Abstract
The repulsive guidance molecule A (RGMa) is a contact-mediated axon guidance molecule that has significant roles in central nervous system (CNS) development. Here we have examined whether RGMa has novel roles in cell migration and cell adhesion outside the nervous system. RGMa was found to stimulate cell migration from Xenopus animal cap explants in a neogenin-dependent and BMP-independent manner. RGMa also stimulated the adhesion of Xenopus animal cap cells, and this adhesion was dependent on neogenin and independent of calcium. To begin to functionally characterize the role of specific domains in RGMa, we assessed the migratory and adhesive activities of deletion mutants. RGMa lacking the partial von Willebrand factor type D (vWF) domain preferentially perturbed cell adhesion, while mutants lacking the RGD motif affected cell migration. We also revealed that manipulating the levels of RGMa in vivo caused major migration defects during Xenopus gastrulation. We have revealed here novel roles of RGMa in cell migration and adhesion and demonstrated that perturbations to the homeostasis of RGMa expression can severely disrupt major morphogenetic events. These results have implications for understanding the role of RGMa in both health and disease.
INTRODUCTION
Repulsive guidance molecule (RGM) was first identified in the embryonic chick retinotectal system as a chemorepulsive molecule for retinal axons (35). Subsequently, three different RGMs (RGMa, RGMb, and RGMc) were identified in mouse, and each was found to have a unique spatiotemporal expression pattern (41, 48). RGMa is a membrane-bound protein with ∼430 amino acid (aa) residues that has a glycosylphosphatidylinositol (GPI)-anchored C-terminal domain and a conventional N-terminal signal peptide (35, 48). It is cysteine rich and contains a putative autoproteolytic cleavage site, a single tri-amino acid motif, Arg-Gly-Asp (RGD), a partial von Willebrand factor type D (vWF) domain, and two hydrophobic domains at the N and C termini. These key molecular structures, except for the RGD domain (which is absent in RGMb in most animal models), are shared by all members of the RGM family (5, 35). A conserved RSDSPEI sequence, 5′ to the partial von Willebrand type D domain, is present within RGMa from mammals, frogs, and birds. To date, the roles of these different RGM domains remain unknown.
The first RGM receptor was identified by cell surface binding of chick RGM to cells expressing neogenin, a member of the immunoglobulin superfamily of transmembrane receptors (31, 35, 43, 54–57). RGMa-neogenin interactions are involved in axon guidance in the developing visual system and in axon tract formation in the embryonic brain in vivo (31, 35, 54, 55, 57, 58). RGMa also has chemorepulsive activity during laminar patterning of the developing mouse dentate gyrus in vitro (4). Interestingly, RGMa knockout mice did not show an abnormality in retinal topography but displayed an exencephalic phenotype in ∼50% of embryos, suggesting that RGMa plays a role in neural tube closure (37). We and others have shown that this role is conserved in Xenopus and zebrafish (19, 32). Neural fold elevation is a crucial step in neural tube closure, and loss of either RGMa or neogenin resulted in failure of neural fold elevation, severe disruption of microtubule networks, and abnormal cell polarity at this developmental stage (19). Neurulation is preceded by complex cell movements during gastrula, such as epiboly and convergent extension and involution of the marginal zone (21). The involvement of RGMa in these earlier gastrula events could contribute to the later role of RGMa in neurulation.
RGMa regulates bone morphogenetic protein (BMP) signaling in vitro in various cell lines (2, 17, 60). RGMa seems to enhance BMP signaling by directly binding to BMP2 and -4 and forming a complex with BMP type I receptors (2). However, further in vitro gain-of-RGMa-function experiments using different cell lines revealed that RGMa-dependent BMP signaling required the BMP receptor type IIA rather than type I receptors (60). More recently, neogenin, RGMc, and BMP2 from primary cultures of chondrocytes have been shown to form superreceptor complexes (61). These results suggest that there are context-dependent differences in RGM-mediated BMP signaling. Apart from these in vitro analyses, the relative roles and significance of interactions among RGMa, neogenin, and BMPs in embryogenesis and organogenesis remain unknown. Recently, perturbed RGMa expression was reported on some tissue cancers and cancer cell lines, suggesting that regulated RGMa expression may be important in suppressing tumorigenesis and metastasis (12, 24, 28, 29).
We report here that RGMa regulates cell adhesion and migration via its vWF and RGD domains. Neogenin but not BMP activity was shown to be responsible for this RGMa-mediated cell migration and adhesion. Finally, we demonstrate that RGMa plays a critical role in modulating major morphogenetic movements during gastrula and early neurula in embryonic vertebrate development.
MATERIALS AND METHODS
Animals.
Xenopus borealis embryos were obtained by induced mating in the physical containment level 2 (PC2) facility within the Animal House at the University of Queensland (Australian Quarantine and Inspection Service permit number 199867909). Embryos were staged according to the method of Nieuwkoop and Faber (38).
All experimental procedures involving the use of Xenopus embryos were approved by the Animal Ethics Committee within the University of Queensland, Australia.
Cloning of Xenopus RGMa deletion mutants and RNA synthesis for RGMa constructs.
We have reported two homologues for RGMa in Xenopus, RGMa1 and RGMa2, which show 96% identity to each other in protein sequences (50). Full-length RGMa1 (FL-RGMa1) for Xenopus borealis (GenBank accession number EF694981) was used to synthesize the deletion mutants which lack either the RGD domain (aa 79 to 81) or the partial von Willebrand factor type D domain (vWF domain, aa 147 to 206). The domains were predicted by the alignment of protein sequences between chick RGM and Xenopus borealis RGMa1 (xRGMa1) (35, 50). RGMa-ΔvWF was synthesized by the following strategy. First, forward and reverse primers were designed to amplify two independent RGMa1 PCR fragments (aa 1 to 146 and aa 207 to 432) using high-performance PCR (Phusion high-fidelity PCR kit; Finnzyme, Vantaa, Finland). For the 5′ end of RGMa-ΔvWF, the forward primer was designed to include an EcoRI restriction site and the start region of xRGMa1, while the reverse primer included the 5′ region upstream of the vWF domain with an Xho restriction site on its 3′ end. For the 3′ end of RGMa-ΔvWF, the forward primer was designed to include an Xho restriction site prior to the region 3′ downstream of the vWF domain, and the reverse primer included 3′-end sequences for xRGMa1 followed by an Xba restriction site. These two fragments were then ligated and inserted into pCS2+ vector for mRNA synthesis of the RGMa-ΔvWF construct. RGMa-ΔRGD was synthesized by a similar strategy. The 5′ end of RGMa-ΔRGD was amplified using high-performance PCR with forward and reverse primers that target between the start site region and the 5′ region upstream of the RGD domain. For the amplification of the 3′ end of RGMa-ΔRGD, a forward primer was designed to cover 5′ upstream and 3′ downstream of the RGD site but lacking 9 nucleotides that corresponded to the RGD domain. The reverse primer for RGMa-ΔRGD targeted the region that included the stop site for xRGMa1 followed by a SalI restriction site. These two fragments were then ligated and inserted into pCS2+ vector for mRNA synthesis of the RGMa-ΔRGD construct. Correct sequences for RGMa-ΔvWF and RGMa-ΔRGD were confirmed by the Australian Genome Research Facility (AGRF, Brisbane, Australia) and analyzed using Vector NTI software (Invitrogen). Capped mRNA was synthesized by in vitro transcription using mMessage mMachine SP6 RNA transcription kits (Ambion Inc., Austin, TX) according to the manufacturer's instructions.
Microinjection of Xenopus blastomeres.
Fertilized Xenopus eggs were prepared as described by Connor and Key (8). A total of 4.6 nl of mRNA was injected into a single blastomere of 2-cell-stage Xenopus embryos. Uninjected embryos were used to monitor the quality of the egg batch used in the experiment. Injected embryos were reared at 18 to 23°C and fixed in 4% paraformaldehyde (PFA) solution at the appropriate developmental stage. For the animal cap stem cell explant assay, two injections were made into each animal side of both blastomeres of 2-cell-stage Xenopus embryos, ensuring the same amount of mRNA used as per in vivo experiments for each embryo. The efficacy, specificity, and toxicity of neogenin untransclated region morpholino (UTR MO), RGMa-ATG MO, and RGMa mRNA were previously described (50, 57). Embryos were cultured in 0.4× Marc's modified Ringer's (MMR) solution (1× MMR is 1 mM MgCl2, 2 mM KCl, 2 mM CaCl2, 5 mM HEPES, 0.1 M NaCl) containing 5% Ficoll at 23°C until control embryos reached stage 7.
Animal cap stem cell explant assay.
Prepared stage 7 embryos were washed three times in 0.7× MMR before being transferred to 0.7× MMR-gentamicin (50 μg/ml)-bovine serum albumin (BSA) (0.1%, wt/vol) for dissection. Animal caps were isolated from stage 8 to 9 embryos and cultured in agarose-coated cell culture plates containing 0.7× MMR-gentamicin (50 μg/ml)-BSA (0.1%, wt/vol) at 18°C. To test BMP activities in relation to RGMa-neogenin signaling, the exogenous BMP ligand blocker Noggin (50 to 500 ng/ml, recombinant human Noggin; R&D Systems, Minneapolis, MN) was used. To examine any change in adhesion property after RGMa overexpression, we isolated animal caps from stage 9 embryos as explained above. Dissected explants were immediately transferred to 1× calcium- and magnesium-free medium (1× CMFM) (88 mM NaCl, 1 mM KCl, 2.4 mM NaHCO3, and 7.5 mM Tris-Cl, pH 8.0). For time lapse, each control and experimental animal cap explant was categorized as either a no-cell migration or a cell migration phenotype. Cell migration was considered to occur when the area of the extruded cell mass was larger than the area of the animal cap. For the cell sorting reaggregation assay, different populations of cells were dissociated and blindly mixed in CMFM before calcium (2 mM) was reintroduced to the medium for the aggregation. Reaggregation was induced by incubation on a horizontal shaker (50 to 60 rpm) for 60 min. Images of the embryos were collected using a Nikon DS-Ri1 camera attached to an Olympus SZX12 compound fluorescence microscope.
Analysis of gastrulation.
To understand the role of RGMa during gastrulation, RGMa mutants and control and experimental embryos were collected and fixed immediately in 4% PFA before the phenotypes were analyzed. To examine external gross anatomical features, images of embryos (vegetal or posterior view) were collected and the area of blastopore was measured using Image J. To analyze internal gross anatomical features, bisection of control and experimental embryos was made using a surgical blade. Internal gross anatomical features of RGMa mutants were compared to those of control embryos collected at the same time point.
Statistical analysis.
Statistical analysis of the results was performed using GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA). For all categorical data, a chi-square test of homogeneity and Fisher's exact test were performed. For quantitative data, one-way analysis of variance (ANOVA) or two-way ANOVA with Bonferroni posttest was performed. All error bars in the figures indicate standard errors of the means (SEM) of the analyzed data. Numbers on the top of each graph indicate the raw number of embryos used in each data set.
Image manipulation.
Collected images were color balanced and oriented using Adobe Photoshop CS4 software, without further digital manipulation.
RESULTS
RGMa induces animal cap spreading.
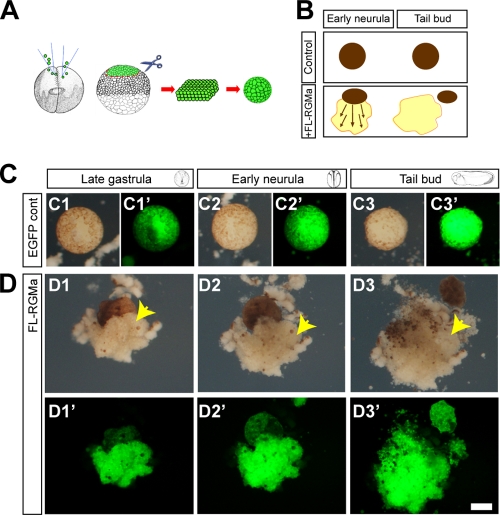
Since RGMa is widely expressed during pregastrula, becomes restricted to the ectoderm of the gastrulating Xenopus embryo, and, by neurulation, is highly localized to the neural plate (14, 45), we predicted that this axon guidance molecule would have early functional roles outside the nervous system. Targeted knockdown of RGMa in Xenopus has already revealed that RGMa causes dysmorphogenesis of the embryo (14). We have also shown previously that overexpression of RGMa in the Xenopus embryo causes severe developmental abnormalities (50). To begin to understand the cell mechanisms regulated by RGMa, we used animal cap explants from late-blastula-stage Xenopus embryos to examine cell adhesion and cell migration (Fig. 1A and B). Xenopus animal cap cells are a pluripotent stem cell population that are normally nonmigratory in vitro (25). These cells require activin A induction to display either migratory behavior in dispersed cultures or convergent extension in explants of animal caps (Fig. 1A). As reported by Kwan and Kirschner (25), our uninjected or enhanced green fluorescent protein (EGFP)-overexpressing control animal caps formed spheroidal bodies ex vivo (Fig. 1A to C). In contrast, animal caps overexpressing RGMa underwent considerable expansion and spreading to form a large mantle of tightly adherent cells in the absence of exogenous activin A (P < 0.01) (Fig. 1B and D). A trypan blue cell exclusion assay confirmed that abnormal spreading was not associated with increased cell death and that spread cells are viable (data not shown). This behavior of uninduced animal cap cells has not been reported previously. These spreading cells clump together and remain unattached to the agar-coated dish and thus do not seem to be dependent on traction forces with an external substratum. This mantle formation can be likened to the initial spreading and expansion of superficial ectodermal cells in activin A-treated animal caps (22). However, overexpression of RGMa seems to produce ubiquitous radial extension from the explants rather than linear extension about a longitudinal axis.
Fig 1.
RGMa causes abnormal cell migration and adhesion in the animal cap explant assay. (A) Schematic of Xenopus animal cap explant assay. mRNA/morpholino, together with tracer EGFP mRNA, was microinjected into both of the blastomeres at 2-cell-stage embryos, and the animal cap was isolated at the late-blastula stage. Animal cap explant is initially a sheet of ectodermal cells that becomes spheroid. (B) Schematic of the effect of full-length RGMa (FL-RGMa) overexpression on animal cap explant. Control animal cap explant expressing EGFP alone forms a spheroidal body without further morphological change between early-neurula and tail bud stages. When RGMa is overexpressed, animal cap explant showed extensive radial extension of aggregated cells over time. (C and D) Time lapse images of animal cap explants were collected when control whole embryos reached late-gastrula (14 h in culture, C1, C1′, D1, and D1′), early-neurula (20 h in culture, C2, C2′, D2, and D2′), or tail bud (40 h in culture, C3, C3′, D3, and D3′) stage. C1′ to C3′ and D1′ to D3′ show fluorescent images for C1 to C3 and D1 to D3, respectively. (C1 to C3′) Time lapse images of an EGFP-injected control animal cap explant (2.3 ng EGFP mRNA per embryo). The animal cap forms a spheroidal body (n = 16/16). (D1 to D3′) Time lapse images of an FL-RGMa-overexpressing animal cap showing excessive migration of aggregated cells (arrows) (1.15 ng each RGMa mRNA/EGFP mRNA per embryo, 76.92%; n = 13). Bar, 100 μm.
Although it appeared that overexpression of RGMa induced the migration of animal cap cells, it was equally possible that convergence extension movements involving intercalation of cells and proliferation were driving the spreading of cells from the explants. In order to more clearly observe cell behavior, we developed chimeric animal cap explants by placing a small piece of an animal cap overexpressing RGMa and labeled with the reporter dextran-Texas Red on top of a larger wild-type animal cap (see Fig. S1A in the supplemental material). These chimera formed balls of cells on a nonadherent substrate similarly to chimeras between uninjected dextran-Texas Red-labeled cells and wild-type caps (see Fig. S1B and C). Consistent with our results described above, only those chimeras overexpressing RGMa exhibited spreading of cells (see the arrow in Fig. S1C). Interestingly, only the red cells overexpressing RGMa spread from the chimeras, whereas the nonfluorescent wild-type cells remained within the main bulk of the explant. These results confirmed the specificity of the phenotype and provided the opportunity to analyze the underlying behavior of these cells as they spread from the explant. Time lapse movies of the explants, taken in a 30-min window during cell spreading, clearly revealed that the RGMa-overexpressing cells were selectively displaying protrusive activity, which was consistent with active migration away from the explant (see Movie S1 in the supplemental material). There was no evidence of intercalation of cells or of cell proliferation during this time. The migratory behavior was not consistent with convergence extension movements that are observed in animal cap explants treated with activin A (21). Together, our results indicate that overexpression of RGMa induced aberrant migration and adhesion of animal cap cells.
RGMa signals through neogenin but is not dependent on the BMP pathway during animal cap spreading.
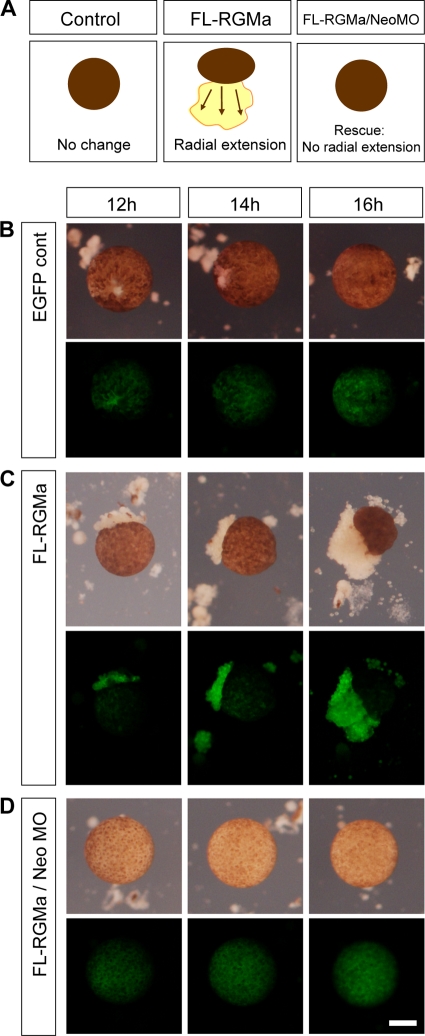
Since neogenin (the receptor for RGMa) is expressed in the ectoderm of gastrulating embryos in a pattern similar to that for RGMa (14, 19), we reasoned that neogenin was mediating the RGMa overexpression phenotype. To determine the role of neogenin in animal cap spreading, we overexpressed RGMa and simultaneously knocked down the expression of neogenin by using previously described antisense morpholinos (MO) (57). This neogenin MO (NeoMO) was shown to specifically knock down the endogenous neogenin protein expression during early Xenopus development (57). Time lapse imaging of RGMa-overexpressing animal caps revealed that cells predominantly spread from the explants between 12 and 16 h after culture, in comparison to control explants, which remained as intact spheroidal bodies (Fig. 2A and B) (P < 0.001). The gain-of-RGMa-function phenotype was inhibited in a dose-dependent manner by knockdown of neogenin. Coinjection of neogenin MO and FL-RGMa mRNA prevented the cell spreading that was observed in RGMa-overexpressing animal caps (Fig. 2D) (P < 0.001). Our results indicate that RGMa signals through neogenin to induce animal cap cell spreading.
Fig 2.
RGMa signals through neogenin during animal cap cell migration. (A) Schematic demonstrating the effect of overexpression of full-length RGMa (FL-RGMa) in animal caps and its rescue by simultaneous neogenin (Neo) downregulation. Like control animal cap explant expressing EGFP alone, neogenin-downregulated explants remain as a spheroidal body without further morphological change. (B to D) Time lapse images were collected at 12 to 16 h in culture postisolation. (B) EGFP control animal cap explants showed no morphological change (2.3 ng EGFP mRNA per embryo, 0%; n = 17). (C) FL-RGMa-overexpressing animal cap explants showed abnormal animal cap cell migration, which increased during the culture period (1.15 ng each RGMa mRNA/EGFP mRNA per embryo, 50%; n = 24). (D) Downregulation of the RGMa receptor neogenin rescued the RGMa gain-of-function phenotype. Images show explants injected with 23 ng of neogenin morpholino (1.15 ng/1.15 ng/23 ng RGMa mRNA/EGFP mRNA/NeoMO per embryo, 0%; n = 17). Bar, 100 μm.
RGMa has been proposed to act as a coreceptor for BMPs and to mediate their signaling in vitro (17, 60). To address the role of BMP in RGMa-neogenin signaling, we treated animal cap explants with exogenous Noggin, which binds to and blocks BMP activity (44). Noggin (50 to 500 ng/ml) was active in our animal cap assay since it caused the induction of the neuroectoderm marker NCAM (26) (see Fig. S2 in the supplemental material). Despite this induction, Noggin had no effect on the morphology of control EGFP-expressing animal caps (cf. Fig. 3A and B with Fig. 2B). In contrast, animal caps overexpressing RGMa demonstrated enhanced cell spreading in the presence of Noggin. Noggin-treated and RGMa-overexpressing explants spread more extensively than similar untreated explants (P < 0.001) (cf. Fig. 3C and D, dotted outlines). These results indicate that the presence of BMP was inhibitory to RGMa-dependent migration of cells from explants. When BMP signaling was antagonized by Noggin, the RGMa-induced cell spreading was significantly enhanced. The Noggin-dependent enhanced spreading of animal caps was rescued by downregulating neogenin (P < 0.001) (Fig. 3E). In conclusion, RGMa-neogenin-mediated cell adhesion and migration are not dependent on BMP activity in our animal cap assay.
Fig 3.
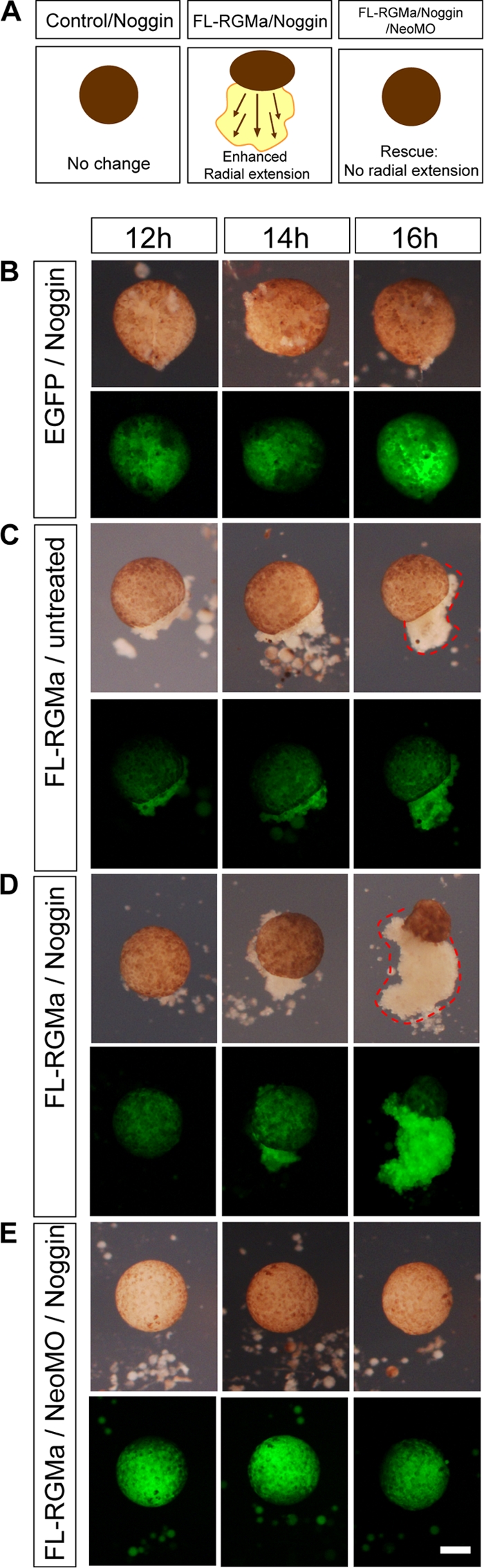
Noggin enhances RGMa-dependent animal cap cell migration. (A) Schematic showing the effect of Noggin treatment on RGMa-mediated animal cap cell migration. Downregulation of BMP activity by exogenous Noggin treatment enhanced, rather than rescued, RGMa-mediated radial extension of animal cap cell migration. Simultaneous neogenin downregulation rescued this radial extension phenotype, suggesting that BMP activity is not necessary in the functional RGMa-neogenin signaling during the animal cap cell migration. (B to E) Time lapse images were collected between 12 and 16 h of culture without (B and C) or with (D and E) exogenous Noggin. (B) EGFP-injected animal cap explants (2.3 ng EGFP mRNA per embryo) cultured in the presence of Noggin showed no cell spreading (0%; n = 14). (C) FL-RGMa-overexpressing animal cap explants (1.15 ng each EGFP mRNA/RGMa mRNA) showed abnormal animal cap spreading. (D) FL-RGMa-overexpressing animal cap explants cultured in the presence of Noggin showed enhanced cell spreading (93.33%; n = 30). (E) The Noggin-dependent enhanced spreading of animal caps was rescued with neogenin downregulation (23 ng NeoMO, 21.43%; n = 14). Dotted lines demarcate abnormal cell spreading at 16 h postisolation. Bar, 100 μm.
RGMa increases cell-cell adhesion in a neogenin-dependent manner.
In order to more clearly decipher the role of RGMa-neogenin in cell adhesion as distinct from that in cell migration, we next used an animal cap disaggregation assay. As previously described (39), control animal caps from stage 9 blastula embryos dissociate within 45 to 60 min of incubation in calcium- and magnesium-free medium (CMFM) (Fig. 4A). As previously reported, the outer ectodermal layer (pigmented) is more resistant to dissociation than the inner layer (51). Interestingly, the RGMa-overexpressing animal cap explants remained intact during the 60-min incubation (Fig. 4B). This adhesion was dependent on neogenin since knocking down expression of neogenin in RGMa gain-of-function embryos caused animal caps to dissociate (Fig. 4C).
Fig 4.
RGMa increases cell-cell adhesion in animal cap cells in a neogenin-dependent and calcium-independent manner. (A to C) Animal cap explants were isolated from stage 9 (St 9) late-blastula embryos and incubated in calcium- and magnesium-free medium (CMFM) for up to 60 min. (A) Control animal cap explants dissociate to form isolated cells in CMFM. (B) RGMa-overexpressing animal cap explants (1.15 ng RGMa mRNA) stayed intact under the same condition. (C) Simultaneous neogenin downregulation (1.15 ng/23 ng RGMa mRNA/NeoMO) rescued the phenotype. Bar, 500 μm.
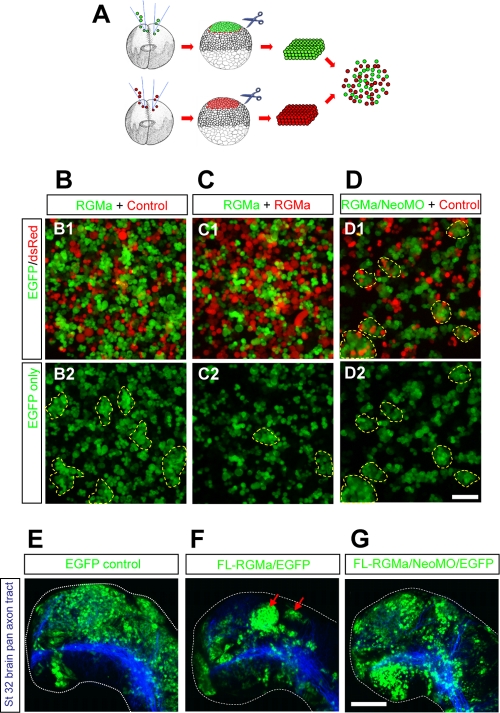
Next, we examined the role of RGMa-neogenin signaling in a cell sorting reaggregation assay using dissociated animal cap cells from late-blastula embryos (1, 18). Different groups of cells were color coded (by using EGFP or DsRed as a tracer) (Fig. 5A) to observe cell-cell interactions between heterogeneous groups of cells (Fig. 5B and C). Mixing RGMa-overexpressing green cells and control red cells showed preferential aggregation of green cells (Fig. 5B1 and B2, dotted outlines). This preferential clumping of green cells was attenuated when both red and green cells expressed RGMa (Fig. 5C1 and C2, dotted outlines). In these cultures, the green and red cells appeared intermixed due to the ubiquitous expression of RGMa, indicating that neither DsRed nor EGFP was responsible for the selective adhesion. This result also suggests that RGMa-mediated adhesion involved only cells overexpressing RGMa. We then examined whether this preferential homophilic interaction was dependent on neogenin. In the absence of neogenin, RGMa-overexpressing green cells no longer bound exclusively to themselves and instead formed heterophilic clumps of green and red cells (Fig. 5D1 and D2, dotted outlines). Taken together, these results indicate that RGMa and neogenin act to increase cell adhesion.
Fig 5.
RGMa-neogenin-mediated cell adhesion affects cell sorting in animal cap cells. (A) Schematic of cell sorting reaggregation assay. (B to D) RGMa mRNA was coinjected with either the tracer EGFP mRNA or DsRed mRNA to both blastomeres of different 2-cell-stage embryos. Animal caps were isolated from late-blastula-stage embryos and dissociated by pipetting in CMFM. Differently color-coded (green and red) cells were mixed in CMFM before calcium was reintroduced (2 mM). The mixed cells were incubated for 60 min on a horizontal shaker (∼50 rpm). RGMa-overexpressing green cells preferentially form cell aggregates when mixed with control red cells (B2, dotted outlines). This activity was attenuated when RGMa-overexpressing cells were mixed homogenously, showing smaller cell aggregates (C2, dotted outlines). RGMa-overexpressing cells lose preferential binding in the absence of neogenin (D1 and D2, dotted outlines). (E to G) Mosaic overexpression was induced by microinjecting EGFP alone (control, 2.3 ng per embryo), RGMa/EGFP (1.15 ng each per embryo), or RGMa/NeoMO/EGFP (1.15 ng/11.5 ng/1.15 ng per embryo) in developing embryos into one of the dorsally fated cells in 8- to 16-cell-stage embryos. RGMa-overexpressing neuroepithelium forms segments of tightly aggregated cells within early brain through neogenin. Z-stacked confocal images show whole-mount forebrains (stage 32 [St 32]) immunolabeled with anti-acetylated alpha-tubulin antibody/Cy3 (blue) to visualize pan-axon tracts. RGMa-overexpressing cells form dense aggregates within the forebrain (F, arrows; 77.42%, n = 31 hemispheres). Knockdown of neogenin rescued this phenotype (G; 33.33%, n = 24 hemispheres) and showed no difference from the results for the control (E; 7.14%, n = 14 hemispheres). Dotted lines demarcate whole-mount brain from background. All images show the dorsal side at the top and the rostral side at the left. Bar, 100 μm.
To address the role of RGMa-neogenin in mediating adhesion and cell sorting during development in vivo, we mosaically overexpressed EGFP alone (control) or RGMa/EGFP or RGMa/NeoMO/EGFP in developing embryos by injection into one of the dorsally fated cells in 8- to 16-cell-stage embryos. Control and experimental embryos were reared until control embryos reached stage 32 to 34, when embryos were fixed. We examined the stage 32 to 34 Xenopus neuroepithelium, since RGMa and neogenin are endogenously expressed and their functional roles are well characterized in the Xenopus forebrain during this stage (57, 58). Discrete RGMa-overexpressing cell aggregates were observed within the neuroepithelium of RGMa-overexpressing embryos (Fig. 5F, arrows) and not controls (Fig. 5E) (P < 0.01). These clumps of cells phenocopied the increased adhesion and clumping of cells that we observed in the animal cap and dissociation assays described above. It also confirmed the cell-autonomous nature of the increased adhesion. The brain phenotype was significantly rescued when neogenin was simultaneously knocked down (Fig. 5G) (P < 0.01). Taken together, these results indicate that RGMa-neogenin is capable of affecting cell adhesion and sorting during embryonic development, as shown in our ex vivo and in vitro cell sorting and reaggregation assays (Fig. 1, 4, and 5A to D).
In summary, we have shown that RGMa induces cell adhesion in a neogenin-dependent manner. Together with the results from Fig. 2D (the rescue of the cell spreading phenotype by knockdown of neogenin), RGMa has been shown to mediate both abnormal cell migration and cell adhesion in gastrulating embryos by interactions with endogenous neogenin.
Specific RGMa domains are responsible for cell migration and adhesion.
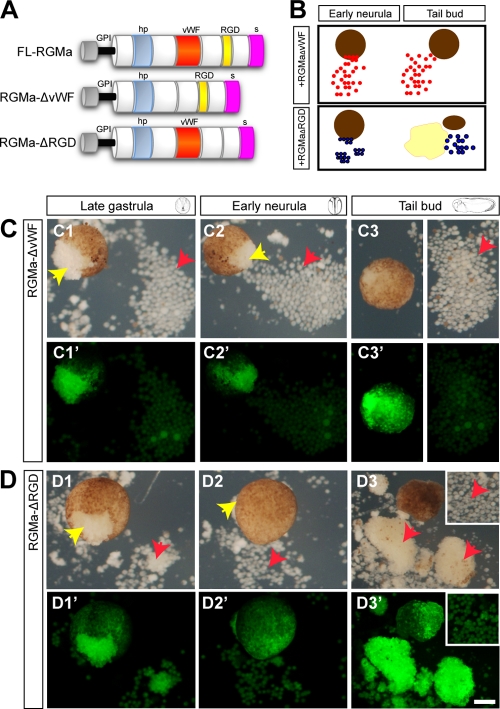
To further dissect the role of RGMa in cell migration and adhesion, we next generated two RGMa deletion mutants lacking either the von Willebrand factor (vWF) or the integrin-binding RGD domain (Fig. 6A). These deletion mutants are referred to as RGMa-ΔvWF and RGMa-ΔRGD, respectively. As shown from Fig. 1, animal cap explants were isolated from late-blastula embryos overexpressing RGMa-ΔvWF or RGMa-ΔRGD and examined by time lapse imaging until the stage equivalent to the tail bud stage (Fig. 6B). We found that, by late gastrula, cells overexpressing RGMa-ΔvWF (Fig. 6C, yellow arrows) spread but failed to remain adherent and form a mantle around animal cap explants. These cells dispersed around the explant (Fig. 6C, red arrows), which indicated that the vWF domain was critical in modulating the adhesiveness of spreading animal cap cells (70%, n = 7/10). In contrast, few cells spread from gain-of-function RGMa-ΔRGD explants until after early neurula (Fig. 6D, yellow arrows). By tail bud stage, these migrating cells formed large clumps resembling those observed for the FL-RGMa-overexpressing caps (cf. Fig. 1D3 and Fig. 6D3; 100%, n = 8/8). Once animal cap cells expressing RGMa-ΔRGD began to migrate, they maintained their adhesiveness and proceeded to spread as in wild-type-RGMa-overexpressing cells. A trypan blue cell exclusion assay confirmed that the loss of cell adhesion or abnormal migration by the overexpression of RGMa deletion mutants is not associated with cell death and that these migrated cells are viable (data not shown). Together, these results revealed that the vWF domain was necessary for adhesion of the spreading cells, while the RGD domain instead regulated the initiation of migratory behavior.
Fig 6.
vWF and RGD domains are involved in cell migration and adhesion. (A) Schematic of FL-RGMa and two RGMa domain deletion mutants, RGMa-ΔvWF and RGMa-ΔRGD. (B) Schematic of the role of the vWF and RGD domains in RGMa-mediated migration of cell aggregates in animal cap explants. In the absence of the vWF domain, RGMa produced a small number of larger and nonadhesive cells from the animal cap explant. In the absence of the RGD domain, RGMa-mediated cell migration was delayed, but cells spread and formed aggregates by the tail bud stage. (C1 to C3′) Time lapse images of an RGMa-ΔvWF-overexpressing animal cap. (D1 to D3′) Time lapse images of an RGMa-ΔRGD-overexpressing animal cap. C1′ to C3′ and D1′ to D3′ show fluorescent images of C1 to C3 and D1 to D3, respectively. Yellow arrows indicate cells migrating from animal cap explant, and red arrows indicate migrated cells. Insets at C3, C3′, D3, and D3′ show cells that are outside the field of view. Bar, 100 μm.
Regulated RGMa expression is critical during gastrula cell movements.
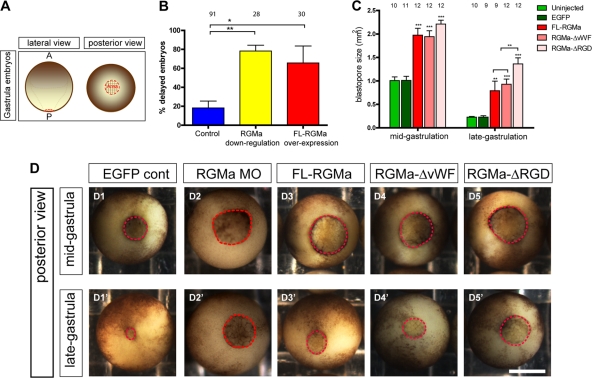
In order to further functionally characterize the cell migration and adhesion activity of RGMa, FL-RGMa was either knocked down (RGMa MO, 23 ng) or ectopically expressed (FL-RGMa mRNA, 1.15 ng) in vivo, together with the reporter EGFP mRNA (1.15 ng), into one blastomere of 2-cell-stage Xenopus embryos. Upon the initiation of gastrulation in Xenopus, the blastopore appears at the posterior end of the embryo (Fig. 7A) (21). As closure of the blastopore is an external indication of normal gastrula-related cell movements, we first examined whether RGMa plays a role in blastopore closure (Fig. 7B). We found that either knockdown of RGMa using previously described antisense morpholinos (19, 57) or overexpression of wild-type Xenopus RGMa in Xenopus embryos caused delayed blastopore closure by late-gastrulation stage (Fig. 7B and D1′ to D3′) (P < 0.05). These results indicated that regulated spatiotemporal expression of RGMa in vivo was essential for gastrula cell movements.
Fig 7.
Embryos overexpressing RGMa mutants exhibit delays in blastopore closure during gastrula. (A) Schematic of Xenopus gastrula embryos, showing lateral and posterior views. Red dotted lines demarcate the blastopore. (B) Regulated expression of RGMa is crucial for blastopore closure during gastrulation. Embryos were injected with either RGMa-ATG morpholino (23 ng of MO) or FL-RGMa (0.6 or 1.15 ng mRNA). Control embryos were either uninjected or injected with EGFP or standard control morpholino. At late-gastrulation stage, embryos were scored for delayed blastopore closure if the blastopore area was larger than the average area of that for control animals at midgastrula (>2-h delay). Error bars indicate the standard errors of the means of the average percentages from multiple repeats (control, 7 repeats; RGMa downregulation, 3 repeats; RGMa overexpression, 3 repeats). **, P < 0.01; *, P < 0.05. (C) Blastopore size in embryos overexpressing FL-RGMa (1.15 ng each RGMa mRNA/EGFP mRNA per embryo) and RGMa mutants (1.15 ng each RGMa-ΔvWF or RGMa-ΔRGD mRNA/EGFP mRNA per embryo). Embryos overexpressing FL-RGMa and RGMa mutants were collected when 90% of control embryos passed midgastrulation stage (stage 11) and again when all control embryos passed late-gastrulation stage (stage 12). In the absence of the RGD domain, RGMa-overexpressing embryos showed a significantly large blastopore by late-gastrulation stage. ***, P < 0.001; **, P < 0.01. (D) Posterior view of a representative control embryo, an RGMa knockdown embryo, and embryos overexpressing FL-RGMa or RGMa mutants at midgastrulation (D1 to D5) and late-gastrulation (D1′ to D5′) stages. The dotted circle outlines the blastopore for each embryo. Bar, 300 μm.
Functional roles of RGMa domains during gastrula cell movements.
To further decipher the role of RGMa domains during blastopore closure, FL-RGMa, RGMa-ΔvWF, or RGMa-ΔRGD mRNA (1.15 ng) was ectopically expressed in vivo, together with the reporter EGFP mRNA (1.15 ng), into one blastomere of 2-cell-stage Xenopus embryos. These injected embryos were analyzed for blastopore closure when control embryos had reached both midgastrulation and late-gastrulation stages. At midgastrula stage, control embryos possess a blastopore with a surface area of ∼1 mm2 (Fig. 7C and D1). However, embryos overexpressing FL-RGMa, RGMa-ΔvWF, or RGMa-ΔRGD have significantly larger blastopores than controls (P < 0.001) (Fig. 7C, D1, and D3 to D5). No significant differences in blastopore size of the RGMa mutants were observed at this stage (Fig. 7C and D3 to D5). By late gastrula, the blastopores of embryos injected with FL-RGMa, RGMa-ΔvWF, or RGMa-ΔRGD had closed only to approximately the size of those of control embryos at midgastrula (Fig. 7C, D1, and D3′ to D5′). However, by late gastrula, RGMa-ΔRGD-overexpressing embryos showed significantly more delay in blastopore closure than FL-RGMa- and RGMa-ΔvWF-overexpressing embryos (P < 0.01) (Fig. 7C and D3′ to D5′). This result is consistent with the role of the RGD domain shown in the animal cap explant assay (Fig. 6D). Interestingly, the vWF domain in RGMa was not responsible for this slowing of blastopore closure, since there was no difference in phenotype between FL-RGMa- and RGMa-ΔvWF-overexpressing embryos. It appears that loss of the vWF domain in RGMa in vivo does not affect the cohesiveness of the embryo, as is observed ex vivo (Fig. 6C). Additional adhesion mechanisms in the differentiating germ layers are probably compensatory.
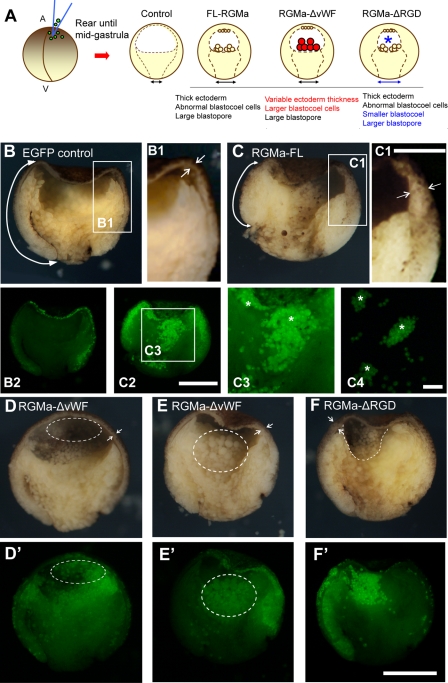
Gastrulation cell movements include epiboly and convergent extension and involution of the marginal zone (prospective mesoderm) and involve tightly regulated cell behaviors to organize the emerging germ layers (21). To determine whether RGMa overexpression could specifically perturb these cell movements during gastrula, we reared mutant embryos until midgastrula (stage 11). Bisection of these gastrulating embryos revealed that overexpression of FL-RGMa, RGMa-ΔvWF, or RGMa-ΔRGD mRNA (1.15 ng) in vivo generated phenotypes comparable to those for ex vivo animal cap explants (Fig. 1 and 6). All affected embryos showed ectopic accumulation of fluorescent cells in the blastocoel (summarized in Fig. 8A; FL-RGMa, n = 22; RGMa-ΔvWF, n = 15; RGMa-ΔRGD, n = 17). For FL-RGMa- and RGMa-ΔRGD-overexpressing embryos, the aggregates of ectopic accumulation of fluorescent cells were easily detached from the blastocoel floor, while they remained adherent to the blastocoel roof (Fig. 8C2 to C4, asterisks). RGMa overexpression also perturbed specific gastrulation cell movements. FL-RGMa overexpression reduced the extent of mesoderm involution (cf. double arrows in Fig. 8B and C), and these mutants also exhibited markedly thicker ectoderm than control EGFP-overexpressing embryos (cf. double arrows in Fig. 8B1 and C1), which is consistent with defects in epiboly (11, 16). RGMa-ΔvWF gain-of-function mutants exhibited two different phenotypes: embryos either showed normal epiboly, with many small ectopic cells adherent to the blastocoel roof (Fig. 8D and D′, double arrows and dotted outline; n = 8/15), or exhibited markedly thicker ectoderm, with abnormal epiboly and large ectopic cells within the blastocoel (Fig. 8E and E′, double arrows and dotted outlines; n = 7/15). In contrast, the RGMa-ΔRGD mutants typically exhibited simultaneous thickening of the ectoderm and a smaller blastocoel cavity (Fig. 8F, double arrows and dotted line).
Fig 8.
Embryos overexpressing RGMa constructs exhibit abnormal internal morphology during gastrulation. (A) Schematic of bisected Xenopus gastrula embryos following overexpression of FL-RGMa, RGMa-ΔvWF, or RGMa-ΔRGD mRNA. Red descriptions indicate the RGMa-ΔvWF-specific phenotypes, whereas blue descriptions indicate the RGMa-ΔRGD-specific phenotypes. A, animal; V, vegetal. (B to F) Bisection of a control EGFP-injected embryo (B, B1, and B2) and RGMa mutant embryos (C to F, C1 to C3) that were collected when control embryos reached midgastrula. Panels B2 and C2 show fluorescent images of the same embryos shown in panels B and C, respectively. All bisected embryos show animal (top), vegetal (bottom), dorsal (left), and ventral (right) sides. (B and C) RGMa-overexpressed embryos show thicker ectoderm and reduced mesoderm involution. (B1 and C1) Magnified images of ectoderm from control and FL-RGMa-overexpressing embryos. Arrows in panels B, B1, C, and C1 indicate the different thicknesses of ectoderm and the different rates of mesoderm involution. (C3 and C4) Magnified images of C2 (C3) and isolated blastocoel cells (C4) showing aggregation of RGMa-positive cells (asterisks). These cell aggregates were not adherent to the blastocoel floor. (D and E) Overexpression of RGMa-ΔvWF generated two phenotypes. (F) An RGMa-ΔRGD mutant embryo exhibiting a phenotype similar to that for FL-RGMa-overexpressed embryos but showing a smaller blastocoel (dotted line). Facing arrows in panels D to F indicate the thickness of ectoderm adjacent to the tip of the involuting mesoderm. Bars, 300 μm (B, C, and D to F), 150 μm (B1 and C1), 300 μm (B2 and C2), and 150 μm (C3 and C4).
These results reveal that the vWF domain has a selective role in epiboly. In the absence of the vWF domain, approximately 50% of the embryos demonstrated normal ectoderm expansion over the embryo surface, indicating that this domain mediated many of the epiboly defects when FL-RGMa was overexpressed. The loss of the RGD domain caused more severe defects in migration than those observed following overexpression of FL-RGMa. Thus, the RGD domain appears to attenuate migration phenotypes when wild-type RGMa (FL-RGMa) is overexpressed. Taken together, RGMa gain-of-function perturbs gastrulation through a combination of abnormal morphogenetic processes.
Perturbation in gastrula migration results in neural tube defects in RGMa mutants.
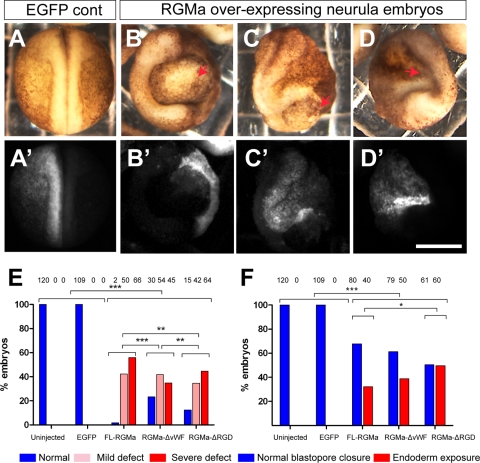
Overexpression of RGMa caused migration defects during gastrulation which could account for the later-developing abnormal neurulation phenotypes. Therefore, we next examined embryos at early-neurula stage (stages 19 to 21) (Fig. 9) unilaterally overexpressing FL-RGMa, RGMa-ΔvWF, or RGMa-ΔRGD mRNA (1.15 ng). Most of these embryos exhibited abnormal gross morphology (∼95% penetrance), typical of previously described gastrulation-related defects (6, 27, 30, 42, 52). Embryos had prominent abnormalities, such as persistently opened blastopore, exposed endoderm, incomplete neural folds, and a reduced body axis (Fig. 9B to D). We classified phenotypes (Fig. 9E) as either mildly abnormal (<50% reduction in neural tube length or persistently opened blastopore) or severely abnormal (>50% reduction in neural tube length or an exposure of endoderm with completely unfused neural tube). RGMa overexpression caused significantly more neurulation defects than were seen with control embryos (P < 0.001) (Fig. 9E).
Fig 9.
Overexpression of wild-type and mutant RGMa perturbs morphogenesis in neurulating Xenopus embryos. (A to D) Unilateral overexpression of FL-RGMa induces severe defects in developing Xenopus embryos by neurulation stages 19 to 21. Panels A′ to D′ show fluorescent images of the same embryos shown in panels A to D, respectively. (A) Control EGFP-injected embryo. (B to D) Unilateral overexpression of RGMa causes neurulation phenotypes. RGMa-overexpressing embryos showed the ring embryo phenotype (B and C) or an underdeveloped neural tube (D). Arrows in panels B to D indicate prominent abnormalities. (E and F) Penetration of neurulation (E) and endoderm-exposing ring (F) phenotypes from control, FL-RGMa, RGMa-ΔvWF, and RGMa-ΔRGD embryos. Bar, 300 μm. ***, P < 0.001; **, P < 0.01; *, P < 0.05.
Loss of the RGD domain had little impact on the penetrance of the neurulation defects (reduced from 100% to ∼90%) (Fig. 9E). Similarly, RGMa lacking the vWF domain continued to produce a neurulation phenotype in ∼80% of embryos. Thus, even in the absence of either of these two domains, RGMa continued to maintain functional activity and produced significant morphogenetic defects. However, it needs to be pointed out that each of these domains caused vastly different preneurula phenotypes that ultimately led to the observed gross neurulation defects (Fig. 7 and 8). To differentiate the roles of RGMa domains in neurulation, we examined differences in expressivity of the previously described “ring” phenotype (20). The ring phenotype arises from defects in cell migration occurring during gastrulation (20) and consists of a prominent neural fold surrounding the blastopore and the exposed underlying endoderm during neurulation (Fig. 9B, C, and F). Thirty percent of embryos injected with FL-RGMa exhibited this ring phenotype. Injection of RGMa-ΔvWF did not significantly affect the penetrance of this particular trait, whereas injection of RGMa-ΔRGD significantly increased the penetrance from 30% to 50% (Fig. 7F). This result suggests that the RGD domain in RGMa selectively attenuates the expression of this neurulation phenotype by facilitating gain-of-RGMa-function migration.
In summary, we have shown here that despite the loss of either the vWF or the RGD domain, RGMa maintains most of its gross neurulation activity in gain-of-function embryos. While the loss of the RGD domain had only modest effects on the overall penetrance of abnormal phenotypes, this domain selectively attenuated the expression of the ring phenotype during neurulation, consistent with our ex vivo and in vivo results showing delayed radial migration of cell aggregates and blastopore closure (Fig. 6D and 7C and D).
Taken together, our results have revealed that regulated RGMa-neogenin signaling is critical in maintaining normal cell migration and adhesion during early embryogenesis. Aberrant ectodermal migration of aggregated RGMa-overexpressing cells ex vivo was recapitulated in developing embryos, resulting in phenotypes with disturbed cell movement during gastrula and neurula. Finally, functional assessment of the RGMa vWF and RGD domains revealed that the vWF domain is more responsible for cell-cell adhesion, whereas the RGD domain facilitates cell migration.
DISCUSSION
We have shown here that overexpression of RGMa induces neogenin-mediated abnormal cell migration and adhesion in animal cap explants. This is the first time that RGMa-neogenin interactions have been shown to regulate cell migration and cell-cell adhesion outside the nervous system during development. We have also assessed the functional roles of the vWF and RGD domains in RGMa and shown that they play a role in RGMa-mediated cell migration and adhesion. In vivo loss- and gain-of-RGMa-function embryos demonstrated severe morphogenetic defects in epiboly, blastopore closure, and neurulation. Thus, regulated expression of RGMa is critical for ensuring normal function of the RGMa-neogenin signaling pathway for cell movements during embryogenesis.
RGMa-neogenin affects migration.
Overexpression of RGMa caused animal cap cells to undergo extension movements and migrate nondirectionally from explants. Subsequently, we found that RGMa gain-of-function embryos showed severe neurulation defects, including incomplete and unfused neural tubes, shortened body axis, and exposed endoderm (ring embryo phenotype). Our ex vivo analyses indicated that these mutant phenotypes probably arise as a result of aberrant migration. This conclusion is consistent with previous reports that similar phenotypes result from aberrant migration during gastrulation (6, 27, 30, 42, 52). For instance, during Xenopus development, loss of Myosin IIb caused defective convergence and extension movements which culminated in abnormal blastopore closure, unfused neural tube, exposed endoderm, and shortened body axis (52). Similarly, overexpression of the Dishevelled-associated protein Hipk1 affected β-catenin-dependent cell migration, which produced many of the same gastrulation and neurulation defects (30). We have shown that morpholino knockdown of RGMa caused significant delay in blastopore closure, while others (14) have revealed that older embryos exhibited severe morphological defects, particularly in the head. Taken together, these loss- and gain-of-function approaches indicate that spatiotemporal control of RGMa expression is essential for cell migration during embryogenesis. Our results suggest that RGMa and neogenin are part of a larger network of regulatory interactions that modulate early embryonic cell migration and hence influence major morphogenetic events. Interestingly, the role of RGMa is clearly distinct from that of the closely related RGMb (Dragon), since overexpression of the latter member of the RGM family in Xenopus revealed that it regulated neural patterning rather than perturbed early morphogenesis (45). Later in development, RGMb-neogenin interactions appear to decrease cell adhesion (9) rather than increase it, as observed here for RGMa. However, the role of RGMb in cell adhesion is contentious since in vitro studies indicate that RGMb can increase cell adhesion through homophilic interactions (46).
A role for RGMa in cell migration is consistent with recent reports that RGMa may be involved in carcinogenesis in a variety of distinct tissues and cell lines (12, 24, 28, 29). This is the not the first example of an axon guidance molecule being implicated in carcinoma. For example, L1-CAM, which plays critical roles in axon guidance and cell migration during the development of the nervous system, also promotes invasion and motility in colon cancer cells as well as tumor formation and the metastasis in nude mice (13, 47). This functional role of L1-CAM is modulated by a family of sheddase called ADAM (a disintegrin and metalloprotease). Interestingly, a recent report indicates that ADAM 17 regulates the chemorepellent axon guidance of RGMa-neogenin in cultured cortical neurons (40). A role for ADAM17 in RGMa-mediated cell migration remains to be determined.
RGMa signals through neogenin to increase cell-cell adhesion.
We have revealed that RGMa regulates cell-cell adhesion in the ectoderm of Xenopus pregastrula embryos. Overexpression of RGMa prevented the dissociation of animal caps in calcium-free medium. However, this phenotype was reversed when neogenin was simultaneously knocked down, indicating that RGMa-neogenin interactions were mediating this behavior. In our reaggregation assay, RGMa-overexpressing cells preferentially formed cell aggregates, suggesting that RGMa was acting to regulate cell adhesion, most likely by interacting with neogenin. While RGMa trans-signaling is responsible for axon guidance and T-cell adhesion (35, 36, 43), others have proposed that RGM-neogenin may signal in cis (2, 60, 61). For instance, RGMc has been proposed to act as a bridge between neogenin and BMP receptors in cis to enhance transmembrane Smad signaling in cell lines (61). In HEK cells, neogenin strongly associated with BMP receptors only in the presence of RGMc coexpression. Similarly, neogenin coimmunoprecipitated with RGMc in lipid raft-enriched detergent-resistant membrane fractions isolated from the cultured chondrocytes following BMP2 stimulation (61). However, we have shown that blocking of the BMP pathway could not rescue RGMa-neogenin-induced cell spreading in ex vivo animal cap explants, suggesting that RGMa-neogenin interactions are not dependent on BMP activity in this context. Instead, as RGMa-neogenin cell spreading was enhanced by Noggin treatment, we report an unexpected deregulating activity of BMP. Our results are consistent with a model whereby BMP activity is not necessary for RGMa-neogenin function, as reported recently in the immune system (34). It remains to be determined whether RGMa interacts with neogenin in cis or in trans to mediate cell adhesion. It should be noted that in cis interactions would require recruitment of coreceptors or cell adhesion molecules to mediate such adhesion. If this were the case, then it is clear that calcium-dependent adhesion molecules would not be involved, since we found that animal caps overexpressing RGMa continued to maintain adhesiveness in calcium-free medium. In trans interactions could easily account for cell sorting if neogenin-RGMa binding between wild-type cells was not saturated. In this case, overexpressing RGMa would increase adhesiveness and lead to cell sorting on the basis of differential adhesiveness.
It is now clear that RGMa-neogenin interactions underlie both adhesive and chemorepulsive events in different tissues. Interestingly, several other chemorepulsive axon guidance receptor-ligand interactions are also capable of both cell adhesion and chemorepulsion. An immunoglobulin receptor, Dscam, mediates repulsive guidance and establishes axon topography simultaneously through homophilic recognition in Drosophila (33, 59). Bidirectional chemorepulsive ligand ephrinB2a and its EphA4 receptor were also shown to control rhombomere boundaries by adhesive homotypic sorting mechanisms (10). Robo-Slit interactions cause commissural axons to be repulsed from the midline in the spinal cord (23), whereas Robo2/Slit1 mediates cell adhesion of neuroepithelial placodes by interacting with N-cadherin (49). Overexpression of Robo2 led to cell aggregates in placodes which were very similar in appearance to those observed in the neural tube when we overexpressed RGMa.
vWF and RGD domains in RGMa play functional roles in cell adhesion and cell migration.
The vWF domain in RGMa is a partial type D domain of von Willebrand factor (vWF) containing most key amino acids of the complete D domain (35). Full-length von Willebrand factor has five type D (D1 to D4 and D′) domains which serve as binding sites for the blood coagulation cofactor factor VIII and enable the formation of interdimer disulfide bonds necessary for its normal function (15). Proteins containing vWF domains are involved in a variety of different biological events, including cell adhesion and migration and signal transduction (3, 7). For example, the partial D domains in Zonadhesin regulate gamete adhesion (53). We have clearly demonstrated using the animal cap explant assay that the vWF domain in RGMa plays a role in cell adhesion. As noted above, animal caps overexpressing full-length RGMa produced adhesive aggregates of cells which spread from the explants. In contrast, when animal caps overexpressed mutant RGMa lacking the vWF domain, spreading cells failed to remain adhesive and instead dispersed as single cells around the explants. This phenotype was also consistently shown in vivo as larger and isolated blastocoel cells of gastrula embryos overexpressing RGMa-ΔvWF. These results strongly implicate a role for the vWF domain in cell adhesion mediated by RGMa.
To begin to understand the role of the RGD motif in the function of RGMa, we created a deletion mutant (RGMa-ΔRGD) lacking this tripeptide. Rather than causing spreading cells to disaggregate from animal caps, as observed with the vWF deletion mutant, the lack of RGD caused cells to delay their migration from the explants. Thus, RGD in RGMa was selectively regulating the migratory activity of animal cap cells and not their adhesiveness. This role is consistent with the phenotype observed in embryos following the in vivo overexpression of RGMa-ΔRGD. In the absence of the RGD domain, blastopore closure was significantly delayed and involuted mesoderm was expanded in gastrulating embryos compared to results for embryos overexpressing full-length RGMa. In summary, we show here that the RGMa vWF and RGD domains are functional and developmentally active in RGMa-mediated cell adhesion and migration ex vivo and during gastrula and neurula stages of Xenopus embryos.
In conclusion, we have identified previously unknown roles of RGMa-neogenin in cell adhesion and migration during early vertebrate embryonic development. Our results support a critical role of RGMa and its receptor neogenin in regulating critical cell behaviors required for key morphogenetic events. These results are consistent with emerging evidence implicating RGMa in carcinogenesis and immune responses (12, 24, 28, 29, 34, 36). Together, roles of RGMa-neogenin in cell migration and adhesion appear to be essential for both embryonic development and postnatal homeostasis.
Supplementary Material
ACKNOWLEDGMENTS
This project was supported by a grant from the National Health and Medical Research Council of Australia to B.K. (number 631598) and an International Postgraduate Research Scholarship of Australia granted to G.J.L.
We thank Christina Claxton for help with designing and cloning RGMa-ΔvWF and RGMa-ΔRGD constructs and Julie Conway for maintaining the Xenopus colony.
We declare that we have no conflict of interest.
Footnotes
Published ahead of print 3 January 2012
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1. Aamar E, Dawid IB. 2008. Protocadherin-18a has a role in cell adhesion, behavior and migration in zebrafish development. Dev. Biol. 318:335–346 [DOI] [PubMed] [Google Scholar]
- 2. Babitt JL, et al. 2005. Repulsive guidance molecule (RGMa), a DRAGON homologue, is a bone morphogenetic protein co-receptor. J. Biol. Chem. 280:29820–29827 [DOI] [PubMed] [Google Scholar]
- 3. Bork P. 1991. Shuffled domains in extracellular proteins. FEBS Lett. 286:47–54 [DOI] [PubMed] [Google Scholar]
- 4. Brinks H, et al. 2004. The repulsive guidance molecule RGMa is involved in the formation of afferent connections in the dentate gyrus. J. Neurosci. 24:3862–3869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Camus LM, Lambert LA. 2007. Molecular evolution of hemojuvelin and the repulsive guidance molecule family. J. Mol. Evol. 65:68–81 [DOI] [PubMed] [Google Scholar]
- 6. Cha JY, et al. 2007. The role of FoxC1 in early Xenopus development. Dev. Dyn. 236:2731–2741 [DOI] [PubMed] [Google Scholar]
- 7. Colombatti A, Bonaldo P. 1991. The superfamily of proteins with von Willebrand-factor type-A-like domains, one theme common to components of extracellular matrix, hemostasis, cellular adhesion, and defense mechanisms. Blood 77:2305–2315 [PubMed] [Google Scholar]
- 8. Connor RM, Key B. 2002. Expression and role of roundabout-1 in embryonic Xenopus forebrain. Dev. Dyn. 225:22–34 [DOI] [PubMed] [Google Scholar]
- 9. Conrad S, et al. 2010. RGMb controls aggregation and migration of neogenin-positive cells in vitro and in vivo. Mol. Cell. Neurosci. 43:222–231 [DOI] [PubMed] [Google Scholar]
- 10. Cooke JE, Kemp HA, Moens CB. 2005. EphA4 is required for cell adhesion and rhombomere-boundary formation in the zebrafish. Curr. Biol. 15:536–542 [DOI] [PubMed] [Google Scholar]
- 11. Cousin H, DeSimone DW, Alfandari D. 2008. PACSIN2 regulates cell adhesion during gastrulation in Xenopus laevis. Dev. Biol. 319:86–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Feys T, et al. 2007. A detailed inventory of DNA copy number alterations in four commonly used Hodgkin's lymphoma cell lines. Haematologica 92:913–920 [DOI] [PubMed] [Google Scholar]
- 13. Gavert N, et al. 2007. Expression of L1-CAM and ADAM10 in human colon cancer cells induces metastasis. Cancer Res. 67:7703–7712 [DOI] [PubMed] [Google Scholar]
- 14. Gessert S, Maurus D, Kuhl M. 2008. Repulsive guidance molecule A (RGM A) and its receptor neogenin during neural and neural crest cell development of Xenopus laevis. Biol. Cell 100:659–673 [DOI] [PubMed] [Google Scholar]
- 15. Goodeve AC. 2010. The genetic basis of von Willebrand disease. Blood Rev. 24:123–134 [DOI] [PubMed] [Google Scholar]
- 16. Goto T, Hasegawa K, Kinoshita T, Kubota HY. 2001. A novel POZ/zinc finger protein, champignon, interferes with gastrulation movements in Xenopus. Dev. Dyn. 221:14–25 [DOI] [PubMed] [Google Scholar]
- 17. Halbrooks PJ, Ding R, Wozney JM, Bain G. 2007. Role of RGM coreceptors in bone morphogenetic protein signaling. J. Mol. Signal. 2:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Karaulanov EE, Bottcher RT, Niehrs C. 2006. A role for fibronectin-leucine-rich transmembrane cell-surface proteins in homotypic cell adhesion. EMBO Rep. 7:283–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kee N, et al. 2008. Neogenin and RGMa control neural tube closure and neuroepithelial morphology by regulating cell polarity. J. Neurosci. 28:12643–12653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Keller R, et al. 2000. Mechanisms of convergence and extension by cell intercalation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 355:897–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Keller R, Davidson LA, Shook DR. 2003. How we are shaped: the biomechanics of gastrulation. Differentiation 71:171–205 [DOI] [PubMed] [Google Scholar]
- 22. Keller R, Winklbauer R. 1992. Cellular basis of amphibian gastrulation. Curr. Top. Dev. Biol. 27:39–89 [DOI] [PubMed] [Google Scholar]
- 23. Kidd T, Bland KS, Goodman CS. 1999. Slit is the midline repellent for the robo receptor in Drosophila. Cell 96:785–794 [DOI] [PubMed] [Google Scholar]
- 24. Kondo Y, et al. 2008. Gene silencing in cancer by histone H3 lysine 27 trimethylation independent of promoter DNA methylation. Nat. Genet. 40:741–750 [DOI] [PubMed] [Google Scholar]
- 25. Kwan KM, Kirschner MW. 2003. Xbra functions as a switch between cell migration and convergent extension in the Xenopus gastrula. Development 130:1961–1972 [DOI] [PubMed] [Google Scholar]
- 26. Lamb TM, et al. 1993. Neural inducion by the secreted polypeptide noggin. Science 262:713–718 [DOI] [PubMed] [Google Scholar]
- 27. Laurent A, et al. 2009. ZFPIP/Zfp462 is maternally required for proper early Xenopus laevis development. Dev. Biol. 327:169–176 [DOI] [PubMed] [Google Scholar]
- 28. Li J, Ye L, Mansel RE, Jiang WG. 2011. Potential prognostic value of repulsive guidance molecules in breast cancer. Anticancer Res. 31:1703–1711 [PubMed] [Google Scholar]
- 29. Li VSW, et al. 2009. Frequent inactivation of axon guidance molecule RGMA in human colon cancer through genetic and epigenetic mechanisms. Gastroenterology 137:176–187 [DOI] [PubMed] [Google Scholar]
- 30. Louie SH, et al. 2009. Modulation of the beta-catenin signaling pathway by the Dishevelled-associated protein Hipk1. Plos One 4:e4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Matsunaga E, et al. 2004. RGM and its receptor neogenin regulate neuronal survival. Nat. Cell Biol. 6:749–755 [DOI] [PubMed] [Google Scholar]
- 32. Mawdsley DJ, et al. 2004. The Netrin receptor Neogenin is required for neural tube formation and somitogenesis in zebrafish. Dev. Biol. 269:302–315 [DOI] [PubMed] [Google Scholar]
- 33. Millard SS, Flanagan JJ, Pappu KS, Wu W, Zipursky SL. 2007. Dscam2 mediates axonal tiling in the Drosophila visual system. Nature 447:720–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mirakaj V, et al. 2011. Repulsive guidance molecule-A (RGM-A) inhibits leukocyte migration and mitigates inflammation. Proc. Natl. Acad. Sci. U. S. A. 108:6555–6560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Monnier PP, et al. 2002. RGM is a repulsive guidance molecule for retinal axons. Nature 419:392–395 [DOI] [PubMed] [Google Scholar]
- 36. Muramatsu R, et al. 2011. RGMa modulates T cell responses and is involved in autoimmune encephalomyelitis. Nat. Med. 17:488–494 [DOI] [PubMed] [Google Scholar]
- 37. Niederkofler V, Salie R, Sigrist M, Arber S. 2004. Repulsive guidance molecule (RGM) gene function is required for neural tube closure but not retinal topography in the mouse visual system. J. Neurosci. 24:808–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nieuwkoop P, Faber J. 1994. Normal table of Xenopus laevis (Daudin). Garland Publishing Inc., New York, NY [Google Scholar]
- 39. Ogata S, Cho KWY. 2007. Dissection of organizer and animal pole explants from Xenopus laevis embryos and assembly of a cell adhesion assay. J. Vis. Exp. doi: 10.3791/187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Okamura Y, Kohmura E, Yamashita T. 2011. TACE cleaves neogenin to desensitize cortical neurons to the repulsive guidance molecule. Neurosci. Res. 71:63–70 [DOI] [PubMed] [Google Scholar]
- 41. Oldekamp J, Kramer N, Alvarez-Bolado G, Skutella T. 2004. Expression pattern of the repulsive guidance molecules RGM A, B and C during mouse development. Gene Expr. Patterns 4:283–288 [DOI] [PubMed] [Google Scholar]
- 42. Popsueva AE, et al. 2001. Overexpression of camello, a member of a novel protein family, reduces blastomere adhesion and inhibits gastrulation in Xenopus laevis. Dev. Biol. 234:483–496 [DOI] [PubMed] [Google Scholar]
- 43. Rajagopalan S, et al. 2004. Neogenin mediates the action of repulsive guidance molecule. Nat. Cell Biol. 6:756–762 [DOI] [PubMed] [Google Scholar]
- 44. Re'em-Kalma Y, Lamb T, Frank D. 1995. Competition between noggin and bone morphogenetic protein 4 activities may regulate dorsalization during Xenopus development. Proc. Natl. Acad. Sci. U. S. A. 92:12141–12145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Samad TA, et al. 2005. DRAGON, a bone morphogenetic protein co-receptor. J. Biol. Chem. 280:14122–14129 [DOI] [PubMed] [Google Scholar]
- 46. Samad TA, et al. 2004. DRAGON: a member of the repulsive guidance molecule-realted family of neuronal- and muscle-expressed membrane proteins is regulated by DRG11 and has neuronal adhesive porperties. J. Nuerosci. 24:2027–2036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schafer MK, Altevogt P. 2010. L1CAM malfunction in the nervous system and human carcinomas. Cell. Mol. Life Sci. 67:2425–2437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schmidtmer J, Engelkamp D. 2004. Isolation and expression pattern of three mouse homologues of chick Rgm. Gene Expr. Patterns 4:105–110 [DOI] [PubMed] [Google Scholar]
- 49. Shiau CE, Bronner-Fraser M. 2009. N-cadherin acts in concert with Slit1-Robo2 signaling in regulating aggregation of placode-derived cranial sensory neurons. Development 136:4155–4164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shin GJ, Wilson NH. 2008. Overexpression of repulsive guidance molecule (RGM) a induces cell death through Neogenin in early vertebrate development. J. Mol. Histol. 39:105–113 [DOI] [PubMed] [Google Scholar]
- 51. Sive HL, Grainger RM, Harland RM. 2007. Dissociation and reaggregation of Xenopus laevis animal caps. CSH Protoc. doi: 10.1101/pdb.prot4778. [DOI] [PubMed] [Google Scholar]
- 52. Skoglund P, Rolo A, Chen XJ, Gumbiner BM, Keller R. 2008. Convergence and extension at gastrulation require a myosin IIB-dependent cortical actin network. Development 135:2435–2444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tardif S, et al. 2010. Zonadhesin is essential for species specificity of sperm adhesion to the egg zona pellucida. J. Biol. Chem. 285:24863–24870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tassew NG, Charish J, Chestopalova L, Monnier PP. 2009. Sustained in vivo inhibition of protein domains using single-chain Fv recombinant antibodies and its application to dissect RGMa activity on axonal outgrowth. J. Neurosci. 29:1126–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tassew NG, et al. 2008. Intraretinal RGMa is involved in retino-tectal mapping. Mol. Cell. Neurosci. 37:761–769 [DOI] [PubMed] [Google Scholar]
- 56. Vielmetter J, Kayyem JF, Roman JM, Dreyer WJ. 1994. Neogenin, an avian cell surface protein expressed during terminal neuronal differentiation, is closely related to the human tumor suppressor molecule deleted in colorectal cancer. J. Cell Biol. 127:2009–2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wilson NH, Key B. 2006. Neogenin interacts with RGMa and Netrin-1 to guide axons within the embryonic vertebrate forebrain. Dev. Biol. 296:485–498 [DOI] [PubMed] [Google Scholar]
- 58. Wilson NH, Key B. 2007. Neogenin: one receptor, many functions. Int. J. Biochem. Cell Biol. 39:874–878 [DOI] [PubMed] [Google Scholar]
- 59. Wojtowicz WM, Flanagan JJ, Millard SS, Zipursky SL, Clemens JC. 2004. Alternative splicing of Drosophila Dscam generates axon guidance receptors that exhibit isoform-specific homophilic binding. Cell 118:619–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Xia Y, et al. 2007. Repulsive guidance molecule RGMa alters utilization of bone morphogenetic protein (BMP) type II receptors by BMP2 and BMP4. J. Biol. Chem. 282:18129–18140 [DOI] [PubMed] [Google Scholar]
- 61. Zhou Z, et al. 2010. Neogenin regulation of BMP-induced canonical Smad signaling and endochondral bone formation. Dev. Cell 19:90–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.