Abstract
Insulin signaling in osteoblasts contributes to whole-body glucose homeostasis in the mouse and in humans by increasing the activity of osteocalcin. The osteoblast insulin signaling cascade is negatively regulated by ESP, a tyrosine phosphatase dephosphorylating the insulin receptor. Esp is one of many tyrosine phosphatases expressed in osteoblasts, and this observation suggests that other protein tyrosine phosphatases (PTPs) may contribute to the attenuation of insulin receptor phosphorylation in this cell type. In this study, we sought to identify an additional PTP(s) that, like ESP, would function in the osteoblast to regulate insulin signaling and thus affect activity of the insulin-sensitizing hormone osteocalcin. For that purpose, we used as criteria expression in osteoblasts, regulation by isoproterenol, and ability to trap the insulin receptor in a substrate-trapping assay. Here we show that the T-cell protein tyrosine phosphatase (TC-PTP) regulates insulin receptor phosphorylation in the osteoblast, thus compromising bone resorption and bioactivity of osteocalcin. Accordingly, osteoblast-specific deletion of TC-PTP promotes insulin sensitivity in an osteocalcin-dependent manner. This study increases the number of genes involved in the bone regulation of glucose homeostasis.
INTRODUCTION
The tenuous cross talk existing between bone remodeling and energy metabolism was first demonstrated in vivo through the realization that leptin, an adipocyte-derived hormone, inhibits both appetite (23, 25, 44) and bone mass accrual (16). The existence of this cross talk was then further substantiated by the observation that, in turn, osteoblasts regulate whole-body glucose metabolism through secretion of the hormone osteocalcin, which favors insulin secretion and insulin sensitivity and increases energy expenditure (20, 33, 37).
Like other peptide hormones, osteocalcin undergoes significant posttranslational modification before being released into general circulation (29, 45). Specifically, osteocalcin, which is secreted by osteoblasts as a γ-carboxylated protein, must be decarboxylated to become activated and able to fulfill its endocrine functions (33). This activation of osteocalcin has been shown to occur outside the osteoblast, in the bone resorption lacunae (21). As the only mechanism known for decarboxylating proteins outside the cell is incubating them at an acidic pH, the passage of osteocalcin through the acidic microenvironment of the resorption lacunae allows it to become decarboxylated and thus activated (18, 21). In effect, the resorbing function of osteoclasts favors glucose homeostasis by activating osteocalcin (21).
In addition to being an endocrine cell, the osteoblast receives many endocrine signals, one of them being insulin. Among other functions, insulin signaling in osteoblast inhibits the expression of Opg, a gene encoding a decoy receptor for the RANKL osteoclast differentiation factor. As a consequence, insulin signals to the osteoblast to promote bone resorption and osteocalcin bioactivity, and thereby its own secretion (21).
As is the case in other insulin-sensitive cells such as the hepatocytes and myocytes, the insulin signaling cascade in the osteoblast is tightly regulated (39, 41, 48). In particular, protein tyrosine phosphatases (PTPs) play a crucial role in attenuating insulin receptor phosphorylation to limit insulin signaling in many cell types and maintain glucose homeostasis (31, 41, 48). To date, the only tyrosine phosphatase expressed in the mouse osteoblast that has been shown to dephosphorylate the insulin receptor is ESP (21). As a result, the deletion of Esp specifically in the osteoblast enhances insulin signaling, increases circulating levels of active osteocalcin, and accordingly favors glucose tolerance, insulin sensitivity, and energy expenditure (21, 33). This and other experiments have identified ESP as a major intracellular regulator of osteocalcin's endocrine function in the mouse.
However, ESP is not the only PTP present in osteoblasts, an observation suggesting that other PTPs in addition to ESP may contribute to the regulation of glucose metabolism through their expression in osteoblasts. If this were the case, it would strengthen the notion that bone is involved in the regulation of glucose metabolism.
To address this question, and since ESP belongs to the family of classical PTPs, which are defined by their specificity for phosphotyrosine (2, 6), we tested all 37 other mammalian classical PTPs for their ability to bind to the endogenous insulin receptor in osteoblasts and to be upregulated by isoproterenol, as is Esp (30). Only one PTP was able to bind to the osteoblast insulin receptor and respond to isoproterenol treatment—T-cell PTP (TC-PTP). We show here that TC-PTP regulates osteocalcin bioactivity by inhibiting bone resorption, thus affecting whole-body glucose metabolism. Hence, these results identify a role for TC-PTP as a novel regulator of energy metabolism through its expression in the osteoblast.
MATERIALS AND METHODS
Animal studies.
All mice studied were age-matched littermate males on a mixed (87.5% C57BL/6J; 12.5% 129/Sv) background. Genotyping was performed by PCR using DNA extracted from tail tips; primer sequences are available upon request. All mice were maintained on a 12-hour light/dark cycle in a barrier facility with free access to standard chow and water and analyzed at 5 to 7 weeks of age.
Metabolic measurements.
Glucose in tail blood was measured using a glucometer (Accucheck). For the glucose tolerance test (GTT), mice were fasted overnight and injected with 2 kg d-glucose per kg body weight. Blood glucose was assayed immediately before and at 15, 30, 60, and 120 min postinjection. For the insulin tolerance test (ITT), mice were fasted for 4 h and injected with 0.5 U/kg insulin (Humulin R; Lilly). Blood glucose was measured immediately before and at 30, 60, 90, and 120 min postinjection. For the glucose-stimulated insulin secretion (GSIS) assay, mice were fasted overnight and injected with 2 kg d-glucose per kg body weight. Tail blood was collected immediately before and at 2, 5, 15, and 30 min postinjection (33). Enzyme-linked immunosorbent assays (ELISAs) were used to determine serum insulin (Mercodia), serum carboxy-terminal collagen cross-links (CTx) (Serum Crosslaps; IDS), and serum uncarboxylated (Glu), carboxylated (GLA), and total osteocalcin as previously described (22). Whole calvaria were collected in mice injected through the inferior vena cava after overnight fasting. Quantification of Western blot data was performed using ImageJ.
Substrate trapping and coimmunoprecipitation.
Glutathione S-transferase (GST)–PTPDA proteins were generated by cloning the nonreceptor PTPs and cytoplasmic phosphatase domains of the receptor PTPs into the BamHI site of pGEX 4T3. Site-directed mutagenesis was used to mutate the catalytic aspartate acid (D) residue to inactive alanine (A), as previously described (24). The expression vectors were then transformed into BL21(DE3)pLysS bacteria (Novagen). Recombinant GST-PTPDA proteins were induced and purified using glutathione-Sepharose beads and then immediately incubated in lysate of pervanadate-treated ROS17/2.8 cells, as previously described (21). The proteins were resolved on SDS-PAGE gels followed by Western blotting. Anti-insulin receptor β-subunit (anti-IRβ) and anti-pTyr were obtained from Cell Signaling Technology. For coimmunoprecipitation assays, PTP1B and TC-PTP and their respective substrate-trapping mutants were cloned into the EcoRI and BamHI sites of the pFLAG-5a expression vector. ROS17/2.8 cells were transfected with FLAG fusion protein or empty vector, and InsR was expressed in pCNDA3.1 using Lipofectamine 2000. Twenty-four hours posttransfection, FLAG fusion proteins were immunoprecipitated overnight and eluted as previously described (21) and resolved on SDS-PAGE gels followed by Western blotting. Anti-FLAG M2 affinity gel and 3×FLAG peptide were obtained from Sigma.
Cell culture.
Mouse primary osteoblasts were isolated as previously described (17). Small interfering RNA (siRNA) knockdown was achieved by transfection with siRNA pools (On-target; Dharmacon) according to the manufacturer's instructions. Anti-Y1150/Y1151 IRβ antibody was obtained from Cell Signaling Technology. Ptpn2+/+ or Ptpn2−/− osteoblasts were generated by infecting Ptpn2flox/flox osteoblasts with either green fluorescent protein (GFP)- or Cre-expressing adenovirus (University of Iowa). For the stimulation with isoproterenol and analysis of mineralized osteoblasts, cells were differentiated 3 days postconfluence in alpha minimal essential medium (α-MEM) containing 10% fetal bovine serum (FBS), 10 mM β-glycerophosphate, and 100 μg/ml ascorbic acid. Osteoblasts differentiated for 5 days were stimulated with isoproterenol for 4 h. Osteoblast/osteoclast cocultures were prepared as previously described (21). In vitro resorption activity of osteoclasts was measured using the BD Biocoat osteologic bone cell culture system according to the manufacturer's instructions. Primary osteoblast mineralization was visualized using Von Kossa staining and quantified by ImageJ.
Gene expression analysis.
RNA isolation, cDNA preparation, and quantitative PCR (qPCR) analysis were performed using standard protocols, and relative threshold cycle (CT) values are standardized to CT values of control β-actin or S18 (for osteoclasts), unless otherwise indicated. Exonic qPCR primers used to compare expression were normalized according to a standard curve of mouse genomic DNA.
Bone histomorphometry.
Static and dynamic histomorphometric analyses were performed on vertebral column specimens collected from 7-week-old mice using undecalcified sections according to standard protocols using the Osteomeasure analysis system (Osteometrics).
Statistics.
Results are given as means ± standard errors of the means. Statistical analyses were performed using an unpaired, two-tailed Student t test. For all experiments shown in the figures, significance is indicated as follows: *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001.
RESULTS
TC-PTP and PTP1B bind to the insulin receptor in osteoblasts.
With the goal of identifying additional protein tyrosine phosphatases that could dephosphorylate the insulin receptor in osteoblasts, we tested members of the classical protein tyrosine phosphatase (PTP) family for in vitro binding of the insulin receptor and expression in osteoblasts.
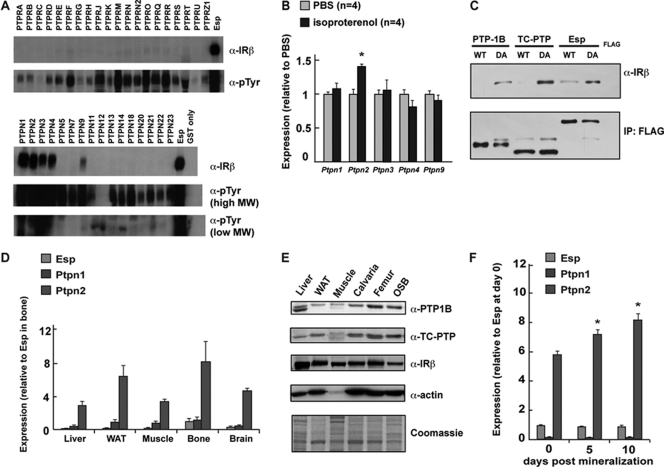
For the first purpose, we generated substrate-trapping mutants of all PTPs by introducing a DA substitution mutation in each phosphatase domain that abolishes catalytic activity but preserves the ability of the PTPs to bind substrate(s) (9, 24). These mutant GST fusion proteins were incubated with extracts from pervanadate-treated ROS17/2.8 osteoblast cells and then pulled down using glutathione beads. As shown in Fig. 1A, Western blot analysis indicated that, in addition to ESP, five PTPs were able to interact directly with the endogenous β subunit of the insulin receptor in osteoblasts: PTP1B (Ptpn1), TC-PTP (Ptpn2), PTPCL (Ptpn3), PTPMEG (Ptpn4), and MEG2 (Ptpn9).
Fig 1.
Identifying PTP(s) that parallels ESP. (A) In vitro substrate trapping. Extracts from pervanadate-treated ROS17/2.8 cells were pulled down using GST only or DA mutants of PTP-GST fusion proteins. IRβ was detected by Western blotting. An antiphosphotyrosine antibody (α-pTyr) was used for detection. MW, molecular weight. (B) Stimulation of primary osteoblasts with isoproterenol (10 μM). (C) In vivo substrate trapping. WT and DA FLAG-tagged PTP1B and TC-PTP proteins were immunoprecipitated from ROS17/2.8 cells. Immunoprecipitated proteins (IP) and total cell lysates were then analyzed by Western blotting. (D) qPCR expression analysis across different tissues. (E) Expression analysis by Western blotting. WAT, white adipose tissue. (F) qPCR expression analysis in proliferative and differentiating mouse primary osteoblasts.
In an effort to further narrow our search, we relied on one particularity of the ESP/osteocalcin metabolic pathway. Esp gene expression in osteoblasts is upregulated upon treatment of osteoblasts with the β2-adrenergic receptor agonist isoproterenol (30). We considered this response a defining feature of the osteocalcin regulation of energy metabolism and thus tested whether expression of Ptpn1, Ptpn2, Ptpn3, Ptpn4, and Ptpn9 was affected by isoproterenol treatment of osteoblasts. Remarkably, Ptpn2 expression was significantly affected by isoproterenol treatment in mineralized osteoblasts (Fig. 1B). These results identify TC-PTP as the leading candidate to be a second PTP involved in the osteoblast regulation of energy metabolism.
To formally demonstrate that the insulin receptor is a physiological substrate of TC-PTP in osteoblasts, we performed coimmunoprecipitation with the TC-PTP substrate-trapping mutant expressed in the osteoblast, using ESP as a positive control in this assay (24). For that purpose, we transfected ROS17/2.8 osteoblast cells with FLAG-tagged wild type (WT) and substrate-trapping mutants of PTP1B, TC-PTP, or ESP. IRβ coimmunoprecipitated with DA substrate-trapping mutants of PTP1B, TC-PTP, and ESP, while it did not with FLAG alone (Fig. 1C). Interestingly, wild-type ESP protein also exhibited binding to the insulin receptor, which was not detected with PTP1B and TC-PTP, suggesting that ESP can bind to the insulin receptor independently of its phosphatase domain (Fig. 1C). These results confirm that the insulin receptor is a substrate of PTP1B and TC-PTP in rodent osteoblasts.
TC-PTP is expressed in insulin-responsive tissues, including osteoblasts.
In view of these results, we then determined the level of expression of TC-PTP in bone cells in vivo. Western blot and qPCR analysis confirmed the presence of mRNAs encoding TC-PTP (Ptpn2) in isolated femur tissue, coinciding with the presence of the insulin receptor (Fig. 1D and E). When comparing the relative levels of Ptpn1, Ptpn2, and Esp expression by qPCR analysis using exonic primers normalized to genomic DNA, we observed that in all tissues tested, Ptpn2 expression exceeded that of both Esp and Ptpn1, with prominent expression in bone (Fig. 1D). Because bone is composed of multiple cell types, we also analyzed expression of the PTPs in primary osteoblasts isolated from newborn calvaria. Consistent with data obtained from whole bone, we observed that Ptpn2 was more highly expressed in primary osteoblasts than were Ptpn1 and Esp (Fig. 1F). Interestingly, Ptpn2 but not Ptpn1 expression was enhanced when osteoblasts were induced to fully differentiate in medium containing β-glycerophosphate and ascorbic acid (Fig. 1F).
Generation of mice lacking TC-PTP or PTP1B specifically in the osteoblast.
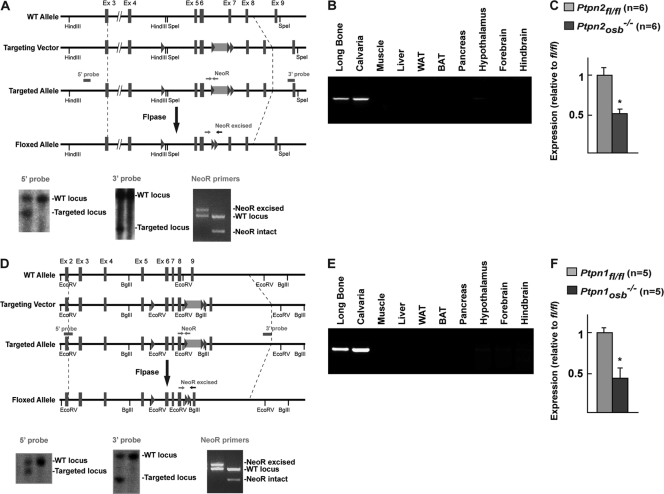
To study the function of TC-PTP in osteoblasts in vivo, we used a cell-specific loss-of-function strategy in the mouse and generated a floxed allele of Ptpn2 and Ptpn1 by homologous recombination in mouse embryonic stem (ES) cells. For the Ptpn2 floxed allele targeting strategy, we used a targeting vector including the genomic sequence from exon 4 to exon 8. Two loxP sites were added to flank exons 5 to 7, which contain the catalytic domain of TC-PTP (Fig. 2A). In addition, a neomycin (NeoR) cassette flanked by two Frt sites was inserted in the intron between exons 6 and 7 (Fig. 2A). Southern blot analysis identified the targeted Ptpn2 allele using a 5′ probe directed to intron 2 and a 3′ probe directed to the region containing exon 9 and a portion of intron 8 (Fig. 2A). The inserted NeoR cassette was subsequently removed by crossing mice containing the targeted allele with mice expressing Flp recombinase. NeoR excision was verified by PCR analysis (Fig. 2A).
Fig 2.
Generation of Ptpn2osb−/− and Ptpn1osb−/− mice. (A) Generation of the Ptpn2 floxed allele. (B) Deletion of the Ptpn2 allele in indicated tissues. (C) qPCR analysis of Ptpn2 expression in bone marrow-derived osteoblasts (normalized to osteocalcin expression). (D) Generation of the Ptpn1 floxed allele. (E) Deletion of the Ptpn1 allele in indicated tissues. (F) qPCR analysis of Ptpn1 expression in bone marrow-derived osteoblasts (normalized to osteocalcin expression). WAT, white adipose tissue; BAT, brown adipose tissue. Asterisks are defined in Materials and Methods.
To delete TC-PTP specifically in osteoblasts (Ptpn2osb−/− mice), mice harboring the floxed allele of Ptpn2 were crossed with mice expressing the α1(I)collagen-Cre transgene (12). PCR analysis confirmed that recombination of the Ptpn2 floxed allele was limited to bone only (Fig. 2B). Ptpn2osb−/− mice were born at the expected Mendelian ratio and appeared phenotypically normal at birth, indicating that Ptpn2 expression in osteoblasts is dispensable for normal embryonic development.
To generate the Ptpn1 floxed allele, we used a targeting vector including the genomic sequence from exon 3 to exon 9, similarly to what has been done previously (7). Two loxP sites were added to flank exons 6 to 8, since the catalytic domain of PTP1B is contained in exons 7 and 8 (Fig. 2A). A neomycin (NeoR) cassette flanked by two Frt sites was inserted in the intron between exons 8 and 9 (Fig. 2A). Southern blot analysis identified the targeted Ptpn1 allele using a 5′ probe directed to intron 2 and a 3′ probe directed to the region containing exon 9 (Fig. 2A). The inserted NeoR cassette was subsequently removed by crossing mice containing the targeted allele with mice expressing Flp recombinase and confirmed by PCR analysis (Fig. 2B). qPCR analysis of bone marrow-derived osteoblasts estimated recombination efficiency of 47.3% (Fig. 2C).
Similarly to Ptpn2osb−/−, mice harboring the floxed allele of Ptpn1 were crossed with mice expressing the α1(I)collagen-Cre transgene (Fig. 2D) (12). PCR and qPCR analysis confirmed that recombination of the Ptpn2 floxed allele was limited to bone only and at approximately 53.6% efficiency (Fig. 2E and F). Ptpn1osb−/− mice were also born at the expected Mendelian ratio and appeared phenotypically normal throughout life.
TC-PTP regulates insulin signaling in osteoblasts.
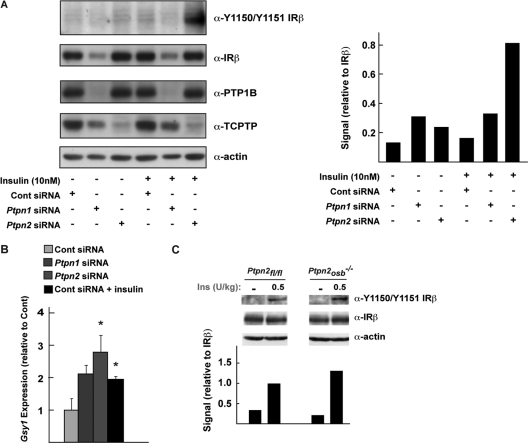
Next, we evaluated the contribution of TC-PTP in the regulation of insulin signaling in isolated osteoblasts and compared it to that of PTP1B. Primary cultures of calvarial osteoblasts were transfected with siRNA to suppress expression of either PTP1B or TC-PTP. In response to insulin stimulation, phosphorylation of the insulin receptor on the Y1150 and Y1151 residues was enhanced in osteoblasts deficient specifically for TC-PTP (Fig. 3A). There was no apparent increase in phosphorylation of the insulin receptor in osteoblasts lacking PTP1B. However, normalization of the signal with the amount of total insulin receptor indicated that phosphorylation was in fact increased nearly 2-fold in these osteoblasts, compared to the 5-fold increase observed in osteoblasts treated with TC-PTP siRNA (Fig. 3A). We also observed that osteoblasts lacking either PTP1B or TC-PTP had increased basal expression of the insulin target gene Gsy1, though this increase reached statistical significance only in TC-PTP-deficient osteoblasts (Fig. 3B). Taken together, these results suggested that TC-PTP might play a more important role in regulating insulin signaling in the mouse osteoblast.
Fig 3.
TC-PTP regulates insulin receptor phosphorylation in osteoblasts. (A) (Left) Phosphorylation of the insulin receptor β-subunit 5 min after treatment with insulin (10 nM) in mouse primary osteoblasts. (Right) Quantification of signal normalized to IRβ using ImageJ. (B) Expression of the insulin target gene Gsy1 in primary osteoblasts. Treatment of control is with 10 nM insulin. Asterisks are defined in Materials and Methods. (C) (Top) Phosphorylation of the insulin receptor β-subunit in whole calvaria after injection of insulin. (Bottom) Quantification of signal normalized to IRβ using ImageJ.
In view of these results, we focused our subsequent work on TC-PTP and asked whether insulin signaling is enhanced in the osteoblasts of Ptpn2osb−/− mice. Western blot analysis of whole calvaria isolated after intravenous injection of 0.5 U/kg insulin revealed that insulin receptor phosphorylation was increased in Ptpn2osb−/− calvaria (Fig. 3C). It is important to underline that because bone is a mixture of multiple cell types and Ptpn2 deletion is specific to osteoblasts only, the difference in insulin receptor phosphorylation in whole calvaria was expected to be subtle. Nevertheless, phosphorylation of the insulin receptor on Y1150 and Y1151 was enhanced more than 30% in the calvaria of Ptpn2osb−/− mice compared to their floxed littermate controls, confirming that TC-PTP regulates insulin receptor phosphorylation in vivo (Fig. 3C).
Mice lacking TC-PTP specifically in osteoblasts demonstrate increased osteocalcin bioactivity and insulin sensitivity.
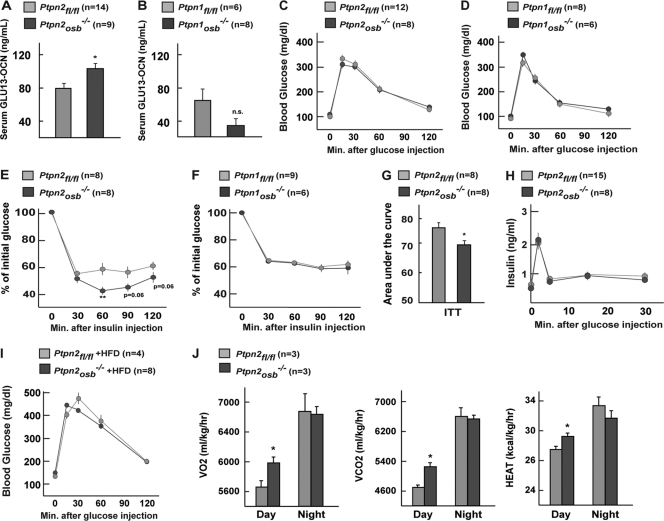
To test if TC-PTP or PTP1B regulates osteocalcin bioactivity, we quantified, using an ELISA developed in the laboratory (22), serum undercarboxylated osteocalcin (GLU13-OCN) levels in Ptpn2osb−/− and Ptpn1osb−/− mice. Compared to their WT littermates, Ptpn2osb−/− mice have increased serum levels of GLU13-OCN, though osteocalcin bioactivity was not significantly altered in Ptpn1osb−/− mice (Fig. 4A and B). This result confirmed that the increase in insulin signaling in the osteoblasts lacking TC-PTP coincided with an increase in serum undercarboxylated, i.e., active, osteocalcin (Fig. 4A). This finding suggested that one function of TC-PTP in osteoblasts is to regulate osteocalcin bioactivity.
Fig 4.
Improved insulin sensitivity in Ptpn2osb−/− mice. (A and B) Total GLU13 osteocalcin levels in male Ptpn2osb−/− (A) and Ptpn1osb−/− (B) mice. (C and D) Glucose tolerance tests (GTTs) on male Ptpn2osb−/− (C) and Ptpn1osb−/− (D) mice. (E and F) Insulin tolerance tests (ITTs) on male Ptpn2osb−/− (E) and Ptpn1osb−/− (F) mice. (G) Area under the curve of panel E. (H) Glucose-stimulated insulin secretion (GSIS) test of Ptpn2osb−/− male mice. (I) GTT on male Ptpn2osb−/− mice on a high-fat diet (HFD). (J) Energy expenditure of Ptpn2osb−/− male mice. Asterisks in bar graphs are defined in Materials and Methods. n.s., not significant.
Given the influence exerted by osteocalcin on glucose metabolism (33), we then asked whether osteoblast-specific deletion of TC-PTP might affect whole-body glucose metabolism. A glucose tolerance test (GTT) performed at 6 weeks of age revealed no difference in glucose tolerance in Ptpn2osb−/− mice (Fig. 4C). As expected, there was similarly no difference in glucose tolerance of Ptpn1osb−/− mice (Fig. 4D). However, Ptpn2osb−/− mice consistently exhibited increased insulin sensitivity as measured by an insulin tolerance test (ITT) (Fig. 4E). This effect was not observed in Ptpn1osb−/− mice (Fig. 4F). The increase in insulin sensitivity of Ptpn2osb−/− mice was also apparent when calculated as the area under the curve of the insulin tolerance test (Fig. 4G). To test whether glucose homeostasis was altered in the insulin-sensitive Ptpn2osb−/− mice under challenge conditions, we performed a glucose tolerance test on mice that were fed a high-fat diet for 5 weeks. Glucose tolerance was also unaffected under these conditions (Fig. 4I). These data indicated that osteoblast expression of TC-PTP but not of PTP1B regulates insulin sensitivity, presumably by influencing osteocalcin bioactivity. A glucose-stimulated insulin secretion (GSIS) test confirmed that insulin secretion was normal in Ptpn2osb−/− mice (Fig. 4H). Energy expenditure was also increased in Ptpn2osb−/− mice during the day cycle (Fig. 4J). These results indicated that although the increase in activated osteocalcin of Ptpn2osb−/− mice was sufficient to affect insulin sensitivity and energy expenditure, it was insufficient to affect insulin secretion, glucose tolerance, and night cycle energy expenditure. The role of TC-PTP is not identical to that of ESP, as Esposb−/− mice suffer from both hypoglycemia and hyperinsulinemia and display increased energy expenditure in both day and night cycles (33).
TC-PTP affects bone resorption through its expression in osteoblasts.
We had previously shown that insulin signaling in osteoblasts is a molecular determinant of osteocalcin bioactivity by promoting bone resorption. Accordingly, bone resorption is higher in Esposb−/− mice that are a model of a gain of function of insulin signaling in osteoblasts and have an increase in active osteocalcin (21). To determine whether TC-PTP regulates osteocalcin activity by utilizing the same mechanism of action, we asked whether TC-PTP influences bone resorption.
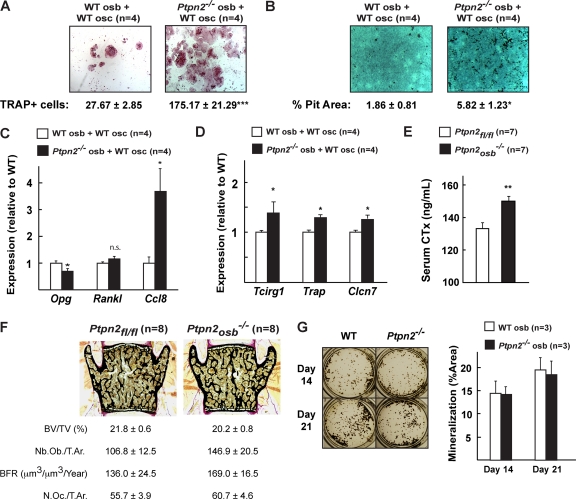
For that purpose, we performed a classical coculture assay (47). In this experiment, wild-type (WT) or Ptpn2−/− osteoblasts were cultured with WT monocytes in the presence of vitamin D3 (VitD3) and prostaglandin E2 (PGE2) for 8 days. At the end of the experiment, we stained for tartrate-resistant acid phosphatase (TRAP), an enzyme expressed only in mature osteoclasts, to evaluate osteoclast differentiation (10). TRAP staining revealed that osteoblasts lacking TC-PTP (Ptpn2−/−) induced greater osteoclast differentiation than did WT osteoblasts, as quantified by number of TRAP-positive cells (Fig. 5A). Functional analysis confirmed that the resorptive function of osteoclasts was also increased, as the resorptive pit area covered by osteoclasts cocultured with Ptpn2−/− osteoblasts was significantly larger than that of osteoclasts cultured with WT osteoblasts (Fig. 5B). Osteoblasts lacking TC-PTP (Ptpn2−/−) demonstrated decreased expression of Opg but no change in Rankl expression, as do Esp−/− osteoblasts (Fig. 5C) (21). In addition, we observed an increase in expression of Ccl8, a monocyte chemoattractant supporting osteoclast formation (Fig. 5C) (49). These results confirmed that TC-PTP, primarily through its regulation of insulin signaling in osteoblasts, influences bone resorption. Consistent with this contention, expression of Tcirg1, a gene expressed in osteoclasts but whose expression is regulated by insulin signaling in osteoblasts (21), was significantly increased in the osteoclasts cocultured with Ptpn2−/− osteoblasts (Fig. 5D). The same was true for Clcn7, a chloride channel also regulating the acidity of the resorption lacunae (27, 40), and Trap, a marker of osteoclasts (4) (Fig. 5D). Taken together, these experiments indicate that in cell culture, TC-PTP, presumably through its ability to inactivate the insulin receptor in the osteoblasts, affects Opg expression and thus regulates osteoclast differentiation and function.
Fig 5.
TC-PTP in osteoblasts regulates osteoclast differentiation. (A) Representative pictures of TRAP staining of osteoclasts cocultured in the presence of WT or Ptpn2−/− osteoblasts. Quantification of the number of TRAP-positive cells is shown below. (B) Representative pictures of resorptive activity of osteoclasts cocultured in the presence of WT or Ptpn2−/− osteoblasts. Quantification of resorptive pit area is shown below. (C) qPCR analysis of Opg, Rankl, and Ccl8 expression in WT or Ptpn2−/− osteoblasts cocultured with osteoclasts. (D) qPCR analysis of Tcirg1, Trap, and Clcn7 expression in osteoclasts cocultured in the presence of WT or Ptpn2−/− osteoblasts. (E) CTx serum levels in 6-week-old mice. Asterisks in panels C to E are defined in Materials and Methods. n.s., not significant. (F) Representative pictures and histomorphometric analysis of Ptpn2osb−/− mice and control Ptpn2fl/fl littermates. BV/TV, bone volume/tissue volume; N.Oc./T.Ar., number of osteoclasts/trabecular area; Nb.Ob./T.Ar., number of osteoblasts/trabecular area; BFR, bone formation rate/bone surface. (G) WT or Ptpn2−/− primary osteoblasts in culture differentiated for 14 and 21 days.
Accordingly, compared to control littermates, Ptpn2osb−/− mice have increased osteoclast activity as demonstrated by increased serum levels of CTx, a marker of bone resorption (Fig. 5E) (38). However, osteoclast number in these mice was not significantly changed, indicating that in vivo deletion of TC-PTP is sufficient to affect osteoclast activity but not osteoclast number (Fig. 5F).
Bone histomorphometry analysis performed in vertebrae revealed that at 6 weeks of age, bone volume was slightly decreased, though this did not reach statistical significance (Fig. 5F). Osteoblast number and bone formation rate were also not significantly altered. Primary osteoblasts lacking Ptpn2 similarly displayed no apparent proliferation and differentiation defect, as demonstrated by Von Kossa staining (Fig. 5F). This result suggests that TC-PTP does not affect osteoblast proliferation and ability to differentiate.
These data indicate that TC-PTP, by modulating insulin signaling in osteoblasts, is a determinant of osteoclast activity. It is through this mechanism that TC-PTP affects osteocalcin bioactivity and thus insulin sensitivity.
DISCUSSION
We demonstrate here that in addition to ESP, another tyrosine phosphatase, TC-PTP, regulates whole-body insulin sensitivity and day cycle energy expenditure by increasing osteocalcin activity through its expression in the osteoblast. These results further our understanding of the complexity of the regulation of osteocalcin activity and underscore the importance of the osteoblast as an endocrine cell type.
Using substrate-trapping assays and loss-of-function models, we show that a function of TC-PTP is to attenuate insulin signaling in the osteoblast. In view of these results, we generated mice lacking TC-PTP specifically in the osteoblast (Ptpn2osb−/−) and observed that the level of circulating active osteocalcin is increased in these mice compared to their littermate controls. This corresponded to an increase in whole-body insulin sensitivity and day cycle energy expenditure in Ptpn2osb−/− mice on a standard chow diet. These mice are therefore a partial phenocopy of Esposb−/− mice, which, in addition, display an increase in insulin secretion and increase in energy expenditure in the night cycle. These results suggest that activated osteocalcin may differentially regulate insulin secretion and insulin sensitivity. Accordingly, target tissues may respond to different activation thresholds of osteocalcin.
Though its role in the mouse osteoblast is more robust, ESP is nonfunctional in humans (11). In contrast, TC-PTP is conserved in humans (PTPN2) and has been implicated as a susceptibility gene in early-onset type I diabetes (19). Though TC-PTP may have a more minor role in mouse osteoblasts, its function of regulating osteocalcin activity is translatable to humans. Further study will be required to investigate whether ESP and TC-PTP regulate insulin signaling cooperatively in the mouse osteoblast.
That deletion of Ptpn2 in osteoblasts is insufficient to affect mineralization and proliferation highlights the dissociative effect of insulin signaling on osteoblast differentiation and osteocalcin activation and suggests the presence of other factors affecting insulin receptor activation (27). It thus remains to be seen if additional regulatory factors in the osteoblast are involved in the control of energy metabolism by bone. In particular, the role of the other tyrosine phosphatases expressed in osteoblasts that can bind the insulin receptor but that are not regulated by isoproterenol stimulation will need to be investigated. That Ptpn2 expression is stimulated upon treatment of osteoblasts with isoproterenol also raises the question of whether osteoblast expression of Ptpn2 contributes to the sympathetic regulation of bone mass and/or glucose homeostasis (30, 32).
As insulin signaling in osteoblasts has been shown to favor bone resorption (21), we also examined how TC-PTP deficiency in these cells affects osteoclast function. A coculture system demonstrated that TC-PTP regulates the differentiation and resorptive activity of osteoclasts though Opg expression in osteoblasts. Previous work had demonstrated a cell-autonomous function of TC-PTP in hematopoietic cell differentiation (42, 43). Mice lacking TC-PTP globally exhibit an increase in osteoclast density and bone resorption (15). The current work expands the importance of TC-PTP by showing that it is able to regulate differentiation cell nonautonomously.
TC-PTP has been previously implicated in the regulation of metabolism, as demonstrated by its role in the liver of regulating gluconeogenesis and function in the hypothalamus of regulating leptin signaling (26, 34). It also shares a phosphatase domain with PTP1B, the prototypical phosphatase of the insulin receptor and functional human homologue of ESP (3, 21). A ubiquitously expressed protein, PTP1B is implicated in the regulation of energy metabolism through its functions in muscle, fat, and liver and in the pro-opiomelanocortin (POMC) neurons of the brain (1, 5, 7, 13, 14). Hence, we explored the possibility that PTP1B expression in the osteoblast may also contribute to the regulation of energy metabolism. In vitro experiments suggest that PTP1B does not seem to affect insulin receptor activation in osteoblasts, at least not to the same extent that TC-PTP does. Additionally, mice specifically lacking Ptpn1 in osteoblasts (Ptpn1osb−/−) have no apparent differences in body weight, glucose tolerance, and insulin sensitivity from those of control littermates when fed on a normal diet.
That TC-PTP, but not PTP1B, regulates the osteocalcin activation pathway in mouse osteoblasts may be due to their relative levels of expression, as quantified by qPCR analysis (Fig. 1F). However, this difference may also be another example of divergent function between the two highly related phosphatases. Such a difference in the roles of PTP1B and TC-PTP has been documented for the regulation of cell spreading and adhesion (46) and pancreatic islet response to endoplasmic reticulum (ER) stress (8), as well as to the specificity of their substrates (36, 42, 43, 50). In addition, PTP1B and TC-PTP have been shown to contribute differentially to glucose homeostasis through their expression in muscle (35). Evidence from previous studies has also indicated that at a molecular level, the difference in PTP1B and TC-PTP regulation of the insulin receptor can be dissected to the level of the phosphorylated residue (28).
This work extends our understanding of the bone regulation of energy metabolism, by identifying TC-PTP as an additional regulator of the osteocalcin activation pathway. The finding that TC-PTP, a bona fide phosphatase of the insulin receptor, functions in osteoblasts to affect insulin sensitivity further establishes the role of insulin signaling in bone remodeling and energy metabolism.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health training grant Hormones: Biochemistry and Molecular Biology-T32DK07328 (T.Z.) and by a grant from the National Institutes of Health (G.K.).
We thank M. Ferron for reagents and help with coculture procedures and T. Yoshizawa for assistance with GSIS.
Footnotes
Published ahead of print 17 January 2012
REFERENCES
- 1. Agouni A, et al. 2011. Liver-specific deletion of protein tyrosine phosphatase (PTP) 1B improves obesity- and pharmacologically-induced endoplasmic reticulum stress. Biochem. J. 438:369–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alonso A, et al. 2004. Protein tyrosine phosphatases in the human genome. Cell 117:699–711 [DOI] [PubMed] [Google Scholar]
- 3. Andersen JN, et al. 2001. Structural and evolutionary relationships among protein tyrosine phosphatase domains. Mol. Cell. Biol. 21:7117–7136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Andersson GN, Marks SC., Jr 1989. Tartrate-resistant acid ATPase as a cytochemical marker for osteoclasts. J. Histochem. Cytochem. 37:115–117 [DOI] [PubMed] [Google Scholar]
- 5. Banno R, et al. 2010. PTP1B and SHP2 in POMC neurons reciprocally regulate energy balance in mice. J. Clin. Invest. 120:720–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barr AJ, et al. 2009. Large-scale structural analysis of the classical human protein tyrosine phosphatome. Cell 136:352–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bence KK, et al. 2006. Neuronal PTP1B regulates body weight, adiposity and leptin action. Nat. Med. 12:917–924 [DOI] [PubMed] [Google Scholar]
- 8. Bettaieb A, et al. 2011. Differential regulation of endoplasmic reticulum stress by protein tyrosine phosphatase 1B and T cell protein tyrosine phosphatase. J. Biol. Chem. 286:9225–9235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Blanchetot C, Chagnon M, Dube N, Halle M, Tremblay ML. 2005. Substrate-trapping techniques in the identification of cellular PTP targets. Methods 35:44–53 [DOI] [PubMed] [Google Scholar]
- 10. Burstone MS. 1959. Histochemical demonstration of acid phosphatase activity in osteoclasts. J. Histochem. Cytochem. 7:39–41 [DOI] [PubMed] [Google Scholar]
- 11. Cousin W, Courseaux A, Ladoux A, Dani C, Peraldi P. 2004. Cloning of hOST-PTP: the only example of a protein-tyrosine-phosphatase the function of which has been lost between rodent and human. Biochem. Biophys. Res. Commun. 321:259–265 [DOI] [PubMed] [Google Scholar]
- 12. Dacquin R, Starbuck M, Schinke T, Karsenty G. 2002. Mouse alpha1(I)-collagen promoter is the best known promoter to drive efficient Cre recombinase expression in osteoblast. Dev. Dyn. 224:245–251 [DOI] [PubMed] [Google Scholar]
- 13. Delibegovic M, et al. 2007. Improved glucose homeostasis in mice with muscle-specific deletion of protein-tyrosine phosphatase 1B. Mol. Cell. Biol. 27:7727–7734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Delibegovic M, et al. 2009. Liver-specific deletion of protein-tyrosine phosphatase 1B (PTP1B) improves metabolic syndrome and attenuates diet-induced endoplasmic reticulum stress. Diabetes 58:590–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Doody KM, et al. 3 October 2011. T cell protein tyrosine phosphatase deficiency results in spontaneous synovitis and subchondral bone resorption in mice. Arthritis Rheum. [Epub ahead of print.] doi:10.1002/art.33399 [DOI] [PubMed] [Google Scholar]
- 16. Ducy P, et al. 2000. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell 100:197–207 [DOI] [PubMed] [Google Scholar]
- 17. Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. 1997. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell 89:747–754 [DOI] [PubMed] [Google Scholar]
- 18. Engelke JA, Hale JE, Suttie JW, Price PA. 1991. Vitamin K-dependent carboxylase: utilization of decarboxylated bone Gla protein and matrix Gla protein as substrates. Biochim. Biophys. Acta 1078:31–34 [DOI] [PubMed] [Google Scholar]
- 19. Espino-Paisan L, et al. 2011. A polymorphism in PTPN2 gene is associated with an earlier onset of type 1 diabetes. Immunogenetics 63:255–258 [DOI] [PubMed] [Google Scholar]
- 20. Ferron M, Hinoi E, Karsenty G, Ducy P. 2008. Osteocalcin differentially regulates beta cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc. Natl. Acad. Sci. U. S. A. 105:5266–5270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ferron M, et al. 2010. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell 142:296–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ferron M, Wei J, Yoshizawa T, Ducy P, Karsenty G. 2010. An ELISA-based method to quantify osteocalcin carboxylation in mice. Biochem. Biophys. Res. Commun. 397:691–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Flier JS, Elmquist JK. 1997. Energetic pursuit of leptin function. Nat. Biotechnol. 15:20–21 [DOI] [PubMed] [Google Scholar]
- 24. Flint AJ, Tiganis T, Barford D, Tonks NK. 1997. Development of “substrate-trapping” mutants to identify physiological substrates of protein tyrosine phosphatases. Proc. Natl. Acad. Sci. U. S. A. 94:1680–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Friedman JM, Halaas JL. 1998. Leptin and the regulation of body weight in mammals. Nature 395:763–770 [DOI] [PubMed] [Google Scholar]
- 26. Fukushima A, et al. 2010. T-cell protein tyrosine phosphatase attenuates STAT3 and insulin signaling in the liver to regulate gluconeogenesis. Diabetes 59:1906–1914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fulzele K, et al. 2010. Insulin receptor signaling in osteoblasts regulates postnatal bone acquisition and body composition. Cell 142:309–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Galic S, et al. 2005. Coordinated regulation of insulin signaling by the protein tyrosine phosphatases PTP1B and TCPTP. Mol. Cell. Biol. 25:819–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hauschka PV, Lian JB, Cole DE, Gundberg CM. 1989. Osteocalcin and matrix Gla protein: vitamin K-dependent proteins in bone. Physiol. Rev. 69:990–1047 [DOI] [PubMed] [Google Scholar]
- 30. Hinoi E, et al. 2008. The sympathetic tone mediates leptin's inhibition of insulin secretion by modulating osteocalcin bioactivity. J. Cell Biol. 183:1235–1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hunter T. 1995. Protein kinases and phosphatases: the yin and yang of protein phosphorylation and signaling. Cell 80:225–236 [DOI] [PubMed] [Google Scholar]
- 32. Kajimura D, et al. 2011. Genetic determination of the cellular basis of the sympathetic regulation of bone mass accrual. J. Exp. Med. 208:841–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee NK, et al. 2007. Endocrine regulation of energy metabolism by the skeleton. Cell 130:456–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Loh K, et al. 2011. Elevated hypothalamic TCPTP in obesity contributes to cellular leptin resistance. Cell Metab. 14:684–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Loh K, et al. 2012. T cell protein tyrosine phosphatase (TCPTP) deficiency in muscle does not alter insulin signalling and glucose homeostasis in mice. Diabetologia 55:468–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nievergall E, et al. 2010. PTP1B regulates Eph receptor function and trafficking. J. Cell Biol. 191:1189–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rached MT, et al. 2010. FoxO1 expression in osteoblasts regulates glucose homeostasis through regulation of osteocalcin in mice. J. Clin. Invest. 120:357–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rosen HN, et al. 2000. Serum CTX: a new marker of bone resorption that shows treatment effect more often than other markers because of low coefficient of variability and large changes with bisphosphonate therapy. Calcif. Tissue Int. 66:100–103 [DOI] [PubMed] [Google Scholar]
- 39. Saltiel AR, Kahn CR. 2001. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 414:799–806 [DOI] [PubMed] [Google Scholar]
- 40. Schaller S, Henriksen K, Sorensen MG, Karsdal MA. 2005. The role of chloride channels in osteoclasts: ClC-7 as a target for osteoporosis treatment. Drug News Perspect. 18:489–495 [DOI] [PubMed] [Google Scholar]
- 41. Schlessinger J. 2000. Cell signaling by receptor tyrosine kinases. Cell 103:211–225 [DOI] [PubMed] [Google Scholar]
- 42. Simoncic PD, et al. 2006. T-cell protein tyrosine phosphatase (Tcptp) is a negative regulator of colony-stimulating factor 1 signaling and macrophage differentiation. Mol. Cell. Biol. 26:4149–4160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Simoncic PD, Lee-Loy A, Barber DL, Tremblay ML, McGlade CJ. 2002. The T cell protein tyrosine phosphatase is a negative regulator of janus family kinases 1 and 3. Curr. Biol. 12:446–453 [DOI] [PubMed] [Google Scholar]
- 44. Spiegelman BM, Flier JS. 2001. Obesity and the regulation of energy balance. Cell 104:531–543 [DOI] [PubMed] [Google Scholar]
- 45. Steiner DF. 2011. On the discovery of precursor processing. Methods Mol. Biol. 768:3–11 [DOI] [PubMed] [Google Scholar]
- 46. Stuible M, Doody KM, Tremblay ML. 2008. PTP1B and TC-PTP: regulators of transformation and tumorigenesis. Cancer Metastasis Rev. 27:215–230 [DOI] [PubMed] [Google Scholar]
- 47. Takahashi N, et al. 1988. Osteoblastic cells are involved in osteoclast formation. Endocrinology 123:2600–2602 [DOI] [PubMed] [Google Scholar]
- 48. Tonks NK. 2006. Protein tyrosine phosphatases: from genes, to function, to disease. Nat. Rev. Mol. Cell Biol. 7:833–846 [DOI] [PubMed] [Google Scholar]
- 49. Winslow MM, et al. 2006. Calcineurin/NFAT signaling in osteoblasts regulates bone mass. Dev. Cell 10:771–782 [DOI] [PubMed] [Google Scholar]
- 50. Xu D, Qu CK. 2008. Protein tyrosine phosphatases in the JAK/STAT pathway. Front. Biosci. 13:4925–4932 [DOI] [PMC free article] [PubMed] [Google Scholar]