Abstract
Cyclase-associated protein (CAP) is an evolutionarily conserved regulator of the G-actin/F-actin ratio and, in yeast, is involved in regulating the adenylyl cyclase activity. We show that cell polarization, F-actin organization, and phototaxis are altered in a Dictyostelium CAP knockout mutant. Furthermore, in complementation assays we determined the roles of the individual domains in signaling and regulation of the actin cytoskeleton. We studied in detail the adenylyl cyclase activity and found that the mutant cells have normal levels of the aggregation phase-specific adenylyl cyclase and that receptor-mediated activation is intact. However, cAMP relay that is responsible for the generation of propagating cAMP waves that control the chemotactic aggregation of starving Dictyostelium cells was altered, and the cAMP-induced cGMP production was significantly reduced. The data suggest an interaction of CAP with adenylyl cyclase in Dictyostelium and an influence on signaling pathways directly as well as through its function as a regulatory component of the cytoskeleton.
INTRODUCTION
CAP/ASP56, a regulator of the F-actin/G-actin ratio has been identified in many species (Hubberstey and Mottillo, 2002). In yeast, CAP/Srv2 was identified as an adenylyl cyclase-associated protein by a biochemical and a genetic approach (Fedor-Chaiken et al., 1990; Field et al., 1990). Mutations in CAP affected the regulation of the adenylyl cyclase and the cytoskeleton. These characteristics led to the suggestion that CAP is a bifunctional protein with roles in signaling and regulation of the cytoskeleton that have been attributed to individual domains of the protein (Goldschmidt-Clermont and Janmey, 1991). The amino terminal region contains the adenylyl cyclase binding site (Nishida et al., 1998). The overall structure of this domain consists of an α-helix bundle composed of six antiparallel helices (Ksiazek et al., 2003). It is followed by a proline-rich region that interacts with proteins containing an SH3 domain, whereas the C terminus is responsible for binding to monomeric actin (Freeman et al., 1995; Gottwald et al., 1996; Wesp et al., 1997). The Drosophila homolog was identified in two independent screens. Benlali et al. (2000) identified CAP in a screen for mutations that disrupted eye development by increasing the F-actin levels and inducing premature photoreceptor differentiation. Baum et al. (2000) discovered CAP when searching for mutations that perturbed actin organization. CAP preferentially accumulated in the oocyte where it inhibited actin polymerization resulting in a loss of asymmetric distribution of mRNA determinants within the oocyte.
Cell polarization is defined as an asymmetry of cell shape and cellular functions that is stable for some time and requires localized signaling, directed cytoskeletal rearrangements, and distinct recruitments of proteins and supramolecular complexes (for reviews, see Bretscher, 2003; Nelson, 2003). During growth Dictyostelium cells do not display a fixed polarity. They constantly change their shape and form new ends in response to external signals, which target them toward a food source. However, after the onset of starvation periodic signals of the chemoattractant cAMP lead to polarization of the cells and initiate the development into a multicellular organism. In response to cAMP proteins such as the cytosolic regulator of adenylyl cyclase (CRAC), a PH-domain-containing protein, and protein kinase B associate temporarily with newly formed polarized regions of the cell and help to initiate extension of pseudopods (Parent et al., 1998; Meili et al., 1999; Comer and Parent, 2002). This process requires distinct signaling molecules, the chemotactic machinery as well as components of the cytoskeleton such as CAP, which in Dictyostelium is involved in actin cytoskeleton rearrangements and relocalizes quickly to newly extending pseudopods upon a cAMP stimulus (Gottwald et al., 1996).
cAMP signaling is essential for the chemotactic aggregation of individual Dictyostelium cells into multicellular aggregates and for progression through late development (Firtel and Meili, 2000). The aggregation centers produce cAMP pulses, which are detected, amplified, and relayed to the surrounding cells. The cAMP is sensed by a cAMP receptor on the cell surface, which couples to a heterotrimeric G protein. The Gβγ complex is set free and, together with CRAC, it activates the adenylyl cyclase (ACA) and leads to synthesis of cAMP (cAMP relay) (Firtel and Chung, 2000). CRAC transiently associates with the plasma membrane at the stimulated edge (Insall et al., 1994; Parent et al., 1998). In addition to CRAC, other factors exist that affect activation of ACA such as pianissimo, extracellular signal-regulated kinase, and aimless (Verkerke-Van Wijk and Schaap, 1997). cAMP also initiates a network of signaling pathways such as cGMP signaling, which is responsible for changes in the cytoskeleton (Liu and Newell, 1988).
CAP bsr, a Dictyostelium mutant in which the CAP gene has been inactivated by homologous recombination in such a way that the expression of the full-length protein was reduced to <5% of the protein concentration in wild-type AX2, revealed changes during growth and development. Growing cells were heterogeneous with regard to cell size and were often multinucleated. The mutant had an endocytosis and a chemotaxis defect. When chemotactic motility was assayed by applying a cAMP gradient, the cells did not properly orientate in the direction of the chemotactic agent. Development was significantly delayed and developmentally regulated genes such as contact site A (csA) and cAMP receptor I were expressed significantly later than in wild type. However, the mutant was able to complete the developmental cycle and to form fruiting bodies containing viable spores (Noegel et al., 1999).
Here, we studied the responses of mutant cells to exogenous cAMP stimuli and tested specifically the cAMP relay and events that are associated with cAMP initiated changes in cell polarity, F-actin accumulation, cGMP production, and directed migration during the slug stage. The data suggest that loss of the cyclase associated protein caused a drastically lowered sensitivity to external signals resulting in reduced cell polarity and altered cAMP waves during aggregation.
MATERIALS AND METHODS
Strains and Developmental Conditions
D. discoideum strain AX2, the CAP-deficient mutant CAP bsr, the adenylyl cyclase-deficient mutant aca (Pitt et al., 1992), and transformants were cultured at 21°C as described previously (Noegel et al., 1999). For developmental studies, exponentially growing cells were harvested from liquid medium, washed twice in Soerensen phosphate buffer (17 mM Na+/K+-phosphate buffer, pH 6.0), and shaking was continued for the indicated times in Soerensen buffer at a density of 1 × 107 cells/ml. EDTA sensitivity of cell-cell contacts was determined as described previously (Faix et al., 1990). For rescue experiments, CAP bsr cells were transformed with vectors allowing the expression of green fluorescent protein (GFP) fusion proteins (Noegel et al., 1999). Full-length CAP cDNA was cloned into pDEXRH vector leading to expression of CAP carrying GFP at its amino terminus (Westphal et al., 1997). N- and C-terminal deletion constructs of CAP, N-CAP (aa 1-215), N-ProCAP (aa 1-254), C-CAP (aa 255-464), and ProC-CAP (aa 216-464), were generated by polymerase chain reaction and cloned into pDdA15GFP (Gerisch et al., 1995). The GFP tag was at the C termini of the proteins. Control of transcription was under the actin15 gene promoter and actin8 gene terminator. Plasmids were introduced using the CaCl2 technique (Nellen et al., 1984); selection was with G418. Transformants were cloned and analyzed by fluorescence microscopy. Protein levels were determined by Western blotting with GFP-specific monoclonal antibodies and antibodies that recognized specifically the N- or the C-terminal domain of CAP (Gottwald et al., 1996).
Mutant Analysis
For determination of the cell size, cells were harvested, washed, and then resuspended at a density of 1 × 107 cells/ml in Soerensen phosphate buffer and shaking was continued for another hour at 21°C and 160 rpm in the presence of 10 mM EDTA. Cell polarity was determined for aggregation competent cells. Cells at the appropriate time points were allowed to settle on coverslips and fixed with cold methanol (-20°C). Cells were stained with monoclonal antibodies. Detection was with Cy3-labeled anti-mouse IgG.
cAMP Relay Experiments
For determination of the cAMP relay response the cells were shaken at a density of 2 × 107 cells/ml in development buffer (DB) as described in Patel et al. (2000). The cells were either not pulsed or pulsed at 6-min intervals with cAMP to a final concentration of 50 nM. The assay of the cAMP relay response and the adenylyl cyclase assay and quantification of adenylyl cyclase amounts were done as described in Patel et al. (2000).
Phototaxis Analysis
To analyze slug behavior, 5 × 106 amoebae were inoculated onto a circular, 0.5-cm2 origin at the center of a water agar plate. Slugs were allowed to form and migrate toward light (Fisher et al., 1983). After 48 h, slugs and slime trails were transferred to nitrocellulose filters (BA85; Schleicher & Schuell, Dassel, Germany) and stained with Amido Black.
Determination of F-Actin Levels
For actin detection in immunoblots and methanol fixed cells we used monoclonal antibody (mAb) act1 (Simpson et al., 1984). F-Actin was detected in picric acid/paraformaldehyde fixed cells with tetramethylrhodamine B isothiocyanate (TRITC)-phalloidin (Sigma Chemie, Deisenhofen, Germany). For analysis of F-actin accumulation after stimulation with cAMP, we used the method described by McRobbie and Newell (1983). Alternatively determination was done with TRITC-phalloidin (Haugwitz et al., 1994). Both methods gave comparable results.
cGMP Determination
Cells were starved for the appropriate time at 2 × 107 cells/ml or 4 × 107 cells/ml followed by stimulation with 0.1 μM cAMP. Responses were terminated by lysing the cells with 0.5% Triton X-100 in 0.1 M HCl (final concentration each). Incubation was for 10 min at room temperature. Cells were viewed under a microscope to control lysis. The samples were centrifuged for 5 min at 10,000 × g, and the cGMP content was determined using a commercially available kit (cyclic GMP [low pH] immunoassay; R&D, Wiesbaden, Germany). Samples were either used directly or stored at -20°C. Determinations were done in duplicate or triplicate and the assays were performed at least three times. To ensure that the appropriate developmental stages had been reached, samples were taken in parallel and assayed for the presence of the csA protein.
Analysis of Cell Shape and Cell Migration
Aggregation competent wild-type and mutant cells were plated onto glass coverslips in small plastic dishes, and cell migration was recorded at intervals of 10 s by using an Axiovert-200 inverted microscope and the Axiovision software (Carl Zeiss, Jena, Germany). The time-lapse movies were analyzed with the DIAS program (Solltech, Oakdale, IA; Wessels et al., 1998). For shape analysis, the outlines of the single cells were drawn manually. Several hundred cells have been recorded under different conditions, including after development in shaking cultures or adhered to the plastic surface in submerged cultures. Chemotaxis experiments were performed with micropipettes and a micromanipulator system (Eppendorf, Hamburg, Germany) essentially as described previously (Gerisch and Keller, 1981).
Yeast Two-Hybrid Interaction
Full-length Dictyostelium CAP was cloned into pAS2 (Harper et al., 1993), a 419-base pair fragment corresponding to the C terminus of the Dictyostelium adenylyl cyclase (position 4394-4813 of the published sequence; Pitt et al., 1992) was amplified by polymerase chain reaction with the following primers 5′ GTT GCA ATT TCA AGA GTA GT 3′ and 5′ TTC TTA ACT TGA AAG ATG GA 3′, and cloned into pACT2.
Miscellaneous Methods and Monoclonal Antibodies Used
Changes of myosin II in detergent-insoluble cytoskeletons were analyzed in aggregation-competent wild-type and mutant cells after treatment with caffeine, stimulation with cAMP, and lysis as described previously (Chung and Firtel, 1999). SDS-PAGE, immunoblotting, and immunofluorescence were done as described previously (Gottwald et al., 1996). mAb 223-445-1 had been generated against the C-terminal domain of CAP and 230-18-8 against the N-terminal domain (Gottwald et al., 1996). Contact site A antibody 33-294-17 (Berthold et al., 1985) was used to monitor the developmental stage, mAb act1 recognized actin (Simpson et al., 1984). Antibody K3-184-2 was raised against recombinant GFP; it recognizes wild-type GFP and the red-shifted isoform S65T. Microscopic analysis was done as described previously (Noegel et al., 1999).
RESULTS
CAP Is Required for Cell Polarization
In Dictyostelium, CAP shows a temporary enrichment in extending pseudopods during chemotaxis. We have therefore analyzed the shape of CAP bsr cells during the aggregation phase in detail. At this developmental stage, cells of the parental strain AX2 elongate and form cell-to-cell contacts. Proteins such as actin or the csA protein accumulate at polar regions of the cells and at cell-to-cell contacts. CAP bsr cells also aggregated, although with a delay, and within these aggregates the cells were more rounded and did not exhibit the typical elongated shape. Taking the developmental delay of the CAP bsr mutant into account, we performed the analysis between 9 and 15 h after the start of development. The developmental stage was monitored by analyzing the presence of the contact site A protein, a developmentally regulated cell adhesion molecule that is expressed at the beginning of aggregation and disappears as soon as tight aggregates are formed (Noegel et al., 1986). Thus, this adhesion molecule is an excellent marker for comparing the developmental stage of CAP bsr and wild-type strains. At all time points tested, the mutant cells did not polarize as well as wild-type cells. We then supplied exogenous pulses of cAMP to enhance development (Noegel et al., 1986). Also under these conditions the mutant cells remained less polarized, although expression of csA occurred earlier as in unpulsed cells (our unpublished data). In general, the data shown in this report all have been obtained with cells that developed without additional cAMP pulses unless indicated.
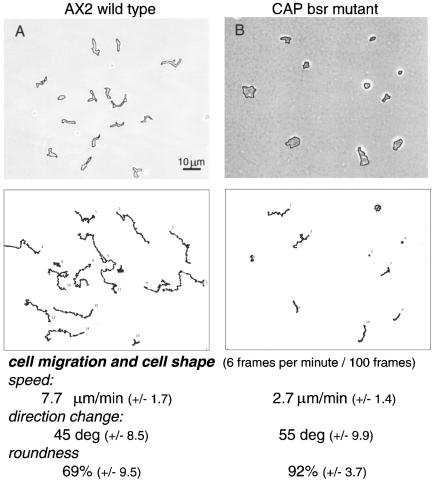
Figure 1 shows the typical migrating pattern of AX2 wild-type and mutant cells after 6 h (AX2) or 12 h (CAP bsr) of starvation in shaking culture. Whereas the AX2 cells were elongated and migrated even without an external cAMP source in a rather directed manner, the CAP mutants were rounded and moved only short distances. Speed, direction change, and roundness reflect this behavior. In contrast to wild-type cells and despite a comparable developmental stage, the mutant cells have a strong tendency to extent pseudopods into all directions and to continuously change the angle of the migration track (Figure 2). As soon as strong cAMP gradients are present either due to the formation of large aggregates during development in submerged culture or by stimulating the cells with microcapillaries that are filled with 10-4 M cAMP, also the CAP bsr cells start to polarize and to migrate toward the cAMP source. This suggests a reduced sensitivity to chemoattractant which leads to a reduced cell polarization.
Figure 1.
Cell migration of AX2 wild-type and CAP bsr mutant cells. After development in shaking culture for 6 h (AX2) or 12 h (CAP bsr) cells were harvested, washed, plated onto glass surfaces and monitored >100 frames at intervals of 10 s. Top, cells and their outlines in the first frame. Bottom, tracks of migration over time. The calculated numbers show that speed of migration, direction change, and roundness of the cells are drastically altered in the CAP mutant.
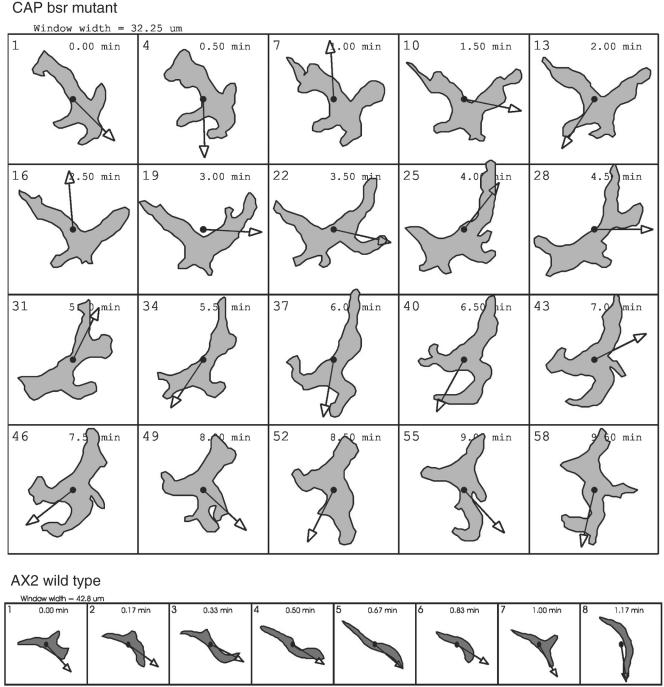
Figure 2.
Analysis of cell polarization. Top, shape changes of a single CAP bsr cell after 12 h of starvation in a submerged culture were recorded at high magnification >10 min at a rate of 10 s/frame. The outlines were traced manually and the changes of direction (arrows) calculated using the DIAS image analysis software. The panel shows every third frame. The cell shapes suggest a lack of polarity and a continuous change of direction. Bottom, for comparison, polarization and directed migration of an AX2 wild-type cell at the same stage of development. Window widths at top panels are 32.25 μm, at the bottom panels 42.8 μm.
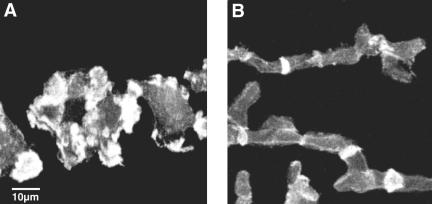
The expression of a GFP-tagged full-length CAP reverted the cell polarity defect completely, and cells after 6 h of starvation were highly elongated like wild-type cells. It also restored the F-actin accumulation at leading edges, whereas in the mutant cells it was present in multiple patches in the cortical region (Figure 3). Furthermore, the developmental defect was reverted and expression of developmentally regulated proteins was as in wild-type (our unpublished data).
Figure 3.
F-Actin distribution in the CAP bsr mutant (A) and in CAP bsr cells expressing a full-length GFP-CAP fusion (B). Cells after 6 h of starvation were fixed with paraformaldehyde/picric acid. F-actin was detected by TRITC-phalloidin. The mutant cells had a more rounded cell shape and F-actin was present in multiple patches at the cortex, whereas cells expressing full-length GFPCAP were elongated and showed a thin F-actin rim along the boundaries of the cell and an enrichment at cell-cell contacts.
Signaling to the Actin Cytoskeleton Is Normal in the CAP Mutant
On application of cAMP, a characteristic pattern of actin polymerization and depolymerization is observed. After an initial increase of the F-actin concentration, the filaments depolymerize again followed by another phase of F-actin accumulation. We studied this response in wild-type AX2 and CAP bsr mutant cells at comparable developmental stages. We found, that the pattern of F-actin accumulation in the mutant exhibited characteristics similar to the wild-type pattern (Figure 4). We conclude from these data that in this process there occurs no involvement of CAP beyond the stimulation of the cAMP-receptor.
Figure 4.
F-Actin polymerization response upon cAMP stimulation is normal in the CAP bsr mutant. The F-actin content was determined using TRITC-phalloidin staining of cells fixed at various times (in seconds) after stimulation with cAMP (10-7 M). The amount of F-actin was normalized relative to the F-actin level of unstimulated cells (0 s). Open symbols, CAP bsr, closed symbols, AX2. The results from a typical experiment are shown.
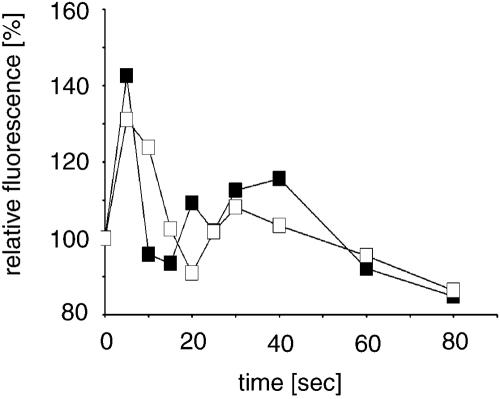
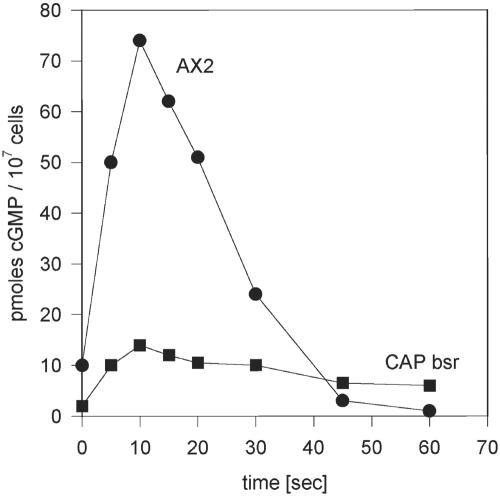
The cAMP-induced cGMP Response Is Altered in CAP bsr
The signal transduction chain leading from cell surface receptors to chemotactically induced cell polarization and pseudopod formation involves also cGMP. cGMP production is responsible for recruiting myosin to the actin cortex that finally leads to the elongated morphology of the cells. When we assayed cGMP production in response to a cAMP pulse, we observed in AX2 cells a cGMP peak at 10 s after stimulation. Basal levels were reached again after 45 s. The mutant showed a similar pattern as AX2 wild-type cells. However, the increase in cGMP was substantially lower and reached only ∼20% of wild-type levels (Figure 5). cGMP has been linked to myosin assembly (Liu and Newell, 1988) and a reduced cGMP response might lead to changes in the actomyosin cortex. Stimulation of aggregation competent CAP bsr mutants with cAMP and determination of myosin assembly over the following 120 s showed that the mutant altered the levels of polymerized myosin; however, this happened in a highly irregular manner. Whereas assembled myosin peaked in wild-type cells after ∼30 s, the peaks in the mutant were smaller and occurred between 20 and 120 s, despite the inhibition of endogenous signal relay by treatment with caffeine. We conclude from these data that regulation of myosin assembly is disorganized in the mutant and might be the reason for the frequent formation of additional pseudopods in aggregation competent cells.
Figure 5.
cGMP response upon stimulation with cAMP. AX2 wild-type and CAP bsr mutants at comparable developmental stages are distinguishable in their cAMP-induced transient increase of intracellular cGMP concentrations. The curve for AX2 represents a single typical experiment. The curve for CAP bsr represents the data from three independent assays. The error bars were too narrow to be visible.
CAP Is Required for cAMP Relay
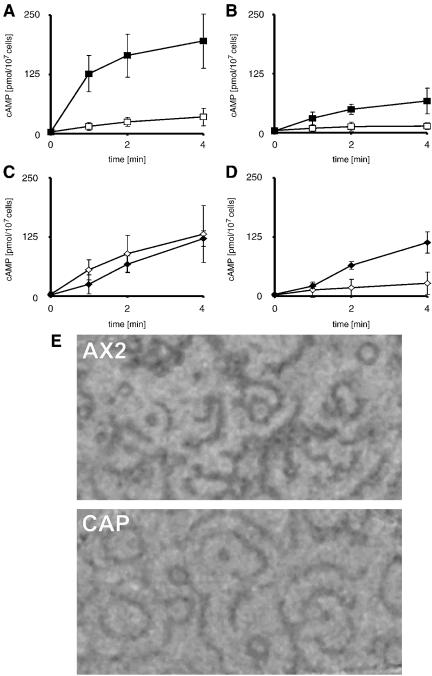
So far, a direct link between CAP and adenylyl cyclase from organisms other than yeast has not been made. We have performed a series of experiments that were designed to unravel a cross talk between both proteins. First, we did cAMP relay experiments. For this, we used cells starved for 3 or 6 h either in the presence or absence of exogenous cAMP pulses. In AX2 cells, which had been starved for 3 h, cAMP pulsing induced a strong relay response. In CAP bsr cells, the relay response is much lower. Although it increases in cells after cAMP pulsing, the kinetics of the cAMP relay response is different from the one in AX2 (Figure 6, A and B). The cAMP relay response after6hinthe presence or absence of cAMP also differs between wild type and mutant. In AX2, the relay response of unpulsed cells is higher than that of pulsed cells (Figure 6C) and the response of pulsed cells after 6 h is lower than that after 3 h of pulsing. Pulsed CAP bsr cells exhibit a different response. After 6 h of pulsing, the cAMP production is higher than after 3 h of pulsing, and CAP bsr and AX2 are similar in their relay response both with regard to magnitude and kinetics (Figure 6, C and D, closed symbols). It is noteworthy, that we never observed such a rapid and dramatic rise in cAMP production in the mutant as is seen in 3-h pulsed AX2 cells. Nonpulsed mutant cells at 6 h show a small relay response very similar to the 3-h result.
Figure 6.
cAMP relay is altered in CAP bsr. cAMP relay was measured in wild-type AX2 and CAP bsr cells that had been starved for 3 or 6 h either in the absence or presence of cAMP pulsing. (A) cAMP relay in AX2 cells starved for 3 h in the absence (open squares) or presence (closed squares) of cAMP pulsing. cAMP pulsing induces a much stronger relay response. (B) cAMP relay in CAP bsr cells starved for 3 h in the absence (open squares) or presence (closed squares) of cAMP pulsing. The relay response is much lower than in AX2 in both cases. It is noticeable that the kinetics of the cAMP relay response is also considerably slower in CAP bsr compared with AX2. (C) cAMP relay after 6 h in AX2 in the presence (closed diamonds) or absence (open diamonds) of cAMP pulsing. The relay response of unpulsed cells is higher than that of unpulsed cells after 3 h; however, the relay response of pulsed cells after 6 h is lower than after 3 h of pulsing (A). There is little difference in relay response between pulsed and nonpulsed cells after 6 h. (D) cAMP relay after 6 h in CAP bsr cells in the presence (closed diamonds) or absence (open diamonds) of cAMP pulsing. Nonpulsed cells show a small relay response just as cells after 3 h of starvation (B), whereas the cells that have been pulsed for 6 h show a slightly stronger relay response than CAP bsr cells that have been pulsed for 3 h. The magnitude and kinetics of relay in CAP bsr and AX2 cells that have been pulsed for 6 h is indistinguishable (C and D). The data shown are the means and standard deviations of four independent experiments performed on different days. (E) Measurement of darkfield waves. Due to a slower oscillation frequency the waves of the mutant are slightly larger than the waves in AX2 wild-type. The measurements of 75 (AX2) and 78 (CAP bsr) wave periods from three independent experiments showed with 270 s (± 37, AX2) and 306 s (± 38, CAP bsr) a statistically significant (p < 0.001) difference.
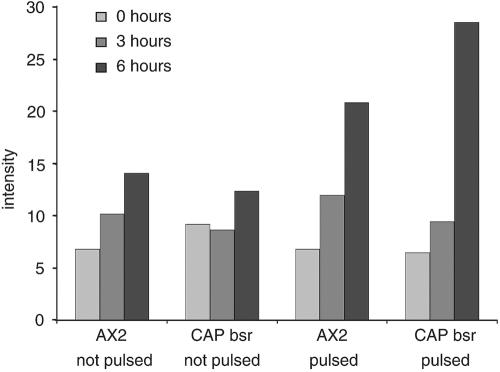
These findings were supported by results from darkfield wave measurements with which one can analyze the cAMP relay (Dormann et al., 2000). In these experiments, the waves occurred at comparable stages of development in AX2 and CAP bsr. However, the waves produced by the mutant were larger compared with AX2, and the slightly slower oscillation frequency in the mutant reflects changes in the cyclase activity as a result of the feedback in cAMP production (Figure 6E).
We also analyzed the level of ACA directly by Western blot analysis followed by a quantitation of the blots to ensure that the differences we observed were not due to lower amounts of the protein. In AX2, the levels of ACA increase during development, whereas pulsing produces much higher levels of protein. In CAP bsr, there is less of an increase during development in unstimulated cells, but after cAMP pulsing ACA expression increases at least to similar amounts as observed in pulsed AX2 cells (Figure 7). This shows that CAP bsr cells can express ACA to normal levels when presented with appropriate cAMP signals.
Figure 7.
CAP bsr has normal amounts of ACA at the protein level. Cells were allowed to develop at 107/ml in DB buffer for various times with or without cAMP pulsing. Samples were collected and solubilized in Laemmli sample buffer and 106 cells were subjected to SDS-PAGE on 8% gels. Immunoblotting was performed on nitrocellulose membranes by using a peptide antibody directed against the last 15 amino acids of ACA (Parent and Devreotes, 1995). The antiserum was diluted 1:3000 in Tris-buffered saline/Tween 20 and detection was performed by chemiluminescence by using horseradish peroxidase-coupled sheep anti-rabbit IgG. Chemiluminescence was recorded and quantitated using a Fujifilm LAS 1000 image reader.
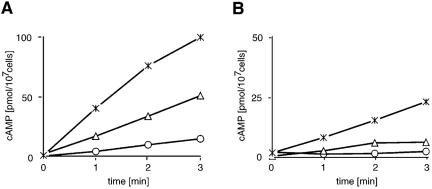
Finally, we measured ACA activity in cell lysates of AX2 wild-type and CAP bsr mutant after 6 h of starvation in the presence of Mg2+, Mn2+, and guanosine 5′-O-(3-thio)triphosphate (GTPγS). We found that cyclase activity in CAP bsr can be stimulated by GTPγS in vitro, which might be an indication of ACA activation by Gβγ. The absolute activity after GTPγS stimulation is, however, much lower in the mutant than in AX2. This is also true for the basal activity, which is measured in the presence of Mg2+ and for the unregulated adenylyl cyclase activity measured in the presence of Mn2+. The lower absolute activation of adenylyl cyclase is probably due to the fact that the cells were not pulsed. However, the degree of stimulation measured as the activity in the presence of GTPγS compared with the basal activity measured in the presence of Mg2+ is very similar in the mutant compared with AX2 (Figure 8). From the data, we conclude that adenylyl cyclase is present in the mutant cells and is functional. The alteration in the cAMP relay response however suggests that CAP/ASP56 participates in this pathway.
Figure 8.
Stimulation of ACA activity in cellular extracts of CAP bsr is normal. The ACA activity of cell lysates was measured for 2 min in the presence of Mg2+ (circles), Mn2+ (triangles), or GTPγS (stars) in AX2 (A) and CAP bsr cells (B) starved for 6 h without pulsing. It is seen that ACA activity can be stimulated in the mutant by GTPγS. The degree of stimulation measured as the activity in the presence of GTPγS compared with the basal activity measured in the presence of Mg2+ is very similar in the mutant compared with AX2, however absolute activity after GTPγS stimulation is much lower in the mutant than in AX2. This is also true for the unregulated adenylyl cyclase activity measured in the presence of Mn2+.
Does CAP Physically Interact with ACA?
To further support these data, we tested the interaction between CAP and ACA directly. In yeast, a physical interaction had been shown between adenylyl cyclase and CAP. The interaction domain in CAP was localized to a short N-terminal stretch that is highly conserved in CAP from other species. In the adenylyl cyclase, the binding site is located at the C terminus (Nishida et al., 1998). This sequence is also conserved in the Dictyostelium protein. Although we could not detect an interaction when we investigated the corresponding domains of the Dictyostelium proteins in the yeast two-hybrid system, we observed an effect in an adenylyl cyclase mutant, aca, when moderately overexpressing GFP-tagged full-length CAP.
We first tested the protein levels in the aca mutant and found that they were comparable with AX2 wild type. At the immunofluorescence level, we detected the protein in the cytosol and at the plasma membrane as has been reported for the wild type (Gottwald et al., 1996). Moreover, GFP-CAP in the aca mutant showed similar dynamics as in wild type. It relocalized during phagocytosis and pinocytosis to the phagocytic or pinocytic cup, respectively, and was enriched in pseudopods (our unpublished data). The levels of the GFP-fusion protein were similar to the ones of the endogenous protein (our unpublished data). In further analysis, we observed that the developmental phenotype of aca cells expressing the GFP-CAP was altered. Normally, aca mutant cells cannot undergo development. In contrast, aca cells expressing GFP-CAP formed aggregates when starved in suspension (our unpublished data). The aggregates were dissociated when EDTA was added, which is indicative of the formation of EDTA-sensitive cell contacts mediated by the adhesion molecule DdCAD-1 (Wong et al., 1996). When we tested the GFP-tagged domains of CAP, we found that N-CAP-ProGFP had a similar effect. From these results, it seems that the block in early development of aca mutant cells can be overcome by increased levels of CAP.
Distinct CAP Domains Restore Cell Morphology and Development
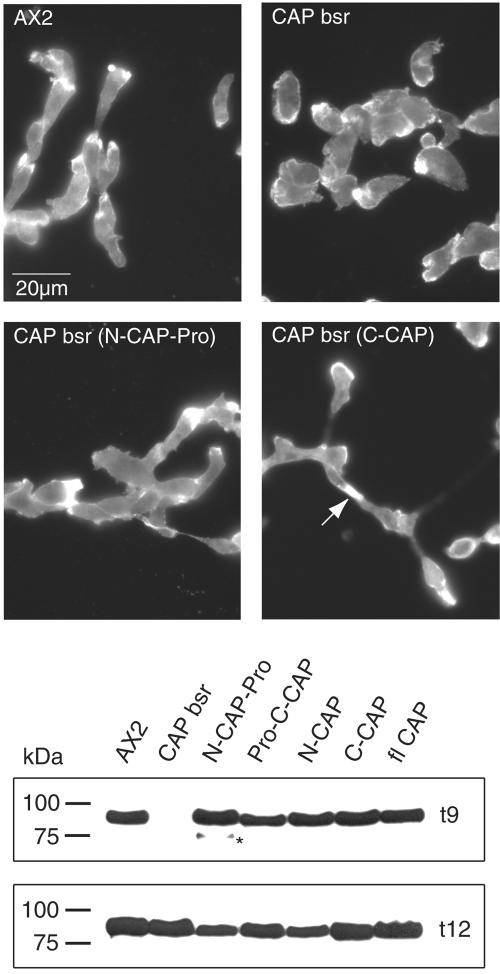
Having shown that expression of full-length CAP in CAP bsr cells restores polarity and reverts the developmental defect, we extended this analysis to the individual CAP domains, which were expressed as GFP fusion proteins. For full-length CAP and ProC-CAP, the levels of the GFP fusion proteins were nearly comparable with wild-type CAP levels; N-CAP, N-CAP-Pro, and C-CAP showed higher levels of expression (our unpublished data). We found that all domains restored the cell polarity defect and cells were elongated when they aggregated (Figure 9, top, shown for NCAP-Pro and C-CAP). They corrected the developmental defect and csA expression exhibited the same pattern as in AX2 wild type (Figure 9, bottom, shown for t9 and t12; Table 1).
Figure 9.
Top, cell polarization defect of the CAP bsr mutant can be rescued by expression of individual domains. Cells harvested after 6 h of starvation in shaken suspension were allowed to settle on coverslips, fixed with cold methanol (-20°C), and stained with actin specific mAb act1 followed by treatment with a secondary Cy3-labeled antibody. Actin staining is mainly seen around the cell boundaries and is enriched in the front and in the cell-cell contact regions. AX2 and mutant cells expressing N-CAP-Pro or C-CAP GFP fusion proteins are elongated and actin is enriched in the polar regions, whereas CAP bsr cells do not polarize properly at any time during development independent of the expression of developmental markers. The arrow in the lower right panel points to a cell cell contact region. Bottom, developmental delay of CAP bsr is rescued by expression of the CAP domains. csA levels were used to monitor the developmental stage. In wild-type cells, csA is present after 6 h of starvation in shaken suspension, in CAP bsr mutants the protein is strongly expressed after 12 h. At t9 AX2 and all CAP bsr transformants expressing N- or C-terminal domains of CAP show maximal accumulation of the csA protein. Total cell homogenates derived from 5 × 105 cells after 9 and 12 h of starvation were subjected to SDS-PAGE (10% acrylamide), and the resulting Western blots were probed with mAb 33-294-17. The star indicates an incompletely processed form of the csA glycoprotein. The sizes in kilodaltons of the molecular mass marker are given at the left.
Table 1.
Rescue activities of individual CAP domains
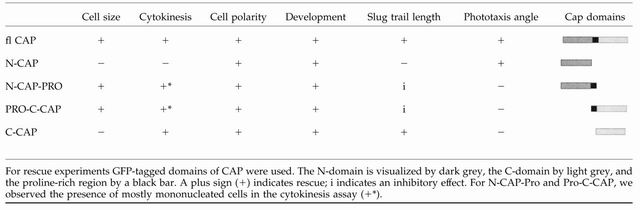
We also tested whether further defects in the mutant like cell morphology, cell size, and the cytokinesis defect could be rescued by any of the domains. CAP bsr cells are heterogeneous in cell size with their diameters being shifted to 15-20 μm from 10-12 μm in AX2 wild-type. Furthermore, mutant cells have three and more nuclei, whereas wild-type cells are mostly mono- and binucleated. N-CAP expression in the mutant did not affect multinuclearity and cell size. The presence of the proline-rich region in either N-CAP or CCAP GFP fusion proteins led to the occurrence of mostly mononucleated cells and to a reduction in cell size. C-CAP-expressing mutant cells resembled AX2 wild type with regard to nuclei number (Table 1); in contrast, cell size was not reduced to normal. We rather observed a broad distribution of cell sizes (our unpublished data). The data from this analysis suggest that the proline-rich region regulates the cell size and nuclei number, whereas the C-terminal domain on its own affects cytokinesis.
CAP Is Indispensable for Correct Phototaxis
The slug stage of development is important for the survival of Dictyostelium in its natural surroundings. Slugs migrate with great sensitivity toward light, i.e., the soil surface from where the spores can be dispersed. Several phototaxis mutants have been described. In some of them, the underlying defect resides in genes encoding cytoskeletal proteins (Fisher et al., 1997; Stocker et al., 1999), another mutant was defective in a Ras gene (Wilkins et al., 2000).
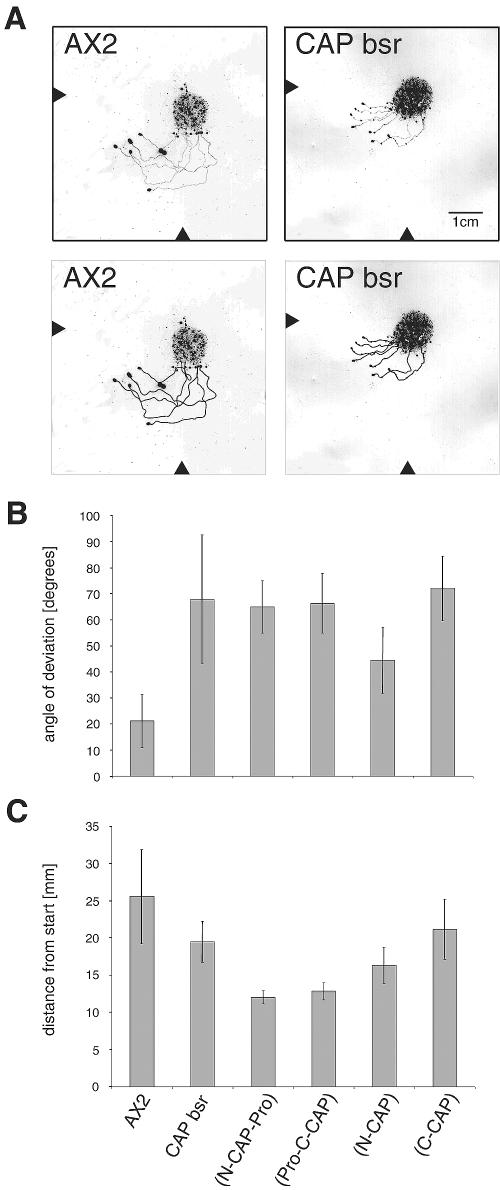
CAP mutant cells show a general delay in development; they do, however, complete the developmental cycle. When we studied the multicellular slug stage we noted a defect in phototaxis. Wild-type slugs migrate almost directly toward a lateral light source, whereas CAP bsr slugs do not orient correctly and do not migrate as far as wild-type slugs in the same period of time (Figure 10). That they do not have a general defect in light sensing was revealed in experiments where we changed the position of the light source after the slugs had migrated for 28 h, which was followed by another migration period of 24 h. CAP bsr slugs showed a turn in the direction of migration as did wild-type slugs (Figure 10A). CAP bsr slugs expressing N-CAP showed an improved orientation. They migrated in an angle of ∼40° toward the light source compared with 20° for wild-type slugs and nearly 60° for CAP bsr (Figure 10B). C-CAP expression led to some improvement in the migratory behavior because these slugs traveled over longer distances. The presence of the proline rich region in the N- or the C-terminal domain had an inhibitory effect on the distance traveled (Figure 10C and Table 1). CAP therefore seems to be involved in two aspects of slug phototaxis, migration and orientation.
Figure 10.
CAP bsr mutant has a phototaxis defect. (A) Phototactic behavior of the mutant is altered in comparison with AX2 wild type. Cells were incubated for 28 h in a phototaxis chamber with a narrow light source (arrowhead at the bottom). After this time, the chamber was rotated by 90° (arrowhead at the left) and the cells incubated for additional 24 h. Subsequently, trails and cell material were blotted and stained with Amido Black. In the bottom panels, the migration tracks are highlighted for better visibility. AX2 slugs migrate directly toward the light source, whereas the mutant is slower and shows only poor directionality. (B) The angle of deviation during slug phototaxis is increased for all mutant strains as compared with AX2. (C) CAP bsr slugs expressing C-CAP-GFP can migrate nearly as far as wild type, whereas the presence of the proline-rich region in any of the domains has a negative effect on the distance traveled. The data are collected from between 6 and 30 experiments per strain.
DISCUSSION
Our studies show that the cyclase associated protein CAP determines cell polarity and affects development. We have also provided evidence that it is required for adenylyl cyclase activity by studying the cAMP relay response in Dictyostelium. Moreover, moderate overexpression of CAP in an adenylyl cyclase mutant led to induction of early developmental stages. Further progression into development, however, did not occur.
CAP in Cell Polarity
Mutation of CAP in the Drosophila eye (acu) leads to a premature differentiation of photoreceptors (Benlali et al., 2000). This is thought to be due to the inability of the cells to undergo shape changes that are required for proper hedgehog signaling. Associated with this inability to change shapes was an accumulation of F-actin. The Dictyostelium mutant that we have described shows defects in many properties and cellular reactions. Most notable is the deficiency of the cells to polarize properly. They share this defect with the Drosophila mutant cap in which oocyte polarity is disrupted and F-actin organization altered (Baum et al., 2000). For Drosophila it was concluded that CAP is required for the correct spatial regulation of actin polymerization and that normal actin organization is required for proper polarization of the oocyte. The central role of the actin cytoskeleton is certainly important for proper polarization of D. discoideum cells as well. Especially, the inhibition of pseudopod formation at the sides of elongated cells requires a very strong acto-myosin cortex and favors the motility at the front and rear areas. CAP plays apparently two roles in cell polarization and migration: 1) As was shown previously, CAP accumulated in actin-rich regions at moving fronts that favor polarization and might be a function of the actin-binding domain in the CAP C terminus (Gottwald et al., 1996). 2) The additional interaction with the cyclase via its N terminus guarantees correct signaling activities. The reduced levels of cGMP in the CAP bsr mutant are sufficient to impair the recruitment of myosin to the actin cortex, which leads to a soft actin meshwork and the formation of additional pseudopods at the sides of polarized cells thus disturbing overall elongation and orientation (Figure 2). The reduced sensitivity to chemotactic signals from outside and the altered relay response in the mutant contribute to the poor polarization behavior.
The data we have collected by performing rescue experiments with separate domains also indicate that CAP does not solely act as actin regulatory protein. We could clearly attribute a role to the proline-rich domain because both the N- and the C-terminal polypeptides containing this stretch corrected the increase in cell size and led to a reduction in nuclei number. Moreover, these polypeptides had an adverse effect on phototaxis and inhibited phototactic migration, whereas the N-domain alone improved the orientation during phototaxis, and the C-domain on its own improved the slug migration. For the yeast protein, it has been shown that the proline-rich domain binds SH3-domain containing proteins and is responsible for CAP's localization at the cortical cytoskeleton (Lila and Drubin, 1997). In contrast, in CAP from Dictyostelium it is an N-terminal stretch that mediates correct localization (Noegel et al., 1999). Surprisingly, all four constructs rescued the developmental defect and led to formation of polarized cells. This confirms the dual function of CAP, rendering similar to the findings in yeast, the N terminus as being responsible for proper signal transduction and the C terminus as being involved in cytoskeletal dynamics at moving fronts. The reexpression of any of the functional domains, therefore, is sufficient to overcome the defects of the mutant under laboratory conditions. We cannot exclude, however, that also the N terminus influences the cytoskeleton in an indirect manner as suggested by Moriyama and Yahara (2002). They found that both domains independently affect F-actin polymerization, whereby the C-domain directly interacts with F-actin, and the N-domain interacted with an actin-cofilin complex. Our results from the rescue experiments suggest a role for the proline rich domain as well which might act in combination with the N- or C-domain.
CAP in cAMP Signaling
Dictyostelium is unique in its ability to use cAMP for initiation and progression through development. The chemotactic aggregation of starving cells is controlled by propagating waves of cAMP. The cAMP signals are periodically initiated by cells in the aggregation centers and relayed by surrounding cells. This results in outward-propagating waves of cAMP that induce inward movement of the cells. The process of cAMP signaling has been studied in depth, and the cAMP receptors and the cAMP synthesizing enzymes are well characterized. Dictyostelium harbors three adenylyl cyclases: an aggregation-specific cyclase, ACA; a germination-specific cyclase, ACG; and ACB, a more recently discovered adenylyl cyclase that has characteristics different from ACA and presumably acts in a G protein-independent way (Pitt et al., 1992; Kim et al., 1998). Responsible for the cAMP relay is the aggregation-specific cyclase ACA. In CAP bsr cells, the protein was present in unaltered amounts and its activity could be assayed in cell homogenates. However, the cAMP relay was altered and the cAMP-induced secretion of cAMP did not occur with the same characteristics as in wild type. The data do not imply, however, that CAP is essential for adenylyl cyclase activity. The situation rather resembles the one in yeast where only one aspect of cyclase activation, the Ras-mediated activation, is affected.
The activity of the Dicytostelium ACA is regulated by the Gβγ complex (Chen et al., 1996) and other factors such as CRAC, pianissimo, aimless, a RasGEF, and extracellular signal-regulated kinase 2, a mitogen-activated protein kinase (Insall et al., 1994; Segall et al., 1995; Insall et al., 1996; Chen et al., 1997). Cells lacking these factors are very similar in their phenotypes, and all have been isolated because they failed to aggregate. These mutants are not only similar among each other with regard to their developmental phenotype but also they are specifically defective in the receptor/G protein-mediated activation of ACA because GTPγS did no longer stimulate ACA activity in cell lysates. The effect of CAP on ACA activity is distinctly different as CAP mutants can aggregate, although with a delay, and as GTPγS stimulation is still effective. Although our results from the yeast two-hybrid analysis were negative, the question is still open whether the CAP-ACA interaction is a direct one or whether it requires one or more proteins linking CAP and cAMP signaling.
CAP in Late Development
The phototactic defect that we have observed also can be linked to CAP's effect on the cAMP relay. Previous work showed that cAMP waves organize the slug (Dormann et al., 1997, 2001), and Miura and Siegert (2000) reported that cAMP mediates cell-cell signaling and chemotaxis of the cells in a slug. cAMP waves are generated in the anterior prestalk zone in response to light and are relayed to the posterior zone. In fact, we have observed a defect in signaling in the mutant during late stages of development and found in dark field measurements that wave formation was altered (our unpublished data). In the phototaxis assay we noted that CAP acts both on the orientation as well as on migration and that both components of phototaxis can be separated. The rescue experiments showed an impact of the N-domain on the efficiency of phototaxis by improving the angle of migration in the direction of the light, whereas the C-domain caused the slugs to migrate over longer distances. The first effect could be due to the activity of the N-domain in signaling, whereas the second one might require CAP's activity as actin associated protein. Taking the results from our analysis together, we conclude that the evolutionarily conserved protein CAP may play a critical part in cell polarity and movement in a diversity of organisms.
Acknowledgments
We thank Daniela Rieger, Marc Borath, and Berthold Gassen for cell culture and technical assistance. The work was supported by grants from the DFG, the Fonds der Chemischen Industrie, and Köln Fortune. The work in C.J.W.'s laboratory is supported by a Wellcome Trust Program Grant.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03-05-0269. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-05-0269.
References
- Baum, B., Li, W., and Perrimon, N. (2000). A cyclase-associated protein regulates actin and cell polarity during Drosophila oogenesis and in yeast. Curr. Biol. 10, 964-973. [DOI] [PubMed] [Google Scholar]
- Benlali, A., Draskovic, I., Hazelett, D.J., and Treisman, J.E. (2000). act up controls actin polymerization to alter cell shape and restrict Hedgehog signaling in the Drosophila eye disc. Cell 101, 271-281. [DOI] [PubMed] [Google Scholar]
- Bertholdt, G., Stadler, J., Bozzaro, S., Fichtner, B., and Gerisch, G. (1985). Carbohydrate and other epitopes of the contact site A glycoprotein of Dictyostelium discoideum as characterized by monoclonal antibodies. Cell Differ. 16, 187-202. [DOI] [PubMed] [Google Scholar]
- Bretscher, A. (2003). Polarized growth and organelle segregation in yeast: the tracks, motors, and receptors. J. Cell Biol. 160, 811-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, M.Y., Insall, R.H., and Devreotes, P.N. (1996). Signaling through chemoattractant receptors in Dictyostelium. Trends Genet. 12, 52-57. [DOI] [PubMed] [Google Scholar]
- Chen, M.Y., Long, Y., and Devreotes, P.N. (1997). A novel cytosolic regulator, Pianissimo, is required for chemoattractant receptor and G protein-mediated activation of the 12 transmembrane domain adenylyl cyclase in Dictyostelium. Genes Dev. 11, 3218-3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung, C.Y., and Firtel, R.A. (1999). PAKa, a putative PAK family member, is required for cytokinesis and the regulation of the cytoskeleton in Dictyostelium discoideum cells during chemotaxis. J. Cell Biol. 147, 559-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer, F.I., and Parent, C.A. (2002). PI 3-kinases and PTEN: how opposites chemoattract. Cell 109, 541-544. [DOI] [PubMed] [Google Scholar]
- Dormann, D., Weijer, C., and Siegert, F. (1997). Twisted scroll waves organize Dictyostelium mucoroides slugs. J. Cell Sci. 110, 1831-1837. [DOI] [PubMed] [Google Scholar]
- Dormann, D., Vasiev, B., and Weijer, C.J. (2000). The control of chemotactic cell movement during Dictyostelium morphogenesis. Phil. Trans. R. Soc. Lond. B. Biol. Sci. 355, 983-991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dormann, D., Kim, J.Y., Devreotes, P.N., and Weijer, C.J. (2001). cAMP receptor affinity controls wave dynamics, geometry and morphogenesis in Dictyostelium. J. Cell Sci. 114, 2513-2523. [DOI] [PubMed] [Google Scholar]
- Faix, J., Gerisch, G., and Noegel, A.A. (1990). Constitutive overexpression of the contact site A glycoprotein enables growth-phase cells of Dictyostelium discoideum to aggregate. EMBO J. 9, 2709-2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedor-Chaiken, M., Deschenes, R.L., and Broach, J.R. (1990). SRV2, a gene required for Ras activation of adenylate cyclase in yeast. Cell 61, 329-340. [DOI] [PubMed] [Google Scholar]
- Field, J., et al. (1990). Cloning and characterization of CAP, the S. cerevisiae gene encoding the 70 kD adenylyl cyclase-associated protein. Cell 61, 319-327. [DOI] [PubMed] [Google Scholar]
- Firtel, R.A., and Chung, C.Y. (2000). The molecular genetics of chemotaxis: sensing and responding to chemoattractant gradients. Bioessays 22, 603-615. [DOI] [PubMed] [Google Scholar]
- Firtel, R.A., and Meili, R. (2000). Dictyostelium: a model for regulated cell movement during morphogenesis. Curr. Opin. Genet. Dev. 10, 421-427. [DOI] [PubMed] [Google Scholar]
- Fisher, P.R., Grant, W.N., Dohrmann, U., and Williams, K.L. (1983). Spontaneous turning behaviour by Dictyostelium discoideum slugs. J. Cell Sci. 62, 161-170. [DOI] [PubMed] [Google Scholar]
- Fisher, P.R., Noegel, A.A., Fechheimer, M., Rivero, F., Prassler, J., and Gerisch, G. (1997). Photosensory and thermosensory responses in Dictyostelium slugs are specifically impaired by absence of the F-actin cross-linking gelation factor (ABP-120). Curr. Biol. 7, 889-892. [DOI] [PubMed] [Google Scholar]
- Freeman, N.L., Chen, Z., Horenstein, J., Weber, A., and Field, J. (1995). An actin monomer binding activity localizes to the carboxyl-terminal half of the Saccharomyces cerevisiae cyclase-associated protein. J. Biol. Chem. 270, 5680-5685. [DOI] [PubMed] [Google Scholar]
- Gerisch, G., and Keller, H.U. (1981). Chemotactic reorientation of granulocytes stimulated with micropipettes containing fMet-Leu-Phe. J. Cell Sci. 52, 1-10. [DOI] [PubMed] [Google Scholar]
- Gerisch, G., Albrecht, R., Heizer, C., Hodgkinson, S., and Maniak, M. (1995). Chemoattractant-controlled accumulation of coronin at the leading edge of Dictyostelium cells monitored using a green fluorescent protein-coronin fusion protein. Curr. Biol. 5, 1280-1285. [DOI] [PubMed] [Google Scholar]
- Goldschmidt-Clermont, P.J., and Janmey, P.A. (1991). Profilin, a weak CAP for actin and RAS. Cell 66, 419-421. [DOI] [PubMed] [Google Scholar]
- Gottwald, U., Brokamp, R., Karakesisoglou, I., Schleicher, M., and Noegel, A.A. (1996). Identification of a cyclase-associated protein (CAP) homologue in Dictyostelium discoideum and characterization of its interaction with actin. Mol. Biol. Cell 7, 261-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper, J.W., Adami, G.R., Wei, N., Keyomarski, K., and Elledge, S.J. (1993). The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G 1 cyclin-dependent kinases. Cell 75, 805-816. [DOI] [PubMed] [Google Scholar]
- Haugwitz, M., Noegel, A.A., Karakesisoglou, J., and Schleicher, M. (1994). Dictyostelium amoebae that lack G-actin sequestering profilins show defects in F-actin content, cytokinesis and development. Cell 79, 303-314. [DOI] [PubMed] [Google Scholar]
- Hubberstey, A.V., and Mottillo, E.P. (2002). Cyclase-associated proteins: CAPacity for linking signal transduction and actin polymerization. FASEB J. 16, 487-499. [DOI] [PubMed] [Google Scholar]
- Insall, R., Kuspa, A., Lilly, P.J., Shaulsky, G., Levin, L.R., Loomis, W.F., and Devreotes, P. (1994). CRAC, a cytosolic protein containing a pleckstrin homology domain, is required for receptor and G protein-mediated activation of adenylyl cyclase in Dictyostelium. J. Cell Biol. 126, 1537-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insall, R.H., Borleis, J., and Devreotes, P.N. (1996). The aimless RasGEF is required for processing of chemotactic signals through G-protein-coupled receptors in Dictyostelium. Curr. Biol. 6, 719-729. [DOI] [PubMed] [Google Scholar]
- Kim, H.J., Chang, W.T., Meima, M., Gross, J.D., and Schaap, P. (1998). A novel adenylyl cyclase detected in rapidly developing mutants of Dictyostelium. J. Biol. Chem. 273, 30859-30862. [DOI] [PubMed] [Google Scholar]
- Ksiazek, D., Brandstetter, H., Israel, L., Bourenkov, G.P., Katchalova, G., Janssen, K.-P., Bartunik, H.D., Noegel, A.A., Schleicher, M., and Holak, T.A. (2003). Structure of the N-terminal domain of the adenylyl cyclase-associated protein (CAP) from Dictyostelium discoideum. Structure 11, 1171-1178. [DOI] [PubMed] [Google Scholar]
- Lila, T., and Drubin, D.G. (1997). Evidence for physical and functional interactions among two Saccharomyces cerevisiae SH3 domain proteins, an adenylyl cyclase-associated protein and the actin cytoskeleton. Mol. Biol. Cell 8, 367-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, G., and Newell, P.C. (1988). Evidence that cyclic GMP regulates myosin interaction with the cytoskeleton during chemotaxis of Dictyostelium. J. Cell Sci. 90, 123-129. [DOI] [PubMed] [Google Scholar]
- Meili, R., Ellsworth, C., Lee, S., Reddy, T.B., Ma, H., and Firtel, R.A. (1999). Chemoattractant-mediated transient activation and membrane localization of Akt/PKB is required for efficient chemotaxis to cAMP in Dictyostelium. EMBO J. 18, 2092-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura, K., and Siegert, F. (2000). Light affects cAMP signaling and cell movement activity in Dictyostelium discoideum. Proc. Natl. Acad. Sci. USA 97, 2111-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRobbie, S.J., and Newell, P.C. (1983). Changes in actin associated with the cytoskeleton following chemotactic stimulation of Dictyostelium discoideum. Biochem. Biophys. Res. Commun. 115, 351-359. [DOI] [PubMed] [Google Scholar]
- Moriyama, K., and Yahara, I. (2002). Human CAP1 is a key factor in the recycling of cofilin and actin for rapid actin turnover. J. Cell Sci. 115, 1591-1601. [DOI] [PubMed] [Google Scholar]
- Nellen, W., Silan, C., and Firtel, A. (1984). DNA-mediated transformation in Dictyostelium discoideum: regulated expression of an actin gene fusion. Mol. Cell. Biol. 12, 2890-2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson, W.J. (2003). Adaptation of core mechanisms to generate cell polarity. Nature 422, 766-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida, Y., Shima, F., Sen, H., Tanaka, Y., Yanagihara, C., Yamawaki-Kataoka, Y., Kariya, K., and Kataoka, T. (1998). Coiled-coil interaction of N-terminal 36 residues of cyclase-associated protein with adenylyl cyclase is sufficient for its function in Saccharomyces cerevisiae ras pathway. J. Biol. Chem. 273, 28019-28024. [DOI] [PubMed] [Google Scholar]
- Noegel, A.A., Gerisch, G., Stadler, J., and Westphal, M. (1986). Complete sequence and transcript regulation of a cell adhesion protein from aggregating Dictyostelium cells. EMBO J. 5, 1473-1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noegel, A.A., Rivero, F., Albrecht, R., Janssen, K.P., Köhler, J., Parent, C.A., and Schleicher, M. (1999). Assessing the role of the ASP56/CAP homologue of Dictyostelium discoideum and the requirements for subcellular localization. J. Cell Sci. 112, 3195-3203. [DOI] [PubMed] [Google Scholar]
- Patel, H., Guo, K., Parent, C., Gross, J., Devreotes, P.N., and Weijer, C.J. (2000). A temperature-sensitive adenylyl cyclase mutant of Dictyostelium. EMBO J. 19, 2247-2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent, C.A., and Devreotes, P.N. (1995). Isolation of inactive and G protein-resistant adenylyl cyclase mutants using random mutagenesis. J. Biol. Chem. 270, 22693-22696. [DOI] [PubMed] [Google Scholar]
- Parent, C.A., Blacklock, B.J., Froehlich, W.M., Murphy, D.B., and Devreotes, P.N. (1998). G protein signaling events are activated at the leading edge of chemotactic cells. Cell 95, 81-91. [DOI] [PubMed] [Google Scholar]
- Pitt, G.S., Milona, N., Borleis, J., Lin, K.C., Reed, R.R., and Devreotes., P. N. (1992). Structurally distinct and stage-specific adenylyl cyclase genes play different roles in Dictyostelium development. Cell 69, 305-315. [DOI] [PubMed] [Google Scholar]
- Segall, J.E., Kuspa, A., Shaulsky, G., Ecke, M., Maeda, M., Gaskins, C., Firtel, R.A., and Loomis, W.F. (1995). A MAP kinase necessary for receptor-mediated activation of adenylyl cyclase in Dictyostelium. J. Cell Biol. 128, 405-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson, P.A., Spudich, J.A., and Parham, P. (1984). Monoclonal antibodies prepared against Dictyostelium actin: characterization and interactions with actin. J. Cell Biol. 99, 287-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker, S., Hiery, M., and Marriott, G. (1999). Phototactic migration of Dictyostelium cells is linked to a new type of gelsolin-related protein. Mol. Biol. Cell 10, 161-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkerke-Van Wijk, I., and Schaap, P. (1997). cAMP, a signal for survival. In: Dictyostelium. A Model System for Cell Biology and Developmental Biology, ed. Y. Maeda, K. Inouye, and I. Takeuchi, Tokyo, Japan: Universal Academy Press, 145-162.
- Wessels, D., Voss, E., v. Bergen, N., Burns, R., Stites, J., and Soll, D.R. (1998). A computer-assisted system for reconstructing and interpreting the dynamic three-dimensional relationships of the outer surface, nucleus and pseudopods of crawling cells. Cell Motil. Cytoskeleton 41, 225-246. [DOI] [PubMed] [Google Scholar]
- Wesp, A., Hicke, L., Palecek, J., Lombardi, R., Aust, T., Munn, A.L., and Riezman, H. (1997). End4p/Sla2p interacts with actin-associated proteins for endocytosis in Saccharomyces cerevisiae. Mol. Biol. Cell 11, 2291-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal, M., Jungbluth, A., Heidecker, M., Mühlbauer, B., Heizer, C., Schwartz, J.M., Marriott, G., and Gerisch, G. (1997). Microfilament dynamics during cell movement and chemotaxis monitored using a GFP-actin fusion protein. Curr. Biol. 7, 176-183. [DOI] [PubMed] [Google Scholar]
- Wilkins, A., Khosla, M., Fraser, D.J., Spiegelman, G.B., Fisher, P.R., Weeks, G., and Insall, R.H. (2000). Dictyostelium RasD is required for normal phototaxis, but not differentiation. Genes Dev. 14, 1407-1413. [PMC free article] [PubMed] [Google Scholar]
- Wong, E.F., Brar, S.K., Sesaki, H., Yang, C., and Siu, C.H. (1996). Molecular cloning and characterization of DdCAD-1, a Ca2+-dependent cell-cell adhesion molecule, in Dictyostelium discoideum. J. Biol. Chem. 271, 16399-16408. [DOI] [PubMed] [Google Scholar]