Abstract
The Mpk1 pathway of Saccharomyces cerevisiae is a key determinant of cell wall integrity. A genetic link between the Mpk1 kinase and the Ppz phosphatases has been reported, but the nature of this connection was unclear. Recently, the Ppz phosphatases were shown to be regulators of K+ and pH homeostasis. Here, we demonstrate that Ppz-deficient strains display increased steady-state K+ levels and sensitivity to increased KCl concentrations. Given these observations and the fact that K+ is the major determinant of intracellular turgor pressure, we reasoned that the connection between PPZ1 and -2 and MPK1 was due to the combination of increased internal turgor pressure in Ppz-deficient strains and cell wall instability observed in strains lacking MPK1. Accordingly, the MPK1 gene was up-regulated, the Mpk1 protein was overexpressed, and the phosphorylated active form was more abundant in Ppz-deficient strains. Moreover, the expression of genes previously identified as targets of the Mpk1 pathway are also up-regulated in strains lacking PPZ1 and -2. The transcriptional and posttranslational modifications of Mpk1 were not observed when the internal K+ concentration (and thus turgor pressure) was lowered by disrupting the TRK1 and -2 K+ transporter genes or when the cell wall was stabilized by the addition of sorbitol. Moreover, we present genetic evidence showing that both the Wsc1 and Mid2 branches of the Mpk1 pathway contribute to this response. Finally, despite its role in G1/S transition, increased levels of activated Mpk1 do not appear to be responsible for the cell cycle phenotype observed in Ppz-deficient strains.
The cell wall is an essential and dynamic organelle of the budding yeast Saccharomyces cerevisiae that is required to maintain cell integrity during vegetative growth, mating, and stress conditions. Several lines of evidence have established an important role for the signal transduction cascade that begins with the Rho1 GTPase, which then activates protein kinase C (Pkc1), and culminates in the activation of a canonical mitogen-activated protein (MAP) kinase cascade, referred to as the Mpk1 pathway (recently reviewed in references 7 and 8). Phosphorylation of the Mpk1 MAP kinase (also known as Slt2) has several consequences in the cell, including activation of the serum response factor-like transcription factor Rlm1, responsible for regulating the expression of a myriad of genes implicated in cell wall biosynthesis (11, 27). Several environmental stimuli are known to induce Mpk1-mediated cell wall integrity signaling, such as elevated temperatures, hypo-osmotic shock, and exposure to mating pheromone (3, 12, 19, 30). Two transmembrane proteins, Wsc1 and Mid2, have been identified as the major sensors of cell wall stress. Wsc1 (also known as Hcs77 and Slg1) has been proposed to sense cell wall stress at the plasma membrane during vegetative growth (6, 9, 24, 26). Mid2, on the other hand, while also located at the plasma membrane, appears to function primarily during pheromone-induced morphogenesis. Although null mutations of either gene have mild effects on growth in normal media, the disruption of both genes causes a severe lytic phenotype, suggesting that the functions of these genes are partially redundant during vegetative growth (14, 24).
A genetic link between the Mpk1 pathway and the type 1-like Ppz protein phosphatases has been established. Specifically, the PPZ1 and PPZ2 genes act as multicopy suppressors of the temperature sensitivity of mpk1 or pkc1 strains (15). In addition, similar lytic phenotypes and caffeine sensitivity have been observed for strains lacking either components of the Mpk1 pathway or the PPZ1 gene (15, 23). Moreover, growth of a mutant strain lacking MPK1, PPZ1, and PPZ2 requires the addition of an osmostabilizer, such as 1 M sorbitol (15). Although these observations were made some time ago, the connection between these genes has remained unclear. Recently, it was reported that the Ppz phosphatases play an important role in the maintenance of K+ and pH homeostasis and that regulation of these physiological parameters has important consequences for the determination of membrane potential, cell cycle progression, and cell wall integrity (28).
Both the Mpk1 pathway and the Ppz phosphatases are known to have effects on G1 cell cycle progression. Specifically, Mpk1 is activated not only during cell wall stress but also during the G1/S transition of the cell cycle and in response to mating pheromone (3, 12, 19, 30). In addition, Mpk1 has been shown to physically associate with and phosphorylate components of the SBF transcription factor, which plays a vital role in the yeast cell cycle (16). Moreover, the function of the upstream activator of the Mpk1 pathway, Pkc1, is partially dependent on the presence of the yeast cyclin-dependent kinase, Cdc28, and overexpression of the PKC1 gene can rescue the bud emergence defect of a mutant lacking swi4, a gene encoding a component of the SBF transcription factor (6, 17). Therefore, the Mpk1 pathway is required for normal progression through the G1/S transition of the cell cycle. On the other hand, the Ppz phosphatases appear to negatively affect the G1 phase of the cell cycle. Overexpression of the PPZ1 gene causes a pronounced slow growth defect and accumulation of cells at G1 (2). Conversely, strains lacking Ppz activity recover faster from α-factor-induced G1 cell cycle arrest (4, 28). However, the mechanism by which Ppz activity affects cell cycle progression has yet to be identified.
In addition to the observed effects on cell cycle progression, strains deficient in Ppz activity display sensitivity to agents that are thought to destabilize the cell wall, such as caffeine and Calcofluor white (23, 28). The sensitivity of Ppz-deficient strains to these agents is relieved by further disruption of the genes encoding the high affinity K+ transporters encoded by the TRK1 and TRK2 genes. Thus, it appeared that a connection must exist between the alterations in K+ homeostasis and cell wall stability observed in these strains. Since K+ is the major determinant of turgor pressure in living cells, we reasoned that if strains lacking Ppz activity display a relative accumulation of K+ under steady-state conditions, then the internal turgor pressure of these strains would also be increased. This increase in turgor pressure would represent a constant stress on the cell walls of these strains. Therefore, constitutive reinforcement of the cell wall, presumably mediated by the Mpk1 pathway, would be required in these cells. Here we present several lines of evidence that support the proposed hypothesis.
MATERIALS AND METHODS
Yeast strains and culture conditions.
All strains of S. cerevisiae used in this work are listed in Table 1. The WSC1 gene was deleted by cloning genomic fragments corresponding to bp −200 to + 100 (relative to ATG) and bp 1 to 424 (after the stop) by PCR, digesting with restriction enzymes specific for sites in the primers, and inserting them into the pJJ252 vector (10), flanking the LEU2 open reading frame. For the MID2 deletion, an identical approach was followed except that genomic PCR fragments corresponding to bp −496 to −120 (before ATG) and bp 29 to 470 (after the stop) of the MID2 gene were used. Correct integration of the disruption cassettes was confirmed by genomic PCR using a 5′ primer upstream of the disruption cassette and a 3′ primer corresponding to the gene used for selection. Complete media contained 2% glucose, 2% peptone, and 1% yeast extract (YPD). Where indicated, YPD was supplemented with the indicated concentrations of sorbitol or KCl. Synthetic medium (SD) contained 2% glucose, 0.7% yeast nitrogen base (Difco) without amino acids, 50 mM MES [2-(N-morpholino)ethanesulfonic acid] adjusted to pH 5.5 with Tris, and the amino acids and purine and pyrimidine bases required by the strains.
TABLE 1.
Yeast strains used in this study
| Strain | Relevant genotype | Reference or source |
|---|---|---|
| DBY746 | Matα ura3-52 leu2-3 leu2-112 his3-Δ1 trp1-289 | A. Rodriguez-Navarro |
| LY 83 | DBY746 ppz1::URA3 ppz2::TRP1 | 22 |
| LY 85 | DBY746 ena1-4::HIS3 | 28 |
| LY 102 | DBY746 ena1-4::HIS3 ppz1::URA3 ppz2::TRP1 | 28 |
| W303-1A | Mataade2-1 can1-100 his3-11,15 leu2-3,112 trp1-1 ura3-1 | 5 |
| WΔ3 | W303-1A trk1::LEU2 trk2::HIS3 | 20 |
| LY 140 | W303-1A trk1::LEU2 trk2::HIS3 ppz1::URA3 ppz2::TRP1 | 28 |
| LY191 | W303-1A mid2::LEU2 | This study |
| LY 192 | W303-1A wsc1::LEU2 | This study |
| LY190 | W303-1A mpk1::LEU2 | This study |
| LY165 | W303-1A ppz1::TRP1 | This study |
| LY194 | W303-1A ppz1::TRP1 mid2::LEU2 | This study |
| LY195 | W303-1A ppz1::TRP1 wsc1::LEU2 | This study |
| LY193 | W303-1A ppz1::TRP1 mpk1::LEU2 | This study |
| LY139 | W303-1A ppz1::URA3 ppz2::TRP1 | 28 |
| LY197 | W303-1A ppz1::URA3 ppz2::TRP1 mid2::LEU2 | This study |
| LY198 | W303-1A ppz1::URA3 ppz2::TRP1 wsc1::LEU2 | This study |
| LY196 | W303-1A ppz1::URA3 ppz2::TRP1 mpk1::LEU2 | This study |
K+ measurements.
Internal [K+] was determined by high-performance liquid chromatography (HPLC) analysis as described previously (28).
Cell size measurements.
Cell size was analyzed in exponentially growing cells (after brief sonication) in the Particle Count and Size Analyzer, model Z2 (Coulter Inc.).
Western blot analysis.
The indicated strains were grown to mid-log phase in YPD supplemented or not with 1 M sorbitol. A total of 107 cells were pelleted by centrifugation and then frozen at −70°C. Cell pellets were resuspended in loading buffer, separated on a sodium dodecyl sulfate-8% polyacrylamide gel electrophoresis gel, transferred to a nitrocellulose membrane, and immunoblotted with either an anti-Mpk1 antiserum (1:1,000; a kind gift from M. Molina) or an anti-phospho-p44/42 MAP kinase monoclonal antibody (1:5,000; Cell Signaling Technology, Inc.). Anti-Mpk1 blots were visualized using the ECL detection system (Amersham), and anti-phospho-p44/42 MAP kinase blots were visualized using alkaline phosphatase.
Northern blot analysis.
Total RNA was isolated from yeast cells that were grown to mid-log-phase in a rich medium supplemented or not with 1 M sorbitol. Approximately 20 μg of RNA per lane was separated in formaldehyde gels and transferred to nylon membranes (Hybond-N; Amersham). Radioactively labeled probes were hybridized in PSE buffer (300 mM sodium phosphate [pH 7.2], 7% sodium dodecyl sulfate, 1 mM EDTA). PCR fragments derived from yeast chromosomal DNA representing nucleotides 1 to 990 of MPK1, 1 to 1016 of SED1, 1 to 977 of PIR3, and 77 to 706 of TBP1 were used as probes.
Pheromone response and recovery.
The indicated strains were grown in YPD medium to mid-log phase, and equal numbers of cells were added to top agar (YPD with 0.7% agar) and spread evenly on YPD plates. Immediately after solidification, sterile cellulose disks (diameter, 0.6 cm; Difco) with 14 μg of synthetic α-factor (Sigma) were placed on the nascent lawn. YPD medium, top agar, and plates were supplemented with the indicated amount of sorbitol. Images of plates were taken after 48 h of incubation at 28°C.
RESULTS
Strains lacking PPZ1 and -2 show alterations in steady-state [K+], are sensitive to high KCl concentrations, and have increased cell sizes.
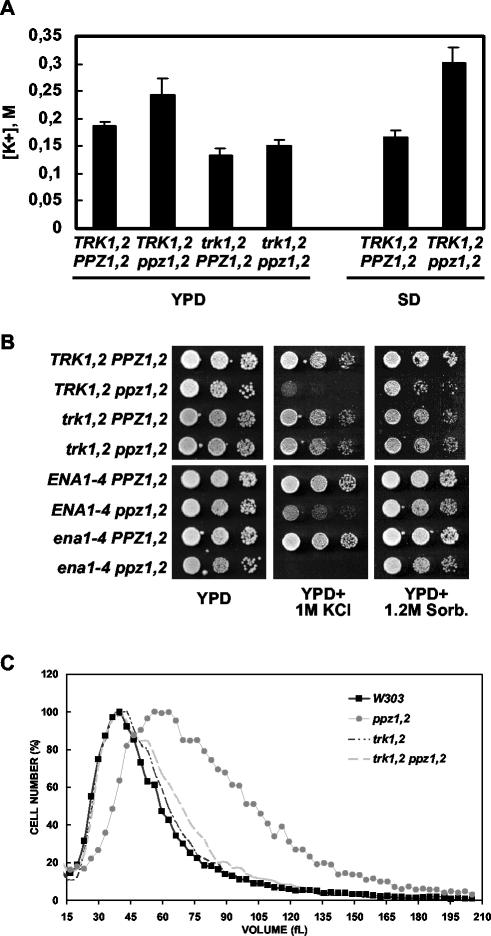
Various lines of evidence suggested TRK1 and -2-dependent alterations in the K+ homeostasis of strains lacking the PPZ1 and PPZ2 genes (28). Accordingly, we detected increases in the steady-state K+ levels of ppz1 ppz2 mutants grown in both rich and minimal media compared to levels in the isogenic wild-type strain (Fig. 1A). In addition, further disruption of the TRK1 and -2 genes in the ppz1 ppz2 background significantly lowered the internal K+ concentration to below wild-type levels. The slight differences observed between the ppz1 ppz2 trk1 trk2 strains and the trk1 trk2 mutant are not statistically significant, suggesting that the effect of the loss of the phosphatases on K+ homeostasis requires the presence of the TRK1 and -2 genes (Fig. 1A). One important prediction of the hypothesis that the Ppz phosphatases are the negative regulators of K+ uptake is that, without the genes, the cell would be unable to impede the entrance of excess K+. We observed that strains lacking the PPZ1 and -2 genes were sensitive to increased concentrations of KCl (Fig. 1B). This effect was specific for K+, as no effect on cell growth was observed in the presence of equivalent osmotic stress due to the addition of sorbitol or NaCl (Fig. 1B and data not shown). In addition, we observed that strains lacking PPZ1 and -2 were larger than the control strain, presumably due to increased turgor pressure (Fig. 1C). In agreement with our previous studies, both the K+ sensitivity of the ppz1 ppz2 strain and the increase in cell size were relieved by further disruption of the high-affinity K+ transporters encoded by the TRK1 and -2 genes. On the other hand, as would be expected, further disruption of the genes encoding the cation extrusion pumps, Ena1p to Ena4p, exacerbated the K+ sensitivity of the ppz1 ppz2 strain (Fig. 1B). Taken together, these data suggest that the absence of Ppz activity results in an inability to regulate the upper limits of K+ uptake, causing an increase in turgor pressure.
FIG. 1.
Analysis of intracellular accumulation of K+, KCl toxicity, and cell size in strains lacking PPZ1 and -2. (A) The indicated strains (W303-1A, LY139, LY140, and WΔ3) were grown to mid-log phase in either rich or minimal medium, as indicated, and processed for HPLC analysis of K+ content as described previously (28). Data are averages of three separate measurements; error bars, standard deviations. Similar results were observed in three different experiments. (B) The indicated strains (W303-1A, LY139, LY140, WΔ3, DBY746, LY83, LY85, and LY102) were grown to saturation in selective media, serially diluted in sterile water, and spotted onto YPD plates containing the indicated amount of KCl or sorbitol. Images were taken after 2 to 3 days of incubation at 28°C. Identical results were obtained in two separate experiments. (C) The indicated strains (as in panel A) were grown to early-log phase, and cell size was determined as described in Materials and Methods. Data are averages from three independent colonies of each genotype. Similar results were observed in two separate experiments.
MPK1 mRNA, protein, and phosphorylation levels in ppz1 ppz2 mutants.
Given the results demonstrating that loss of PPZ1 and 2 results in a marked increase in the internal K+ concentration and KCl sensitivity, we reasoned that the additive lytic phenotype of a ppz1 ppz2 mpk1 strain may be due to the combination of turgor pressure (caused by PPZ1 and -2 disruption) and a weakened cell wall (observed in mpk1 mutants). If this hypothesis is true, then strains lacking PPZ1 and -2 should display increased activity of the Mpk1 pathway due to the augmented internal turgor pressure. Therefore, we assayed the expression of the MPK1 gene, protein, and phosphorylation levels in ppz1 ppz2 mutant strains. We observed a marked up-regulation of the MPK1 mRNA in ppz1 ppz2 mutants compared to levels in the wild-type strain (sixfold induction [Fig. 2A]). Similar results were also observed in the DBY746 background (data not shown). The increase in MPK1 expression was removed by adding an osmotic stabilizer to the external medium (1 M sorbitol) or by lowering the internal K+ concentration in the ppz1 ppz2 mutant by further disruption of the TRK1 and -2 genes.
FIG. 2.
Northern and Western blot analyses of Mpk1 expression and phosphorylation. (A) The indicated strains (W303-1A, LY139, LY140, WΔ3, and LY190) were grown to mid-log phase in complete media supplemented where indicated with 1 M sorbitol (SORB). Northern blot analysis was performed as described in Materials and Methods by using the indicated probes. TBP1 was used as an internal loading control. (B) The same strains described above were grown to mid-log phase in complete media supplemented with the indicated amount of sorbitol, and immunoblots were prepared as described in Materials and Methods. The upper panel represents an immunoblot using an antiserum raised against Mpk1, and the center panel represents an immunoblot with a monoclonal antibody specific for the dually phosphorylated form of Mpk1 (Mpk1*). Similar results were observed in three separate experiments. The Ponceau S staining of the membrane is shown in the bottom panel as a control for protein loading.
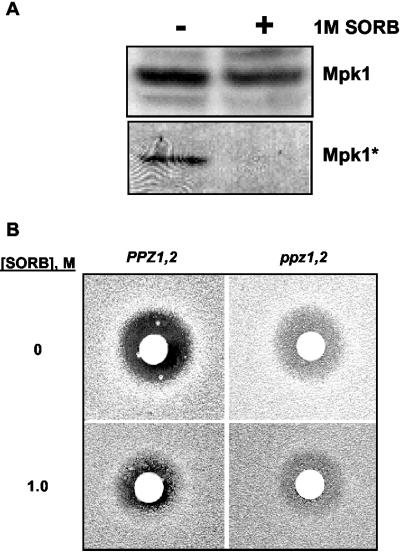
We next examined the steady state levels of the Mpk1 protein in the same strains. In agreement with the results from Northern blot analysis, higher levels of Mpk1 protein were detected in Ppz-deficient strains (an increase of approximately fourfold [Fig. 2B]). As observed at the level of mRNA, this increase in Mpk1 expression was not observed in Ppz-deficient strains lacking TRK1 and -2. In the presence of 1 M sorbitol, the expression levels of Mpk1 were decreased in ppz1 ppz2 strains but remained slightly higher than those in the isogenic wild-type strain. Since dual phosphorylation of Mpk1 has been thoroughly shown to correlate with activation of the kinase activity, we used a dual phosphorylation-specific monoclonal antibody to assay the levels of Mpk1 activation (18, 26). We observed that under steady-state conditions, higher levels of phosphorylated Mpk1 were detectable in strains lacking PPZ1 and -2 than in the wild type (Fig. 2B, lanes a and c). The Mpk1 phosphorylation was not observed upon addition of an osmotic stabilizer (1 M sorbitol) to the medium to counteract the internal turgor pressure and remove the cell wall stress (Fig. 2B, lanes c and d). As indicated by Fig. 7A below, phosphorylation was not observed in the presence of sorbitol when equal amounts of Mpk1 protein were analyzed. Moreover, in agreement with previous results, Mpk1 phosphorylation was not detectable in Ppz-deficient strains lacking TRK1 and -2 (Fig. 2B, lane e). These analyses demonstrate that in strains lacking PPZ1 and -2, under steady-state conditions, more Mpk1 protein is present in the cell and increases in the phosphorylated form of Mpk1 are readily detectable, suggesting that this pathway may be more active in ppz1 ppz2 strains.
FIG. 7.
Strains lacking PPZ1 and -2 continue to display accelerated α-factor recovery in the presence of sorbitol. (A) Strain LY139 was grown to mid-log phase in YPD medium containing the indicated amounts of sorbitol (SORB), processed as described in Materials and Methods. Nitrocellulose membranes were immunoblotted with the anti-Mpk1 antibody (upper panel) and the phosphospecific anti-Mpk1 antibody (lower panel). Similar results were observed in two different experiments. (B) The indicated strains (W303-1A and LY139) were grown to an optical density of 0.4 at 660 nm in complete medium containing the indicated concentration of sorbitol, and the assay was performed as described in Materials and Methods. Images of the plates were taken after 48 h. Identical results were observed in two separate experiments.
Transcriptional regulation of targets of the Mpk1 pathway in strains lacking PPZ1 and -2.
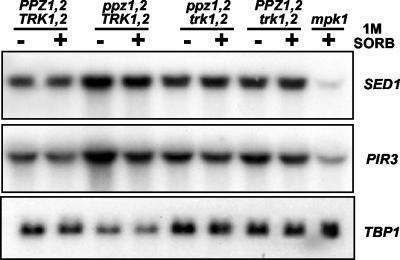
Having observed that the protein and phosphorylation levels of Mpk1 are higher in strains lacking Ppz activity, we wanted to use an independent experimental approach to determine whether this pathway is activated. To this end, we examined the transcriptional regulation of some representative targets of the Mpk1 pathway. Based on a previous study that identified many genes regulated by the Mpk1 pathway (11), we chose two representative genes encoding putative cell wall proteins. The SED1 gene encodes a protein of unknown function, but it is predicted to be a glycosylphosphatidylinositol (GPI)-modified protein and a component of the plasma membrane and cell wall (1). The PIR3 gene is a member of the PIR family of cell wall proteins implicated in resistance to the antifungal protein osmotin (29). This class of proteins has been shown to differ from GPI-linked proteins in being attached to the cell wall directly through β-1,3-glucan, and to date, two members of this family, PIR1 and PIR2, have been implicated in tolerance to heat shock (13). We observed increases in the steady-state transcript levels of these genes in strains lacking PPZ1 and -2 (5- and 3.5-fold induction of SED1 and PIR3, respectively [Fig. 3]). Moreover, this response was not observed in Ppz-deficient strains lacking TRK1 and -2. These data are in good agreement with those observed at the level of Mpk1 protein expression and phosphorylation, and they provide strong evidence that the Mpk1 pathway is activated in Ppz-deficient strains in a TRK1- and -2-dependent manner.
FIG. 3.
Target genes of the Mpk1 pathway are up-regulated in Ppz-deficient strains. The indicated strains (W303-1A, LY139, LY140, WΔ3, and LY190) were grown to mid-log phase in complete media supplemented, where indicated, with 1 M sorbitol (SORB). Northern blot analysis was performed as described in Materials and Methods by using the indicated probes. TBP1 was used as an internal loading control.
Exacerbation of lytic phenotypes by exogenous KCl.
To establish phenotypic confirmation of the model presented, we examined the growth of various mutants in media supplemented with KCl. Our hypothesis would contend that the addition of exogenous KCl should exacerbate the lytic phenotype of ppz1 ppz2 mpk1 strains. We clearly observed that the lytic phenotype of the compound mutant was augmented upon addition of exogenous KCl (Fig. 4). The growth of strains lacking MPK1 is equivalent to that of the wild-type strain in rich media supplemented with 0.8 M KCl. But, as would be predicted, strains lacking MPK1 and PPZ1 and -2 grow very poorly in media supplemented with KCl. Identical results were observed in ppz1 mpk1 strains (data not shown). However, this effect is not observed when an equivalent osmotic stress, in the form of sorbitol, is added to the medium, demonstrating that the sensitivity of these strains is KCl specific.
FIG. 4.
The KCl sensitivity of the ppz1 ppz2 strain is exacerbated by the further disruption of MPK1. The indicated strains (W303-1A, LY190, LY139, and LY196) were grown to saturation in YPD plus 1 M sorbitol (SORB.), serially diluted in sterile 1 M sorbitol, and spotted onto YPD plates containing the indicated amount of KCl or sorbitol. Images were taken after 2 to 3 days of incubation at 28°C. Identical results were obtained in two separate experiments. Identical results were also observed in minimal media (data not shown).
The Wsc1- and Mid2-mediated branches of the Mpk1 pathway contribute to the response to internal turgor pressure.
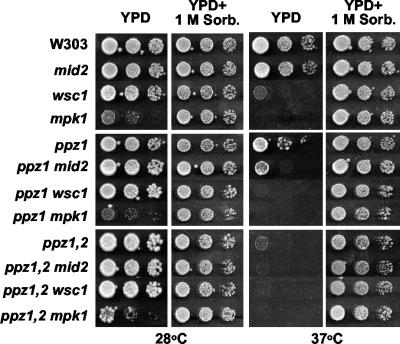
Recent work from several laboratories has demonstrated that Mpk1 can be stimulated through two upstream pathways, one mediated by Wsc1 and the other by Mid2 (6, 9, 14, 24, 26). The Wsc1 (also known as Hsc77 or Slg1) part of the pathway has been shown to sense cell wall stress during vegetative growth, whereas Mid2 functions principally in signaling cell wall stress during pheromone-induced morphogenesis. Both pathways converge on the Rho1 guanine nucleotide exchange factor, Rom2, and lead to the activation of the Mpk1 pathway via Pkc1 (21). Because we were interested in establishing which of these sensors is responsible for the response to increased internal turgor pressure, we constructed the corresponding strains lacking WSC1 or MID2 alone or in combination with disruption of the PPZ1 and -2 genes. Interestingly, disruption of either WSC1 or MID2 in the ppz1 ppz2 background showed no phenotypes at 28°C. However, both the ppz1 ppz2 wsc1 strain and the ppz1 ppz2 mid2 strain displayed a severe lytic phenotype at 37°C, which could be rescued by the addition of 1 M sorbitol to the medium (Fig. 5). However, since disruption of both PPZ1 and PPZ2 causes a severe cell lysis defect at 37°C, we went on to construct disruptions of WSC1 and MID2 in the ppz1 single-mutant background, where the lytic phenotype at 37°C is not as severe. In these strains, we observed that disruption of either MID2 or WSC1 exacerbated the lytic phenotype observed for the ppz1 mutant. Thus, both Wsc1 and Mid2 contribute to the sensing of internal turgor pressure.
FIG. 5.
Disruption of either WSC1 or MID2 exacerbates the lytic phenotype of the ppz1 mutant. The indicated strains (W303-1A, LY191, LY192, LY190, LY165, LY194, LY195, LY193, LY139, LY197, LY198, and LY196) were grown to saturation in YPD medium supplemented with 1 M sorbitol (Sorb.), serially diluted in sterile 1 M sorbitol, and spotted onto YPD plates containing the indicated amount of sorbitol. Images were taken after 2 to 3 days of incubation at 28 or 37°C. Identical results were obtained in two separate experiments.
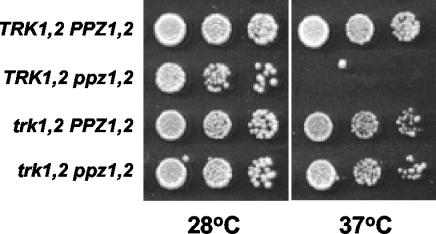
The temperature sensitivity of ppz1 ppz2 strains is rescued by further disruption of TRK1 and -2.
The temperature-sensitive phenotype of mutants lacking PPZ1 and -2 was originally cited as one of the observations supporting the model that these phosphatases participate in the cell wall integrity pathway. According to our present hypothesis, this Ppz-deficient phenotype may also be explained by increased turgor pressure provoking lysis during cell wall stress caused by high temperatures. To examine this possibility, we assayed the growth of various mutant strains at both 28 and 37°C. We observed that further disruption of the TRK1 and -2 genes, which would decrease the turgor pressure, relieved the lytic phenotype observed for ppz1 ppz2 mutants at 37°C (Fig. 6). These data suggest that cell wall stress caused by increased turgor pressure is responsible for the temperature-dependent lytic phenotype of Ppz-deficient strains.
FIG. 6.
The lytic phenotype of the ppz1 ppz2 mutant is relieved by further disruption of TRK1 and -2. The indicated strains (W303-1A, LY139, LY140, WΔ3, and LY190) were grown to saturation in rich media, serially diluted in sterile water, and spotted onto YPD plates. Images were taken after 2 to 3 days of incubation at 28 or 37°C. Identical results were obtained in two separate experiments.
Strains lacking PPZ1 and -2 continue to show accelerated recovery from α-factor in the presence of sorbitol.
The overexpression and disruption of PPZ1 have been shown to have opposite effects on G1/S transition. Specifically, the disruption of the PPZ genes leads to an accelerated G1/S transition, as indicated by accelerated recovery from α-factor arrest, whereas overexpression of PPZ1 causes a G1 block (2). In addition, previous reports have demonstrated that Mpk1 is activated during the G1/S transition and that this activation is important for bud emergence (19, 30). Therefore, we tested whether the accelerated G1/S transition observed after α-factor treatment in ppz1 ppz2 mutant strains was due to the constitutive activation of Mpk1. First, we confirmed the lack of Mpk1 phosphorylation in Ppz-deficient strains grown in the presence of an osmotic stabilizer (1 M sorbitol). Since Mpk1 is overexpressed in strains lacking PPZ1 and -2 grown in rich media, we first empirically determined the amount of extracts required for equal Mpk1 loading in samples grown with or without sorbitol (Fig. 7A, top panel). The levels of phosphorylated Mpk1 were then assayed in the same samples (Fig. 7A, bottom panel). The absence of detectable phosphorylated Mpk1 in the presence of 1 M sorbitol presented in Fig. 2B was confirmed under conditions of equal loading of Mpk1 protein. In addition, phosphorylated Mpk1 can still be detected in a fivefold dilution of the sample not treated with sorbitol, suggesting that, in the presence of sorbitol, phosphorylation of Mpk1 is less than 20% of that observed without sorbitol (data not shown). Under these conditions, where Mpk1 activation is not detectable (1 M sorbitol), ppz1 ppz2 mutant strains continue to recover faster from α-factor treatment (Fig. 7B). Although these results do not completely discard a role for Mpk1 in this ppz1- and -2-dependent accelerated α-factor recovery, it appears that other cell cycle components are largely responsible for this PPZ-dependent phenotype.
DISCUSSION
Elucidating the connection between signal transduction pathways established using genetic approaches is currently an important task in molecular and cellular biology. Some time ago, two lines of evidence established a genetic interaction between a MAP kinase, Mpk1 (Slt2), and the type 1-related phosphatases, Ppz1 and -2. Specifically, PPZ1 and -2 were identified as multicopy suppressors of the temperature sensitivity of mpk1 and pkc1 mutant strains (15). In addition, strains lacking either PPZ1 and -2 or MPK1 display similar temperature-dependent cell lysis defects, and these effects are additive when all three genes are disrupted (15). These observations were initially interpreted as evidence that both Mpk1 and Ppz phosphatases contributed to a pathway(s) governing the maintenance of cell wall stability. Here, we demonstrate that the link between these enzymes is not due to changes in the phosphorylation state of proteins in a common pathway required for cell wall stability. Rather, these enzymes affect very different but interrelated cellular properties. On the one hand, the Ppz phosphatases regulate K+/pH homeostasis within the cell in a Trk1- and -2-dependent manner (28; this work). This regulation has important implications for ion homeostasis, cell cycle progression, and determination of the internal turgor pressure. On the other hand, the Mpk1 pathway has been shown to up-regulate a number of genes required for cell wall stability in response to cell stresses and during normal cell growth and differentiation (11). The combined effects on turgor pressure and cell wall strength explain all of the previously reported connections between these enzymes.
Several lines of evidence support this hypothesis. First, we show that mutants lacking PPZ1 and -2 accumulate more intracellular KCl under both normal growth conditions and NaCl stress (Fig. 1) (28). Ppz-deficient strains are also specifically sensitive to exogenous KCl. Interestingly, we could observe this sensitivity of ppz1 ppz2 strains in minimal medium containing only 0.2 M KCl (data not shown). Additionally, we show that these mutants have an augmented cell size. This increase in cell size is observed in all stages of the cell cycle and does not therefore reflect an accumulation of cells at the G2/M phase of the cell cycle (data not shown). Moreover, we observed that addition of KCl to the external medium greatly exacerbates the lytic phenotype of mpk1 ppz1 ppz2 strains. Taken together, these results demonstrate that the internal turgor pressure of strains lacking Ppz activity is increased over that of the wild-type strain.
As would be expected for a strain under constant cell wall stress, the MPK1 mRNA is up-regulated, the Mpk1 protein is overexpressed, and the phosphorylated form is more abundant in strains lacking PPZ1 and -2. Moreover, two representative target mRNAs of the Mpk1 pathway, SED1 and PIR3, are also up-regulated in ppz1 ppz2 mutants. Taken together, these data provide strong evidence that the cell wall integrity pathway is activated in response to the increased turgor pressure caused by disruption of the PPZ1 and PPZ2 genes. These effects on Mpk1 are removed either by addition of an osmotic stabilizer, presumably to counteract the cell wall stress, or by further disruption of the genes encoding the high-affinity K+ uptake system, TRK1 and -2.
Removal of Trk1 and -2 greatly decreases the capability of the cell to import K+ from the external medium and would therefore relieve the internal turgor pressure in strains lacking PPZ1 and -2. Accordingly, removal of the TRK1 and -2 genes from the ppz1 ppz2 mutant reduces the overexpression and activation of Mpk1 and relieves the temperature-dependent cell lysis defect observed for the ppz1 ppz2 mutant. These data add to the list of Ppz-deficient phenotypes that depend on the presence of TRK1 and -2. Biochemical studies are in progress to establish whether the Ppz protein phosphatases directly regulate these potassium transporters. As we have also observed that Ppz-deficient mutants that do not express TRK1 and -2 display a slight but reproducible relative tolerance to LiCl and NaCl, it is very likely that these protein phosphatases have more than one target in the cell (28). Further studies are aimed at identifying these proteins, but one recent study points to a possible role for the calcineurin pathway (25).
We also present evidence demonstrating that both the Wsc1 and Mid2 branches of the Mpk1 pathway contribute to the sensing of internal turgor pressure. This result is not surprising, considering the previously reported functions of Wsc1 and Mid2 as sensors of cell wall stress. However, our results add to the definition of the function of these sensors by demonstrating that the response to cell wall stress is not limited in its duration, since it is a chronic response in the case of the ppz1 ppz2 mutant, and that changes in the external environment are not required. These observations suggest that the cell has a size limit and that when cells are presented with chronically increased internal K+ concentrations, they respond by activating the Mpk1 pathway to reinforce the cell wall in order to counteract an increase in cell volume and avoid lysis.
The genes encoding Mpk1 and Ppz1 and -2 have been implicated in cell cycle progression (2, 30). Owing to its important role in stimulating the expression of several cell wall components, Mpk1 is thought to contribute mainly to the dynamic reorganization of the cell wall required for bud emergence. Accordingly, Mpk1 is activated upon addition of α-factor, presumably to facilitate cell wall changes necessary for mating (30). However, Mpk1 has also been reported to bind to and phosphorylate components of the SBF transcription factor, which is a key regulator in the yeast G1/S transition (16), suggesting perhaps a more general role for Mpk1 in cell cycle progression. Indeed, not only has the upstream activator of Mpk1, Pkc1, been identified as a multicopy suppressor of a mutant of one of the components of the SBF transcription factor, Swi4, but this suppression requires the G1 cyclins, Cln1 and Cln2 (6). Thus, one plausible explanation for the accelerated G1/S transition observed in ppz1 ppz2 mutants treated with α-factor was the relative increase in levels of activated Mpk1. However, under conditions where no Mpk1 phosphorylation is detected, ppz1 ppz2 mutant strains continue to display the same α-factor recovery phenotype. Although it is clear that recovery from an α-factor-induced cell cycle block does not necessarily indicate changes in the G1/S transition during vegetative growth, our results provide evidence that Mpk1 activation is not likely to fully account for this phenotype and that other cell cycle components may respond to increases in internal K+/pH to drive G1 progression in Ppz-deficient strains.
Acknowledgments
L.Y. was supported by a postdoctoral fellowship from the European Molecular Biology Organization and is presently an investigator sponsored by the “Ramón y Cajal” Program (Spanish Ministerio de Ciencia y Tecnología, Madrid, Spain). S.M. is supported by a predoctoral grant from the Ministerio de Ciencia y Tecnología (Madrid, Spain). This work was supported by grants PB98-0565-C04-01, BMC2000-1129, and PB98-0565-C04-02 from the Spanish Ministerio de Ciencia y Tecnología.
We thank José Ramón Murguía for helpful discussions and Maria Molina for anti-Mpk1 antibodies.
REFERENCES
- 1.Caro, L. H., H. Tettelin, J. H. Vossen, A. F. Ram, H. van den Ende, and F. M. Klis. 1997. In silicio identification of glycosyl-phosphatidylinositol-anchored plasma-membrane and cell wall proteins of Saccharomyces cerevisiae. Yeast 13:1477-1489. [DOI] [PubMed] [Google Scholar]
- 2.Clotet, J., E. Gari, M. Aldea, and J. Ariño. 1999. The yeast Ser/Thr phosphatases Sit4 and Ppz1 play opposite roles in regulation of the cell cycle. Mol. Cell. Biol. 19:2408-2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davenport, K. R., M. Sohaskey, Y. Kamada, D. E. Levin, and M. C. Gustin. 1995. A second osmosensing signal transduction pathway in yeast. Hypotonic shock activates the PKC1 protein kinase-regulated cell integrity pathway. J. Biol. Chem. 270:30157-30161. [DOI] [PubMed] [Google Scholar]
- 4.Ferrando, A., S. J. Kron, G. Rios, G. R. Fink, and R. Serrano. 1995. Regulation of cation transport in Saccharomyces cerevisiae by the salt tolerance gene HAL3. Mol. Cell. Biol. 15:5470-5481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goossens, A., N. de La Fuente, J. Forment, R. Serrano, and F. Portillo. 2000. Regulation of yeast H+-ATPase by protein kinases belonging to a family dedicated to activation of plasma membrane transporters. Mol. Cell. Biol. 20:7654-7661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gray, J. V., J. P. Ogas, Y. Kamada, M. Stone, D. E. Levin, and I. Herskowitz. 1997. A role for the Pkc1 MAP kinase pathway of Saccharomyces cerevisiae in bud emergence and identification of a putative upstream regulator. EMBO J. 16:4924-4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heinisch, J. J., A. Lorberg, H. P. Schmitz, and J. J. Jacoby. 1999. The protein kinase C-mediated MAP kinase pathway involved in the maintenance of cellular integrity in Saccharomyces cerevisiae. Mol. Microbiol. 32:671-680. [DOI] [PubMed] [Google Scholar]
- 8.Hohmann, S. 2002. Osmotic stress signaling and osmoadaptation in yeasts. Microbiol. Mol. Biol. Rev. 66:300-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacoby, J. J., S. M. Nilius, and J. J. Heinisch. 1998. A screen for upstream components of the yeast protein kinase C signal transduction pathway identifies the product of the SLG1 gene. Mol. Gen. Genet. 258:148-155. [DOI] [PubMed] [Google Scholar]
- 10.Jones, J. S., and L. Prakash. 1990. Yeast Saccharomyces cerevisiae selectable markers in pUC18 polylinkers. Yeast 6:363-366. [DOI] [PubMed] [Google Scholar]
- 11.Jung, U. S., and D. E. Levin. 1999. Genome-wide analysis of gene expression regulated by the yeast cell wall integrity signalling pathway. Mol. Microbiol. 34:1049-1057. [DOI] [PubMed] [Google Scholar]
- 12.Kamada, Y., U. S. Jung, J. Piotrowski, and D. E. Levin. 1995. The protein kinase C-activated MAP kinase pathway of Saccharomyces cerevisiae mediates a novel aspect of the heat shock response. Genes Dev. 9:1559-1571. [DOI] [PubMed] [Google Scholar]
- 13.Kapteyn, J. C., P. Van Egmond, E. Sievi, H. van den Ende, M. Makarow, and F. M. Klis. 1999. The contribution of the O-glycosylated protein Pir2p/Hsp150 to the construction of the yeast cell wall in wild-type cells and β1,6-glucan-deficient mutants. Mol. Microbiol. 31:1835-1844. [DOI] [PubMed] [Google Scholar]
- 14.Ketela, T., R. Green, and H. Bussey. 1999. Saccharomyces cerevisiae Mid2p is a potential cell wall stress sensor and upstream activator of the PKC1-MPK1 cell integrity pathway. J. Bacteriol. 181:3330-3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee, K. S., L. K. Hines, and D. E. Levin. 1993. A pair of functionally redundant yeast genes (PPZ1 and PPZ2) encoding type 1-related protein phosphatases function within the PKC1-mediated pathway. Mol. Cell. Biol. 13:5843-5853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Madden, K., Y. J. Sheu, K. Baetz, B. Andrews, and M. Snyder. 1997. SBF cell cycle regulator as a target of the yeast PKC-MAP kinase pathway. Science 275:1781-1784. [DOI] [PubMed] [Google Scholar]
- 17.Marini, N. J., E. Meldrum, B. Buehrer, A. V. Hubberstey, D. E. Stone, A. Traynor-Kaplan, and S. I. Reed. 1996. A pathway in the yeast cell division cycle linking protein kinase C (Pkc1) to activation of Cdc28 at START. EMBO J. 15:3040-3052. [PMC free article] [PubMed] [Google Scholar]
- 18.Martin, H., J. M. Rodriguez-Pachon, C. Ruiz, C. Nombela, and M. Molina. 2000. Regulatory mechanisms for modulation of signaling through the cell integrity Slt2-mediated pathway in Saccharomyces cerevisiae. J. Biol. Chem. 275:1511-1519. [DOI] [PubMed] [Google Scholar]
- 19.Mazzoni, C., P. Zarov, A. Rambourg, and C. Mann. 1993. The SLT2 (MPK1) MAP kinase homolog is involved in polarized cell growth in Saccharomyces cerevisiae. J. Cell Biol. 123:1821-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mulet, J. M., M. P. Leube, S. J. Kron, G. Rios, G. R. Fink, and R. Serrano. 1999. A novel mechanism of ion homeostasis and salt tolerance in yeast: the Hal4 and Hal5 protein kinases modulate the Trk1-Trk2 potassium transporter. Mol. Cell. Biol. 19:3328-3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Philip, B., and D. E. Levin. 2001. Wsc1 and Mid2 are cell surface sensors for cell wall integrity signaling that act through Rom2, a guanine nucleotide exchange factor for Rho1. Mol. Cell. Biol. 21:271-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Posas, F., M. Camps, and J. Ariño. 1995. The PPZ protein phosphatases are important determinants of salt tolerance in yeast cells. J. Biol. Chem. 270:13036-13041. [DOI] [PubMed] [Google Scholar]
- 23.Posas, F., A. Casamayor, and J. Ariño. 1993. The PPZ protein phosphatases are involved in the maintenance of osmotic stability of yeast cells. FEBS Lett. 318:282-286. [DOI] [PubMed] [Google Scholar]
- 24.Rajavel, M., B. Philip, B. M. Buehrer, B. Errede, and D. E. Levin. 1999. Mid2 is a putative sensor for cell integrity signaling in Saccharomyces cerevisiae. Mol. Cell. Biol. 19:3969-3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruiz, A., L. Yenush, and J. Ariño. 2003. Regulation of the expression of the ENA1 Na+-ATPase gene by the Ppz1 protein phosphatase is mediated by the calcineurin pathway. Eukaryot. Cell 2:937-948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verna, J., A. Lodder, K. Lee, A. Vagts, and R. Ballester. 1997. A family of genes required for maintenance of cell wall integrity and for the stress response in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 94:13804-13809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watanabe, Y., K. Irie, and K. Matsumoto. 1995. Yeast RLM1 encodes a serum response factor-like protein that may function downstream of the Mpk1 (Slt2) mitogen-activated protein kinase pathway. Mol. Cell. Biol. 15:5740-5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yenush, L., J. M. Mulet, J. Arino, and R. Serrano. 2002. The Ppz protein phosphatases are key regulators of K+ and pH homeostasis: implications for salt tolerance, cell wall integrity and cell cycle progression. EMBO J. 21:920-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yun, D. J., Y. Zhao, J. M. Pardo, M. L. Narasimhan, B. Damsz, H. Lee, L. R. Abad, M. P. D'Urzo, P. M. Hasegawa, and R. A. Bressan. 1997. Stress proteins on the yeast cell surface determine resistance to osmotin, a plant antifungal protein. Proc. Natl. Acad. Sci. USA 94:7082-7087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zarzov, P., C. Mazzoni, and C. Mann. 1996. The SLT2(MPK1) MAP kinase is activated during periods of polarized cell growth in yeast. EMBO J. 15:83-91. [PMC free article] [PubMed] [Google Scholar]