Abstract
G protein-coupled receptor kinases (GRKs) regulate numerous G protein-coupled receptors (GPCRs) by phosphorylating the intracellular domain of the active receptor, resulting in receptor desensitization and internalization. GRKs also regulate GPCR trafficking in a phosphorylation-independent manner via direct protein-protein interactions. Emerging evidence suggests that GRK2, the most widely studied member of this family of kinases, modulates multiple cellular responses in various physiological contexts by either phosphorylating non-receptor substrates or by directly interacting with signaling molecules. In this review, we discuss traditional and newly discovered roles of GRK2 in receptor internalization and signaling as well as its impact on non-receptor substrates. We also discuss novel exciting roles of GRK2 in the regulation of dopamine receptor signaling and in the activation and trafficking of the atypical GPCR, Smoothened (Smo).
GRKs and β-arrestins attenuate GPCR signal transduction
GPCRs are seven-transmembrane receptors (7TMRs) encoded by approximately 950 genes, representing the largest family of cell-surface receptors [1]. They transmit a wide range of extracellular stimuli into cells, regulating the majority of biological processes. Upon agonist stimulation, GPCRs activate heterotrimeric G proteins, which exchange bound GDP for GTP, leading to the dissociation of the G protein into activated Gα and Gβγ subunits. This dissociation promotes downstream signaling through specific effector proteins and second messengers [2]. Because ~30% of the therapeutic drugs used in the clinic directly target GPCRs, it is of paramount importance to understand the mechanisms by which GPCRs are regulated. The process of GPCR desensitization (physical uncoupling of the G protein from the cognate receptor) is initiated by various G protein-coupled receptor kinases (GRKs). These kinases phosphorylate intracellular domains of activated receptors, leading to the recruitment of the multifunctional adaptor proteins, arrestins, to the receptors and the attenuation of intracellular G protein-dependent signaling [3]. In humans, seven GRKs are grouped and classified in three subfamilies: the GRK1-like subfamily includes GRK1 and GRK7, which primarily regulate photoreceptors in the retina. The GRK2-like subfamily includes GRK2 and GRK3, both of which are ubiquitously expressed. The GRK4-like subfamily includes GRK4, whose expression is primarily in the testis, cerebellum and kidney, and GRK5 and GRK6, both of which are widely expressed [4]. Four genes encode for arrestins: arrestin-1 and arrestin-4 are restricted to the retina, whereas arrestin-2 (β-arrestin-1) and arrestin-3 (β-arrestin-2) are ubiquitously expressed [5].
GRK-dependent recruitment of β-arrestins to the phosphorylated receptor plays several roles in the attenuation of GPCR signaling. As mentioned above, it promotes rapid receptor desensitization; however, by interacting with components of the endocytic machinery such as clathrin and the AP2 adaptor complex, β-arrestins target GPCRs for clathrin-mediated endocytosis and internalization [6, 7]. As scaffold proteins, β-arrestins also interact with various signaling molecules (e.g. mitogen-activated protein kinase (MAPK) cascade components and non-receptor tyrosine kinases), promoting β-arrestin-dependent and G protein-independent signaling pathways. β-arrestin-dependent signaling has been shown to modulate a wide range of cellular processes such as cell motility, chemotaxis and cell survival. A complete list of β-arrestin interacting signaling molecules, as well as the physiological relevance of such interactions has been extensively reviewed [3, 8].
Emerging evidence suggests that GRKs modulate multiple cellular responses in diverse tissues and physiological contexts independent of β-arrestins [9–13]. In particular, recent studies have described the ability of GRK2 to phosphorylate non-receptor substrates and interact with a diverse repertoire of protein partners (Table 1). Additionally, new evidence suggests that GRK2 mediates signaling from the atypical GPCR Smoothened (Smo), thereby playing an important role in the Hedgehog (Hh) signaling pathway during embryonic development [14–17]. In this review, we discuss recent understandings regarding GRK2’s complex roles in phosphorylation-dependent and independent signaling as well as its implications on various cellular processes and pathologies.
Table 1.
GRK2 interacting proteins and non-receptor substrates
| Tissue/Cell | Function | References | |
|---|---|---|---|
|
Phosphorylation-independent interaction |
|||
| Gαq | In vitro, COS-1 cells,HEK293 cells |
|
[18, 19] [39] [40, 41] |
| Gβγ | HEK293 cells, HL-1 cells |
|
[36, 37] [38] |
| Raf Kinase Inhibitor Protein (RKIP) | HEK293 cells |
|
[24] |
| Phosphoinositide 3-kinase (PI3K) | HEK293 cells, NIH3T3 cells | AP2 recruitment to the plasma membrane and GPCR internalization |
[25, 26] |
| Clathrin | HEK293 cells | β-arrestin independent, dynamin-dependent GPCR internalization |
[28, 29] |
| GRK Interactor-1 and 2 (GIT-1, GIT-2) |
HEK293 cells HeLa cells, MEFs derived from GRK2 KO mice |
|
[33–35] [45, 46] |
| RalA GTPase | HEK293 cells | Phosphorylation-independent desensitization of the lysophosphatidic acid (LPA1) receptor |
[43] |
| Akt | Sinusoidal endothelial cells isolated from normal and injured rat livers |
Inhibition of Akt activity and endothelial cell nitric oxide synthase (eNOS) phosphorylation, leading to reduced NO production |
[47] |
| MEK | HEK293 cells, splenocytes from GRK2 heterozygous |
Inhibition of ERK activation upon chemokine induction |
[48] |
| Caveolin | In vitro, COS-1 cells, A431 cells, NIH- 3T3 cells |
|
[49] |
| EPAC | Nociceptor GRK2 heterozygous (SNS- GRK2+/−)mice |
Signaling switch to MEK/ERK pathway and activation of RAP1 |
[50] |
| Pin1 | HeLa cells | down-regulation of GRK2 levels during G2/M checkpoint |
[70] |
| Ptch1 | HEK293 cells zebrafish |
reduced association between Ptch1 and cyclin B1, leading to increased cell proliferation |
[72] |
| Ffragile X Mental Retardation Protein (FMRP) |
FMRP KO mice | Inhibition of GRK2 translocation to the plasma membrane |
[82] |
| Non-receptor substrates | |||
| Tubulin | In vitro, HEK293, COS-1 cells, Porcine brain preparations |
Regulation of microtubule assembly and agonist-induced GPCR internalization |
[53–56] |
| Radixin | MDCK cells | Stimulation of Rac1 activity, membrane protrusion and cell motility |
[58] |
| Ezrin | In vitro, HEK293 cells, Hep2 cells | Induction of membrane ruffling and actin cytoskeleton reorganization |
[59] |
| IRIS | In vitro, cardiac specific GRK2 transgenic mice, cardiac specific GRK2 KO mice |
Reducing cardiac glucose uptake and insulin sensitivity |
[61] |
| Smad2 and Smad3 | In vitro, Human hepatocarcinoma cells, CHO cells |
Attenuation of TGFβ-mediated signaling by preventing Smad nuclear translocation |
[62] |
| α- and β-synuclein | In vitro, COS-1 cells | Inhibition of synuclein interactions with phospholipids and phospholipase D2 |
[64] |
| IκBα | In vitro, macrophages | TNFα-induced activation of NFκB in macrophages |
[66] |
| P38 | In vitro, HEK293 cells, 3T3L1- preadipocytes, macrophages from GRK2 heterozygous KO mice |
Inhibition of P38 MAPK activity | [67] |
| Atypical GPCR substrates | |||
| Smo | HEK393 cells, C3H10T1/2 cells, Shh- LIGHT cells, Zebrafish, Mice |
|
[14–17, 85] |
GRK2 domains
All GRKs share a common structure which consists of a highly conserved, centrally located catalytic domain of ~270 residues, flanked by a ~185 amino acids N-terminal domain and a C-terminal domain with variable length and structure. Both the N-terminal and the C-terminal domains are involved in the regulation of GRK targeting to the membrane and activity. The N-terminus harbors several regulatory motifs, including an RH domain (regulator of G protein signaling homology domain). The C-terminus of GRKs mediates their interactions with lipids and membrane proteins and thereby controls the subcellular distribution of these kinases [4, 10]. The N-terminus of GRK2 selectively interacts with Gαq. This interaction stimulates the weak GTPase activity of Gαq in a receptor-dependent manner; however, it inhibits the Gαq-mediated activation of phospholipase C (PLC) by sequestering Gα [18, 19]. This was one of the first examples of a phosphorylation-independent role for GRK2 in the regulation of GPCR signaling. The C-terminus of GRK2 is relatively long as compared with other GRKs. It contains a pleckstrin homology domain (PH), which interacts with phosphatidylinositol 4,5-biphosphate (PIP2) and free Gβγ subunits. These interactions mediate the agonist-dependent translocation of GRK2 to the plasma membrane, a process that results in enhanced phosphorylation of activated GPCRs. GRK2 and GRK3 are the only isoforms that interact with the released Gβγ subunit and translocate to the membrane upon agonist stimulation. The crystal structures of GRK2 in complex with either Gαq or Gβγ have been solved, shedding light on the different functions exerted by GRK2 regulatory domains and their intra- and inter-molecular interactions [20, 21].
GRK2 regulates GPCR internalization and trafficking
Endocytosis of 7TMRs leads to their removal from the plasma membrane, after which they are sorted to either lysosomes for degradation or to recycling endosomes for subsequent reinsertion into the plasma membrane. GRK-dependent recruitment of β-arrestins to the phosphorylated receptor is crucial for clathrin-dependent endocytosis, which is the predominant internalization pathway described for the majority of GPCRs. Interestingly, some receptors are regulated by a single GRK, whereas others appear to be regulated by many GRKs. In fact, recent reports suggest that distinct phosphorylation patterns by different GRKs establish a signaling barcode that determines β-arrestin functionality [22, 23].
GRK2 also utilizes β-arrestin-independent mechanisms to mediate receptor internalization. Recent studies have shown that GRK2 associates with a growing number of protein partners with known roles in receptor internalization and signaling (Table 1). In some cases, this interaction interferes with normal receptor down-regulation by blocking the kinase activity of GRK2 (e.g. GRK2 association with RKIP, an inhibitor of Raf kinase [24]). However, in other reported cases, the interaction between GRK2 and its protein partner directly affects receptor endocytosis or internalization. For instance, GRK2 binds phosphoinositide 3-kinase (PI3K) and recruits it to the cell surface upon ligand stimulation of the β-adrenergic receptor (βAR, [25]). This interaction has been shown to be important for βAR endocytosis, most likely via enhanced recruitment of AP2 to the receptor [26]. Blocking the interaction of GRK2 with PI3K using a dominant negative version of the GRK2-binding domain for PI3K improves contractile function during heart failure by reversing βAR desensitization abnormalities and restoring βAR signaling [27]. Moreover, the C-terminus of GRK2 directly binds clathrin, an interaction that facilitates certain receptor internalization, resulting in the co-localization of the receptor and GRK2 in endosomes [28, 29]. It has been argued that the interaction between GRK2 and clathrin could be involved in β-arrestin-independent but dynamin-dependent internalization [29]. This notion supports previous observations showing that GRK2 and GRK3 are more efficient than GRK5 and GRK6 in triggering clathrin-mediated endocytosis [30–32]. Other GRK2 binding proteins are the multi-domain proteins GRK Interactor-1 and 2 (GIT-1 and GIT-2) that have been implicated in the trafficking of internalized receptors. GRK2 facilitates GIT-1 and GIT-2 binding to the membrane, where they inhibit the agonist-promoted clathrin-mediated endocytosis of several receptors, attenuating downstream signaling and leading to the accumulation of phosphorylated inactive receptors at the plasma membrane [33–35]. Altogether, GRK2 plays an active role in receptor internalization, a role that extends well beyond just the recruitment of β-arrestins.
Phosphorylation-independent roles of GRK2 in GPCR signaling
The direct interactions of GRK2 with various signaling molecules can affect the levels of downstream activity of certain receptors or initiate alternative signal transduction cascades independent of receptor phosphorylation. A prime example of such interaction is the one between GRK2 and Gβγ. This interaction does not only stimulate a transient translocation of GRK2 to the membrane [36, 37], but also leads to the desensitization of the G protein-coupled inwardly-rectifying potassium channel (GIRK) downstream of adenosine type 1 receptor (A1R) and µ-opioid receptor (µOR) via the direct association of Gβγ with GIRK channels - a process that occurs independent of GRK2 kinase activity [38]. Similarly, the RH domain of GRK2 can sequester Gαq and interfere with Gαq–coupled receptor signaling. In striatal neurons, Gαq binding was reported to desensitize the metabotropic glutamate receptor 5 (mGluR5) and target it for internalization in a phosphorylation-independent manner [39]. In 3T3-L1 adipocytes, Gαq scavenging by GRK2 inhibits insulin-induced glucose transport [40] and mediates insulin resistance following chronic treatment with endothelin-1 [41]. A very recent study has suggested that GRK2-mediated desensitization of the Gαs-coupled histamine H2 receptor (H2R) in cells requires the RH domain of GRK2 rather than its kinase activity [42]. However, whether the mechanism involves sequestering of Gαs has not been determined. In another study, overexpressed GRK2 was reported to interact with RalA GTPase and to interfere with RalA interaction with the lysophosphatidic acid (LPA1) receptor, promoting a phosphorylation-independent desensitization of the LPA1 receptor in HEK293 cells [43]. It will be interesting to investigate the physiological relevance of this reported interaction.
A role for GRK2 in epithelial and immune cell migration is also evident. This role involves both phosphorylation-dependent and -independent control of GPCRs expressed in these tissues and has a direct impact on the development of inflammatory diseases. Hence, GRK2-mediated desensitization of sphingosine-1-phosphate receptor (S1PR) has been recently reported to be important for lymphocyte migration into infected tissue [44]. Interestingly, elevated GRK2 levels in epithelial cells and fibroblasts facilitate their migration in response to fibronectin, through activation of S1PR but independent of receptor phosphorylation. At least part of the stimulatory effect of GRK2 on epithelial cell migration involves the dynamic GRK2/GIT-1interaction described above [34], which promotes the activation of the Rac/PAK/MEK/ERK signaling cascade [45, 46]. Nevertheless, excess GRK2 levels in endothelial cells of injured liver were shown to directly inhibit the activity of the signaling molecule Akt, reducing nitric oxide synthase (eNOS) phosphorylation and nitric oxide (NO) production and leading to portal hypertension [47]. This inhibition of Akt happens in addition to the classical role of GRK2 in the agonist-induced desensitization of the endothelial receptor type B (ETb) and attenuation of the downstream PI-3 kinase-Akt-eNOS pathway.
By establishing phosphorylation-independent interactions, GRK2 also serves as a negative regulator of immune response. A key indication of this role is the ability of GRK2 to regulate MAPK signaling via direct association with MEK (MAPK kinase) [48]. Increased levels of GRK2 inhibit ERK activation upon chemokine induction, whereas decreased GRK2 levels stimulate its activity. Similar inhibition was achieved with a catalytically inactive K220R mutant construct of GRK2 and was shown to take place downstream of G protein activation [48]. Notably, both the PH domain and the N-terminal domain of GRK2 contain binding sites for the scaffold protein, caveolin. The association with caveolin blocks the basal activity of GRK2 in cellular systems [49] and could potentially modulate GRK2 interactions with different MAPK and other signaling molecules. However, it is not clear whether the interaction between GRK2 and caveolin is functional beyond the regulation of GRK2 basal activity.
A novel interaction between GRK2 and EPAC (cyclic AMP (cAMP)-activated guanine nucleotide exchange protein) has been suggested to induce a signaling switch following conditions of inflammatory hyperalgesia (enhanced response to nociceptive stimuli, [50]). It has been shown in heterozygous mice that reduction of GRK2 levels specifically in neurons that express receptors for nociception (nociceptor) triggers a switch from protein kinase A (PKA)/cAMP- to MEK/ERK-dependent signaling through the activation of EPAC and the small GTPase, RAP1, which, in turn results in prolonged hyperalgesia [50]. In light of their previous reports showing that GRK2 levels are reduced under neuropathic pain [51, 52], Eijkelkamp et al speculated that GRK2 can serve as a risk factor for prolonged pain in conditions of chronic inflammation or nerve damage [50]. These examples illustrate that GRK2 can establish its own protein-protein interactions downstream of the receptor, initiating alternative signaling pathways independent of its kinase activity and affecting various cellular functions. Although many of these interactions were proven to be functional, in some cases the data consists largely of co-immunoprecipitation of overexpressed proteins. To achieve a reliable picture of the cellular responses regulated by GRK2, it will be important in future work to validate these reported interactions in a physiological context.
Regulation of non-receptor substrates
A growing body of evidence has shown that GRK2 is capable of phosphorylating non-receptor substrates (Table 1). GRK2 is a microtubule-associated kinase that directly phosphorylates tubulin following βAR stimulation [53–56], suggesting a functional link between GRK2 and the cytoskeleton. Accordingly, GRK2 levels can affect agonist-induced βAR internalization in a mechanism involving microtubule stability [57]. GRK2-mediated phosphorylation of the membrane-cytoskeleton linkers, radixin [58] and ezrin [59], provides another indication for this functional link to the cytoskeleton. Another important target of GRK2 kinase activity is the insulin receptor substrate 1 (IRIS). It has been reported that increased GRK2 levels mediate insulin resistance in myoblasts and adipocytes via a mechanism, which involves sequestration of Gαq and IRIS [41, 60]. Interestingly, it was later shown that GRK2 directly phosphorylates IRIS in cardiomyocytes, a process that negatively affects cardiac glucose uptake and insulin sensitivity following ischemic injury and ultimately leads to the development of heart failure [61]. These findings demonstrate that lowering GRK2 in myocytes after ischemic injury will contribute to restore cardiac metabolism and prevent the development of subsequent heart failure.
GRK2 also phosphorylates Smad2 and 3, the intracellular mediators of the activin/transforming growth factor beta (TGFβ) signaling pathway [62]. GRK2 expression levels are up-regulated upon activin/TGFβ stimulation, initiating a negative feedback loop which results in the phosphorylation of Smad and impaired activation and translocation of this protein into the nucleus. This regulation of Smad results in reduced expression of target genes, many of which are important for activin/TGFβ-mediated cell growth arrest and apoptosis in both normal and malignant liver cells. Furthermore, it has been reported that the TGFβ–mediated increase in GRK2 levels antagonizes the angiotensin II (AngII)-induced vascular smooth muscle cell proliferation and migration. Thus, GRK2 potentially mediates the cross talk between the activin/TGFβ and the AngII signaling cascades [63]. Other reported substrates are α- and β-synuclein [64] which have been linked to neurodegenerative disorders such as Parkinson’s and Alzheimer’s diseases [65].
In line with the complex role of GRK2 in the immune system, GRK2-mediated phosphorylation of IκBα promotes tumor necrosis factor α (TNFα)-induced activation of nuclear factor kappa B NFκB transcription factor [66], whereas phosphorylation of p38 MAPK blocks p38 activity and lead to reduced cytokines release [67], both in macrophages. The potential role of GRK2 in cell cycle progression has been further suggested by the observation that overexpression of GRK2 reduces cell proliferation in smooth muscle cells and thyroid cancer cells [68, 69]. In addition, Penela et al. demonstrated that phosphorylation of GRK2 by cyclin-dependent kinase 2 (CDK2) followed by GRK2 interaction with the cell cycle regulator Pin1 promotes down-regulation of GRK2 levels during G2/M checkpoint, a critical event for proper cell cycle progression [70]. However, whether the kinase activity of GRK2 is required for this effect on the cell cycle has yet to be determined. Overexpression of GRK2 has been recently reported to attenuate hepatocellular carcinoma cell (HCC) proliferation through inducing G2/M phase cell cycle arrest [71]. This growth arrest is accompanied by increased levels of p53 phosphorylation and cyclin B and was shown to be GRK2 kinase activity dependent. In HEK293 cells, both GRK2 and GRK2 K220R interact with Ptch1, an interaction that reduces the association between Ptch1 and cyclin B1, leading to increased cyclin B1-mediated cell proliferation [72]. Rescue experiments in zebrafish embryos revealed that the kinase independent regulation of GRK2 on Ptch1/cyclin B1 pathway is important for early development [72]. However, whether a physical interaction between these two proteins occurs in vivo remains to be to be elucidated. Collectively these results suggest that modulating GRK2 levels may offer a new therapeutic strategy to control proliferation and cell growth in various cancer cells.
Emerging role for GRK2 in dopamine receptor signaling
The neurotransmitter dopamine (DA) plays a central role in many physiological processes, such as motor output and reward, via its modulation of dopamine receptors–a family of GPCRs that are highly expressed in the brain. Numerous studies have demonstrated that GRK2 protein levels dynamically regulate the signaling and trafficking of multiple dopamine receptor subtypes [73–75]. For example, the co-expression of GRK2 with the dopamine type 1 receptor (D1R) in HEK293 cells results in enhanced receptor phosphorylation and agonist-induced desensitization [75, 76]. Similarly, the overexpression of GRK2 in the same expression system has also been shown to enhance both phosphorylation and agonist-induced internalization of the dopamine type 2 receptor (D2R), [73, 77]. Although the earlier study of D2R endocytosis presumed that this phenomenon was directly mediated by a phosphorylation-dependent mechanism, more recent investigations of D2R mutants suggest a much different story.
Namkung et al. demonstrated that GRK2/3 phosphorylation-null mutant D2 receptors undergo normal agonist-induced desensitization, internalization and recruitment of β-arrestin-2 in HEK293T cells (Figure 1, [77]). Interestingly, they found that GRK-mediated phosphorylation was required instead, for the post-endocytic trafficking of D2 receptors [77]. Subsequent work further revealed that the overexpression of GRK2 alone decreased the surface expression (via enhanced agonist-induced internalization) of both wild-type and a phosphorylation-null mutant D2Rs [78]. This phenomenon was independent of GRK2-mediated phosphorylation because the overexpression of GRK2 K220R also resulted in decreased D2R expression. Although these studies provide a new phosphorylation-independent role for GRK2 in the regulation of D2R trafficking, it will be important in future work to investigate this mechanism in a system that expresses more physiologically-relevant levels of GRK2, such as in primary neurons.
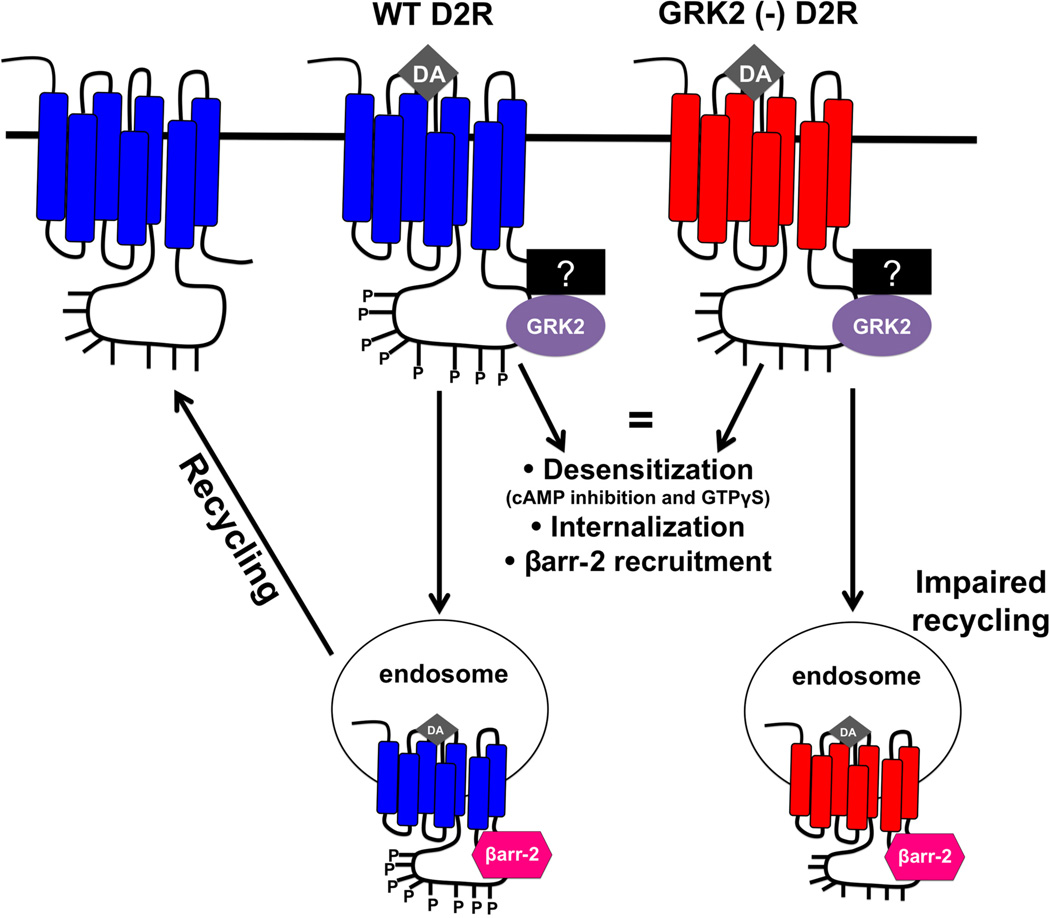
Figure 1. Phosphorylation-independent role for GRK2 in the regulation of D2R trafficking.
Namkung et al. identified eight serine and threonine residues located within the third intracellular loop of the rat D2 long receptor that are all GRK2 phosphorylation sites [77]. Mutation of all of these sites to either alanine or valine residues generates a D2 receptor that cannot be phosphorylated by GRK2 (GRK2 (−) D2R). Stimulation of wild-type (WT) or GRK2 (−) D2Rs with dopamine (DA) results in an equivalent amount of receptor desensitization (evidenced by the extent of inhibition of forskolin-induced cAMP levels and GTPγS binding), internalization and β-arrestin-2 (βarr-2) recruitment. However, GRK2 (−) D2Rs do not recycle as efficiently (50% reduction) compared to wild-type receptors [77]. GRK2 was found to be associated with both the wild-type and mutant D2Rs under both basal conditions and in the presence of agonist [78]. The association of GRK2 with either the wild-type or phosphorylation-null D2R may be direct or may be mediated indirectly by currently unknown interacting proteins (denoted as a question mark (?) in the figure).
GRK2-mediated regulation of dopamine receptor signaling and trafficking in vivo is a much less developed field. One interesting finding that potentially links GRK2 to the dopamine receptor system is the observation that chronic depletion of extracellular dopamine levels by administration of neurotoxins, such as 6-hydroxdopamine (6-OHDA) or 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) hydrochloride, promotes region-specific changes in the expression of GRK2 in the brain [79, 80]. Specifically, in a primate model of MPTP-lesioning of dopamine neurons, GRK2 protein levels are robustly increased in the caudal caudate nucleus [80]. This effect was reversed by increasing dopamine levels via chronic treatment with levodopa (L-DOPA), a dopamine precursor, indicating that dopamine levels inversely correlate with the levels of GRK2 in selected brain regions [80]. Interestingly, 6-OHDA lesioning in a rodent model resulted in the opposite effect on GRK2 levels (i.e. dopamine depletion reduced the levels of GRK2 in the caudal caudate nucleus [79]). Although further work is needed to explain these inconsistencies across animal models, it is clear that GRK2 levels can be profoundly modulated by manipulations of the dopamine system. Additional support for a role for GRK2 in the regulation of dopamine receptor signaling comes from the observation that the acute or chronic administration of the dopamine transporter inhibitor, cocaine, to rodents alters the levels of GRK2 in the striatum [81]. Importantly, it was found in this study that the acute effect of cocaine was completely prevented by co-administration of D1 or D2 receptor antagonists, indicating a central requirement for these receptors in this phenomenon.
Perhaps the most compelling evidence to date implicating GRK2 as a regulator of D1R or D2R signaling in vivo comes from the study of fragile X mental retardation protein (FMRP) deficient mice [82]. Wang et al. reported that the deletion of FMRP promotes hyperphosphorylation of D1R in both the frontal cortex and striatum, which results in impaired receptor-mediated signaling and numerous behavioral phenotypes consistent with a dysfunctional dopamine system [82]. Intriguingly, they report that FMRP interacts with GRK2 within the cytosol and that this described interaction prevents the translocation of GRK2 to the plasma membrane. Thus, they suggest that in FMRP deficient mice, D1R hyperphosphorylation and impaired signaling may be due to an enhanced localization of GRK2 at the plasma membrane [82]. It is tempting to speculate that the membrane-associated GRK2 may be regulating D1 receptor responsiveness in a phosphorylation-independent manner as observed for the D2R. Future work with phosphorylation-null D1 receptors and the various GRK2 mutants, as well as with novel GRK2 conditional knockout (KO) mice, will likely reveal previously unrecognized actions of this kinase in the brain.
GRK2 in development: novel roles in the signaling and trafficking of the atypical GPCR, Smo
Smo is an atypical GPCR that serves as the main transducer of the conserved hedgehog (Hh) signaling pathway, thereby regulating many aspects of embryonic development and growth control [83, 84]. Binding of Hh ligand to its transmembrane receptor Ptch1 relieves Ptch1 inhibition of Smo and allows Smo to signal to a complex of proteins that includes the transcription factors Ci (in Drosophila) / Gli (in vertebrates) [83]. Similar to classic GPCRs, phosphorylation of active Smo by GRK2 and recruitment of β-arrestin results in Smo internalization through clathrin-mediated endocytosis in transfected cells [85]. GRK2 has also been shown to promote signaling through phosphorylation of Smo C-terminus at multiple sites [14, 17] and enhancement of the direct interaction between Smo and β-arrestin [16]. In mammalian cells, the levels of Smo phosphorylation by GRK2 and the casein kinase, CK1α, are induced by the Sonic Hh (Shh) ligand and correlate with Smo activity levels [14]. Mutating GRK2 and CK1α phosphorylation sites in Smo C-terminus as well as overexpressing GRK2 K220R effectively prevents Smo signaling [14, 16, 17]. Interestingly, Gβγ is required for both GRK2 activation and Smo phosphorylation by GRK2, emphasizing the role of GRK2 in Smo signaling and the idea that Smo acts through mechanisms common to classical GPCRs [17, 84]. This notion is also consistent with previous GTPγS binding experiments in a Drosophila cell line that demonstrate Smo coupling to Gi [86]. β-arrestin-2 and GRK2/3 knockdown in zebrafish embryos recapitulates Hh loss of function phenotypes, including misshaped somite, impaired muscle development and neural patterning, providing further evidence for their association with Hh signaling in vertebrates [15, 17, 87]. Moreover, GRK2 KO mice display multiple developmental abnormalities apart from the well-described ones in cardiac development [88, 89]. At embryonic day 11.5 (E11.5), these mice display defects characteristic of impaired Hh signaling, including growth arrest and impaired neural tube patterning [17].
An essential step in vertebrate Hh signaling is Smo trafficking into the primary cilium by intra-flagellar transport (IFT) particles (reviewed in [90, 91]). Consistent with the positive role of GRK2 and β-arrestin in the Hh signaling pathway, β-arrestin was shown to promote Smo translocation into cilia by mediating its interaction with the kinesin motor protein Kif3A in mammalian cell culture [92]. Notably, Chen et al. reported that the interaction between Smo and β-arrestin is facilitated by GRK2 and CK1α phosphorylation of Smo, preferentially near or at the primary cilia [14]. They also found that effective phosphorylation of Smo depends on ciliary kinesin-II motor [14]. Recently we reported that cells with stable expression of both GRK2 and Smo assemble longer cilia, where higher levels of Smo can be accumulated, leading to induced signaling (Figure 2a). This phenomenon was shown to be kinase-dependent and not to involve GRK2 translocation into the cilia [15]. The observation that stable over-expression of Smo and GRK2 can affect cilia length is consistent with a previous suggestion that the Hh pathway by itself may influence the length of primary cilia [93] and that GRK2 regulates microtubule assembly [53]. It is possible that GRK2 phosphorylates tubulin in proximity to the cilia or other proteins that would enter the cilium and modulate microtubule organization. Alternatively, reports that a temporary down-regulation of GRK2 is required for proper cell cycle progression [70] and that depletion of β-arrestin results in uncontrolled cell proliferation and reduced cilia numbers [94] suggest an indirect mechanism involving cell cycle control.
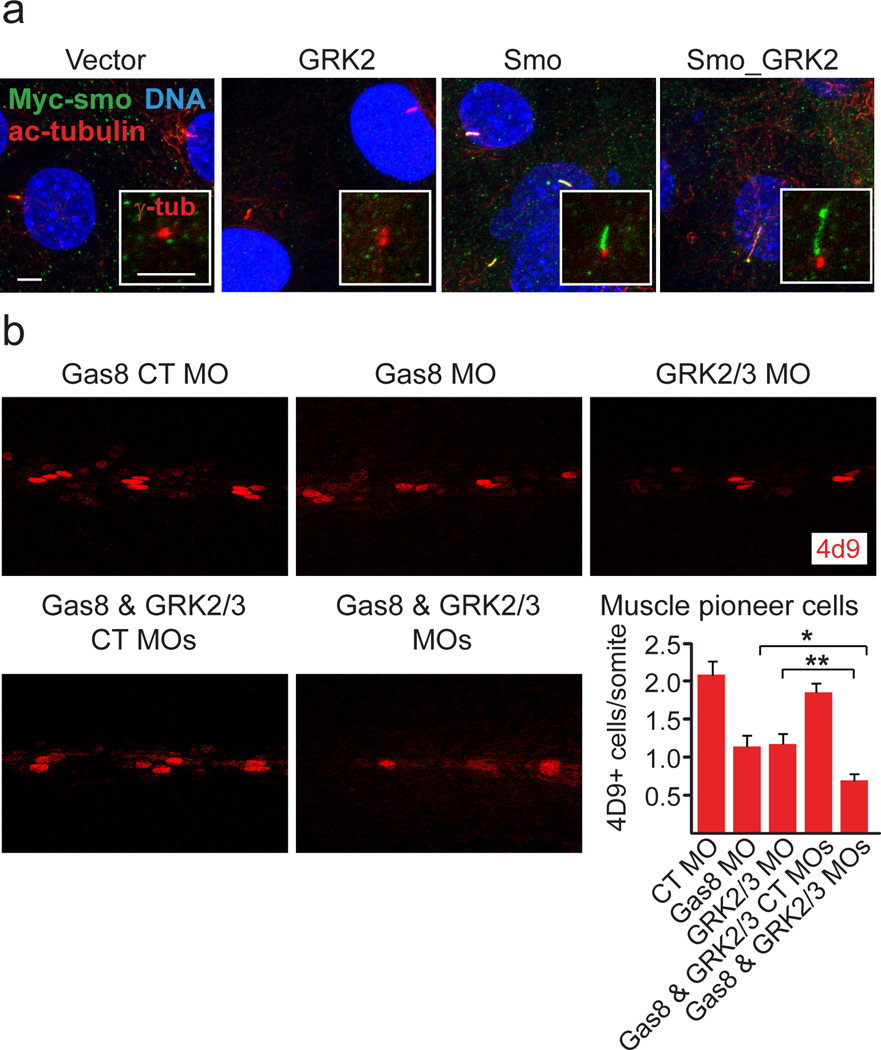
Figure 2. GRK2 effects on cilia elongation and zebrafish slow muscle development.
a. Stable overexpression of GRK2 and Smo leads to increased cilia length and accumulation of Smo within the cilia shaft. Cilia of C3H10T1/2 cells stably expressing GRK2, Myc tagged Smo (Smo), Myc tagged Smo and GRK2 (Smo_GRK2) or two empty vectors are labeled using antibodies against acetylated tubulin and the Myc tag. Insets show co-staining of γ-tubulin (basal body of the cilia, red) and anti Myc (green). Scales are 5 µm. b. A synergistic effect of zebrafish Gas8 and GRK2/3 knockdown on muscle pioneer cells. Analysis of engrailed+ muscle pioneer cells (4d9) in 27 hours post fertilization embryos injected with the indicated antisense morpholinos (MOs). Bar graphs represent numbers of engrailed+ cells ± SEM measured in several somites. Separate injections of Gas8 MO and GRK2/3 MO lead to a reduction in muscle pioneer cells as compared with Gas8 control (CT) MO. Co-injection of Gas8 and GRK2/3 MO leads to a more pronounced reduction in muscle pioneer cells as compared to separate injections of either Gas8 MO or GRK2/3 MO. *, P<0.01; **, P<0.05. Embryos are shown in lateral view, anterior to the left. Modified from [15].
Importantly, we have also discovered that GRK2 synergizes with a novel regulator of Smo, the growth arrest specific 8 (Gas8) [15]. Gas8 is a microtubule-associated protein that is normally localized to the basal body of the primary cilium [95, 96]. Although knocking down Gas8 by lentiviral shRNA is sufficient for attenuating Smo signaling and preventing Smo translocation into the primary cilium, overexpression of Gas8 results in enhanced signaling only in the presence of catalytically active GRK2 [15]. Similar to GRK2/3, knocking down Gas8 in zebrafish embryos using a translation-blocking morpholino leads to various developmental abnormalities and attenuated Hh signaling [15, 97]. Accordingly, Gas8 morphants display impaired slow muscle development characterized by moderate reduction in slow muscle and muscle pioneer cells [15]. Moreover, simultaneous knock down of GRK2/3 and Gas8 appears to have a synergistic effect on muscle development as muscle pioneer cells are even more decreased (Figure 2b). In contrast to our observations in mammalian cells, GRK2/3 does not seem to have a role in cilia formation or elongation in zebrafish embryos [15]. Based on these collective findings, we postulate a hypothetic model in Figure 3 for the roles of GRK2 in the activation of vertebrate Hh signaling pathway.
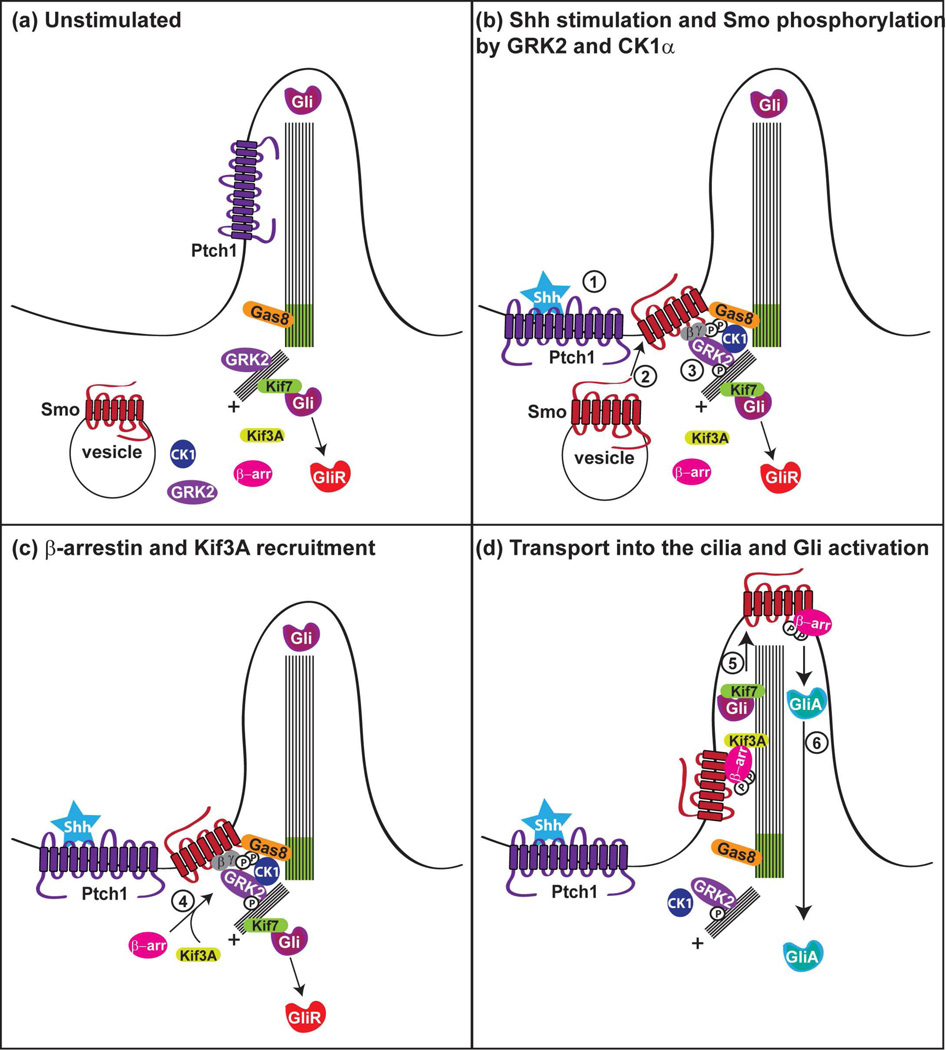
Figure 3. Proposed model for GRK2 regulation of vertebrate Hh signaling.
a. In the absence of ligand, Ptch1 is localized to the cilium while Smo remains in cytoplasmic vesicles (outside of the cilium). Gli transcription factors are held outside of the cilium in a complex of proteins. This complex likely includes the kinesin motor protein, Kif7, which prevents the entry of Gli into the cilium and promotes the processing of Gli repressors (GliR). b. In response to Shh ligand stimulation (step 1), Ptch1 leaves the cilium whereas Smo translocates to the ciliary membrane (step 2). The kinases CK1α and GRK2 phosphorylate Smo at the basal body of the cilium (step 3), a critical step for the recruitment of β-arrestin (β -arr) to the receptor and their consequent interaction with the kinesin motor protein, Kif3A (step 4 in c). GRK2 also cooperates with Gas8 to promote efficient trafficking and signaling of Smo (step 5 in d). Kif7 translocates to the cilium, thereby allowing Gli accumulation in the cilia. Additionally, the GRK2-mediated phosphorylation of tubulin is likely important for ciliary microtubule maintenance. Successful activation of Smo leads to the activation of Glis (GliA) at the cilia tip and their subsequent trafficking out of the cilium toward the nucleus (step 6 in d).
Further examination of the potential relationship between Gas8, GRK2 and other components of Smo signaling is an attractive avenue of future research. It would be interesting to know whether GRK2 and CK1α employ distinct mechanisms to regulate Smo, for example, by interacting with different effector molecules. It is possible that Gas8 and GRK2 cooperate to regulate β-arrestin recruitment to phosphorylated Smo. It is also possible that GRK2-mediated phosphorylation is necessary to relieve the interaction between Gas8 and Smo prior to Smo transport into the cilia, because Gas8 itself remains associated with the cilia basal body following stimulation.
Concluding remarks
GRK2 has long been considered a classic GPCR kinase in that its mechanism of action involves the direct phosphorylation of the receptor upon agonist stimulation– a process that ultimately leads to the attenuation of at least one arm of GPCR intracellular signaling. Receptor internalization via clathrin-mediated endocytosis was believed to occur by a single mechanism whereby the recruitment of β-arrestins to the receptor represented a central requirement. Subsequent evidence has revealed a much more complex role for GRK2 downstream of various GPCRs in several different cellular contexts. It is now evident that GRK2 is not only capable of phosphorylating GPCRs, but it can interact with a variety of endocytic proteins, ultimately leading to the regulation of receptor trafficking in a β-arrestin-independent manner. The GRK2 interactome has also been extended beyond endocytic proteins to include multiple signaling molecules. GRK2 modulation of various signaling cascades in a kinase-independent fashion has been shown to influence a wide range of cellular processes. Lastly, the discovery of non-receptor substrates for GRK2 has prompted a reconsideration of the ever complex role of this one kinase on various biological functions varying from cell motility and growth control, signaling during embryonic development to dopamine receptor signaling. Selectively targeting GRK2 may therefore represent a new therapeutic strategy to tackle human pathologies involving uncontrolled cell proliferation, deregulated signaling pathways or even dopamine-related neurological disorders.
Acknowledgements
We are grateful to Drs. Richard T. Premont and Melanie Philipp for critically reading this manuscript. The research in our lab is supported in part by NIH NS19576 and MH073853. T.D is a recipient of an NRSA grant from NIDA 1F32-DA030026. T.E was a Recipient of a postdoctoral fellowship from The Machiah Foundation, a supporting foundation of the Jewish Community Federation of San Francisco, the Peninsula, Marin & Sonoma Counties.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict: The authors declare no conflict of interest.
References
- 1.Takeda S, et al. Identification of G protein-coupled receptor genes from the human genome sequence. FEBS Lett. 2002;520:97–101. doi: 10.1016/s0014-5793(02)02775-8. [DOI] [PubMed] [Google Scholar]
- 2.Pierce KL, et al. Seven-transmembrane receptors. Nat Rev Mol Cell Biol. 2002;3:639–650. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- 3.Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by beta-arrestins. Science. 2005;308:512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- 4.Pitcher JA, et al. G protein-coupled receptor kinases. Annu Rev Biochem. 1998;67:653–692. doi: 10.1146/annurev.biochem.67.1.653. [DOI] [PubMed] [Google Scholar]
- 5.Shenoy SK, Lefkowitz RJ. Multifaceted roles of beta-arrestins in the regulation of seven-membrane-spanning receptor trafficking and signalling. Biochem J. 2003;375:503–515. doi: 10.1042/BJ20031076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferguson SS, et al. Role of beta-arrestin in mediating agonist-promoted G protein-coupled receptor internalization. Science. 1996;271:363–366. doi: 10.1126/science.271.5247.363. [DOI] [PubMed] [Google Scholar]
- 7.Goodman OB, Jr, et al. Beta-arrestin acts as a clathrin adaptor in endocytosis of the beta2-adrenergic receptor. Nature. 1996;383:447–450. doi: 10.1038/383447a0. [DOI] [PubMed] [Google Scholar]
- 8.Shenoy SK, Lefkowitz RJ. beta-Arrestin-mediated receptor trafficking and signal transduction. Trends Pharmacol Sci. 2011;32:521–533. doi: 10.1016/j.tips.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferguson SS. Phosphorylation-independent attenuation of GPCR signalling. Trends Pharmacol Sci. 2007;28:173–179. doi: 10.1016/j.tips.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 10.Penela P, et al. The complex G protein-coupled receptor kinase 2 (GRK2) interactome unveils new physiopathological targets. Br J Pharmacol. 2010;160:821–832. doi: 10.1111/j.1476-5381.2010.00727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Premont RT, Gainetdinov RR. Physiological roles of G protein-coupled receptor kinases and arrestins. Annu Rev Physiol. 2007;69:511–534. doi: 10.1146/annurev.physiol.69.022405.154731. [DOI] [PubMed] [Google Scholar]
- 12.Reiter E, Lefkowitz RJ. GRKs and beta-arrestins: roles in receptor silencing, trafficking and signaling. Trends Endocrinol Metab. 2006;17:159–165. doi: 10.1016/j.tem.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 13.Ribas C, et al. The G protein-coupled receptor kinase (GRK) interactome: role of GRKs in GPCR regulation and signaling. Biochim Biophys Acta. 2007;1768:913–922. doi: 10.1016/j.bbamem.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 14.Chen Y, et al. Sonic Hedgehog Dependent Phosphorylation by CK1alpha and GRK2 Is Required for Ciliary Accumulation and Activation of Smoothened. PLoS Biol. 2011;9 doi: 10.1371/journal.pbio.1001083. e1001083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evron T, et al. Growth Arrest Specific 8 (Gas8) and G Protein-coupled Receptor Kinase 2 (GRK2) Cooperate in the Control of Smoothened Signaling. J Biol Chem. 2011;286:27676–27686. doi: 10.1074/jbc.M111.234666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meloni AR, et al. Smoothened signal transduction is promoted by G protein-coupled receptor kinase 2. Mol Cell Biol. 2006;26:7550–7560. doi: 10.1128/MCB.00546-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Philipp M, et al. Smoothened signaling in vertebrates is facilitated by a G protein-coupled receptor kinase. Mol Biol Cell. 2008;19:5478–5489. doi: 10.1091/mbc.E08-05-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carman CV, et al. Selective regulation of Galpha(q/11) by an RGS domain in the G protein-coupled receptor kinase, GRK2. J Biol Chem. 1999;274:34483–34492. doi: 10.1074/jbc.274.48.34483. [DOI] [PubMed] [Google Scholar]
- 19.Sallese M, et al. Selective regulation of Gq signaling by G protein-coupled receptor kinase 2: direct interaction of kinase N terminus with activated galphaq. Mol Pharmacol. 2000;57:826–831. [PubMed] [Google Scholar]
- 20.Boughton AP, et al. Heterotrimeric G protein beta1gamma2 subunits change orientation upon complex formation with G protein-coupled receptor kinase 2 (GRK2) on a model membrane. Proc Natl Acad Sci U S A. 2011;108:E667–E673. doi: 10.1073/pnas.1108236108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tesmer VM, et al. Snapshot of activated G proteins at the membrane: the Galphaq-GRK2-Gbetagamma complex. Science. 2005;310:1686–1690. doi: 10.1126/science.1118890. [DOI] [PubMed] [Google Scholar]
- 22.Butcher AJ, et al. Differential G-protein-coupled receptor phosphorylation provides evidence for a signaling bar code. J Biol Chem. 2011;286:11506–11518. doi: 10.1074/jbc.M110.154526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nobles KN, et al. Distinct Phosphorylation Sites on the {beta}2-Adrenergic Receptor Establish a Barcode That Encodes Differential Functions of {beta}-Arrestin. Science signaling. 2011;4:ra51. doi: 10.1126/scisignal.2001707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lorenz K, et al. Protein kinase C switches the Raf-kinase inhibitor from Raf-1 to GRK-2. Nature. 2003;426:574–579. doi: 10.1038/nature02158. [DOI] [PubMed] [Google Scholar]
- 25.Naga Prasad SV, et al. Agonist-dependent recruitment of phosphoinositide 3-kinase to the membrane by beta-adrenergic receptor kinase 1. A role in receptor sequestration. J Biol Chem. 2001;276:18953–18959. doi: 10.1074/jbc.M102376200. [DOI] [PubMed] [Google Scholar]
- 26.Naga Prasad SV, et al. Phosphoinositide 3-kinase regulates beta2-adrenergic receptor endocytosis by AP-2 recruitment to the receptor/beta-arrestin complex. J Cell Biol. 2002;158:563–575. doi: 10.1083/jcb.200202113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perrino C, et al. Restoration of beta-adrenergic receptor signaling and contractile function in heart failure by disruption of the betaARK1/phosphoinositide 3-kinase complex. Circulation. 2005;111:2579–2587. doi: 10.1161/CIRCULATIONAHA.104.508796. [DOI] [PubMed] [Google Scholar]
- 28.Ruiz-Gomez A, Mayor F., Jr Beta-adrenergic receptor kinase (GRK2) colocalizes with beta-adrenergic receptors during agonist-induced receptor internalization. J Biol Chem. 1997;272:9601–9604. doi: 10.1074/jbc.272.15.9601. [DOI] [PubMed] [Google Scholar]
- 29.Shiina T, et al. Clathrin box in G protein-coupled receptor kinase 2. J Biol Chem. 2001;276:33019–33026. doi: 10.1074/jbc.M100140200. [DOI] [PubMed] [Google Scholar]
- 30.Gup Q, et al. Regulation of c3a receptor signaling in human mast cells by g protein coupled receptor kinases. PLoS ONE. 2011;6:e22559. doi: 10.1371/journal.pone.0022559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim J, et al. Functional antagonism of different G protein-coupled receptor kinases for beta-arrestin-mediated angiotensin II receptor signaling. Proc Natl Acad Sci U S A. 2005;102:1442–1447. doi: 10.1073/pnas.0409532102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ren XR, et al. Different G protein-coupled receptor kinases govern G protein and beta-arrestin-mediated signaling of V2 vasopressin receptor. Proc Natl Acad Sci U S A. 2005;102:1448–1453. doi: 10.1073/pnas.0409534102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Claing A, et al. Multiple endocytic pathways of G protein-coupled receptors delineated by GIT1 sensitivity. Proc Natl Acad Sci U S A. 2000;97:1119–1124. doi: 10.1073/pnas.97.3.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Premont RT, et al. beta2-Adrenergic receptor regulation by GIT1, a G protein-coupled receptor kinase-associated ADP ribosylation factor GTPase-activating protein. Proc Natl Acad Sci U S A. 1998;95:14082–14087. doi: 10.1073/pnas.95.24.14082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Premont RT, et al. The GIT family of ADP-ribosylation factor GTPase-activating proteins. Functional diversity of GIT2 through alternative splicing. J Biol Chem. 2000;275:22373–22380. doi: 10.1074/jbc.275.29.22373. [DOI] [PubMed] [Google Scholar]
- 36.Li J, et al. Agonist-induced formation of opioid receptor-G protein-coupled receptor kinase (GRK)-G beta gamma complex on membrane is required for GRK2 function in vivo. J Biol Chem. 2003;278:30219–30226. doi: 10.1074/jbc.M302385200. [DOI] [PubMed] [Google Scholar]
- 37.Pitcher JA, et al. Role of beta gamma subunits of G proteins in targeting the beta-adrenergic receptor kinase to membrane-bound receptors. Science. 1992;257:1264–1267. doi: 10.1126/science.1325672. [DOI] [PubMed] [Google Scholar]
- 38.Raveh A, et al. Nonenzymatic rapid control of GIRK channel function by a G protein-coupled receptor kinase. Cell. 2010;143:750–760. doi: 10.1016/j.cell.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 39.Ribeiro FM, et al. Phosphorylation-independent regulation of metabotropic glutamate receptor 5 desensitization and internalization by G protein-coupled receptor kinase 2 in neurons. J Biol Chem. 2009;284:23444–23453. doi: 10.1074/jbc.M109.000778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Usui I, et al. GRK2 is an endogenous protein inhibitor of the insulin signaling pathway for glucose transport stimulation. EMBO J. 2004;23:2821–2829. doi: 10.1038/sj.emboj.7600297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Usui I, et al. G protein-coupled receptor kinase 2 mediates endothelin-1-induced insulin resistance via the inhibition of both Galphaq/11 and insulin receptor substrate-1 pathways in 3T3-L1 adipocytes. Mol Endocrinol. 2005;19:2760–2768. doi: 10.1210/me.2004-0429. [DOI] [PubMed] [Google Scholar]
- 42.Fernandez N, et al. Roles of Phosphorylation-dependent and -independent Mechanisms in the Regulation of Histamine H2 Receptor by G Protein-coupled Receptor Kinase 2. J Biol Chem. 2011;286:28697–28706. doi: 10.1074/jbc.M111.269613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aziziyeh AI, et al. Dual regulation of lysophosphatidic acid (LPA1) receptor signalling by Ral and GRK. Cell Signal. 2009;21:1207–1217. doi: 10.1016/j.cellsig.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 44.Arnon TI, et al. GRK2-dependent S1PR1 desensitization is required for lymphocytes to overcome their attraction to blood. Science. 2011;333:1898–1903. doi: 10.1126/science.1208248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Penela P, et al. G protein-coupled receptor kinase 2 positively regulates epithelial cell migration. EMBO J. 2008;27:1206–1218. doi: 10.1038/emboj.2008.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Penela P, et al. New roles of G protein-coupled receptor kinase 2 (GRK2) in cell migration. Cell adhesion & migration. 2009;3:19–23. doi: 10.4161/cam.3.1.7149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu S, et al. A crucial role for GRK2 in regulation of endothelial cell nitric oxide synthase function in portal hypertension. Nature medicine. 2005;11:952–958. doi: 10.1038/nm1289. [DOI] [PubMed] [Google Scholar]
- 48.Jimenez-Sainz MC, et al. G protein-coupled receptor kinase 2 negatively regulates chemokine signaling at a level downstream from G protein subunits. Mol Biol Cell. 2006;17:25–31. doi: 10.1091/mbc.E05-05-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carman CV, et al. Regulation of G protein-coupled receptor kinases by caveolin. J Biol Chem. 1999;274:8858–8864. doi: 10.1074/jbc.274.13.8858. [DOI] [PubMed] [Google Scholar]
- 50.Eijkelkamp N, et al. Low nociceptor GRK2 prolongs prostaglandin E2 hyperalgesia via biased cAMP signaling to Epac/Rap1, protein kinase Cepsilon, and MEK/ERK. J Neurosci. 2010;30:12806–12815. doi: 10.1523/JNEUROSCI.3142-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eijkelkamp N, et al. GRK2: a novel cell-specific regulator of severity and duration of inflammatory pain. J Neurosci. 2010;30:2138–2149. doi: 10.1523/JNEUROSCI.5752-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kleibeuker W, et al. IL-1 beta signaling is required for mechanical allodynia induced by nerve injury and for the ensuing reduction in spinal cord neuronal GRK2. Brain, behavior, and immunity. 2008;22:200–208. doi: 10.1016/j.bbi.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 53.Carman CV, et al. Binding and phosphorylation of tubulin by G protein-coupled receptor kinases. J Biol Chem. 1998;273:20308–20316. doi: 10.1074/jbc.273.32.20308. [DOI] [PubMed] [Google Scholar]
- 54.Haga K, et al. GTP-binding-protein-coupled receptor kinase 2 (GRK2) binds and phosphorylates tubulin. European journal of biochemistry / FEBS. 1998;255:363–368. doi: 10.1046/j.1432-1327.1998.2550363.x. [DOI] [PubMed] [Google Scholar]
- 55.Pitcher JA, et al. The G protein-coupled receptor kinase 2 is a microtubule-associated protein kinase that phosphorylates tubulin. J Biol Chem. 1998;273:12316–12324. doi: 10.1074/jbc.273.20.12316. [DOI] [PubMed] [Google Scholar]
- 56.Yoshida N, et al. Identification of sites of phosphorylation by G-protein-coupled receptor kinase 2 in beta-tubulin. European journal of biochemistry / FEBS. 2003;270:1154–1163. doi: 10.1046/j.1432-1033.2003.03465.x. [DOI] [PubMed] [Google Scholar]
- 57.Vroon A, et al. Taxol normalizes the impaired agonist-induced beta2-adrenoceptor internalization in splenocytes from GRK2+/− mice. Eur J Pharmacol. 2007;560:9–16. doi: 10.1016/j.ejphar.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 58.Kahsai AW, et al. G protein-coupled receptor kinase 2 activates radixin, regulating membrane protrusion and motility in epithelial cells. Biochim Biophys Acta. 2010;1803:300–310. doi: 10.1016/j.bbamcr.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cant SH, Pitcher JA. G protein-coupled receptor kinase 2-mediated phosphorylation of ezrin is required for G protein-coupled receptor-dependent reorganization of the actin cytoskeleton. Mol Biol Cell. 2005;16:3088–3099. doi: 10.1091/mbc.E04-10-0877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Garcia-Guerra L, et al. G protein-coupled receptor kinase 2 plays a relevant role in insulin resistance and obesity. Diabetes. 2010;59:2407–2417. doi: 10.2337/db10-0771. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 61.Ciccarelli M, et al. G protein-coupled receptor kinase 2 activity impairs cardiac glucose uptake and promotes insulin resistance after myocardial ischemia. Circulation. 2011;123:1953–1962. doi: 10.1161/CIRCULATIONAHA.110.988642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ho J, et al. The G protein-coupled receptor kinase-2 is a TGFbeta-inducible antagonist of TGFbeta signal transduction. EMBO J. 2005;24:3247–3258. doi: 10.1038/sj.emboj.7600794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guo J, et al. TGFbeta-induced GRK2 expression attenuates AngII-regulated vascular smooth muscle cell proliferation and migration. Cell Signal. 2009;21:899–905. doi: 10.1016/j.cellsig.2009.01.037. [DOI] [PubMed] [Google Scholar]
- 64.Pronin AN, et al. Synucleins are a novel class of substrates for G protein-coupled receptor kinases. J Biol Chem. 2000;275:26515–26522. doi: 10.1074/jbc.M003542200. [DOI] [PubMed] [Google Scholar]
- 65.Surguchov A. Molecular and cellular biology of synucleins. International review of cell and molecular biology. 2008;270:225–317. doi: 10.1016/S1937-6448(08)01406-8. [DOI] [PubMed] [Google Scholar]
- 66.Patial S, et al. G-protein-coupled-receptor kinases mediate TNFalpha-induced NFkappaB signalling via direct interaction with and phosphorylation of IkappaBalpha. Biochem J. 2010;425:169–178. doi: 10.1042/BJ20090908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Peregrin S, et al. Phosphorylation of p38 by GRK2 at the docking groove unveils a novel mechanism for inactivating p38MAPK. Curr Biol. 2006;16:2042–2047. doi: 10.1016/j.cub.2006.08.083. [DOI] [PubMed] [Google Scholar]
- 68.Metaye T, et al. Immunohistochemical detection, regulation and antiproliferative function of G-protein-coupled receptor kinase 2 in thyroid carcinomas. The Journal of endocrinology. 2008;198:101–110. doi: 10.1677/JOE-07-0562. [DOI] [PubMed] [Google Scholar]
- 69.Peppel K, et al. Overexpression of G protein-coupled receptor kinase-2 in smooth muscle cells attenuates mitogenic signaling via G protein-coupled and platelet-derived growth factor receptors. Circulation. 2000;102:793–799. doi: 10.1161/01.cir.102.7.793. [DOI] [PubMed] [Google Scholar]
- 70.Penela P, et al. G protein-coupled receptor kinase 2 (GRK2) modulation and cell cycle progression. Proc Natl Acad Sci U S A. 2010;107:1118–1123. doi: 10.1073/pnas.0905778107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wei Z, et al. Growth inhibition of human hepatocellular carcinoma cells by overexpression of G protein-coupled receptor kinase 2. J Cell Physiol. 2011 doi: 10.1002/jcp.22972. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jiang X, et al. Kinase activity-independent regulation of cyclin pathway by GRK2 is essential for zebrafish early development. Proc Natl Acad Sci U S A. 2009;106:10183–10188. doi: 10.1073/pnas.0812105106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ito K, et al. Sequestration of dopamine D2 receptors depends on coexpression of G-protein-coupled receptor kinases 2 or 5. European journal of biochemistry / FEBS. 1999;260:112–119. doi: 10.1046/j.1432-1327.1999.00125.x. [DOI] [PubMed] [Google Scholar]
- 74.Iwata K, et al. Dynamin and rab5 regulate GRK2-dependent internalization of dopamine D2 receptors. European journal of biochemistry / FEBS. 1999;263:596–602. doi: 10.1046/j.1432-1327.1999.00549.x. [DOI] [PubMed] [Google Scholar]
- 75.Tiberi M, et al. Differential regulation of dopamine D1A receptor responsiveness by various G protein-coupled receptor kinases. J Biol Chem. 1996;271:3771–3778. doi: 10.1074/jbc.271.7.3771. [DOI] [PubMed] [Google Scholar]
- 76.Sedaghat K, Tiberi M. Cytoplasmic tail of D1 dopaminergic receptor differentially regulates desensitization and phosphorylation by G protein-coupled receptor kinase 2 and 3. Cell Signal. 2011;23:180–192. doi: 10.1016/j.cellsig.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 77.Namkung Y, et al. G protein-coupled receptor kinase-mediated phosphorylation regulates post-endocytic trafficking of the D2 dopamine receptor. J Biol Chem. 2009;284:15038–15051. doi: 10.1074/jbc.M900388200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Namkung Y, et al. G protein-coupled receptor kinase-2 constitutively regulates D2 dopamine receptor expression and signaling independently of receptor phosphorylation. J Biol Chem. 2009;284:34103–34115. doi: 10.1074/jbc.M109.055707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ahmed MR, et al. Altered expression and subcellular distribution of GRK subtypes in the dopamine-depleted rat basal ganglia is not normalized by l-DOPA treatment. Journal of neurochemistry. 2008;104:1622–1636. doi: 10.1111/j.1471-4159.2007.05104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bezard E, et al. L-DOPA reverses the MPTP-induced elevation of the arrestin2 and GRK6 expression and enhanced ERK activation in monkey brain. Neurobiology of disease. 2005;18:323–335. doi: 10.1016/j.nbd.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 81.Schroeder JA, et al. Regulation of dynamin 2 and G protein-coupled receptor kinase 2 in rat nucleus accumbens during acute and repeated cocaine administration. Synapse. 2009;63:863–870. doi: 10.1002/syn.20669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang H, et al. FMRP acts as a key messenger for dopamine modulation in the forebrain. Neuron. 2008;59:634–647. doi: 10.1016/j.neuron.2008.06.027. [DOI] [PubMed] [Google Scholar]
- 83.Huangfu D, Anderson KV. Signaling from Smo to Ci/Gli: conservation and divergence of Hedgehog pathways from Drosophila to vertebrates. Development. 2006;133:3–14. doi: 10.1242/dev.02169. [DOI] [PubMed] [Google Scholar]
- 84.Philipp M, Caron MG. Hedgehog signaling: is Smo a G protein-coupled receptor? Curr Biol. 2009;19:R125–R127. doi: 10.1016/j.cub.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 85.Chen W, et al. Activity-dependent internalization of smoothened mediated by beta-arrestin 2 and GRK2. Science. 2004;306:2257–2260. doi: 10.1126/science.1104135. [DOI] [PubMed] [Google Scholar]
- 86.Riobo NA, et al. Activation of heterotrimeric G proteins by Smoothened. Proc Natl Acad Sci U S A. 2006;103:12607–12612. doi: 10.1073/pnas.0600880103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wilbanks AM, et al. Beta-arrestin 2 regulates zebrafish development through the hedgehog signaling pathway. Science. 2004;306:2264–2267. doi: 10.1126/science.1104193. [DOI] [PubMed] [Google Scholar]
- 88.Jaber M, et al. Essential role of beta-adrenergic receptor kinase 1 in cardiac development and function. Proc Natl Acad Sci U S A. 1996;93:12974–12979. doi: 10.1073/pnas.93.23.12974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Matkovich SJ, et al. Cardiac-specific ablation of G-protein receptor kinase 2 redefines its roles in heart development and beta-adrenergic signaling. Circ Res. 2006;99:996–1003. doi: 10.1161/01.RES.0000247932.71270.2c. [DOI] [PubMed] [Google Scholar]
- 90.Goetz SC, Anderson KV. The primary cilium: a signalling centre during vertebrate development. Nat Rev Genet. 2010;11:331–344. doi: 10.1038/nrg2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wilson CW, Stainier DY. Vertebrate Hedgehog signaling: cilia rule. BMC Biol. 2010;8:102. doi: 10.1186/1741-7007-8-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kovacs JJ, et al. {beta}-Arrestin-Mediated Localization of Smoothened to the Primary Cilium. Science. 2008;320:1777–1781. doi: 10.1126/science.1157983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kim HR, et al. Gli2a protein localization reveals a role for Iguana/DZIP1 in primary ciliogenesis and a dependence of Hedgehog signal transduction on primary cilia in the zebrafish. BMC Biol. 2010;8:65. doi: 10.1186/1741-7007-8-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Molla-Herman A, et al. Targeting of beta-arrestin2 to the centrosome and primary cilium: role in cell proliferation control. PLoS ONE. 2008;3:e3728. doi: 10.1371/journal.pone.0003728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bekker JM, et al. Direct interaction of Gas11 with microtubules: implications for the dynein regulatory complex. Cell Motil Cytoskeleton. 2007;64:461–473. doi: 10.1002/cm.20196. [DOI] [PubMed] [Google Scholar]
- 96.Colantonio JR, et al. Expanding the role of the dynein regulatory complex to non-axonemal functions: association of GAS11 with the Golgi apparatus. Traffic. 2006;7:538–548. doi: 10.1111/j.1600-0854.2006.00411.x. [DOI] [PubMed] [Google Scholar]
- 97.Colantonio JR, et al. The dynein regulatory complex is required for ciliary motility and otolith biogenesis in the inner ear. Nature. 2009;457:205–209. doi: 10.1038/nature07520. [DOI] [PMC free article] [PubMed] [Google Scholar]