Abstract
Detection of Toxoplasma gondii infection with sensitive and specific methods is a key step in the prevention and treatment of toxoplasmosis. Among the available diagnostic tests, serology is commonly used. Although serological tests give satisfactory results, the production of reliable reagents remains laborious and expensive. There is therefore a real need to acquire specific and effective recombinant antigens for the serodiagnosis of T. gondii infection. In this study, a multiepitope peptide was designed and successfully expressed in Escherichia coli, and then IgG and IgM enzyme-linked immunosorbent assays (ELISAs) were developed and evaluated. Our results showed that the new multiepitope antigen is one of the most promising recombinant antigens which could be used in routine screening of human toxoplasmosis.
INTRODUCTION
The coccidian protozoan Toxoplasma gondii is an obligate intracellular parasite of humans and other warm-blooded animals. Up to one-third of the human population in the world is chronically infected (29). Diagnosis of T. gondii infection is of great medical importance for humans, especially pregnant women and immunosuppressed patients. Primary infection of pregnant women is often associated with fetal infection, which can lead to abortion or severe neonatal malformations. In immunocompromised adults (e.g., AIDS patients), toxoplasmosis (acute or, most often, reactivation of chronic infection) frequently causes a life-threatening encephalitis (22). The development of simple, sensitive, and rapid methods for the detection and identification of T. gondii is crucial for diagnosis and epidemiological studies of the zoonotic disease toxoplasmosis.
In the past few decades, many diagnostic techniques have been applied for the detection of T. gondii in clinical samples, including the Sabin-Feldman dye test (25), enzyme-linked immunosorbent assays (ELISAs) (23), the direct agglutination test (4, 6), and PCR (2). Among the available diagnostic techniques, serological tests are commonly used and have the following advantages. First, the detection of specific immunoglobulin G (IgG) antibodies and the absence of the acute-phase markers IgM and IgA allow diagnosis of the chronic stage of infection or of past exposure to T. gondii. On the other hand, in spite of the difficulty of determining the time of acquisition, the detection of IgM and IgA could suggest active infection (26, 31). Moreover, studies on the value of specific IgE antibody detection for serological diagnosis of acute T. gondii infection have also been done, with promising results (8, 24).
At present, the detection of specific antibodies based on the recognition of crude Toxoplasma antigens requires mass production of the parasite either from peritoneal fluids of infected mice or from tissue cultures. The production of parasites of reliable and high quality remains laborious and expensive. In addition, the use of whole-tachyzoite antigens can result in false-positive reactions (9, 28). The use of an Escherichia coli recombinant antigen(s) would be greatly beneficial in improving standardization of the tests and reducing their production costs. Thus, recent advances in generating recombinant antigens of T. gondii for IgG and IgM serological tests have been made (10, 12, 13, 17, 19, 32). However, in contrast to the case for the current serological tests, none of these recombinant antigens has allowed detection of all serologically positive individuals. Although the use of two or several recombinant antigens could improve the sensitivity of these ELISAs, it would increase the difficulty of antigen preparation and the complexity of the antigen component and lower the specificity of the tests. It is imperative to generate specific and effective recombinant antigens for the serodiagnosis of T. gondii infection.
In this study, to identify immunodominant epitopes that might be serotype specific and useful for serodiagnosis of T. gondii infection, we analyzed the antigens SAG1, SAG2, SAG3, GRA5, GRA6, and P35 of T. gondii using the BioSun and DNAstar software. Two potential epitopes for each antigen with high predicted antigenicity and reactivity were chosen based on the parameters of hydrophilicity, accessibility, flexibility, secondary structure, and polarity. The 12 epitopes were expressed in E. coli and purified for identification using Western immunoblot analysis with a pool of T. gondii-positive human sera. Three recombinant epitopes (rEPs), cloned from SAG1 antigen (rSAG1_EP2), SAG2 antigen (rSAG2_EP1), and SAG3 antigen (rSAG3_EP2), could be strongly recognized by T. gondii-positive human sera but not by T. gondii-negative human sera. A recombinant multiepitope fusion peptide (rMEP) composed of these three epitopes was then cloned, purified, and tested with diverse groups of human sera. Here we assess the diagnostic value of this multiepitope-peptide-based detection of T. gondii-specific IgG and IgM during T. gondii infection and evaluate its potential application as a serological tool.
MATERIALS AND METHODS
Serum samples.
The Institutional Review Board of the Second Affiliated Hospital of Nanjing Medical University approved this study, and written informed consent was obtained from all subjects. All 150 sera used in this study were received from a routine toxoplasmosis screening by IgG ELISA and IgM ELISA (Shenzheng Haitai Co., Ltd., China) in our lab and were further analyzed with highly sensitive and referenced methods, i.e., IgG and IgM indirect immunofluorescence (IIF) and Toxo-ISAGA plus IgM/IgA tests (bioMérieux, China). None of the patients providing serum samples were human immunodeficiency virus positive. Serum samples were classified into three groups. Group A consisted of 32 human serum samples from patients in the acute phase of toxoplasmosis. The presence of specific IgM antibodies was measured with the IgM IIF test and Toxo-ISAGA plus IgM/IgA tests. All sera had positive IgG antibodies with the IgG IIF test and low avidity obtained with a commercial antibody avidity test (Vidas Toxo IgG Avidity; bioMérieux, China). Group B consisted of 76 human serum samples from patients with indicative infections acquired in the distant past (chronic toxoplasmosis). All of those sera had positive IgG antibodies with high avidity and an absence of specific IgM antibodies. Group C (the control group) included 42 human serum samples from seronegative individuals.
Prediction of immunodominant epitopes and construction of rEP expression plasmid.
The immunodominant epitopes of the antigens SAG1 (accession no. AY661791), SAG2 (accession no. FJ825705), SAG3 (accession no. L21720), P35 (accession no. AF310261), GRA5 (accession no. L06091), and GRA6 (accession no. L33814) of T. gondii were analyzed with the BioSun and DNAstar software. Two potential epitopes for each antigen with high predicted antigenicity and reactivity were chosen based on the parameters of hydrophilicity, accessibility, flexibility, secondary structure, and polarity. Twelve pairs of complementary single-stranded DNA oligonucleotides were synthesized (Invitrogen, Shanghai, China) according to the DNA sequences of the predicted epitopes. Two short oligonucleotide sequences containing NcoI and XhoI restriction enzymes sites were added to the 5′ and 3′ ends of each epitope oligonucleotide. The single-stranded DNA oligonucleotides were annealed to generate double-stranded oligonucleotides and then cloned into NcoI and XhoI (Promega, Shanghai, China)-digested plasmid pET-32c to create plasmid pET-epitope. The plasmids encoding recombinant epitopes were characterized by EcoRI digestion and the sequences of inserts confirmed by sequencing (Invitrogen, Shangha, China).
Expression and purification of rEPs.
E. coli BL21(DE3) cells containing recombinant plasmid pET-epitope from an overnight culture diluted 1:100 were grown in Luria-Bertani (LB) broth containing 100 mg/liter of ampicillin for 3 h at 37°C. Each recombinant epitope (rEP) was then induced by adding isopropyl-β-d-thiogalactopyranoside (IPTG) at a final concentration of 1 mmol/liter. The bacteria were harvested by centrifugation at 4°C after 4 h of induction. For purification of the rEPs, the cell pellet from 50 ml of culture was resuspended in 10 ml of lysis buffer (20 mmol/liter Tris-HCl [pH 7.0], 10 mmol/liter iminazole) and then sonicated in an ice bath using a Branson Sonifier 250 (Branson, Danbury, CT) on output setting factors (500 V, 5 s of bursting, 5 s of cooling, 50 × 2 cycles). The sample was centrifuged at 12,000 rpm for 30 min at 4°C. The supernatant was collected and transferred into a balanced column of Ni2+ HiTrap chelating HP (GE Healthcare, Shanghai, China). The column was washed 3 times with washing buffer (20 mmol/liter Tris-HCl [pH 7.0], 50 mmol/liter imidazole), and the recombinant protein was then eluted with elution buffer (20 mmol/liter Tris-HCl, 500 mmol/liter iminazole). The eluted proteins were desalted with a HiTrap desalting column (GE Healthcare, Shanghai, China) and transferred into phosphate-buffered saline (PBS, pH 7.2). The protein concentrations of recombinant epitopes were determined with a protein and nucleotide calculator (Bio-Rad).
SDS-PAGE and Western immunoblot analysis.
The purified rEPs were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on 10% acrylamide gels in the Mini-Protean system (Bio-Rad). After electrophoresis, the proteins were transferred to nitrocellulose membranes (Bio-Rad, Shanghai, China) for Western immunoblot analysis as described previously (20). Briefly, the membrane was incubated with a pool of T. gondii-positive human sera (1:200 dilution in PBS with 0.05% Tween 20) at 4°C overnight with mild shaking and then washed in washing buffer (Tris-buffered saline–Tween 20 [TBS-T] [20 mmol/liter Tris-HCl {pH 7.5}, 500 mmol/liter NaCl, and 0.05% Tween 20]) 4 times for 5 min each. After that, the membrane was incubated with anti-human IgG or IgM conjugated to horseradish peroxidase (HRP) (1:2,000 dilution) for 1 h at room temperature with mild shaking and then washed 4 times as mentioned above. The membrane was developed in 3,3′-diaminobenzidine (DAB)–H2O2 at a dark chamber for 10 min, and the reaction was stopped by washing with distilled water.
Construction, expression, purification, and Western immunoblot analysis of an MEP.
Three epitopes identified from SAG1, SAG2, and SAG3 (named SAG1_EP2, SAG2_EP1, and SAG3_EP2, respectively) were used to construct a multiepitope peptide (MEP). The linker (Gly4Ser)3 was used to connect these epitopes. A pair of complementary single-stranded DNA oligonucleotides were synthesized (Invitrogen, Shanghai, China) according to the DNA sequences of the MEP. The artificial synthesized MEP gene was then cloned into plasmid pET-32c to generate recombinant expression plasmid pET-MEP. The procedure for construction, expression, purification, and Western immunoblot analysis of the MEP was same as described above. The sequence of the recombinant plasmid pET-MEP was confirmed by sequencing (Invitrogen, Shanghai, China). The protein concentration of the recombinant MEP (rMEP) was determined as described above.
ELISA with rSAG1_EP2, rSAG2_EP1, rSAG3_EP2, and rMEP.
Each well of the microtiter plate was coated overnight at 4°C with 100 μl of the recombinant protein diluted in 0.05 M carbonate buffer (pH 9.6) at the optimal concentrations of 5 μg/ml for rSAG1_EP2, rSAG2_EP1, and rSAG3_EP2 and 2 μg/ml for rMEP. After being coated, the wells were washed five times with PBS–0.25% Tween 20 (PBS-T), blocked with 200 μ1 of TBS containing 5% bovine serum albumin (BSA), and incubated at 37°C for 2 h. The plates were then washed as described above, and 100 μl of test or control serum was applied to each well. To test for IgG, the sera were diluted 1:200 in blocking solution. In the case of IgM detection, the sera were diluted at the optimal 1:100 dilution in blocking solution. The plates were incubated for 2 h at room temperature and washed as described above. Horseradish peroxidase-conjugated anti-human IgG or IgM (H+L) (Perkin-Elmer, Shanghai, China) diluted 1:2,000 was used as the secondary antibody. After incubation for 1 h at room temperature and washing, color was developed by the addition of 100 μl per well of a substrate solution containing 3,3′,5,5′-tetramethylbenzidine (TMB) and H2O2. After 5 min of incubation in the dark at 37°C, the reaction was stopped by the addition of 50 μl of 1 N HCl to each well. The optical densities (ODs) were measured at 450 nm with an automatic enzyme-linked immunosorbent assay (ELISA) reader (Bio-Rad). ELISA results were determined for each serum in duplicate. At least two independent ELISAs were performed for each serum. The cutoff point was established as the mean value of reactivity (plus 3 standard deviations) of the negative controls.
RESULTS
Identification and characterization of immunodominant epitopes.
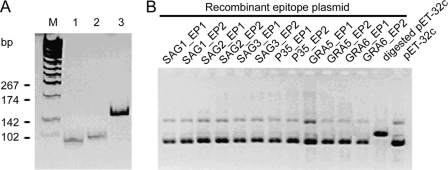
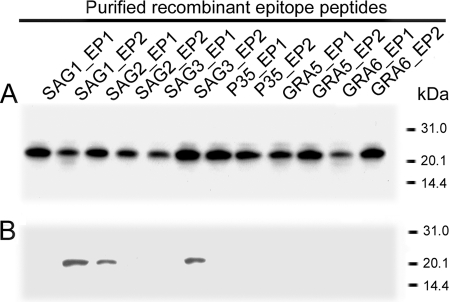
Through the analysis of hydrophilicity, accessibility, flexibility, secondary structure, and polarity for six genes of T. gondii, two potential epitopes for each antigen with high predicted antigenicity and reactivity were obtained (Table 1), even though the potential antigenic epitopes were widely distributed along the entire amino acid sequence of each gene. Twelve pairs of complementary single-stranded DNA oligonucleotides were synthesized according to the DNA sequences of the predicted epitopes. The single-stranded DNA oligonucleotides were annealed to generate double-stranded oligonucleotides (Fig. 1A) and then cloned into plasmid pET-32c to create plasmid pET-epitope. The recombinant pET-epitope plasmids were characterized by EcoRI digestion (Fig. 1B), and the sequence of each insert was confirmed by sequencing. pET-32 was constructed for cloning and high-level expression of protein peptides fused with the 109-amino-acid (aa) Trx-Tag thioredoxin protein. In the blank plasmid pET-32c, thioredoxin and poly-His-Tag were expressed under the control of the T7 promoter; the molecular size of the expression product was around 21 kDa. In the recombinant plasmid pET-epitope, each epitope fused with thioredoxin and His-Tag linker was expressed under the control of the T7 promoter. The predicted molecular size of the epitope was around 22 kDa. SDS-PAGE analysis showed that the epitope and thioredoxin were highly expressed in E. coli with the predicted molecular size after IPTG induction (data not shown). The majority of the epitope was expressed in a soluble form and could be purified easily with an Ni2+ HiTrap chelating HP column. With the optimized culture, when the OD at 600 nm (OD600) was 0.5 and grown for 4 h after induction with 1 mmol/liter IPTG, the quantity of recombinant protein reached 40% of the whole-cell lysate. After purification with a Ni-nitrilotriacetic acid (Ni-NTA) column, the purity of recombinant protein reached 90% (Fig. 2A). Western immunoblot analysis showed that three epitopes, named SAG1_EP2, SAG2_EP1, and SAG3_EP2, were recognized by a pool of T. gondii-positive human sera (Fig. 2B).
Table 1.
Sequence of each of the 12 predicted epitopes from six Toxoplasma gondii genes
| Gene | Predicted epitope | Sequence | Position |
|---|---|---|---|
| SAG1 | SAG1_EP1 | QGNASSDKGA | 239–248 |
| SAG1_EP2 | GLIGSFAACV | 309–318 | |
| SAG2 | SAG2_EP1 | SYDGTPEKPQ | 109–118 |
| SAG2_EP2 | GRNNDGSSAPTP | 133–144 | |
| SAG3 | SAG3_EP1 | KDKGDCERNK | 125–134 |
| SAG3_EP2 | QPGTEGESQA | 347–356 | |
| P35 | P35_ EP1 | GMPKPENPVR | 48–57 |
| P35_ EP2 | QPGTTTTTTS | 211–220 | |
| GRA5 | GRA5_ EP1 | FVGVAGSTRD | 21–30 |
| GRA5_ EP2 | EESKESATAE | 103–112 | |
| GRA6 | GRA6_ EP1 | GRRSPQEPSG | 175–184 |
| GRA6_ EP2 | EGGAEDDRRP | 210–219 |
Fig 1.
Construction of recombinant epitope plasmid. (A) The single-stranded DNA oligonucleotides (lanes 1 and 2) were annealed to the double-stranded DNA oligonucleotides (lane 3). Lane M, DNA molecular marker. (B) The double-stranded DNA oligonucleotides were cloned into NcoI- and XhoI-digested pET-32c and characterized by EcoRI digestion.
Fig 2.
Purification and identification of recombinant epitope peptides. (A) SDS-PAGE analysis of purified recombinant epitope peptides. (B) Western immunoblot analysis of recombinant epitope peptides with a pool of T. gondii-positive human sera.
Construction and characterization of a multiepitope thioredoxin fusion peptide.
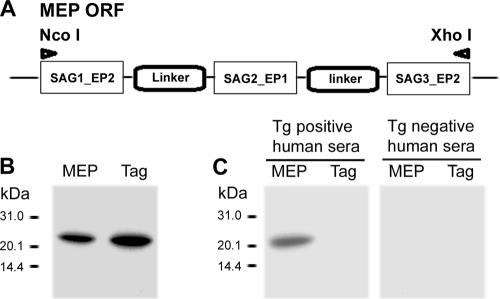
The artificially synthesized multiepitope gene was successfully cloned into plasmid pET-32c (Fig. 3A), The generated recombinant plasmid pET-MEP was then transformed into E. coli BL21 and expressed a soluble thioredoxin fusion protein of approximately 23 kDa, as expected, which could be readily purified by affinity to an Ni2+ HiTrap chelating HP column. Coomassie blue staining showed that the MEP fusion protein represents more than 95% of the stainable material (Fig. 3B). Immunoblot analysis demonstrated that this recombinant MEP and thioredoxin fusion protein was recognized by a pool of T. gondii-positive human sera, whereas no reactivity was detected using a pool of T. gondii-negative human sera (Fig. 3C). As a system background control, the tag protein of thioredoxin was probed with the same serum samples, and no reactivity was noted Taken together, these results indicate that the rMEP may be used as diagnostic antigen for serodiagnosis of T. gondii infection.
Fig 3.
Construction, purification, and identification of the multiepitope peptide (MEP). (A) Diagram of the MEP open reading frame (ORF). (B) SDS-PAGE analysis of the purified recombinant MEP and Tag (thioredoxin). (C) Western immunoblot analysis of recombinant MEP and Tag with T. gondii (Tg)-positive and -negative human sera.
Reactivity of human sera against rSAG1_EP2, rSAG2_EP1, rSAG3_EP2, and rMEP in IgG and IgM ELISA.
Four different IgG ELISAs and four IgM ELISAs were developed using rSAG1_EP2, rSAG2_EP1, rSAG3_EP2, or rMEP as a coating antigen to evaluate the potential of recombinant antigens for the serodiagnosis of toxoplasmosis. The sensitivities of the IgG ELISA determined with 32 sera from patients in the acute phase of toxoplasmosis (group A; IgM+ and IgG+ with low avidity) and 76 sera from patients with chronic infection (group B; IgM− and IgG+ with high avidity) were 87.5% (28 out of 32) and 97.4% (74 out of 76), respectively, for rMEP. The sensitivities of IgG ELISA determined with all serum samples (groups A and B) were 77.8% for rSAG1_EP2, 75.0% for rSAG2_EP1, 65.7% for rSAG3_EP2, and 94.4% for rMEP (Table 2). These results showed that the sensitivity of the rMEP-based IgG ELISA was significantly higher than the sensitivity of the single-recombinant-epitope-based IgG ELISA. In addition, to estimate the specificity of the IgG ELISA, serum samples from 42 seronegative individuals from group C (IgM− and IgG−) were tested. None of these samples was found to yield positivity, resulting in a specificity of 100% for all IgG ELISAs (data not shown). For IgM ELISAs, the sensitivities determined with group A samples was 68.6% (22 out of 32), 56.3% (18 out of 32), 46.8% (15 out of 32), and 96.9% (31 out of 32), respectively, for the rSAG1_EP2-, rSAG2_EP1-, rSAG3_EP2-, and rMEP-based IgM ELISAs (Table 2). None of the serum samples from group B and group C showed a positive result, and thus the specificity for IgM ELISAs was 100% (data not shown).
Table 2.
Reactivity of acute- and chronic-phase serum samples with rSAG1_EP2, rSAG2_EP1, SAG3_EP2, and rMEP in IgG and IgM ELISA
| ELISA | Antigen | No. (%) of positive serum samples from group(s): |
||
|---|---|---|---|---|
| A (n = 32) | B (n = 76) | A + B (n = 108) | ||
| IgG | rSAG1_EP2 | 18 (56.3) | 66 (86.8) | 84 (77.8) |
| rSAG2_EP1 | 19 (59.4) | 62 (81.6) | 81 (75.0) | |
| rSAG3_EP2 | 16 (50.0) | 55 (72.4) | 71 (65.7) | |
| rMEP | 28 (87.5) | 74 (97.4) | 102 (94.4) | |
| IgM | rSAG1_EP2 | 22 (68.6) | ||
| rSAG2_EP1 | 18 (56.3) | |||
| rSAG3_EP2 | 15 (46.8) | |||
| rMEP | 31 (96.9) | |||
DISCUSSION
Currently, many serological kits commercially available for diagnosis of T. gondii infection are based primarily on the use of whole extracts of tachyzoites. However, these extracts contain large amount of proteins and other macromolecules, and most of them can influence the results of the test. Therefore, pseudopositive concordance often happens when these kits are used. The use of purified recombinant proteins obtained via molecular biology is an alternative for the detection of serum antibodies and would allow better standardization of the immunoassays. Furthermore, the use of a combination of recombinant antigens may enhance the sensitivity of an antibody-based assay. Several previous studies have found that recombinant antigens improve the serological diagnosis of T. gondii infections (3, 5, 11, 14, 16, 30). Moreover, recombinant antigens have the potential to be used in the creation of new tests capable of differentiating recently acquired infections from those acquired in the more distant past (1, 15, 20, 27).
However, the exact composition and association of recombinant antigens to be used in immunoassays to detect Toxoplasma antibodies are still open questions. The identification of human immunodominant B-cell epitopes within the T. gondii antigens can help to find those antigens involved in the specific B-cell response, and they will be useful for immunoassays to detect anti-Toxoplasma antibodies (7, 18, 21). Using the B-cell epitopes of those antigens for the serodiagnosis of toxoplasmosis present several advantages, such as the composition of diagnostic antigen being precisely known, being able to use more than one identified B-cell epitope, and easy standardization of the method. Thus, multiepitope peptides may be an alternative source of recombinant antigens that are characteristic of the acute or chronic stages of the infection, serving as a tool for the serodiagnosis of human toxoplasmosis.
To our knowledge, the diagnostic utility of a multiepitope peptide for the serodiagnosis of human toxoplasmosis was examined for the first time in our present study. The data showed that the use of B-cell epitopes in the form of recombinant proteins provides the means of developing very sensitive and specific assays for the detection of antibodies to T. gondii in human sera. Three B-cell epitopes, from SAG1, SAG2, and SAG3, were identified using a pool of human toxoplasmosis-positive sera. We then used an efficient E. coli expression system developed in our laboratory for obtaining a new multiepitope antigen composed of these epitopes. The expression system used allows for the production of immunologically active recombinant antigen, which was used for IgG and IgM ELISA to study the usefulness of this antigen for the serological diagnosis of T. gondii infections in human sera. The sensitivities were 94.4% and 96.9% for the IgG ELISA and IgM ELISA, respectively, based on this recombinant multiepitope antigen, while the specificities for IgG ELISA and IgM ELISA were both 100%. In conclusion, our results indicate that the newly synthesized multiepitope antigen is one of the most promising recombinant antigens for the development of diagnostic kits for routine screening of toxoplasmosis. Further work is needed before an immunoassay with recombinant products will be reliably available for clinical purposes.
ACKNOWLEDGMENT
This work was supported by a grant from Nanjing Medical University (08NMUZ024).
Footnotes
Published ahead of print 4 January 2012
REFERENCES
- 1. Buffolano W, et al. 2005. Use of recombinant antigens for early postnatal diagnosis of congenital toxoplasmosis. J. Clin. Microbiol. 43:5916–5924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Burg JL, Grover CM, Pouletty P, Boothroyd JC. 1989. Direct and sensitive detection of a pathogenic protozoan, Toxoplasma gondii, by polymerase chain reaction. J. Clin. Microbiol. 27:1787–1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Coceres VM, et al. 2010. Evaluation of the antigenic value of recombinant Toxoplasma gondii HSP20 to detect specific immunoglobulin G antibodies in Toxoplasma infected humans. Exp. Parasitol. 126:263–266 [DOI] [PubMed] [Google Scholar]
- 4. Desmonts G, Remington JS. 1980. Direct agglutination test for diagnosis of Toxoplasma infection: method for increasing sensitivity and specificity. J. Clin. Microbiol. 11:562–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ferrandiz J, et al. 2004. Limited value of assays using detection of immunoglobulin G antibodies to the two recombinant dense granule antigens, GRA1 and GRA6 Nt of Toxoplasma gondii, for distinguishing between acute and chronic infections in pregnant women. Clin. Diagn. Lab. Immunol. 11:1016–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fulton JD. 1965. Micro-agglutination test for Toxoplasma antibodies. Immunology 9:491–495 [PMC free article] [PubMed] [Google Scholar]
- 7. Gao SD, et al. 2010. B cell epitopes within VP1 of type O foot-and-mouth disease virus for detection of viral antibodies. Virol. Sin. 25:18–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gross U, Keksel O, Darde ML. 1997. Value of detecting immunoglobulin E antibodies for the serological diagnosis of Toxoplasma gondii infection. Clin. Diagn. Lab. Immunol. 4:247–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hassl A, Muller WA, Spock H. 1991. An identical epitope in Pneumocystis carinii and Toxoplasma gondii causing serological cross reactions. Parasitol. Res. 77:351–352 [DOI] [PubMed] [Google Scholar]
- 10. Holec L, Gasior A, Brillowska-Dabrowska A, Kur J. 2008. Toxoplasma gondii: enzyme-linked immunosorbent assay using different fragments of recombinant microneme protein 1 (MIC1) for detection of immunoglobulin G antibodies. Exp. Parasitol. 119:1–6 [DOI] [PubMed] [Google Scholar]
- 11. Holec L, Hiszczynska-Sawicka E, Gasior A, Brillowska-Dabrowska A, Kur J. 2007. Use of MAG1 recombinant antigen for diagnosis of Toxoplasma gondii infection in humans. Clin. Vaccine Immunol. 14:220–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Holec-Gasior L, Kur J. 2010. Toxoplasma gondii: recombinant GRA5 antigen for detection of immunoglobulin G antibodies using enzyme-linked immunosorbent assay. Exp. Parasitol. 124:272–278 [DOI] [PubMed] [Google Scholar]
- 13. Huang X, et al. 2004. Rapid immunochromatographic test using recombinant SAG2 for detection of antibodies against Toxoplasma gondii in cats. J. Clin. Microbiol. 42:351–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jiang T, et al. 2008. Evaluation of a recombinant MIC3 based latex agglutination test for the rapid serodiagnosis of Toxoplasma gondii infection in swines. Vet. Parasitol. 158:51–56 [DOI] [PubMed] [Google Scholar]
- 15. Johnson AM, Roberts H, Tenter AM. 1992. Evaluation of a recombinant antigen ELISA for the diagnosis of acute toxoplasmosis and comparison with traditional antigen ELISAs. J. Med. Microbiol. 37:404–409 [DOI] [PubMed] [Google Scholar]
- 16. Lau YL, Fong MY. 2008. Toxoplasma gondii: serological characterization and immunogenicity of recombinant surface antigen 2 (SAG2) expressed in the yeast Pichia pastoris. Exp. Parasitol. 119:373–378 [DOI] [PubMed] [Google Scholar]
- 17. Lecordier L, et al. 2000. Enzyme-linked immunosorbent assays using the recombinant dense granule antigens GRA6 and GRA1 of Toxoplasma gondii for detection of immunoglobulin G antibodies. Clin. Diagn. Lab. Immunol. 7:607–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu X, et al. 2010. Identification of a novel linear B-cell epitope in the UL26 and UL26.5 proteins of duck enteritis virus. Virol. J. 7:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lu B, et al. 2006. Toxoplasma gondii: expression pattern and detection of infection using full-length recombinant P35 antigen. Exp. Parasitol. 113:83–90 [DOI] [PubMed] [Google Scholar]
- 20. Martin V, et al. 1998. Detection of human Toxoplasma-specific immunoglobulins A, M, and G with a recombinant Toxoplasma gondii rop2 protein. Clin. Diagn. Lab. Immunol. 5:627–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Montagnani F, De Paolis F, Beghetto E, Gargano N. 2010. Use of recombinant chimeric antigens for the serodiagnosis of Mycoplasma pneumoniae infection. Eur. J. Clin. Microbiol. Infect. Dis. 29:1377–1386 [DOI] [PubMed] [Google Scholar]
- 22. Montoya JG, Liesenfeld O. 2004. Toxoplasmosis. Lancet 363:1965–1976 [DOI] [PubMed] [Google Scholar]
- 23. Naot Y, Remington JS. 1980. An enzyme-linked immunosorbent assay for detection of IgM antibodies to Toxoplasma gondii: use for diagnosis of acute acquired toxoplasmosis. J. Infect. Dis. 142:757–766 [DOI] [PubMed] [Google Scholar]
- 24. Pinon JM, et al. 1990. Detection of specific immunoglobulin E in patients with toxoplasmosis. J. Clin. Microbiol. 28:1739–1743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sabin AB, Feldman HA. 1948. Dyes as microchemical indicators of a new immunity phenomenon affecting a protozoon parasite (Toxoplasma). Science 108:660–663 [DOI] [PubMed] [Google Scholar]
- 26. Stepick-Biek P, Thulliez P, Araujo FG, Remington JS. 1990. IgA antibodies for diagnosis of acute congenital and acquired toxoplasmosis. J. Infect. Dis. 162:270–273 [DOI] [PubMed] [Google Scholar]
- 27. Suzuki LA, Rocha RJ, Rossi CL. 2001. Evaluation of serological markers for the immunodiagnosis of acute acquired toxoplasmosis. J. Med. Microbiol. 50:62–70 [DOI] [PubMed] [Google Scholar]
- 28. Taylor DW, Evans CB, Aley SB, Barta JR, Danforth HD. 1990. Identification of an apically-located antigen that is conserved in sporozoan parasites. J. Protozool. 37:540–545 [DOI] [PubMed] [Google Scholar]
- 29. Tenter AM, Heckeroth AR, Weiss LM. 2000. Toxoplasma gondii: from animals to humans. Int. J. Parasitol. 30:1217–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tumurjav B, et al. 2010. Serodiagnosis of ovine toxoplasmosis in Mongolia by an enzyme-linked immunosorbent assay with recombinant Toxoplasma gondii matrix antigen 1. Jpn. J. Vet. Res. 58:111–119 [PubMed] [Google Scholar]
- 31. Wong SY, Remington JS. 1994. Toxoplasmosis in pregnancy. Clin. Infect. Dis. 18:853–861 [DOI] [PubMed] [Google Scholar]
- 32. Wu K, et al. 2009. Diagnosis of human toxoplasmosis by using the recombinant truncated surface antigen 1 of Toxoplasma gondii. Diagn. Microbiol. Infect. Dis. 64:261–266 [DOI] [PubMed] [Google Scholar]