Abstract
We examined the uptake and intracellular trafficking of F. tularensis Live Vaccine Strain (LVS) and LVS with disruptions of wbtDEF and wbtI genes essential for synthesis of the O antigen of lipopolysaccharide. Unlike parental bacteria, O-antigen-deficient LVS is efficiently killed by serum with intact complement but not by serum lacking terminal complement components. Opsonization of O-antigen-deficient LVS in serum lacking terminal complement components allows efficient uptake of these live bacteria by macrophages. In the presence of complement, whereas parental F. tularensis LVS is internalized within spacious pseudopod loops, mutant LVS is internalized within tightly juxtaposed multiple onion-like layers of pseudopodia. Without complement, both parental and mutant LVSs are internalized within spacious pseudopod loops. Thus, molecules other than O antigen are important in triggering dramatic pseudopod extensions and uptake by spacious pseudopod loops. Following uptake, both parental and mutant LVSs enter compartments that show limited staining for the lysosomal membrane glycoprotein CD63 and little fusion with secondary lysosomes. Subsequently, both parental and mutant LVSs lose their CD63 staining. Whereas the majority of parental LVS escapes into the cytosol by 6 h after uptake, mutant LVS shows a marked lag but does escape by 1 day after uptake. Despite the altered kinetics of phagosome escape, both mutant and parental strains grow to high levels within human macrophages. Thus, the O antigen plays a role in the morphology of uptake in the presence of complement and the kinetics of intracellular growth but is not essential for escape, survival, altered membrane trafficking, or intramacrophage growth.
INTRODUCTION
Francisella tularensis is a Gram-negative bacterium that causes a zoonotic infection, tularemia, in small animals (13, 23). Humans can contract the infection by contact with infected animals, insect bites, ingestion of contaminated food or water, or inhalation of aerosols. F. tularensis consists of 4 subspecies—F. tularensis subsp. tularensis (type A), F. tularensis subsp. holarctica (type B), F. tularensis subsp. mediasiatica, and F. tularensis subsp. novicida—that differ in their geographic distributions and their virulence in humans (13, 23). F. tularensis subsp. tularensis, found almost exclusively in North America, is highly virulent for humans. As few as 10 organisms introduced into the skin or 25 organisms inhaled can lead to a severe infection (30, 31). F. tularensis subsp. holarctica (found in North America and in Europe) and subsp. mediasiatica (found in Asia) are of lower virulence. F. novicida, found in North America and Australia, is virulent in mice and has occasionally been reported to cause a mild disease in humans (38). Because of its high infectivity and capacity to cause severe morbidity and mortality, F. tularensis subsp. tularensis is considered a potential agent of bioterrorism and is classified as a category A select agent.
We have previously shown that complement and complement receptors (10) play a dominant role in mediating the efficient uptake of F. tularensis type A and B strains. Additional ligand-receptor interactions have also been shown to play a role in promoting the adherence and uptake of the bacteria by macrophages, including mannose receptor (4, 34), Fc-gamma receptors, and pulmonary surfactant SP-A (4) and class A scavenger (24) receptors. The bacteria are internalized by the morphologically novel process of looping phagocytosis (10), and the bacteria subsequently enter phagosomes that exhibit arrested maturation, transiently acquiring staining for early endosomal antigens and late endosomal/lysosomal membrane glycoproteins (CD63, LAMP-1, and LAMP-2) but not acquiring lysosomal hydrolases or endocytosed markers of secondary lysosomes (12). The bacteria subsequently lyse their phagosomes and reside and multiply free within the host cell cytosol (12, 14). At later time points (more than 20 h), Checroun et al. have shown that in mouse macrophages, many of the bacteria reenter LAMP-1-positive, LC3-positive autophagic compartments with double membranes (6).
We have found that treatment of the bacteria with heat, formaldehyde, or proteases does not alter the morphology of uptake of the bacteria (10). However, treatment of the bacteria with the carbohydrate-oxidizing agent periodic acid abolishes looping phagocytosis and converts the uptake process entirely to one of conventional phagocytosis (10). These results suggested the possibility that bacterial carbohydrate material, such as the O-antigen polysaccharide or capsular material, might play a role in triggering the unusual uptake morphology of F. tularensis. The O antigen of F. tularensis is unusual in its structure and properties (37, 39), raising the possibility that it might also play a role in subsequent intracellular-trafficking events. To test these hypotheses, we examined the uptake and intracellular fate of O-antigen-deficient mutants of the Live Vaccine Strain (LVS) of F. tularensis.
MATERIALS AND METHODS
Bacteria.
F. tularensis subsp. holarctica Live Vaccine Strain (LVS) was obtained from the Centers for Disease Control and Prevention (Atlanta, GA). The bacteria were passaged, stored, and scraped from agar plates after overnight culture as described previously (11, 12). O-antigen-deficient LVS with a mutation in the sugar transamine/perosamine synthetase gene, wbtI (FTL_0601), designated LVS WbtIG191V, and the complementation vector pTZ819 were provided by T. Inzana at Virginia Polytechnic Institute and State University.
Preparation of an F. tularensis LVS mutant deficient in O antigen (ΔwbtDEF LVS).
Three consecutive genes, wbtD (FTL_0595), wbtE (FTL_0596), and wbtF (FTL_0597), on the O-antigen-biosynthetic gene clusters of F. tularensis were targeted to generate an LVS mutant that is disrupted in biosynthesis of a functional O antigen. Escherichia coli S17-1 (ATCC, Manassas, VA) was transformed with the suicide plasmid pSMT22-RT1, a kind gift from Richard Titball at Defense Science and Technology Laboratory, Porton Down, Salisbury, United Kingdom (36) (Fig. 1). An overnight culture of E. coli was diluted 50-fold in Luria-Bertani medium containing chloramphenicol (25 μg/ml) and grown at 37°C to an optical density at 600 nm of 0.3 to 0.4. F. tularensis LVS was cultured on GC chocolate agar plates (BD BBL, Sparks, MD), and colonies were scraped into Chamberlain medium. The E. coli and LVS suspensions were each pelleted by centrifugation and washed twice with Chamberlain medium. Conjugal transfer of the suicide plasmid from the E. coli donor to the LVS recipient was carried out by procedures similar to those of Golovliov et al. (15). E. coli (108 CFU) and LVS (109 CFU) were mixed in a final volume of 0.1 ml, spotted 25 μl at a time repeatedly onto the same confined area of a chocolate agar plate, and incubated at 25°C overnight. Following incubation, the bacteria were recovered from the plate, suspended in 0.5 ml normal saline, and selected on GC chocolate agar containing chloramphenicol (2.5 μg/ml) and polymyxin B (100 μg/ml), to which LVS is resistant, for counterselection of the E. coli donor strain. LVS colonies appeared 4 to 6 days after plating on the selective medium. The colonies were screened by PCR amplification of the chloramphenicol acetyltransferase (cat) gene and internal and flanking regions of the wbtDEF genes to confirm integration of the suicide plasmid into the bacterial chromosome via homologous recombination. A second recombination event to exclude the plasmid was performed by plating LVS transconjugants on GC chocolate agar containing 5% sucrose.
Fig 1.
Generation and confirmation of the LVS ΔwbtDEF mutant. (A) The 8.9-kb suicide plasmid pSMP22-RT1 contains the 3′ two-thirds of the wbtD gene with no stop codon, a frame-shifted wbtF gene missing both the start and stop codons, and a chloramphenicol resistance gene cassette (camR) between the mutated wbtD and wbtF genes. The locations of the primer pairs (F and R) used for amplification of the various regions on the plasmid vector or the chromosome and ampR, a gene cassette conferring resistance to ampicillin, are indicated. (B) Genetic organization of a portion (9 kb) of the F. tularensis LVS O-antigen gene cluster. H, HindIII. The location of the primer pair used to amplify the wbtE gene is indicated. (C) PCR analysis of the LVS ΔwbtDEF mutant (lane 1), the parental LVS (lane 2), and the suicide plasmid pSMP22-RT1 (lane 3). Lane M, 1-kb plus DNA ladder. FR, flanking region. (D) Southern hybridization analysis of the genomic DNA of the LVS ΔwbtDEF mutant (lane 1) and the parental LVS (lane 2). A 3-kb HindIII genomic DNA fragment was detected in the parental LVS with an oligonucleotide probe for wbtE but not in the ΔwbtDEF mutant (left gel), whereas a 2.5-kb HindIII genomic DNA fragment was detected in the ΔwbtDEF mutant with an oligonucleotide probe to the cat cassette but not in the ΔwbtDEF mutant (right gel). M, biotin-labeled 1-kb plus DNA ladder.
In the mutant prepared as described above, because the wbtD gene is missing a stop codon, it could potentially gain an additional 750 nucleotides (i.e., the promoter and coding sequence for the cat gene) before the reading sequence encounters the next stop codon. As a result, wild-type WbtD (363 amino acids) may be altered to a fusion protein of 613 amino acids with uncertain functionality.
PCR and Southern hybridization analysis.
Total genomic DNA was prepared from F. tularensis LVS parental and O-antigen-deficient strains for use in Southern hybridization and as PCR templates. PCRs were carried out at 1 cycle of 95°C for 1 min; 30 cycles of 95°C for 30 s, 50°C for 1 min, and 72°C for 90 s; and 1 cycle of 72°C for 10 min with Taq DNA polymerase according to instructions provided by the manufacturer (Fermentas, Glen Burnie, MD). As depicted in Fig. 1, primer pairs F1 and R1 were used to amplify a 594-bp internal region of the wbtD gene. Primer pairs F2 and R2 were used to amplify 1,230 bp of the truncated wbtD gene from its immediate upstream and downstream flanking regions on the suicide plasmid pSMT22-RT1. Primer pairs F3 and R3 were used to amplify a 739-bp internal region of the cat gene. Primer pairs F4 and R4 were used to amplify a 753-bp internal region of the wbtE gene. Primer pairs F5 and R5 were used to amplify a 956-bp internal region of the wbtF gene. Primer pairs F6 and R6 were used to amplify 1,052 bp of the mutated wbtF gene from its immediate upstream and downstream flanking regions on the suicide plasmid. For Southern hybridization analysis, biotin-labeled oligonucleotide probes for wbtE and cat, respectively, were hybridized to HindIII-digested genomic DNA, followed by streptavidin-horseradish peroxidase conjugate. The signals from hybridized nucleic acid fragments were detected by reaction with chemiluminescence substrate (Pierce, Rockford, IL) and exposure to X-ray film. Nucleotide sequences for the primers and probes are provided in Table 1.
Table 1.
Strains, plasmids, and oligonucleotides
| Strain, plasmid, or oligonucleotide | Descriptiona | Source or reference |
|---|---|---|
| E. coli | ||
| S17-1 | recA pro hsdR RP4-2-Tc::Mu-kan::Tn7 | ATCC |
| F. tularensis subsp. holarctica | ||
| LVS | Live vaccine strain | CDC |
| LVS-GFP | LVS carrying pFNLTP6 gro-gfp | 11 |
| LVS ΔwbtDEF | O-antigen-deficient mutant with deleted or defective wbtD, wbtE, and wbtF | This study |
| LVS ΔwbtDEF-GFP | LVS ΔwbtDEF carrying pFNLTP6 gro-gfp | This study |
| LVS WbtIG191V | O-antigen-deficient mutant with an amino acid substitution in WbtI | 19 |
| Plasmids | ||
| pSMP22-RT1 | Suicide plasmid used for allelic replacement of wbtD, wbtE, and wbtF on O-antigen gene cluster; Ampr Camr | 36 |
| pFNLTP6 gro-gfp | E. coli-Francisella shuttle vector with gfp driven by Francisella groE promoter; Ampr Kanr | 21 |
| pTZ819 | Complementation shuttle vector for wbtI; Ampr Kanr | 19 |
| Primers | ||
| F1 | 5′-ATGTAACAGGCTTAGGAAGTG-3′ | |
| F2 | 5′-ACGCTTGAGTTGCGCCTCCTG-3′ | |
| F3 | 5′-GTAAGAGGTTCCAACTTTCAC-3′ | |
| F4 | 5′-GAGTCTGGCTTGAGGTCTG-3′ | |
| F5 | 5′-GGCTTACGATAATGTTAAATTTCCTCATG-3′ | |
| F6 | 5′-AACAGTACTGCGATGAGTGG-3′ | |
| R1 | 5′-ATGATAAGCCAGAGAGACCATC-3′ | |
| R2 | 5′-CATTATGGTGAAAGTTGGAACC-3′ | |
| R3 | 5′-GCCACTCATCGCAGTACTG-3′ | |
| R4 | 5′-GTGACTAACAGCAATAATGATC-3′ | |
| R5 | 5′-ACCACTCAACAGCATGCTTTATGC-3′ | |
| R6 | 5′-GGCTTCACCTTCAACCCAAC-3′ | |
| Probes | ||
| Biotin-wbtE | 5′-TTGACGCCGATTATTAAGGCAAGTGAGACGGTTGG-3′ | |
| Biotin-camR | 5′-ATTATACGCAAGGCGACAAGGTGCTGATGCCGCTG-3′ |
Ampr, Camr, and Kanr indicate resistance to ampicillin, chloramphenicol, and kanamycin respectively.
Human serum, cells, and cell lines.
Human AB serum (AB serum) was prepared and handled in a manner that would preserve complement activity (17). Heat-inactivated AB serum (HI-ABS) was prepared by incubation of the serum at 56°C for 30 min. C7-deficient AB serum was obtained from Sigma Chemical Company (St. Louis, MO).
Peripheral blood mononuclear cells were isolated (12), adjusted to 3 × 106 cells/ml in RPMI 1640 with glutamine (Mediatech, Manassas, VA) and 20% autologous serum, and incubated for 5 days in sterile screw-cap Teflon wells (Savillex Corp., Minnetonka, MN) at 37°C, 5% CO2 prior to use. Teflon wells were chilled on ice, and the mononuclear cells were resuspended, washed, and allowed to adhere to coverslips or plastic tissue culture plates (12) or used in suspension for phagocytosis experiments studied by transmission electron microscopy (TEM). The UCLA Institutional Review Board approved the participation of healthy human blood donors in our research.
The human monocytic cell line THP-1 (ATCC TIB 202), was grown in RPMI 1640 medium supplemented with 2 mM glutamine and 10% heat-inactivated fetal bovine serum (HI-FBS). Prior to use in an infection experiment, the THP-1 cells were added (1.5 × 105 cells/cm2) to glass coverslips in 2-cm2 tissue culture wells or to plastic tissue culture plates and differentiated with phorbol 12-myristate 13-acetate (PMA) (100 nM) in RPMI 1640 with 10% HI-FBS for 3 days at 37°C in air containing 5% CO2.
Immunodetection of F. tularensis lipopolysaccharide (LPS) O antigen and proteins.
Whole-cell lysates of the parental and O-antigen-deficient F. tularensis LVS mutant strains (107 CFU) were boiled in SDS sample buffer, and the molecules were separated by 15% SDS-PAGE and transblotted onto a nitrocellulose membrane. The nitrocellulose membrane was probed with a 1:2,000 dilution of murine monoclonal antibody FB11 specific to F. tularensis LPS (Abcam, Cambridge, MA) and subsequently with horseradish peroxidase-conjugated goat anti-mouse IgG (Sigma). Signals were developed by reacting with chemiluminescence substrate (Pierce) and detected by exposure to X-ray film. The nitrocellulose membrane was treated with Restore Plus Western Blot Stripping Buffer (Pierce) and reprobed with rabbit polyclonal antibodies specific for F. tularensis catalase/peroxidase (KatG) or bacterioferritin (Bfr) (18) and subsequently with goat anti-rabbit IgG (Bio-Rad, Hercules, CA), and signals were developed by reacting with chemiluminescence substrate as described above.
Examination of the serum sensitivity of F. tularensis LVS and O-antigen-deficient mutants.
Bacteria scraped from agar plates were suspended in RPMI 1640 with 20 mM HEPES and 10% HI-FBS, C7-deficient serum, C7-deficient serum supplemented with purified complement factor C7, or AB serum. Samples were incubated at 37°C for 10 min, chilled on ice, and serially diluted with 0.9% saline containing 0.1% bovine serum albumin (BSA), and the numbers of surviving bacteria were determined by plating serial dilutions on GC chocolate agar and enumerating CFU. Purified C7 was purchased from Quidel (San Diego, CA) and used at a final concentration of 7 μg/ml.
Measurement of uptake of O-antigen-deficient F. tularensis LVS by human macrophages in HI-AB serum versus C7-deficient AB serum.
O-antigen-deficient LVS mutants were suspended in Dulbecco's modified Eagle's medium (DMEM) containing either 10% HI-ABS or 10% C7-deficient AB serum and incubated with monolayers of THP-1 cells on glass coverslips in 24-well plates for 1.5 h at 37°C at a multiplicity of infection (MOI) of 5:3 (bacteria/macrophages). The monolayers were washed 3 times with Hanks balanced salt solution (HBSS) to remove nonadherent bacteria, incubated for 5 min at 18°C with Alexa Fluor 633-labeled wheat germ agglutinin (WGA) (Invitrogen) to stain macrophage plasma membranes, washed, fixed with 4% paraformaldehyde in 0.1 M PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid)], pH 7.4, containing 6% sucrose, and permeabilized with 0.1% saponin in phosphate-buffered saline (PBS) containing 10 mM lysine, and F. tularensis bacteria were stained with a combination of rabbit antibody to KatG and rabbit antibody to Bfr (18). Both antibodies were used at a dilution of 1:1,000. Monolayers were washed and incubated with Texas Red-X-conjugated goat anti-rabbit antibody (Invitrogen), and host and bacterial nucleic acids were stained with DAPI (4′,6-diamidino-2-phenylindole) (10 μg/ml), mounted with Prolong Gold anti-fade mounting medium, and viewed by confocal fluorescence microscopy. Bacteria that were inside the contours of the WGA-stained macrophages were scored as being intracellular. Under these conditions, which employed incubation at a very low MOI and no centrifugation, extracellular bacteria were rare and represented less than 1% of the bacteria.
Measurement of growth of F. tularensis LVS and O-antigen mutants in monolayers of human macrophages.
Bacterial suspensions were preopsonized by incubation in 10% C7-deficient AB serum in Hanks balanced salt solution at 37°C for 10 min, chilled on ice, and added to monolayers of THP-1 macrophages in 6-well plates at an MOI of 1:1 (bacteria/macrophage). The bacteria were centrifuged onto the monolayers at 1,000 × g for 30 min at 4°C; incubated at 37°C for 60 min to allow uptake; washed three times with DMEM with 20 mM HEPES, pH 7.4; and incubated in DMEM containing 10% HI-FBS and 0.1 μg/ml of gentamicin. We had determined that none of the bacterial strains are able to grow extracellularly in this culture medium, i.e., DMEM containing 10% HI-FBS and 0.1 μg/ml gentamicin. We have observed, however, that use of higher concentrations of gentamicin (1 to 10 μg/ml) over the 1-day period of observation does kill intracellular bacteria. DMEM, rather than RPMI, was used as the culture medium in experiments that assessed intracellular growth of F. tularensis because we have found that DMEM is much less supportive of extracellular growth of F. tularensis than RPMI. At sequential times after infection, the culture supernatant was removed and the monolayers were washed with PBS and scraped with a Costar cell scraper into 1.5 ml of 0.25 M sucrose containing 20 mM HEPES, pH 7.4. The cell suspension was transferred to 1-cm-diameter glass tubes containing 20 glass beads, and the macrophages were disrupted by vortexing 10 times with a 2-s pulse each time. Examination of DAPI-stained samples revealed that bacteria were efficiently liberated from the macrophages by this process of scraping and vortexing. Bacterial CFU were determined by plating serial dilutions on GC chocolate agar. We have found that hypotonic lysis of macrophage monolayers by distilled water or by detergent does not lead to optimal recovery of bacterial CFU due to incomplete lysis of macrophages by distilled water and the impact of detergent on bacterial viability.
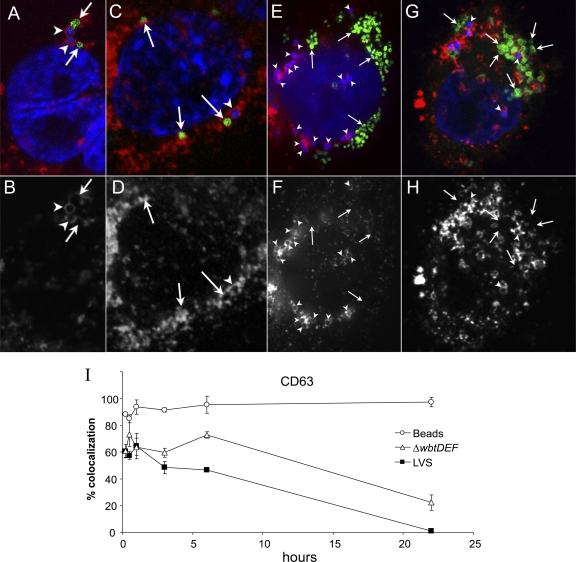
Assessment of colocalization of F. tularensis with CD63 by fluorescence microscopy.
F. tularensis LVS-green fluorescent protein (GFP) or ΔwbtDEF LVS-GFP and fluorescent blue latex beads (1-μm diameter) were preopsonized by incubation with human C7-deficient AB serum for 10 min at 37°C, chilled on ice, and adjusted to an MOI of 10:1 to 2:1 (bacteria/macrophages) for the bacteria and the latex beads (adjusted to a final concentration of 2.5 × 10−4% solids). MOIs of 10:1 were used when the monolayers were fixed within 6 h of infection, and MOIs of 2:1 were used when the monolayers were fixed at times later than 6 h. The bacteria and latex beads were centrifuged onto monolayers of PMA-differentiated THP-1 cells on glass coverslips in 24-well plates at 4°C and 1,000 × g for 30 min. The monolayers were incubated at 37°C for 20 min and washed, the medium was replaced with fresh culture medium, and the monolayers were incubated for an additional 15 min to 24 h and fixed in freshly prepared 4% paraformaldehyde in 0.075 M sodium phosphate, pH 7.4, for 30 min at room temperature. The monolayers were washed twice with PBS and permeabilized by incubation in 0.1% saponin in PBS containing 10 mM glycine for 30 min, and nonspecific antigenic sites were blocked by incubation in 5% goat serum in PBS containing 1% BSA. The monolayers were then incubated with a mouse anti-human CD63 monoclonal antibody (0.1 μg/ml; University of Iowa Hybridoma Bank), followed by a Texas Red-conjugated goat-anti-mouse antibody (Molecular Probes). Host and bacterial nucleic acids were stained with 2.5 μM DAPI (Sigma), and the coverslips were mounted on Prolong Gold Antifade mounting medium (Molecular Probes) and viewed by confocal and epifluorescence microscopy. Epifluorescence was examined with an Eclipse TE2000-S microscope equipped with an X-Cite 120 light source (Nikon), and images were acquired with a Spot RT-KE monochrome camera and SPOT software (Diagnostic Instruments, Sterling Heights, MI). Confocal fluorescence images were acquired with a Leica Confocal SP2 1P-FCS microscope using Leica software. Images were assembled with Adobe Photoshop CS software.
Assessment of colocalization of F. tularensis with Texas Red-dextran-labeled lysosomal compartments.
Monolayers of PMA-differentiated THP-1 cells were incubated overnight with 0.05 mg/ml Texas Red-dextran (10,000 Da; lysine fixable; Molecular Probes) to label lysosomal compartments. The macrophages were washed, and uptake of LVS-GFP or ΔwbtDEF LVS-GFP and fluorescent blue latex beads (1-μm diameter) was synchronized as described above. The monolayers were washed and incubated at 37°C for 15 min to 24 h and fixed in freshly prepared 4% paraformaldehyde in 0.1 M PIPES, pH 7.4, with 6% sucrose for 30 min at room temperature. The fixed monolayers were washed in PBS, stained with DAPI to label host and bacterial DNA, mounted in Prolong Gold Antifade mounting medium, and imaged as described above.
Transmission electron microscopy. (i) Analysis of uptake morphology.
To examine the ultrastructure of the uptake of parental or O-antigen-deficient F. tularensis by human monocyte-derived macrophages (MDM) in suspension or by monolayers of adherent THP-1 cells, we preopsonized the bacteria with 10% C7-deficient AB serum for 10 min at 37°C or left them unopsonized in 10% HI-ABS, chilled the bacteria on ice, and added them to a suspension of ice-cold human MDM or a plate of PMA-differentiated THP-1 cells at an MOI of 1,670:1 (bacteria/macrophages). The use of this relatively high MOI is standard for the examination of uptake at very early time points (≤5 min) by TEM (17). Bacterial-uptake profiles are rare in TEM thin sections if lower MOIs are used. The tube or plate was centrifuged sequentially at 215 × g and 860 × g (10 min each) at 4°C, a temperature at which phagocytosis does not occur. For the MDM in suspension, the supernatant was removed and the pelleted cells and bacteria were warmed to 37°C for 5 min prior to fixation and processing for TEM (17). For monolayers of THP-1 cells, the supernatant was replaced with prewarmed RPMI 1640 containing 10% HI-FBS, and the plates were incubated for 5 min at 37°C prior to fixation and processing.
We scored ultrastructural aspects of the interaction between bacteria and macrophages after 5 min of incubation at 37°C by the following criteria. We scored a bacterium as being engulfed within a “spacious, asymmetric pseudopod loop” (or “spacious looping phagocytosis”) if the bacterium was partly or wholly encircled by a single loosely fitting pseudopod extension. (“Loosely fitting” was defined as an apparent distance of greater than 100 nm between pseudopod and bacterium.) If an additional pseudopod extension was present on the other side of the bacterium, this extension was less than 1/3 the length of the main pseudopod extension. If the distance between the pseudopod and the bacterium was less than 100 nm, we scored the uptake as “tight looping phagocytosis.” Conversely, we scored a bacterium as being internalized within “symmetric ruffles” if it resided within loosely fitting, symmetrically arranged pseudopod pairs. If the pseudopod pairs were of disparate lengths, then the difference was by a factor of 3 or less. We scored bacteria as being internalized by “conventional phagocytosis” if they resided within symmetrical pseudopod extensions that were tightly adherent (within 100 nm) to the surface of the bacterium. We scored uptake as being by “overlapping pseudopod loops” if the uptake profile revealed more than one layer of overlapping pseudopodia embracing the bacterium in an onion-like array of redundant pseudopodia. In most cases, the symmetry or asymmetry of uptake and the spaciousness or tightness of vacuoles was immediately obvious (well within the above-stated criteria), so that formal measurement was rarely required.
(ii) Analysis of phagosome escape by electron microscopy.
We examined the time course of phagosome escape by F. tularensis parental LVS and O-antigen-deficient strains in THP-1 macrophages with and without opsonization. Opsonized bacteria were prepared with C7-deficient AB serum, and nonopsonized bacteria were prepared with HI-FBS as described above. Experiments using nonopsonized bacteria employed 10- to 20-fold-higher MOIs than experiments using opsonized bacteria to compensate for the greatly reduced efficiency of uptake in the absence of opsonization. Uptake of bacteria (either opsonized or nonopsonized) was synchronized by centrifuging the bacteria onto monolayers of PMA-differentiated macrophages at 1,000 × g for 30 min at 4°C. The monolayers were incubated at 37°C for 30 min to allow uptake, washed to remove extracellular bacteria, incubated in DMEM with 10% HI-FBS for 30 min to 21 h at 37°C, fixed, and processed for electron microscopy (12). Bacteria were scored as being “free” in the cytosol if less than 50% of the bacterial circumference was surrounded by a membrane bilayer. In most cases, the distinctions were unequivocal, with the “free” bacteria being totally free and the “bound” bacteria being enclosed within easily discernible membrane bilayers. (Only 10% of the bacteria encountered were intermediate between the two states, and most of these were either 90% free or 90% bound.) Specimens were viewed and photographed with a JEOL 100 CX transmission electron microscope at 80 kV using Kodak electron microscope film, and the images were scanned and assembled with Adobe Photoshop CS.
RESULTS
The F. tularensis LVS ΔwbtDEF mutant is deficient in immunoreactive O antigen.
wbtD, wbtE, and wbtF are consecutive genes located within the O-antigen-biosynthetic gene cluster spanning a 17-kb chromosomal region of F. tularensis (25). We used allelic exchange as described by Golovliov et al. (15) to introduce the suicide plasmid pSMP22-RT1 (36) into F. tularensis LVS to replace wild-type wbtDEF genes on the chromosome with defective wbtD, wbtF, and a chloramphenicol acetyltransferase expression cassette (camR) (Fig. 1A and B). In contrast to the parental LVS and the suicide plasmid control, PCR amplification of the LVS ΔwbtDEF mutant yielded no products for the wbtE gene and the respective plasmid flanking region of wbtD and wbtF genes, indicating that a double-crossover recombination event occurred in the allelic-exchange mutant (Fig. 1C). Southern hybridization analysis confirmed chromosomal integration of the wbtD-camR-wbtF cassette and the loss of the plasmid vector sequence in the LVS ΔwbtDEF mutant (Fig. 1D).
We analyzed the disruption of O-antigen biosynthesis in the LVS ΔwbtDEF mutant by immunoblotting using the FB11 mouse monoclonal antibody specific to F. tularensis LPS. We detected the characteristic ladder pattern of LPS, resulting from the incremental numbers of O-antigen repeating units present, for the parental LVS, but not for the ΔwbtDEF mutant (Fig. 2A, lanes 1 and 3, respectively). Similarly, the LPS ladder was absent from another LVS O-antigen mutant, WbtIG191V (Fig. 2A, lane 2), previously shown to have a glycine-to-valine substitution at amino acid residue 191 in WbtI, a sugar transaminase/perosamine synthetase required for synthesis of the sugar quinovosamine present in the O-antigen oligosaccharide repeating unit of F. tularensis (19). Complementation of the WbtIG191V mutant with an intact copy of the wbtI gene restored the characteristic LPS ladder pattern, though some of the highest-molecular-weight bands were fainter in the complemented strain than in the parental strain (Fig. 2A, lane 4). The absence of O-antigen staining for the WbtIG191V mutant and the ΔwbtDEF mutant (Fig. 2A, lanes 2 and 3, respectively) was not due to inadequate loading of sample onto the gel, since we detected similar intensities of catalase-peroxidase (KatG) and bacterioferritin (Bfr) staining for the parental LVS and the O-antigen-deficient mutant strains (Fig. 2A).
Fig 2.
Characterization of properties of F. tularensis LVS ΔwbtDEF and WbtIG191V mutant strains. (A) O-antigen biosynthesis is disrupted in the ΔwbtDEF and WbtIG191V mutants. Whole-cell lysates from the parental LVS (lane 1), the WbtIG191V (lane 2) and ΔwbtDEF (lane 3) mutant strains, and the WbtIG191V mutant strain complemented with an intact copy of the wbtI gene (lane 4) were subjected to immunoblot analysis using monoclonal antibody FB11 specific for F. tularensis LVS or polyclonal antibodies to F. tularensis proteins KatG and Bfr. While the parental LVS lane exhibits the characteristic ladder pattern of LPS immunoreactivity, no O-antigen-immunoreactive material was detected in the WbtIG191V or ΔwbtDEF lane despite comparable amounts of KatG and Bfr. The O-antigen ladder is restored in the WbtIG191V mutant strain by complementation with an intact copy of the wbtI gene (lane 4). The molecular masses of the protein standards are indicated. (B) O-antigen-deficient mutants are killed by serum with an intact complement (comp) pathway but not by heat-inactivated serum or C7-deficient AB serum. Parental LVS or O-antigen-deficient LVS (either WbtIG191V or ΔwbtDEF) was suspended in RPMI 1640 medium with or without either 10% human AB serum with intact complement, 10% heat-inactivated human AB serum, C7-deficient [C7(−)] AB serum, or C7-deficient serum supplemented with purified C7 (7 μg/ml) and incubated for 10 min at 37°C. The number of CFU surviving the 10-min incubation was determined by plating serial dilutions (the values shown are means and standard errors). Treatment of the WbtIG191V and the ΔwbtDEF O-antigen-deficient strains with AB serum or C7-deficient serum supplemented with purified C7 reduced CFU by more than 3 log units compared with HI-ABS or C7-deficient-serum treatment (P < 0.001; one-way ANOVA). In contrast, the parental LVS and wbtI-complemented strains were resistant to treatment with AB serum and C7-deficient serum supplemented with purified C7. (C and D) Uptake of O-antigen-deficient F. tularensis LVS is promoted by C7-deficient AB serum. O-antigen-deficient LVS mutants were suspended in DMEM containing either 10% HI-AB serum or 10% C7-deficient AB serum and incubated with monolayers of THP-1 cells for 90 min at 37°C at an MOI of 5:3 (bacteria/macrophages). The monolayers were washed 3 times to remove nonadherent bacteria. Plasma membranes were stained by incubation with Alexa Fluor 633-labeled WGA for 5 min at 18°C, and the monolayers were fixed with formaldehyde, permeabilized with saponin, and stained for F. tularensis with a red fluorescent antibody. (C) Numbers of intracellular bacteria per macrophage were enumerated by fluorescence microscopy. Opsonization of the bacteria with C7-deficient serum greatly enhances the uptake of all 4 strains (P < 0.01; one-way ANOVA). The data shown are means and standard errors. (D) A representative confocal image in the xz plane shows an intracellular WbtIG191V mutant (arrow, red) beneath the macrophage plasma membrane (arrowhead, pseudocolor green), and the macrophage nucleus (stained blue by DAPI). (E) O-antigen-deficient F. tularensis LVSs show a substantial delay in growth but ultimately are able to grow to high levels in monolayers of THP-1 cells, and complementation of the WbtIG191V mutant with an intact copy of the wbtI gene eliminates the lag and restores intracellular growth kinetics to those of the parental LVS. Opsonized or unopsonized parental F. tularensis LVS or O-antigen-deficient mutant LVSs were incubated with THP-1 cells in DMEM containing either C7-deficient AB serum or heat-inactivated AB serum, and uptake was synchronized by pelleting the bacteria onto the monolayers by centrifugation. The macrophage monolayers were washed to remove nonadherent bacteria and incubated at 37°C in DMEM containing 10% HI-FBS and gentamicin to prevent extracellular growth of the bacteria, and bacterial CFU in the monolayers were determined at sequential times thereafter. Under both the opsonized and unopsonized conditions, CFU for the WbtIG191V and ΔwbtDEF O-antigen-deficient strains were significantly lower than for the complemented and parental strains at the 6-h to 24-h time points (P < 0.01; two-way ANOVA with Bonferroni posttests). The data shown are means and standard errors. The experiments were performed at least twice with similar results.
O-antigen mutants of F. tularensis are sensitive to serum with intact complement.
We have found that efficient uptake of F. tularensis by human macrophages is mediated by complement receptors and is critically dependent on opsonization by serum with an intact complement pathway (10). However, whereas the parental F. tularensis LVS is resistant to complement lysis, the ΔwbtDEF and WbtIG191V O-antigen-deficient LVS mutants are highly sensitive to complement lysis; incubation of the mutants in 10% serum with an intact complement pathway reduces their viability by more than 3 log units (Fig. 2B). On the other hand, incubation of the bacteria in C7-deficient AB serum (which allows opsonization of the bacteria with complement factor C3 without the formation of a terminal membrane attack complex [1, 22]), does not cause killing of the LVS ΔwbtDEF and WbtIG191V O-antigen-deficient mutants (Fig. 2B). Results with C7-deficient serum supplemented with purified complement factor C7 were similar to those with fresh whole serum. Complementation of the WbtIG191V O-antigen mutant with an intact copy of the wbtI gene restores its resistance to serum. Whereas the viability of the WbtIG191V mutant is reduced by more than 3 log units by incubation in 10% AB serum (P < 0.001; one-way analysis of variance [ANOVA]), the complemented strain is serum resistant, and its viability after incubation in 10% AB serum is not significantly reduced compared with incubation in heat-inactivated serum (Fig. 2B).
Uptake and intracellular growth of O-antigen-deficient LVS is enhanced by incubation in C7-deficient AB serum.
Efficient uptake of F. tularensis by human macrophages requires serum with an intact complement pathway, and it is likely that complement is present in vivo at sites of F. tularensis infection and that it plays a role in the pathophysiology of tularemia. We have found that the O-antigen mutant LVS, like the parental strain, is not taken up efficiently by human macrophages in serum in which complement activity has been heat inactivated (Fig. 2C). However, opsonization of the bacteria in C7-deficient AB serum does support the uptake of the O-antigen-deficient LVS (Fig. 2C and D).
To examine the growth of the O-antigen-deficient LVSs in PMA-differentiated THP-1 cells, we opsonized the bacteria with C7-deficient AB serum and infected the THP-1 cells with the parental, mutant, or wbtI-complemented strain and measured CFU in the monolayer at sequential times after infection. Uptake of the bacteria was synchronized by centrifuging the bacteria onto the macrophage monolayers. Both the parental and the O-antigen mutant strains were able to grow significantly within the macrophages over the 1-day period of observation (Fig. 2E). Under the conditions of this assay (which includes gentamicin in the culture medium), neither the LVS nor the O-antigen-deficient bacteria are able to grow in the culture medium. The O-antigen deletion mutants show a marked lag in growth for the first 6 h compared with the parental LVS. In four independent experiments, whereas LVS on average increased 2.8- ± 0.6-fold (mean ± standard error) from 1 to 6 h, the ΔwbtDEF mutant strain slightly decreased over this period to 0.9 ± 0.2 times its level at 1 h, and the WbtIG191V mutant strain slightly decreased to 0.6 ± 0.2 times its level at 1 h. After 6 h, both mutant strains grew approximately 1 log unit within the macrophages but continued to lag behind the parental strain (Fig. 2E), which grew approximately 2.5 log units over the 1-day period of observation. Complementation of the WbtIG191V mutant with the wbtI gene restored its intramacrophage growth nearly to the level of the parental strain (Fig. 2E).
To determine whether the delayed growth of the O-antigen mutants might be due to injury of the bacteria by deposition of complement factor C3 or by events subsequent to complement receptor-mediated uptake, we examined growth of the bacteria in THP-1 macrophages following nonopsonic uptake. Bacterial adherence and uptake were promoted by centrifugation of the bacteria onto the monolayers. We continued to observe a marked lag in intramacrophage growth even for the nonopsonized O-antigen mutants (Fig. 2E), indicating that complement- and complement receptor-mediated uptake are not responsible for the lag in growth of the O-antigen mutants. The rates of intracellular growth of the parental strain and the wbtI-complemented strain were also similar in the presence and absence of serum opsonization.
O-antigen-deficient LVSs are internalized in tightly fitting asymmetric pseudopod loops and onion-like tightly adherent layers of overlapping pseudopodia in the presence of complement and in spacious pseudopod loops in the absence of complement.
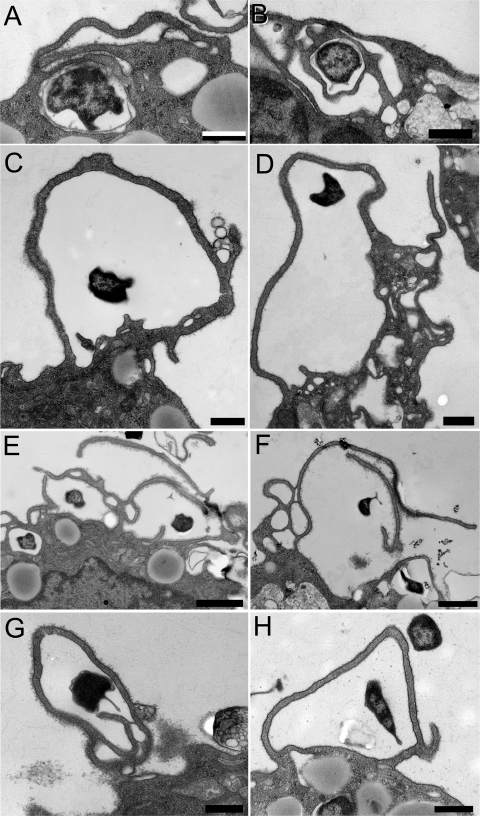
As reported previously (10), we found that uptake of the complement-opsonized parental LVS by human monocyte-derived macrophages is predominantly by spacious, asymmetric pseudopod loops (Fig. 3A and B); 83% of the profiles showed uptake by spacious, asymmetric pseudopod loops; 3% showed uptake by tight (i.e., less than 100 nm between pseudopod and bacterium), asymmetric pseudopod loops; and 11% showed uptake within symmetric, loosely fitting pseudopod extensions (“symmetric ruffles”). Uptake of LVS by symmetric, tightly adherent pseudopodia (conventional phagocytosis) was rare. In contrast, uptake of the ΔwbtDEF and WbtIG191V mutant LVS (preopsonized with complement using C7-deficient serum) was predominantly by relatively tight, asymmetric pseudopod loops (71% and 46%, respectively) (Fig. 3C) and by tightly juxtaposed, overlapping (onion-like) layers of pseudopodia (28% and 54%, respectively) (Fig. 3D to G). As with the parental LVS, uptake of the opsonized O-antigen-deficient LVS by conventional phagocytosis was very rare. For comparison, an uptake profile of E. coli DH5α by conventional phagocytosis is shown in Fig. 3H.
Fig 3.
Complement-opsonized O-antigen-deficient F. tularensis LVSs are internalized by asymmetric and often redundant tightly adherent pseudopod loops. The uptake of C7-deficient serum-opsonized parental LVS (A and B) and O-antigen-deficient LVSs (ΔwbtDEF [C, E, and G]; WbtIG191V [D and F]) by human monocyte-derived macrophages was examined by TEM. Whereas the parental LVS bacteria are engulfed within spacious, asymmetric pseudopod loops (A and B), the O-antigen-deficient mutants are internalized with more tightly adherent asymmetric loops (C and D) and within overlapping, onion-like arrays of adherent pseudopodia (E to G). (H) For comparison, uptake of E. coli by conventional phagocytosis is shown. The experiment was conducted three times with similar results. Bars, 1 μm.
In the absence of serum opsonization, O-antigen-deficient mutant LVSs are internalized within spacious pseudopod loops.
Whereas uptake of complement-opsonized O-antigen mutants (using C7-deficient serum) proceeds via tight loops and onion skin-like redundant loops (Fig. 4A and B), uptake of the O-antigen mutants in the absence of complement opsonization is mediated almost exclusively by spacious pseudopod loops (Fig. 4C and D). In the case of the parental LVS, however, uptake was via spacious, asymmetric pseudopod loops regardless of whether the bacteria were serum opsonized. Complementation of the WbtIG191V mutant with an intact copy of the wbtI gene restored its uptake morphology to that of the LVS, so that it was internalized by spacious pseudopod loops both in the presence (Fig. 4E and F) and in the absence (Fig. 4G and H) of complement opsonization.
Fig 4.
Uptake morphology of O-antigen-deficient mutant F. tularensis LVS is influenced by complement opsonization. (A and B) C7-deficient serum-opsonized WbtIG191V O-antigen-deficient LVS is internalized by human monocyte-derived macrophages via tightly adherent and often redundant pseudopod loops. (C and D) In contrast, in the absence of complement opsonization, the WbtIG191V O-antigen-deficient LVS is internalized in spacious, asymmetric pseudopod loops. (E to H) As in the case of the parental F. tularensis LVS, the complemented mutant is internalized via spacious, asymmetric pseudopod loops both in the presence (E and F) and in the absence (G and H) of serum opsonization. The experiment was conducted twice with similar results. Bars, 0.5 μm (A to D, G, and H) and 1.0 μm (E and F).
Phagosomes containing O-antigen mutant LVS exhibit arrested maturation and have limited interaction with the endosomal-lysosomal pathway.
We have previously shown that the parental LVS and virulent type A F. tularensis have restricted interaction with the endosomal-lysosomal pathway (12). Whereas inert particles, such as 1-μm latex beads, rapidly acquire intense staining for the late endosomal marker CD63 and maintain that level of staining for at least 1 day after uptake, phagosomes containing LVS and the O-antigen-deficient mutant ΔwbtDEF also acquire CD63 but have less intense and less uniform staining for CD63 than latex beads at all time points examined, from 15 min to 1 day (Fig. 5). For example, in the first 6 h after uptake, whereas latex beads show more than 90% colocalization with CD63, LVS and the ΔwbtDEF O-antigen mutant show 60 to 70% colocalization with CD63. However, the ΔwbtDEF O-antigen mutants show a trend toward more staining for CD63 at early times and a subsequent slower decline in CD63 staining than the parental LVS, suggesting a greater degree of phagosomal maturation (Fig. 5I). Similar results were obtained using the WbtIG191V mutant strain (data not shown). We obtained results for LAMP-1 similar to those obtained for CD63.
Fig 5.
Immunofluorescence evaluation of colocalization of CD63 and Texas Red-dextran with parental F. tularensis LVS, O-antigen-deficient LVS ΔwbtDEF, and latex beads. Uptake of fluorescent blue 1-μm latex beads (arrowheads) and either LVS-GFP (A, B, E, and F, arrows) or LVS ΔwbtDEF-GFP (C, D, G, and H, arrows) by human THP-1 cells was synchronized by centrifuging the bacteria and beads onto the monolayer at 4°C and warming the monolayers to 37°C for 30 min. The monolayers were washed to remove nonadherent beads and bacteria, incubated in fresh medium at 37°C, fixed at sequential times thereafter, permeabilized, and stained for CD63 with a Texas Red-conjugated antibody and for host and bacterial DNA with DAPI (blue fluorescence). (A, C, E, and G) Merged color images of the green-fluorescent bacteria, red CD63 fluorescence, and blue fluorescence of the beads and nuclei. (B, D, F, and H) CD63 red fluorescence shown in black and white. (A to D) At the 30-min time point, the LVS-GFP (A and B, arrows) and LVS ΔwbtDEF-GFP (C and D, arrows) are partially rimmed by CD63 staining, and the latex beads (arrowheads) are more uniformly rimmed with CD63 staining. (E to H) At 22 h, the LVS-GFP (E and F, arrows) and LVS ΔwbtDEF-GFP (G and H, arrows) have multiplied extensively, and the majority no longer colocalize with CD63 fluorescence. In contrast, the latex beads (arrowheads) in these heavily infected cells continue to colocalize strongly with CD63. (I) Colocalization of latex beads and LVS-GFP or ΔwbtDEF-GFP with CD63 was evaluated for at least 40 bacteria or beads for each time point from 30 min to 22 h postinfection. The LVS ΔwbtDEF-GFP and the parental strain had significantly less colocalization with CD63 than the latex beads at the 3-h to 22-h time points (P < 0.01; two-way ANOVA with Bonferroni posttests). The data represent means ± standard errors. The experiment was performed twice with similar results.
Whereas the 1-μm latex beads colocalize intensely with the endocytic tracer Texas Red-dextran, phagosomes containing the parental LVS show relatively little colocalization with Texas Red-dextran at all time points (Fig. 6), indicating that latex bead phagosomes fuse with secondary lysosomes but LVS phagosomes do not. The ΔwbtDEF O-antigen mutants also show relatively little colocalization with Texas Red-dextran, but they show a trend toward greater colocalization (as much as 30% at 3 h) than the parental LVS (which has only 10% colocalization at 3 h [Fig. 6I]). For the parental LVS and the ΔwbtDEF O-antigen mutants, it is apparent by fluorescence microscopy that both have proliferated extensively within the macrophages by 1 day postinfection (Fig. 5 and 6). We obtained results with the WbtIG191V mutant similar to those obtained using the ΔwbtDEF O-antigen mutant (data not shown).
Fig 6.
Immunofluorescence evaluation of colocalization of Texas Red-dextran with parental F. tularensis LVS, O-antigen-deficient ΔwbtDEF LVS, and latex beads. Lysosomes of PMA-differentiated THP-1 macrophages were prelabeled by incubation of the macrophages with lysine-fixable Texas Red-dextran (10,000 MW) prior to synchronizing uptake of fluorescent blue 1-μm latex beads and LVS-GFP (A, B, E, and F) or LVS ΔwbtDEF-GFP (C, D, G, and H) as described above. The macrophages were washed, incubated at 37°C, fixed at sequential times thereafter, stained with DAPI, and evaluated by fluorescence microscopy as described above. (A, C, E, and G) Merged fluorescence images showing green-fluorescent bacteria, red-fluorescent Texas Red-dextran, and blue-fluorescent beads and nuclei. (B, D, F, and H) Texas Red-dextran fluorescence is shown in black and white. (A to H) LVS-GFP (A, B, E, and F, arrows) and LVS ΔwbtDEF-GFP (C, D, G, and H, arrows) bacteria have little or no colocalization with Texas Red-dextran at either 30 min (A to D) or 22 h (E to H) after infection. In contrast, the 1-μm fluorescent blue latex beads (arrowheads) colocalize intensely with Texas Red-dextran at both the 30-min and 22-h time points. (I) Colocalization of latex beads and LVS-GFP or ΔwbtDEF-GFP with Texas Red-dextran was evaluated for at least 40 bacteria or beads for each time point from 30 min to 22 h postinfection. LVS ΔwbtDEF-GFP and the parental strain had significantly less colocalization with Texas Red-dextran than the latex beads at all time points (P < 0.001; two-way ANOVA with Bonferroni posttests). The data represent means ± standard errors. The experiment was performed twice with similar results.
O-antigen-deficient F. tularensis LVSs show delayed phagosomal escape compared with the parental LVS.
We examined the time course of phagosome escape by the parental LVS and the ΔwbtDEF O-antigen mutant by TEM. When the bacteria are opsonized in C7-deficient AB serum, 70% of the LVS bacteria escape by 11 h after infection (Fig. 7A). In marked contrast, in the first 11 h after infection, the LVS ΔwbtDEF mutant rarely escapes (Fig. 7A). However, by 1 day after infection, the number of LVS ΔwbtDEF mutant bacteria that have escaped into the cytosol is comparable to that of the parental LVS. We observed a similar delay of escape into the cytosol in parallel experiments conducted with the WbtIG191V mutant, with bacteria only rarely observed in the cytosol in the first 11 h but escape into the cytosol readily apparent by 1 day after infection. To determine whether the delayed escape might be due to injury to the bacteria in fresh serum despite the deficiency of complement factor C7, we also examined the kinetics of escape when the macrophages were infected in culture medium containing heat-inactivated fetal bovine serum and no active source of complement. We employed a 50-fold-higher multiplicity of infection to obtain a level of infection comparable to that achieved when the bacteria were opsonized with complement. Despite the absence of complement opsonization, the kinetics of escape of both the parental LVS and the O-antigen-deficient mutant were comparable to that seen when we used C7-deficient AB serum (Fig. 7B and 8A to H). Whereas the WbtIG191V O-antigen mutant shows greatly delayed escape, the wbtI-complemented WbtIG191V mutant escapes into the cytosol with kinetics similar to those of the LVS, and this holds true regardless of whether the bacteria are opsonized (Fig. 7C).
Fig 7.
Ultrastructural analysis of the time course of phagosome escape in human THP-1 cells by parental F. tularensis LVS and O-antigen-deficient LVS in human THP-1 cells. Monolayers of PMA-differentiated human THP-1 cells were infected with LVS or LVS ΔwbtDEF that had been opsonized with C7-deficient AB serum (A) or without complement opsonization (B) or infected with WbtIG191V O-antigen-deficient LVS or the complemented mutant in the presence or absence of opsonization with C7-deficient serum (C). The macrophages were fixed at 1 h to 21 h and processed for transmission electron microscopy. Bacteria were scored as having escaped into the cytosol if less than 50% of their circumference was surrounded by a membrane bilayer. The difference between the ΔwbtDEF mutant and the parental LVS in mean percent escaped was statistically significant at both the 6- and 12-h time points for both the opsonized (A) and unopsonized (B) conditions (P < 0.01; two-way ANOVA with Bonferroni posttests). In panel C, the difference between the WbtIG191V O-antigen-deficient LVS and the complemented mutant in mean percent escaped at the 8-h time point was statistically significant (P < 0.01; two-way ANOVA with Bonferroni posttests) under both the opsonized and unopsonized conditions. The data represent means ± standard errors. The experiments were performed twice with similar results.
Fig 8.
Ultrastructural analysis of LVS and O-antigen-deficient LVS in human THP-1 cells in the absence of complement opsonization. (A and B) In the first 6 h after uptake of LVS bacteria, even in the absence of complement opsonization, the majority of LVS bacteria (indicated by asterisks) are found within membrane-bound vacuoles. (C to G) However, by 12 h after uptake (C and D), the majority of the LVS bacteria (asterisks) have escaped into the cytosol, while in the absence of serum opsonization, the majority of LVS ΔwbtDEF bacteria remain within membrane-bound vacuoles in the first 12 h after uptake (6 h [E] and12 h [F and G]). By 12 h after uptake, many of the LVS ΔwbtDEF phagosomes are decorated with a characteristic electron-dense fibrillar coat (faint in panel F and more obvious in panel G), which we also observed on the parental LVS phagosomes at earlier time points. (H) Blebbing and disruption of the fibrillar coat appear to accompany escape of the LVS ΔwbtDEF bacteria into the cytosol. (I and J) By 21 h after uptake, the majority of the LVS ΔwbtDEF bacteria have escaped and are free in the cytosol. (K and L) We observed similar features for the WbtIG191V mutant LVS. Panel L shows WbtIG191V mutant LVS bacteria at 16 h postinfection that have escaped into the cytosol. The wbtI-complemented strain (K) exhibits morphological interactions with the host cell similar to those of the LVS, whereas the WbtIG191V mutant strain (L) lacks the electron-lucent zone that is seen in the parental LVS. The electron-lucent zone surrounding the bacteria (K, arrowheads) is restored in the complemented strain. The experiment was conducted twice with similar results. Bars, 1 μm (A to E and J to L) and 0.5 μm (F to I).
The ultrastructural features of phagosome escape by the O-antigen-deficient mutant also resemble those that we documented previously for the parental LVS (9, 11, 12). Just as we observed the formation of a fibrillar coat around the parental LVS (9, 11, 12), we also observed the formation of fibrillar coats on the phagosomes of the O-antigen-deficient mutant F. tularensis (Fig. 8G). In addition, escape of the bacteria into the cytosol appears to be accompanied by blebbing and fragmentation of the fibrillar coat (Fig. 8H).
One interesting difference between the parental and O-antigen-deficient strains is related to the electron-lucent zone around the bacteria. Following escape of the parental LVS into the cytosol, we and others observed an electron-lucent zone around the bacterium (9, 11, 12, 14) (Fig. 8C and D). However, in the case of the O-antigen-deficient mutants, there is little or no electron-lucent zone between the bacterium and the cytosol (Fig. 8I, J, and L), indicating that the O-antigen polysaccharide is required for the electron-lucent zone. As expected, complementation of the WbtIG191V mutant with an intact copy of the wbtI gene restored the electron-lucent zone (Fig. 8K).
DISCUSSION
Our study shows several interesting differences between O-antigen mutants and the parental F. tularensis LVS in their interactions with human macrophages, especially with respect to the morphology of uptake, kinetics of phagosome escape, and sensitivity to serum.
When we opsonized the bacteria with complement, we observed striking differences between the O-antigen mutants and the parental LVS in the morphology of uptake. Whereas the complement-opsonized parental LVS is internalized within spacious, exuberant, asymmetric pseudopod loops, a process we have termed looping phagocytosis, the complement-opsonized O-antigen mutants are internalized either within relatively tight asymmetric loops or within multiple onion-like layers of pseudopodia that are tightly juxtaposed to the bacteria. However, in the absence of complement opsonization, the O-antigen mutants are internalized within spacious loops similar to those of the parental strain. We hypothesize that bacterial molecules other than the O-antigen trigger dramatic pseudopod extensions and that the degree of intimacy established between bacterium and pseudopod depends upon the abundance of receptor-ligand interactions between host and bacterium. Clay et al. (7) have demonstrated that serum-sensitive O-antigen polysaccharide-deficient F. tularensis strains have markedly greater deposition of complement component C3 and C3 fragments, including C3bi, which binds the complement receptor to mediate opsonophagocytosis, than serum-resistant strains with intact O-antigen polysaccharides. We hypothesize that the greater C3bi deposition in the absence of the O-antigen polysaccharide promotes greater complement receptor-complement interaction, thereby promoting more intimate contact, much tighter loops, and the onion skin-like structures. In the absence of complement opsonization, there are fewer receptor-ligand interactions to support the intimate contact between the bacterium and the vigorously extended pseudopod loop.
Apicella et al. (2) have recently shown that F. tularensis has a capsule-like material containing the same tetrasaccharide repeat that is present in the O antigen. These authors have shown that the F. tularensis WbtIG191V mutant, which we also used in this study, is deficient in both O-antigen-immunoreactive material and capsular material (2). Because our ΔwbtDEF mutant is unable to synthesize 2-acetamido-2-deoxygalacturonamide (GalNAcAN), which is part of both the O antigen and the capsule-like material and also lacks the glycosyl transferase required for linking GalNAcAN to N-acetyl-quinovosamine (QuiNAc), our ΔwbtDEF mutant is unable to synthesize the capsular material described by Apicella et al. (2). Thus, it is likely that the O-antigen polysaccharide and the capsule-like material are essential for enabling the spaciousness that we observe in looping phagocytosis of complement-opsonized F. tularensis.
We have found that the ultrastructural morphology of uptake of the O-antigen-deficient bacteria is influenced by the presence or absence of complement opsonization. In the absence of complement opsonization, the O-antigen mutants are internalized by a looping phagocytosis process that resembles that of the parental LVS, and in the presence of complement, they are internalized by tightly adherent and often redundant pseudopod loops. In both cases, they are internalized by a process that differs markedly from the conventional zipper-like phagocytosis that we have observed for other bacteria, such as E. coli, Mycobacterium tuberculosis, and Mycobacterium leprae (8, 16, 32, 33), indicating that factors other than the O antigen are important in the altered phagocytic process. The uptake of the complement-opsonized, O-antigen-deficient LVS by relatively tight asymmetric loops or by multiple layers of tightly juxtaposed pseudopods resembles a process that has been called “overlapping phagocytosis” by Rittig et al. in their description of the uptake of Leishmania promastigotes and of the spirochete Borrelia burgdorferi (27, 28). Common to both looping phagocytosis of the parental LVS and the “overlapping phagocytosis” that we observe for the O-antigen-deficient mutants is absent or delayed fusion of the pseudopod tips and redundancy or overexuberance of the pseudopod extensions. Rittig et al. (28) have proposed that the failure of fusion of pseudopod tips in the phenomenon of “overlapping phagocytosis” could reflect disorganization of regulatory signals. For example, macrophages treated with the phosphatidylinositol 3-kinase inhibitor wortmannin or LY294002 (3, 35) exhibit defects in completion of FcR-gamma-triggered phagocytosis manifested as failure of fusion of the pseudopod tips. Rittig et al. (28) have also proposed that fusion of the pseudopod tips during phagocytosis has a negative-feedback effect upon further pseudopod extension and that the absence of fusion causes continued pseudopod extension. For both the parental LVS and the O-antigen-deficient mutants, the extension of exuberant asymmetric loops and the deployment of multiple onion-like layers of pseudopodia, respectively, is consistent with the hypothesis of impaired fusion of pseudopod tips triggering continued pseudopod extensions. Thus, it is tempting to speculate that F. tularensis has preformed molecules that impair the capacity of the macrophage to generate phosphatidylinositol 3-phosphate (PI3P), thereby causing the unusual uptake profiles that we observe for the parental LVS and the O-antigen-deficient LVS.
Another major difference between the O-antigen mutant and the parental LVS in their interaction with macrophages is in the kinetics of phagosome escape. The LVS O-antigen-deficient mutants that we have studied resemble the parental strain with regard to membrane trafficking, formation of a fibrillar coat, and ultimate escape into the cytosol, but they show a considerable lag period in formation of the fibrillar coat and escape into the cytosol. Resolution of the multiple onion skin-like layers of pseudopodia into a single phagosomal membrane bilayer occurs extremely rapidly—within seconds to minutes—and possible transitional structures between the two are only rarely observed. Therefore, the delayed phagosomal escape of the O-antigen mutants cannot be ascribed to a delay in conversion of the multiple onion-like layers into a single phagosomal membrane bilayer. Moreover, phagosome escape of the O-antigen mutants is delayed even when infection is conducted in the absence of complement preopsonization, a condition in which the onion-like uptake profiles are not observed and the morphology of uptake resembles that of the parental strain.
One possible explanation for the delayed kinetics of escape is that the O-antigen mutants suffer injury from host defenses at the time of phagocytosis and a lag period is required for their recovery. Consistent with this, we have observed that the O-antigen mutants are more sensitive to the human cathelicidin antimicrobial peptide than the parental strain (data not shown). However, it is not known whether the bacteria encounter this antimicrobial peptide after uptake by human macrophages. The lag period could also reflect killing of a major proportion of the bacteria by macrophage defenses shortly after uptake and phagosome escape and cytosolic replication by a minor subpopulation of survivors. In this case, the delay would represent the time required for the minor subpopulation to become the dominant population that is observed within the macrophages. Evidence that this is not a complete explanation for the altered kinetics is that the majority of LVS F. tularensis O-antigen mutants that we observe in the first 12 h after uptake have excellent morphology despite remaining within clearly defined membrane vacuoles. In addition, unlike vacuoles containing dead bacteria, the vacuoles containing the O-antigen mutants acquire relatively little staining for lysosomal membrane glycoproteins and do not fuse with Texas Red-dextran-containing secondary lysosomes. Moreover, since we observe delayed formation of fibrillar coats and delayed appearance of the membrane blebbing and fragmentation that accompany phagosome escape, it appears that even the surviving or uninjured bacteria exhibit delayed kinetics of phagosome escape.
While the serum sensitivity of O-antigen-deficient mutants (5, 7, 19, 26, 36) and the importance of the terminal complement membrane attack complex have been shown previously (5, 7), we show here that the altered intracellular trafficking, cytosolic escape, and capacity for intracellular growth of F. tularensis do not require the O-antigen polysaccharide.
Some investigators have reported more rapid escape of F. tularensis and F. tularensis subsp. novicida than we have observed (6, 29). One difference between our studies and others that might account for the discrepancy is our use of serum to opsonize the bacteria, which are subsequently ingested via complement receptors (10). However, the present study suggests otherwise, since phagosome escape and intracellular growth by F. tularensis LVS (and the wbtI-complemented mutant LVS) in human macrophages proceeds with the same time course regardless of whether the bacteria are opsonized with complement. Instead, it is likely that the differences between our studies and others with respect to the kinetics of phagosome escape lie in the method of measuring the escape. We scored phagosome escape by ultrastructural appearance, whereas others have used accessibility of the phagosomes to antibodies after first disrupting the plasma membranes with digitonin detergent or glass beads. Membranes that appear ultrastructurally intact may nevertheless be porous to antibody. In addition, disruption of the macrophage plasma membrane by digitonin or glass beads may alter the integrity of the phagosome itself, thus causing overestimation of the percentage of permeabilized phagosomes.
O-antigen-deficient mutants of F. tularensis have been studied previously and have consistently been shown to be highly attenuated in mouse models of tularemia, but there have been conflicting results regarding their capacity for growth in macrophages in vitro. Li et al. (19) showed that the WbtIG191V mutant of F. tularensis LVS was completely deficient in O antigen, serum sensitive, and highly attenuated in a mouse model of tularemia and exhibited impaired growth in mouse J774 macrophages that could be partially complemented by providing an intact copy of the wbtI gene in trans. In contrast, a study of a transposon mutant library of F. tularensis LVS identified a wbtA mutant (the first gene in the O-antigen gene cluster) that similarly exhibited complete loss of O-antigen immunoreactivity, serum sensitivity, and severely attenuated virulence in mice, but this mutant showed no growth in mouse J774 macrophages (26). Thomas et al. (36) made a targeted disruption of the wbtDEF genes of F. tularensis subsp. tularensis and subsp. novicida and reported that while the resulting O-antigen mutants of both F. tularensis subsp. tularensis and subsp. novicida were attenuated for virulence in mice, the F. tularensis subsp. tularensis mutant was defective for intramacrophage survival in mouse J774 macrophages, whereas the F. tularensis subsp. novicida wbtDEF mutant grew in the J774 macrophages as well as the parental strain. Lindemann et al. (20) generated O-antigen mutants of Schu S4 by transposon mutagenesis and observed a reduced rate of intramacrophage growth of the mutants. The impaired intracellular growth of these Schu S4 O-antigen mutants was attributed to the capacity of the mutants to cause rapid death of their human macrophage hosts. While Lindemann et al. (20) reported absence of LAMP-1 colocalization with their mutants at 1 h and interpreted this as suggesting rapid escape of the mutants into the cytosol, no ultrastructural studies were reported, and it is unclear whether absence of LAMP-1 immunofluorescence is an accurate measure of escape into the cytosol. In our study, we conducted extensive ultrastructural evaluations of our LVS O-antigen mutants and consistently observed a marked delay in their escape from the phagosome compared with the parental strain. The different observations regarding intracellular growth of O-antigen-deficient mutants of F. tularensis may reflect differences in the bacterial strains and mutants studied, differences in host cells, and differences in the conditions used to assess intramacrophage growth. In human THP-1 macrophages, we observed a substantial lag in growth for the LVS ΔwbtDEF and WbtIG191V mutants compared with the parental strain and the wbtI-complemented mutant strain.
In summary, our studies show that the O antigen is essential for complement resistance of F. tularensis LVS, enables phagocytosis via spacious pseudopod loops (classical looping phagocytosis) in the presence of serum complement, and confers protection against host defenses that allows a relatively high rate of phagosomal escape. However, the O antigen is not essential for F. tularensis survival and growth within human macrophages or for the altered phagosome membrane trafficking and phagosome escape that are prominent features of the intramacrophage life of the pathogen.
ACKNOWLEDGMENTS
We are grateful to Melissa Thomas and Sarah Syke McNees for expert technical assistance. We thank Birgitta Sjostrand, Marianne Ciluffo, and Sirus Kohan of the UCLA Brain Research Institute Electron Microscope Services Center and Microscopic Techniques Core Facility for assistance with electron microscopy and Matthew Schibler of the Advanced Light Microscopy core facility at the UCLA California NanoSystems Institute for assistance with confocal microscopy.
This work was supported by National Institutes of Health grant AI065359.
Footnotes
Published ahead of print 27 December 2011
REFERENCES
- 1. Adinolfi M, Zenthon J. 1982. Complement and disease: a review. J. R. Soc. Med. 75:121–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Apicella MA, et al. 2010. Identification, characterization and immunogenicity of an O-antigen capsular polysaccharide of Francisella tularensis. PLoS One 5:e11060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Araki N, Johnson MT, Swanson JA. 1996. A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J. Cell Biol. 135:1249–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Balagopal A, et al. 2006. Characterization of the receptor-ligand pathways important for entry and survival of Francisella tularensis in human macrophages. Infect. Immun. 74:5114–5125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ben Nasr A, Klimpel GR. 2008. Subversion of complement activation at the bacterial surface promotes serum resistance and opsonophagocytosis of Francisella tularensis. J. Leukoc. Biol. 84:77–85 [DOI] [PubMed] [Google Scholar]
- 6. Checroun C, Wehrly TD, Fischer ER, Hayes SF, Celli J. 2006. Autophagy-mediated reentry of Francisella tularensis into the endocytic compartment after cytoplasmic replication. Proc. Natl. Acad. Sci. U. S. A. 103:14578–14583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clay CD, Soni S, Gunn JS, Schlesinger LS. 2008. Evasion of complement-mediated lysis and complement C3 deposition are regulated by Francisella tularensis lipopolysaccharide O antigen. J. Immunol. 181:5568–5578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Clemens DL, Horwitz MA. 1992. Membrane sorting during phagocytosis: selective exclusion of major histocompatibility complex molecules but not complement receptor CR3 during conventional and coiling phagocytosis. J. Exp. Med. 175:1317–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clemens DL, Horwitz MA. 2007. Uptake and intracellular fate of Francisella tularensis in human macrophages. Ann. N. Y. Acad. Sci. 1105:160–186 [DOI] [PubMed] [Google Scholar]
- 10. Clemens DL, Lee BY, Horwitz MA. 2005. Francisella tularensis enters macrophages via a novel process involving pseudopod loops. Infect. Immun. 73:5892–5902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Clemens DL, Lee BY, Horwitz MA. 2009. Francisella tularensis phagosomal escape does not require acidification of the phagosome. Infect. Immun. 77:1757–1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Clemens DL, Lee BY, Horwitz MA. 2004. Virulent and avirulent strains of Francisella tularensis prevent acidification and maturation of their phagosomes and escape into the cytoplasm in human macrophages. Infect. Immun. 72:3204–3217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ellis J, Oyston PCF, Green M, Titball RW. 2002. Tularemia. Clin. Microbiol. Rev. 15:631–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Golovliov I, Baranov V, Krocova Z, Kovarova H, Sjostedt A. 2003. An attenuated strain of the facultative intracellular bacterium Francisella tularensis can escape the phagosome of monocytic cells. Infect. Immun. 71:5940–5950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Golovliov I, Sjostedt A, Mokrievich A, Pavlov V. 2003. A method for allelic replacement in Francisella tularensis. FEMS Microbiol. Lett. 222:273–280 [DOI] [PubMed] [Google Scholar]
- 16. Horwitz MA. 1982. Phagocytosis of microorganisms. Rev. Infect. Dis. 4:104–123 [DOI] [PubMed] [Google Scholar]
- 17. Horwitz MA. 1984. Phagocytosis of the Legionnaires' disease bacterium (Legionella pneumophila) occurs by a novel mechanism: engulfment within a pseudopod coil. Cell 36:27–33 [DOI] [PubMed] [Google Scholar]
- 18. Lee BY, Horwitz MA, Clemens DL. 2006. Identification, recombinant expression, immunolocalization in macrophages, and T-cell responsiveness of the major extracellular proteins of Francisella tularensis. Infect. Immun. 74:4002–4013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li J, et al. 2007. Attenuation and protective efficacy of an O-antigen-deficient mutant of Francisella tularensis LVS. Microbiology 153:3141–3153 [DOI] [PubMed] [Google Scholar]
- 20. Lindemann SR, et al. 2011. Francisella tularensis Schu S4 O-antigen and capsule biosynthesis gene mutants induce early cell death in human macrophages. Infect. Immun. 79:581–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maier TM, et al. 2004. Construction and characterization of a highly efficient Francisella shuttle plasmid. Appl. Environ. Microbiol. 70:7511–7519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mold C. 1999. Role of complement in host defense against bacterial infection. Microbes Infect. 1:633–638 [DOI] [PubMed] [Google Scholar]
- 23. Oyston PC, Sjostedt A, Titball RW. 2004. Tularaemia: bioterrorism defence renews interest in Francisella tularensis. Nat. Rev. Microbiol. 2:967–978 [DOI] [PubMed] [Google Scholar]
- 24. Pierini LM. 2006. Uptake of serum-opsonized Francisella tularensis by macrophages can be mediated by class A scavenger receptors. Cell. Microbiol. 8:1361–1370 [DOI] [PubMed] [Google Scholar]
- 25. Prior JL, et al. 2003. Characterization of the O antigen gene cluster and structural analysis of the O antigen of Francisella tularensis subsp. tularensis. J. Med. Microbiol. 52:845–851 [DOI] [PubMed] [Google Scholar]
- 26. Raynaud C, et al. 2007. Role of the wbt locus of Francisella tularensis in lipopolysaccharide O-antigen biogenesis and pathogenicity. Infect. Immun. 75:536–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rittig MG, Burmester GR, Krause A. 1998. Coiling phagocytosis: when the zipper jams, the cup is deformed. Trends Microbiol. 6:384–388 [DOI] [PubMed] [Google Scholar]
- 28. Rittig MG, Wilske B, Krause A. 1999. Phagocytosis of microorganisms by means of overshooting pseudopods: where do we stand? Microbes Infect. 1:727–735 [DOI] [PubMed] [Google Scholar]
- 29. Santic M, Asare R, Skrobonja I, Jones S, Abu Kwaik Y. 2008. Acquisition of the vATPase proton pump and phagosome acidification is essential for escape of Francisella tularensis into the macrophage cytosol. Infect. Immun. 76:2671–2677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Saslaw S, Eigelsbach HT, Prior JA, Wilson HE, Carhart S. 1961. Tularemia vaccine study. II. Respiratory challenge. Arch. Intern. Med. 107:702–714 [DOI] [PubMed] [Google Scholar]
- 31. Saslaw S, Eigelsbach HT, Wilson HE, Prior JA, Carhart S. 1961. Tularemia vaccine study. I. Intracutaneous challenge. Arch. Intern. Med. 107:689–701 [DOI] [PubMed] [Google Scholar]
- 32. Schlesinger LS, Bellinger-Kawahara CG, Payne NR, Horwitz MA. 1990. Phagocytosis of Mycobacterium tuberculosis is mediated by human monocyte complement receptors and complement component C3. J. Immunol. 144:2771–2780 [PubMed] [Google Scholar]
- 33. Schlesinger LS, Horwitz MA. 1990. Phagocytosis of leprosy bacilli is mediated by complement receptors CR1 and CR3 on human monocytes and complement component C3 in serum. J. Clin. Invest. 85:1304–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schulert GS, Allen LA. 2006. Differential infection of mononuclear phagocytes by Francisella tularensis: role of the macrophage mannose receptor. J. Leukoc. Biol. 80:563–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Swanson JA, et al. 1999. A contractile activity that closes phagosomes in macrophages. J. Cell Sci. 112:307–316 [DOI] [PubMed] [Google Scholar]
- 36. Thomas RM, et al. 2007. The immunologically distinct O antigens from Francisella tularensis subspecies tularensis and Francisella novicida are both virulence determinants and protective antigens. Infect. Immun. 75:371–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vinogradov E, Perry M, Conlan JW. 2002. Structural analysis of Francisella tularensis lipopolysaccharide. Eur. J. Biochem. 269:6112–6118 [DOI] [PubMed] [Google Scholar]
- 38. Whipp MJ, et al. 2003. Characterization of a novicida-like subspecies of Francisella tularensis isolated in Australia. J. Med. Microbiol. 52:839–842 [DOI] [PubMed] [Google Scholar]
- 39. White JD, et al. 1964. Pathogenesis of experimental respiratory tularemia in monkeys. J. Infect. Dis. 114:277–283 [DOI] [PubMed] [Google Scholar]