkatesan K., Ayivi-Guedehoussou N., Solé X., Hernández P., Lázaro C., Nathanson K. L., Weber B. L., Cusick M. E., Hill D. E., Offit K., Livingston D. M., Gruber S. B., Parvin J. D., Vidal M. (2007) Network modeling links breast cancer susceptibility and centrosome dysfunction. Nat. Genet. 39, 1338–1349 [DOI] [PubMed] [Google Scholar]
- 57. Shimomura A., Miyoshi Y., Taguchi T., Tamaki Y., Noguchi S. (2009) Association of loss of BRCA1 expression with centrosome aberration in human breast cancer. J Cancer Res. Clin Oncol. 135, 421–430 [DOI] [PubMed] [Google Scholar]
- 58. Hsu L. C., White R. L. (1998) BRCA1 is associated with the centrosome during mitosis. Proc. Natl. Acad. Sci. U.S.A. 95, 12983–12988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lotti L. V., Ottini L., D'Amico C., Gradini R., Cama A., Belleudi F., Frati L., Torrisi M. R., Mariani-Costantini R. (2002) Subcellular localization of the BRCA1 gene product in mitotic cells. Genes Chromosomes Cancer 35, 193–203 [DOI] [PubMed] [Google Scholar]
- 60. Hashizume R., Fukuda M., Maeda I., Nishikawa H., Oyake D., Yabuki Y., Ogata H., Ohta T. (2001) The RING heterodimer BRCA1-BARD1 is a ubiquitin ligase inactivated by a breast cancer-derived mutation. J. Biol. Chem. 276, 14537–14540 [DOI] [PubMed] [Google Scholar]
- 61. Coene E. D., Hollinshead M. S., Waeytens A. A., Schelfhout V. R., Eechaute W. P., Shaw M. K., Van Oostveldt P. M., Vaux D. J. (2005) Phosphorylated BRCA1 is predominantly located in the nucleus and mitochondria. Mol. Biol. Cell 16, 997–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Thompson H. M., Cao H., Chen J., Euteneuer U., McNiven M. A. (2004) Dynamin 2 binds γ-tubulin and participates in centrosome cohesion. Nat. Cell Biol. 6, 335–342 [DOI] [PubMed] [Google Scholar]
- 63. Stenoien D. L., Sen S., Mancini M. A., Brinkley B. R. (2003) Dynamic association of a tumor amplified kinase, Aurora-A, with the centrosome and mitotic spindle. Cell Motil. Cytoskeleton 55, 134–146 [DOI] [PubMed] [Google Scholar]
- 64. Hames R. S., Crookes R. E., Straatman K. R., Merdes A., Hayes M. J., Faragher A. J., Fry A. M. (2005) Dynamic recruitment of Nek2 kinase to the centrosome involves microtubules, PCM-1, and localized proteasomal degradation. Mol. Biol. Cell 16, 1711–1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kallio M. J., Beardmore V. A., Weinstein J., Gorbsky G. J. (2002) Rapid microtubule-independent dynamics of Cdc20 at kinetochores and centrosomes in mammalian cells. J. Cell Biol. 158, 841–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Khodjakov A., Rieder C. L. (1999) The sudden recruitment of γ-tubulin to the centrosome at the onset of mitosis and its dynamic exchange throughout the cell cycle do not require microtubules. J. Cell Biol. 146, 585–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ouchi T. (2006) BRCA1 phosphorylation. Biological consequences. Cancer Biol. Ther. 5, 470–475 [DOI] [PubMed] [Google Scholar]
- 68. Roghi C., Giet R., Uzbekov R., Morin N., Chartrain I., Le Guellec R., Couturier A., Dorée M., Philippe M., Prigent C. (1998) The Xenopus protein kinase pEg2 associates with the centrosome in a cell cycle-dependent manner, binds to the spindle microtubules, and is involved in bipolar mitotic spindle assembly. J. Cell Sci. 111, 557–572 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.