Abstract
Mitochondria are cytoplasmic organelles responsible for life and death. Extensive evidence from animal models, postmortem brain studies of and clinical studies of aging and neurodegenerative diseases suggests that mitochondrial function is defective in aging and neurodegenerative diseases, such as Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, and amyotrophic lateral sclerosis. Several lines of research suggest that mitochondrial abnormalities, including defects in oxidative phosphorylation, increased accumulation of mitochondrial DNA defects, impaired calcium influx, accumulation of mutant proteins in mitochondria, and mitochondrial membrane potential dissipation are important cellular changes in both early and late-onset neurodegenerative diseases. Further, emerging evidence suggests that structural changes in mitochondria, including increased mitochondrial fragmentation and decreased mitochondrial fusion, are critical factors associated with mitochondrial dysfunction and cell death in aging and neurodegenerative diseases. This paper discusses research that elucidates features of mitochondria that are associated with cellular dysfunction in aging and neurodegenerative diseases and discusses mitochondrial structural and functional changes, and abnormal mitochondrial dynamics in neurodegenerative diseases. It also outlines mitochondria-targeted therapeutics in neurodegenerative diseases.
Keywords: Abnormal mitochondrial dynamics, Aging, Alzheimer’s disease, Huntington’s disease, Mitochondria, Mitochondria-targeted antioxidants, Neurodegenerative Disease, Parkinson’s disease
Introduction
Mitochondrial abnormalities and aging are the primary risk factors in the development of age-related neurodegenerative diseases, such as Alzheimer’s (AD), Parkinson’s (PD), Huntington’s (HD), and amyotrophic lateral sclerosis (ALS). Tremendous progress has been made in better understanding the role of genetic mutations in the onset of age-related neurodegenerative diseases, and progress has also been made in developing cell and animal models that have been developed to research the progression of neurodegenerative diseases [1]. Most neurodegenerative diseases share similarities: 1) the diseases manifestation in the brain, 2) they have early and late onset states, 3) aging plays a key role in neurodegenerative disease progression, in both early- and late-onset disease states, 4) mitochondrial dysfunction and oxidative stress are involved in the etiology of these diseases, and 5) mitochondria have been found to be associated with mutant protein(s) in persons with these diseases, and differences among neurodegenerative diseases, including AD, PD, HD and ALS are: 1) affected populations of neurons, 2) disease causing genetic mutations, and 3) clinical symptoms. Despite the tremendous progress that has been made in elucidating similar and different features of neurodegenerative diseases, we still do not have a clear understanding of their etiology and of the cellular mechanisms that are involved in their progression. For example, Aβ has been associated with AD; mutant fSOD1 with ALS; mutant parkin, mutant α-synuclein, mutant DJ1 with PD and mutant Htt with Huntington’s [reviewed in 1–5]. This article discusses the latest developments of role of aging and mitochondria, in the progression of neurodegenerative diseases, particularly AD, PD, HD, and ALS. This article also discusses the recent progress of mitochondrial therapeutics for neurodegenerative diseases.
Aging
In recent years, aging has been defined in several ways. A basic definition is that aging is the process of gradual and spontaneous change, leading to maturation through childhood, puberty, and young adulthood and then a decline through middle and late ages [6]. Aging in mammals has also been defined as a complex process of the accumulation of molecular, cellular, and organ damage leading to a loss of function and an increased vulnerability to disease and death [7]. The human phenotype of aging is the failure of one tissue or organ [8,9].
Healthy aging in humans, a universal and natural phenomenon, has been of particular interest to many researchers who study features of healthy lifespans and mechanisms of aging and age-related diseases. Recently, researchers have demonstrated that calorie-restricted diets can extend the healthy lifespan of lower organisms (e.g., yeast, worms), as can manipulations of the genes of lower organisms [7]. Recent epidemiological studies of humans who lived over 110 years of age suggest that humans age uniformly, with multiple similar pathologies (e.g., diabetes, heart disease, cancer, arthritis, and kidney disease) and with neurodegenerative diseases (e.g., AD, PD, HD, ALS), and that the number of these pathologies increases with age. However, some pathologies, such as sinusitis, remain relatively constant with age, while others, such as asthma, decline with age.
Several hypotheses have been proposed to explain biochemical causes of aging, including mitochondrial abnormalities, reactive oxygen species (ROS) production (or the free radical theory of aging), the shortening of telomerase, DNA methylation, and epigenetics [10–14]. Among these, the free radical theory of aging has been given much attention recently because of its connection with mitochondrial defects and oxidative damage. This theory hypothesizes that ROS – generated by an increase in mitochondria, over time – causes defects in mitochondrial DNA and in mitochondrial structural components, resulting in accelerated cell death and senescence [15–17].
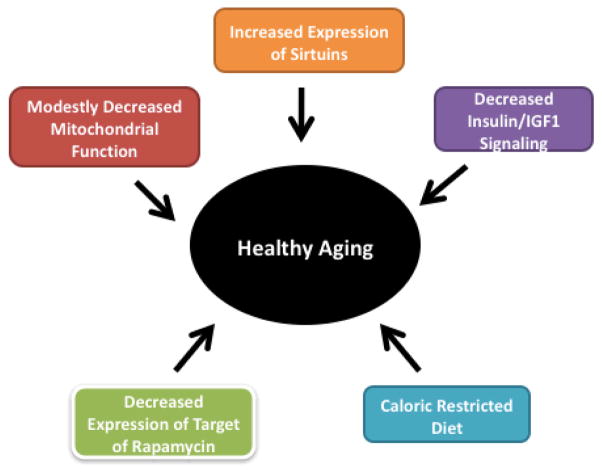
Recent research in lower and higher organisms revealed several important cellular pathways in healthy aging: 1) insulin/IGF – 1 Signaling, in which decreased signaling promotes increased stress resistance and lifespan; 2) sirtuins – an increase in the expression of sirtuins can extend the healthy lifespan; 3) target of rapamycin (TOR) - decreased TOR signaling results in increased lifespan; 4) calorie-restricted diets or optimal diets result in increased lifespan; and 5) modestly decreased mitochondrial function causes increased lifespan [18] (Fig 1). Further, increased telomere length may also contribute to increased lifespan in all organisms [7,19] (Fig. 1). These cellular pathways still needs to be examined in higher organisms such as nonhuman primates and humans. Extensive experimental evidence points to a connection between aging and mitochondria in rodents, nonhuman primates, and humans. Mitochondrial abnormalities have been found to be associated with age. Such abnormalities include accumulations of mtDNA changes, increased production of free radicals, decreased respiratory function, and low ATP production in the brains of aged people [2,3,20–26]. These age-dependent mitochondrial abnormalities may contribute to the development of age-related neurodegenerative diseases such as AD and PD.
Figure 1.
Factors those are critical for healthy and extended lifespan in humans.
Mitochondrial structure and function
Mitochondria are cytoplasmic organelles that are essential for the life and death of the cells. They are usually transmitted maternally, but in rare situations, they can be transmitted paternally [27]. They perform or contribute to the performance of several cellular functions, including: 1) the regulation of intracellular calcium, 2) ATP production, 3) the release of proteins that activate the caspase family of proteases, 4) alteration of the reduction-oxidation potential of cells, and 5) free-radical scavenging [1, 21].
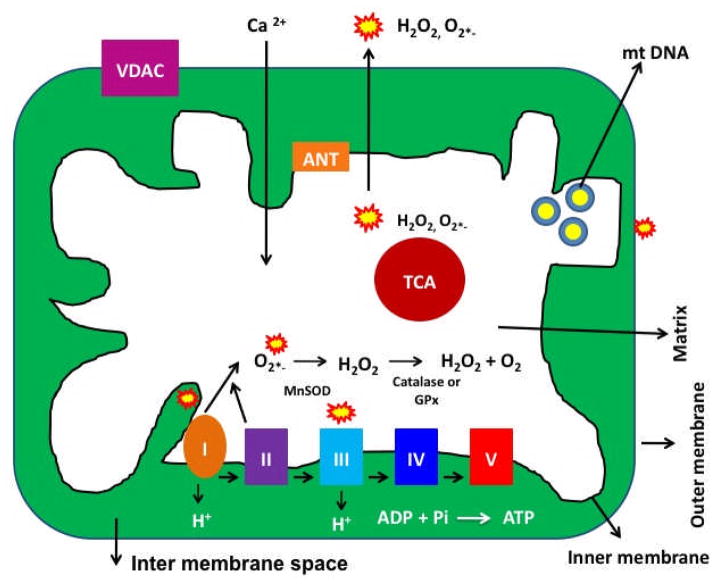
Mitochondria are compartmentalized into 2 lipid membranes: the outer mitochondrial membrane and the inner mitochondrial membrane. The outer membrane is porous and allows the passage of low molecular-weight substances between the cytosol and the inter-membrane space of mitochondria. The inner membrane provides a highly efficient barrier to ionic flow, houses the mitochondrial respiratory chain or electron transport chain (ETC), and covers the mitochondrial matrix. The mitochondrial matrix contains tricarboxylic acid (TCA) and beta-oxidation (see Fig. 2) [27].
Figure 2. Structure of mitochondria.
Mitochondria are bag like structures, comprised of 2 lipid membranes (outer and inner), and matrix that harbor components of tricarboxylic acid cycle, beta-oxidation. The electron transport chain is localized in the inner mitochondrial membrane. Outer mitochondrial membrane is porous, whereas mitochondrial inner membrane restricts ionic flow and protects the contents of matrix. Mitochondrial ATP is generated via oxidative phosphorylation within the inner mitochondrial membrane. Free radicals are generated as a byproduct of oxidative phosphorylation, primarily due to electron leaks in complex I and III of respiratory chain. The components of tricarboxylic acid, including α-ketoglutarate dehydrogenase, generate superoxide radicals in the matrix. These mitochondrially generated free radicals and superoxide radicals are carried to the cytoplasm via voltage-dependent anion channels and participate in lipid peroxidation, and protein and DNA oxidation.
Mitochondria are controlled by both nuclear and mitochondrial genomes. MtDNA consists of a 16,571 base pair, double-stranded, circular DNA molecule [28]. A mitochondrion contains 2–10 copies of mtDNA [1]. mtDNA contains 13 polypeptide genes that encode essential components of the ETC. mtDNA also encodes the 12S and 16S rRNA genes and the 22 tRNA genes required for mitochondrial protein synthesis [27]. Nuclear genes encode the remaining mitochondrial proteins, metabolic enzymes, DNA and RNA polymerases, ribosomal proteins, and mtDNA regulatory factors, such as mitochondrial transcription factor A. Nuclear mitochondrial proteins are synthesized in the cytoplasm and are subsequently transported into mitochondria.
Mitochondrial ATP is generated via oxidative phosphorylation (OXPHOS) within the inner mitochondrial membrane (Fig. 2) [21]. Free radicals are generated as a byproduct of OXPHOS. In the respiratory chain, complexes I and III leak electrons to oxygen, producing primarily superoxide radicals. The superoxide radicals are dismutated by manganese superoxide dismutase (Mn-SOD), generating H2O2 and oxygen. Complex I generates only toward the mitochondrial matrix, but complex III generates toward both the inter-membrane space and the matrix. In addition, the components of tricarboxylic acid, including α-ketoglutarate dehydrogenase, generate superoxide radicals in the matrix [21]. These mitochondrially generated free radicals and superoxide radicals are carried to the cytoplasm via voltage-dependent anion channels and participate in lipid peroxidation, and protein and DNA oxidation.
Mitochondria and aging
Studies of mitochondria using a mitochondria-targeted antioxidant transgenic model of aging suggest that healthy mitochondria and mitochondrial DNA play a large role in perpetuating healthy aging and extending lifespan. In contrast, abnormalities in mitochondria and mtDNA accelerate premature aging.
Mitochondrial dysfunction has been well documented in aging and neurodegenerative diseases [1,3]. To understand the mechanisms of aging, particularly the role of mtDNA in the brain, several researchers studied mtDNA, free radical production, and mitochondrial function in young and aged tissues from rodents, nonhuman primates, and humans [reviewed in 5, 7, 18, 19, 23 and 29, 30]. In aged tissues relative to young tissues from rodents, nonhuman primates, and humans, researchers found an accumulation of mtDNA defects, an increased production of ROS, and decreased mitochondrial function in the brain tissues from old rodents, nonhuman primates and humans [7, 18, 19, 29, 30], indicating that age-related accumulation of mtDNA defects is involved in aging. It has been reported that mitochondrial ETC is responsible for the transfer of electrons from NADH or FADH, to electron acceptors, and to oxygen, the final transfer of which leads to the production of H2O. These biochemical events lead to a small amount (0.5–5%) of electron leakage and, subsequently, to ROS production [reviewed in 1].
MtDNA is localized close to the source of ROS production and may be vulnerable to DNA damage. Oxidized guanosine levels are higher in mtDNA relative to nuclear DNA. It has been reported that several DNA repair mechanisms may operate within mitochondria, but one such repair mechanism – nucleotide excision repair – may be absent in mtDNA, leaving mtDNA vulnerable to a number of DNA changes [1]. MtDNA defects that reduce the accuracy of electron transfer may increase the production of ROS and decrease the production of ATP. An increase in the production of ROS may further damage mtDNA.
mtDNA changes and aging
Changes in mtDNA have been found to be responsible for aging phenotypes [29, 31, 32]. For example, many tissues from aged rodents have lower respiratory function compared to those from younger rodents [29] (Cooper et al. 1992). Both mtDNA single-nucleotide mutations and deletions are highly prevalent in aged cells. There is evidence that damaged mtDNA is more prevalent in aged tissues [33, 34, 35].
To understand the role of mtDNA mutations in aging, two investigators independently created mouse lines containing a point mutation in the proofreading region of DNA polymerase gene, the catalytic subunit of mtDNA polymerase [31, 32]. The mutant mice in both studies exhibited normal DNA polymerase activity but lacked the exonuclease activity necessary for proofreading the mutated mice did not live as long as control mice and experienced an early onset of age-associated features, including weight loss, reduction in subcutaneous fat, hair loss, curvature of the spine, and osteoporosis. Such similar findings from two independent studies suggest that mtDNA changes are involved in the aging process.
Contrary to these studies, a recent study supports the involvement of mitochondria-targeted antioxidants in the healthy aging [36]. To determine the protective effects of catalase, Schriner and colleagues [36] created transgenic mouse lines that over-expressed human catalase localized to peroxisomes (PCAT mice), nuclei (NCAT mice), and mitochondria (MCAT mice). The transgenic mice that were targeted to mitochondria showed about a 20% increase in median and maximal life span compared to the life span of non-transgenic, age-matched wild-type littermates. That catalase increases longevity was most apparent when catalase was targeted to mitochondria (MCAT mice) but not in nuclear (NCAT mice) or in peroxisomal targeted mice (PCAT mice) [36].
Overall, findings from these studies suggest that mutations in mitochondrial DNA play a large role in premature aging. On the contrary, over-expression of mitochondria-targeted antioxidants extend healthy lifespan.
Mitochondrial dysfunction in Neurodegenerative diseases
Several lines of evidence suggests that mitochondria play a large role in neurodegenerative diseases, including AD, PD, HD and ALS: 1) defective mitochondrial enzyme activities, 2) mitochondrial DNA defects, 3) mitochondrial dysfunction and oxidative stress, and 4) mitochondrial structural changes [1–4]. Further, in 1990s, Swerdlow and colleagues studied the role of mtDNA in mitochondrial function using cytoplasmic hybrids (cybrids) of peripheral cells from patients with AD, PD and ALS and control subjects. They found that peripheral cells from AD, PD and ALS lacking mtDNA did not exhibit mitochondrial dysfunction, suggesting that mitochondria are important for mitochondrial function in disease progression in AD, PD and ALS [37–39].
Mitochondrial dysfunction in Alzheimer’s disease
AD is a late-onset, progressive neurodegenerative disease, characterized by the progressive decline of memory, cognitive functions, and changes in behavior and personality [21, 24]. The prevalence of AD is high among aged persons with AD: 13% of individuals 65 years old have AD and 50% of individuals 85 years of age and older have AD [40]. By the year 2050, 50% of people worldwide will have AD. Currently, 5.3 million Americans suffer from AD [41]. In addition to the personal and family hardships that AD creates, these numbers translate into extremely high health care costs. With increasing lifespan in humans, AD is a growing health concern in the society.
Two major pathological features have been observed in postmortem brains from AD patients: 1) intracellular neurofibrillary tangles and 2) extracellular Aβ deposits in the regions of the brain that are responsible for learning and memory. AD is also associated with the loss of synapses, synaptic function, mitochondrial structural and functional abnormalities, inflammatory responses, and neuronal loss [24, 37, 38, 42]. Genetic mutations in APP, PS1, and PS2 genes cause a small proportion (about 1%) of all AD cases; however, causal factors are still unknown for the vast majority of AD patients. Several factors – including lifestyle, diet, environmental exposure, apolipoprotein allele E4, and a genetic variant in sortilin-related receptor 1 gene, clusterin, and complement component receptor 1 – may contribute to late-onset AD [24, 43, 44]. Above all, aging has been considered ‘number one’ risk factor in the progression and development of AD.
AD affects brain regions involved in learning, memory, and cognitive functions: the hippocampus, entorhinal cortex, temporal cortex, frontoparietal cortex, and, subcortical nuclei [45]. Aβ secretion also occurs mainly in these regions; however, the reasons for Aβ secretion and the formation of Aβ deposits in these areas are not fully understood, nor are the relationship between Aβ deposits and AD understood.
Although AD pathogenesis involves a large number of molecular and cellular events, two events that are known to occur early in AD development are: 1) synaptic damage and 2) mitochondrial dysfunction [4, 24, 45–47]. It is generally accepted that an age-dependent accumulation of Aβ at synapses and in synaptic mitochondria probably interferes with synaptic activities, including the release of vesicles and neurotransmitters, and interferes with the production of ATP at the synapses. For normal communication across neurons, and normal cognitive and memory functions, it is critical that synaptic activities and the supply of ATP are normal [24]. However, it is these events that are interrupted more and more frequently in elderly individuals and in AD patients.
In normal, healthy neurons, mitochondria move from cell body to axons, dendrites, and synapses by an anterograde mechanism, supplying ATP at nerve terminals. Mitochondria then travel back to the cell body from synapses through a retrograde mechanism. However, in AD neurons, these mechanisms are abnormal primarily due to defective or functionally inactive mitochondria [4, 22].
Recently, mitochondrial abnormalities have been linked to AD: 1) changes in mtDNA, 2) decreased mitochondrial enzyme activities, 3) abnormal mitochondrial gene expressions, 4) the association of mutant APP and Aβ with mitochondrial membranes in neurons affected by AD, 5) increased mitochondrial structural alterations – increased fragmentation and decreased mitochondrial fusion [4], 6) abnormal mitochondrial trafficking in neurons affected by AD, 7) decreased mitochondrial function and 8) defective synaptic mitochondria.
1. Mitochondrial DNA changes and AD
Mitochondrial DNA defects were found increased in postmortem brain tissue from AD patients and in aged-matched healthy subjects, compared to DNA changes in postmortem brain tissue from young, healthy subjects, suggesting that the accumulation of mitochondrial DNA in AD pathogenesis is age-related [48, 49].
Recently, Coskun and colleagues [50] investigated whether the mtDNA copy number was related to disease progression in AD. Using molecular methods, they investigated the mtDNA copy number in DNA from patients with AD and Downs syndrome. They found increased mtDNA changes and decreased mtDNA copy number in postmortem brains from AD and Downs syndrome patients. Further, in the brain tissues from aged control patients who did not have AD, the researchers found that mutations in the control region of mtDNA increased; and in patients with Downs syndrome, mutations in the control region of mtDNA were associated with a reduced mtDNA copy number and L-strand transcripts. The increase in mtDNA mutations was also seen in peripheral blood DNA and in lymphoblastoid cell DNAs of AD and Downs syndrome patients [50]. In aging, Down syndrome, and Down syndrome AD, mtDNA mutations positively correlated with β-secretase activity, and the copy number of mtDNA was inversely correlated with the levels of Aβ 40 and Aβ 42. Therefore, mtDNA mutations may be responsible for neuropathological changes observed in AD and Down syndrome AD [50].
Lakatos et al [51] investigated mitochondrial DNA variations (haplotypes) in 138 mitochondrial polymorphisms in 358 subjects in the Caucasian Alzheimer’s Disease Neuroimaging Initiative subjects. They found that the mitochondrial ‘haplogroup UK’ may confer genetic susceptibility to AD independently of the apolipoprotein E4 allele [51]
2. Defective mitochondrial enzyme activities and AD
Studies of mitochondrial enzyme activities found decreased levels of cytochrome oxidase activity, pyruvate dehydrogenase, and α-ketodehydrogenase in fibroblasts, lymphoblasts, and postmortem brains from AD patients, compared to neurons, fibroblasts, and lymphoblasts from age-matched healthy subjects [reviewed in 24]. Further, decreased levels of cytochrome oxidase activity were found in transgenic mouse models of AD [52–54].
3. Abnormal mtDNA expressions and AD
Recently, our laboratory investigated mitochondrial gene expressions in postmortem brains from AD patients [55] and in brain specimens from AD transgenic mice [56] and mouse neuroblastoma cells incubated with Aβ peptide (25–35) [57]. We found mitochondrially encoded genes abnormally expressed in the diseased brain specimens. A recent, time-course global gene expression study of Tg2576 mice and age-matched non-transgenic littermates revealed an up-regulation of mitochondrial genes in the Tg2576 mice, suggesting that mitochondrial metabolism is impaired by mutant APP/Aβ and that the up-regulation of mitochondrial genes may be a compensatory response to mutant APP/Aβ [56]. Further, We also found an abnormal expression of mitochondrial-encoded genes in postmortem AD brains compared to the brains from healthy subjects [55], suggesting impaired mitochondrial metabolism in AD.
Very recently, the Reddy laboratory investigated mitochondrial-encoded electron transport chain genes in mouse neuroblastoma (N2a) cells treated and untreated with the Aβ peptide 25–35 [57]. In N2a cells treated with the Aβ peptide, they found significantly increased mRNA expression of mitochondrial-encoded electron transport chain genes in complex 1 (subunit 1, subunit 5), complex III (CytB), complex IV (COX1, COX III) and complex V (ATPase 6) relative to N2a cells untreated with Aβ peptide, indicating that mitochondrial function is impaired by Aβ peptide.
4. Mutant APP and Aβ association with mitochondria
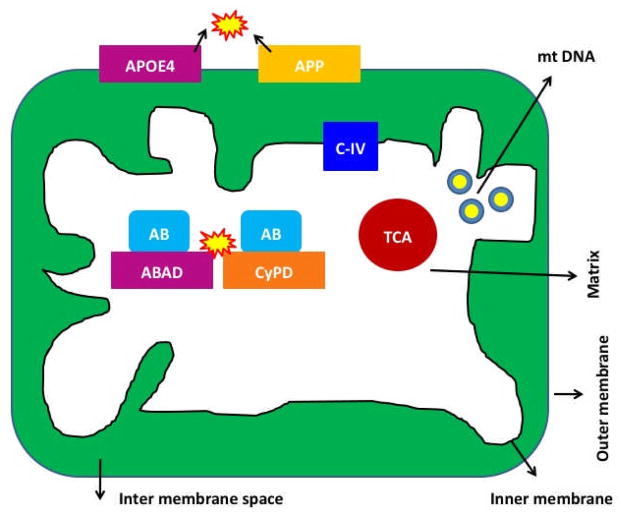
We [52] and others [53, 54, 58–61] reported that AβPP, and monomeric and oligomeric forms of Aβ have been found in mitochondrial membranes (Fig. 3) Lustbader et al [58]. found Aβ normally interacting with the mitochondrial matrix protein ABAD, with this interaction leading to mitochondrial dysfunction. Caspersen et al. [54] found an accumulation of Aβ in the mitochondria from postmortem brain specimens of AD patients and AβPP transgenic mice. Recently, the Reddy laboratory found Aβ monomers and oligomers in mitochondria isolated from the cerebral cortex of AβPP transgenic mice and from N2a cells expressing AβP [52]. A digitonin fractionation analysis of isolated mitochondria from AβPP transgenic mice revealed Aβ in the outer and inner membranes and matrix of mitochondria. We found that mitochondrial Aβ decreases cytochrome oxidase activity and increases free radicals and carbonyl proteins. Du et al [61] found Aβ interaction with mitochondrial matrix protein, cypclophilin D, and this abnormal interaction causes mitochondrial dysfunction in the brains of AD transgenic mice. More recently, Yao et al. [53] found Aβ in mitochondrial membranes of cortical tissues from triple transgenic mice. Figure 3 summarizes the localization of Aβ in mitochondrial membranes.
Figure 3. Localization of mutant proteins of AD in the mitochondria in neurons affected by AD.
In early-onset AD, genetic mutations of APP, PS1 and PS2 genes activate beta and gamma secretases, cleave from c-terminal region of amyloid beta precursor protein, and produce Aβ peptides. The toxic Aβ peptides enter mitochondria, interact with mitochondrial proteins, including cyclophilin D and ABAD --induce free radicals, decrease cytochrome oxidase activity, inhibit ATP generation and damage mitochondria both structurally and functionally. Further, in late-onset AD, APP is transported to outer mitochondrial membrane, blocks the import of nuclear cytochrome oxidase proteins to mitochondria and may be responsible for decreased cytochrome oxidase activity. In addition, N-terminal portion of ApoE4 is associated with mitochondria, induce free radicals and cause oxidative damage. In AD, complex IV of mitochondrial respiratory chain is affected.
5. Abnormal mitochondrial dynamics in AD
Increasing evidence suggests that mitochondrial dynamics are impaired in neurons affected by AD.
Using confocal and electron microscopy, and human neuroblastoma (M17) cells transfected with wild-type or mutant AβPP, Wang and co-workers [62] investigated the effects of AβPP and Aβ on mitochondrial structural changes. Confocal and electron microscopic analysis revealed that about 40% of M17 cells overexpressing wild-type AβPP and more than 80% of M17 cells overexpressing mutant AβPP displayed alterations in mitochondrial morphology, particularly fragmented mitochondria. They also found that increased levels of Fis1 are critical for mitochondrial fission in AβPPwt and AβPPswe M17 cells [62].
Using electron and confocal microscopy, gene expression analysis, and biochemical methods, the Reddy laboratory studied mitochondrial structure and function, and neurite outgrowth in neurons treated with Aβ [57]. In neurons treated with only Aβ, we found increased expressions of mitochondrial fission genes (Drp1 and Fis1) and decreased expressions of fusion genes (Mfn1, Mfn2, and Opa1), indicating abnormal mitochondrial dynamics in AD neurons. mRNA expression of antioxidant enzyme-encoded genes (peroxiredoxins 1–6) was significantly decreased in neurons treated with Aβ relative to untreated neurons. Our electron microscopy of neurons treated with Aβ revealed a significant increase in mitochondrial fragmentation, further supporting abnormal mitochondrial dynamics. We also found significantly decreased neurite outgrowth and decreased mitochondrial function in cells treated with Aβ [57]. These findings suggest that Aβ fragments mitochondria and causes abnormal mitochondrial dynamics, leading to mitochondrial dysfunction.
Using neurons from adult fruit flies, Zhao and colleagues [63] studied the effects of wild-type and an arctic form of Aβ42. They performed extensive time-course analyses to determine the function and structure of both axon and presynaptic terminals of individual neurons. They found Aβ accumulated intracellularly, and they found a wide range of changes typically associated with aging, including the depletion of presynaptic mitochondria, a slow-down of bi-directional transports of axonal mitochondria, decreased synaptic vesicles, increased large vacuoles, and elevated synaptic fatigue [63].
Taken together, these findings suggest Aβ enters mitochondria and causes abnormal mitochondrial dynamics in neurons that are affected by AD, and that such abnormal mitochondrial dynamics cause mitochondrial dysfunction and abnormal mitochondrial trafficking in AD neurons.
In addition, it has been proposed that defective synaptic mitochondria may cause synaptic abnormalities and cognitive decline in AD [24]. Recently, the Reddy laboratory investigated the effects of the mitochondria-targeted antioxidants, MitoQ and SS31, and the anti-aging agent resveratrol on neurons from a mouse model (Tg2576 line) of AD and on mouse neuroblastoma (N2a) cells incubated with the Aβ peptide [57]. Using electron and confocal microscopy, gene expression analysis, and biochemical methods, we studied mitochondrial structure and function and neurite outgrowth in N2a cells treated with MitoQ, SS31, and resveratrol, and then incubated with Aβ. In N2a cells treated with MitoQ, SS31, and resveratrol, and then incubated with Aβ, abnormal expression of peroxiredoxins and mitochondrial structural genes did not develop, and mitochondrial function was normal; intact mitochondria were present and neurite outgrowth was significantly increased. In primary neurons from amyloid-β precursor protein transgenic mice that were treated with MitoQ and SS31, neurite outgrowth was significantly increased and cyclophilin D expression was significantly decreased. These findings suggest that MitoQ and SS31 prevent Aβ toxicity, which would warrant the study of MitoQ and SS31 as potential drugs to treat patients with AD [57].
6. Impaired mitochondrial trafficking and AD
Mitochondrial shape and morphology are regulated and maintained by mitochondrial fission and mitochondrial fusion [4, 22, 62, 64]. In a healthy neuron, fission and fusion mechanisms balance equally. Through mitochondrial trafficking, mitochondria alter their shape and size to move from the cell body to the axons, dendrites, and synapses, and back to the cell body. Fission and fusion are controlled by evolutionary conserved, large GTPases belonging to the dynamin family. Fission is controlled and regulated by the dynamin-related protein Drp1 and the mitochondrial fission 1, the latter of which is localized to the outer membrane of mitochondria [22]. The increase in mitochondrial free radicals activates Fis1, which is also critical for mitochondrial fission.
Three GTPase proteins control mitochondrial fusion: two outer-membrane localized proteins Mfn1 and Mfn2, and one inner-membrane-localized protein Opa1 [4, 22]. The C-terminal part of Mfn1 mediates oligomerization between Mfn1 molecules of adjacent mitochondria and facilitates mitochondrial fusion. In AD neurons, mitochondrially generated free radicals activate Fis1 and promote an increase in mitochondrial fragmentation, which in turn produces defective mitochondria that ultimately damage neurons.
In AD neurons, mitochondrial dynamics are impaired mainly because of fragmentation of mitochondria caused by Aβ. Further, Aβ interacts with mitochondrial proteins and causes structural damage to mitochondria, ultimately leading to the increased production of defective mitochondria. These defective mitochondria might not move from cell body to the nerve terminals and might not produce sufficient levels of ATP at synapses. Such abnormal mitochondrial trafficking and decreased production of ATP at synapses may lead to synaptic degeneration, axonal damage in AD neurons, and may be responsible for cognitive decline in AD patients.
7. Mitochondrial dysfunction, oxidative stress and AD
Mitochondrial dysfunction has been observed in postmortem brain tissue from patients with AD, in platelets from AD patients, in brain tissues from AD transgenic mice, in cell lines that express mutant APP, and in cells treated with Aβ [4]. Several biochemical and cellular studies found increased free radical production, lipid peroxidation, oxidative DNA damage, oxidative protein damage, decreased ATP production, and decreased cell viability in postmortem brain specimens from AD patients, compared to brain specimens from age-matched healthy subjects [60, 65–68].
8. Defective synaptic mitochondria, Aβ and synaptic damage
Very recently, Dragicevic et al [69] studied synaptic mitochondrial abnormities in the AβPPsw and AβPP+PS1 mouse lines, focusing on the hippocampus, cortex, striatum, and amygdala of 12-month-old AβPPsw and AβPP+PS1 mice as well as nontransgenic mice. They measured mitochondrial respiratory rates, ROS production, membrane potential, and cytochrome c oxidase activity. Hippocampal and cortical mitochondria showed the highest levels of mitochondrial dysfunction, while striatal mitochondria were moderately affected, and amygdala mitochondria were minimally affected. Mitochondria in affected brain tissues from AβPP+PS1 mice were more impaired than those from AβPP mice. Synaptic mitochondria were more impaired than nonsynaptic mitochondria in both the AβPPsw and AβPP+PS1 mouse models. The AβPP/PS1 mice showed more impairment in the cognitive interference task of working memory than did the AβPP mice. The correspondence between levels of mitochondrial Aβ and levels of mitochondrial dysfunction in AD mouse models supports a primary role for mitochondrial Aβ in AD pathology. Dragicevic et al [69] studied the relationship between mitochondrial Aβ levels and mitochondrial dysfunction in AD mouse models. Moreover, the degree of cognitive impairment in AD transgenic mice was linked to the extent of mitochondrial dysfunction and mitochondrial Aβ, suggesting that a mitochondrial Aβ-induced signaling cascade may contribute to cognitive impairment [69].
Du et al [46] also investigated APP transgenic mice for synaptic mitochondria and Aβ in AD progression. They detected synaptic mitochondrial pool of Aβ in APP mice as early as 4 months of age, and well before the extracellular Aβ accumulation. Aβ-insulted synaptic mitochondria revealed early deficits in mitochondrial function, as shown by increased mitochondrial permeability transition, decline in both respiratory function and activity of cytochrome c oxidase, and increased mitochondrial oxidative stress [46].
Overall, as described above, mitochondria are damaged both structurally and functionally, in AD mice and AD patients, and critically involved in the progression and development of AD. Further, damaged mitochondria at synapses, may be responsible for synaptic damage and cognitive decline in patients with AD.
Mitochondrial dysfunction in Huntington’s disease
HD is a midlife, neurodegenerative disease with an autosomal-dominant inheritance. HD is characterized by involuntary movements, chorea, dystonia, changes in personality, and cognitive decline [70–72]. Four to 10 people per 100,000 suffer from HD, and they are mostly of Caucasian origin. Key features found in postmortem brain tissues from patients with HD are the loss of medium spiny neurons in the caudate and putamen of the striatum of basal ganglia [70, 73, 74]. In patients in advanced stages of HD, neuronal loss has also been reported in the cortex and hippocampus.
HD is a genetic disease, caused by a genetic mutation in the exon 1 of the HD gene, resulting in an expanded polyglutamine encoding repeat. In persons affected by HD, the number of polyglutamine repeats ranges from 36–120, whereas in unaffected persons, polyglutamine repeats range from 6–35 [75]. Htt, a product of the HD gene, is a 350 kDa protein, ubiquitously expressed in the brain and peripheral tissues [76–82]. In the HD brain, Htt is localized mainly in the cytoplasm of neurons 77, 80–82].
Causes of neuronal loss that characterizes HD are not known, and also not well understood is how the mutant Htt causes HD progression. Several mechanisms and pathways have been proposed to explain HD progression, including: 1) transcriptional dysregulation, 2) the interaction of expanded polyglutamine repeat Htt proteins with other proteins in the central nervous system [83, 84], 3) caspase activation [85–88], 4) NMDAR activation [89–90], 5) calcium dyshomeostasis [90–98], and 6) abnormal mitochondrial bioenergetics and 7) abnormal axonal trafficking [80, 99–103].
Several lines of evidence suggest that mitochondrial abnormalities are involved in the brains of HD patients and HD mouse models: 1) decreased mitochondrial enzyme activities have been found in HD brain, 2) mitochondrial DNA defects, 3) abnormal mitochondrial dynamics 4) mitochondrial trafficking abnormalities, 5) calcium dyshomeostatis, and 6) mitochondrial dysfunction and oxidative stress.
1. Decreased mitochondrial enzyme activities and HD
Biochemical studies of mitochondria in brain tissues from HD patients revealed reduced mitochondrial enzyme activities [99, 104–107]. Further, in studies of HD transgenic and knock-in mice, and in experimental HD rodent models, decreases in enzyme activities of complexes I, II, III, and IV were found in postmortem brain tissues from HD patients [108], suggesting that mitochondria are involved in HD pathogenesis. Recent studies of HD knock-in striatal cells and lymphoblasts from HD patients found expanded polyglutamine repeats associated with decreased mitochondrial ATP and decreased mitochondrial ADP-uptake, suggesting that in HD, mutations in the HD gene may be associated with functional defects in mitochondria [109].
2 Mitochondrial DNA defects and HD
Several recent studies found age-dependent mitochondrial DNA (mtDNA) defects in postmortem brain tissues from HD patients and HD mouse models, and in peripheral cells from HD patients [110–112].
Acevedo-Torres et al [110] investigated mitochondrial DNA defects in two HD mouse models: the chemically induced 3-nitropropionic acid model and the HD transgenic mouse model of the R6/2 strain containing 115–150 polyglutamine repeats in the HD gene [110]. Using quantitative PCR, they measured nuclear and mtDNA damage in the striatum of 5- and 24-month-old untreated and 3-NPA-treated C57BL/6 mice. They found an increase in damage in both nuclear and mitochondrial DNA in the untreated 24-month-old mice, and in the 5-month-old mice, 3-NPA induced 4 to 6 times more damage in the mtDNA than in the nuclear DNA. These data suggest that mtDNA damage may be an early biomarker for HD-associated neurodegeneration, and they support the hypothesis that mtDNA lesions may contribute to the pathogenesis observed in patients with HD [110].
Chen et al. [111] investigated whether pathological changes in HD brains may also be present in peripheral tissues. Deleted and total mtDNA copy numbers were increased, whereas the mRNA expression levels of mtDNA-encoded mitochondrial enzymes were not increased in the HD leukocytes compared to the leukocytes from normal humans [111].
Banoei et al [112] investigated 4 mtDNA deletions based on the size of deletion: 9 kb, 7.5 kb, 7 kb, and 5 kb in the mitochondrial DNA of HD patients and found mitochondrial DNA deletions are present in brains and peripheral tissues of HD patients. Findings from these studies suggest that mutant Htt cause mitochondrial DNA defects in HD brains and peripheral tissues from HD patients.
Overall, these mtDNA studies suggest that mtDNA defects are present in the brains and peripheral cells from HD patients.
3. Abnormal mitochondrial dynamics and HD
In HD neurons, mitochondrially generated free radicals activate Fis1 and promote an increase in mitochondrial fragmentation, which in turn produces defective mitochondria that ultimately damage neurons. Mitochondrial fusion protects cells from the toxic effects of mitochondrial DNA and from mitochondrial mutant Htt by allowing functional complementation of mitochondrial DNA, proteins, and metabolites. Cell hybrids resulting from the fusion of parental cells carrying pathogenic mutations have been found to restore mitochondrial ETC activity 113]. Increased mitochondrial fission may be due to mutant Htt in HD neurons, which may in turn decrease mitochondrial fusion and ultimately damage HD neurons.
4. Abnormal mitochondrial trafficking and HD
Recently, several studies have reported that mutant Htt, interacting with mitochondria and microtubules, impairs the axonal transport of mitochondria to nerve terminals [101–103]. Figure 4 summarizes the localization of mutant Htt in mitochondrial in neurons affected by HD. This defective mitochondrial transport ultimately impairs neural transmission and results in synaptic damage and selective neuronal damage or loss.
Figure 4. The association of mutant huntingtin with mitochondria of HD neurons.
In HD neurons, mutant Htt binds to outer mitochondrial membrane and induces free radical production, and may interrupt with calcium uptake. In HD, complex II of mitochondrial respiratory chain is affected.
Trushina et al [101] investigated the involvement of mutant Htt in the impairment of fast axonal trafficking in HD neurons. The expression of full-length mutant Htt in these neurons was found to impair vesicular and mitochondrial trafficking and in whole animals. In neurons from HD mice, mitochondria, became progressively immobilized and stopped more frequently than in neurons from control mice. These defects occurred early in the development of HD, before the onset of measurable mitochondrial abnormalities. Findings indicated that the loss of Htt function may cause toxicity; mutant Htt-mediated aggregation sequestered Htt and components of trafficking machinery, leading to a loss of mitochondrial mobility and eventually to mitochondrial dysfunction [101].
Chang et al [102] investigated whether mutant Htt alters mitochondrial trafficking and morphology in primary cortical neurons taken from HD mice. They demonstrated that the full-length mutant Htt was more effective than the N-terminal mutant Htt in blocking mitochondrial movement. Cytosolic Htt aggregates impaired the passage of mitochondria along neuronal processes, causing mitochondria to accumulate adjacent to the aggregates and to become immobilized. Further, mitochondrial trafficking was reduced specifically at sites of aggregates but remained unaltered in regions lacking aggregates. They concluded that in primary cortical neurons, an early event in HD pathophysiology may be the aberrant mobility and trafficking of mitochondria caused by cytosolic Htt aggregates [102].
Orr and colleagues [103] investigated the association of N-terminal mutant Htt fragments with mitochondria in HD knockin mice expressing N-terminal 150 polyglutamine repeats. They found the N-terminal expands the polyglutamine repeat protein that is associated with mitochondria and that this association increases with age in knock-in mice [103]. They also found that the interaction between soluble N-terminal mutant Htt and mitochondria interferes with the in vitro association of microtubule-based transport of proteins and mitochondria. Mutant Htt reduced the distribution and transport rate of mitochondria in the processes of cultured neuronal cells. These findings suggest that before aggregates form, N-terminal mutant Htt fragments directly impair mitochondrial function.
These studies indicated that abnormal axonal transport and mitochondrial trafficking early events in HD progression.
5. Calcium dyshomeostatis and HD
Biochemical studies of cell lines in HD mice revealed that calcium-induced mitochondrial permeability is a major factor in HD pathogenesis [91, 92]. Calcium dyshomeostatis was found in patients with HD [88, 90–97] (Fig. 4).
6. Mitochondrial dysfunction and oxidative stress and HD
Increasing evidence suggests that mitochondrial dysfunction is involved in HD progression.
Recently, Solans et al. [114] investigated mitochondrial respiration in yeast cells using an expanded polyglutamine repeat protein. They found cell respiration was reduced after 4–6 h of induction with galactose, and after 10 h of induction, cell respiration further reduced to 50% compared to that of the control yeast cells. They also found defects in cell respiration when the function and the amount of mitochondrial respiratory chain complex II+III were altered during HD progression. The production of ROS was also found significantly enhanced in yeast cells expressing expanded polyglutamines. Mitochondrial morphology and distribution were also altered in HD neurons, possibly from the interaction of aggregates and portions of the mitochondrial network and from a progressive disruption of the actin cytoskeleton. Interactions of misfolded aggregated polyglutamine domains with the mitochondrial and actin networks led to disturbances in mitochondrial distribution and function and to an increase in ROS production. Oxidative damage preferentially affected the function of enzymes containing iron-sulfur clusters, such as complexes II and III. Findings from this mitochondrial study further supported the involvement of mitochondrial dysfunction in HD [114].
With strong evidence indicating the involvement of mitochondrial dysfunction in HD, treating mitochondria with mitochondria-targeted antioxidants is important option to treat patients with HD.
Mitochondrial dysfunction in Parkinson’s disease
PD is characterized by muscle rigidity, tremors, and a slowing of physical movement. Pathological changes in PD are the loss of dopaminergic neurons in the pars compacta region of the substantia nigra and the presence of cytoplasmic inclusions, or Lowy bodies, containing α-synuclein [115–118]. PD is both chronic and progressive. One percent of people 65–69 years of age suffer from PD. Both genetic and environmental toxins are involved in the development of PD [1].
Recent studies revealed that environmental toxins induce PD in humans. The exposure of humans to the environmental toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) results in permanent PD syndrome [119]. Thus, the MPTP mouse model is now used as an experimental model for PD since it rapidly develops PD-like symptoms. The chronic administration of the ETC complex I inhibitor, rotenone, resulted in an model that replicates the loss of dopaminergic nigral neurons, the PD phenotype, and α-synuclein inclusions found in humans with PD.
Several recent genetic studies in PD have revealed DNA mutations in α-synuclein [120], Parkin [121], DJ1 [122], PTEN- induced kinase 1 [123], OMI/HTRA2 [124], leucine rich repeat kinase LRRK2 [125], and ubiquitin carboxy-terminal esterase L1 [126] linked to PD.
Multiple lines of evidence suggest that mitochondrial abnormalities are involved in PD progression: 1) abnormalities in mitochondrial ETC complex I, 2) mitochondrial DNA mutations and DNA defects and PD, 3) abnormal mitochondrial dynamics, and 4) mutant proteins association with mitochondria.
1. Mitochondrial electron transport chain complex 1 defects and PD
Complex I defects were found in PD patients, and complex 1 activity was found to be reduced by 30% in brain and muscle tissues and in platelets from idiopathic PD patients [127, 128]. Complex I defects were found to induce the generation of free radicals that cause oxidative stress and the depletion of ATP [115, 129]. Elevated levels of lipid peroxidation and protein nitration have been found in substantia nigral cells and Lewy bodies [130]. Reduced levels of glutathione and oxidized glutathione, which act as antioxidants, are the earliest marker of nigral cell loss in the brains of PD patients [131].
2. MtDNA mutations and DNA alterations in patients with PD
Mutations in mtDNA are known to cause PD. A point mutation in the mitochondrial 12SrRNA has been found in patients with PD [see review in 1], and the G11778A mutation in the complex I gene (ND4) has been found in a PD family associated with Leber’s optic neuropathy [132]. In persons with PD, mutations in the nuclear-encoded mtDNA polymerase-γ gene impair mtDNA replication and result in multiple mtDNA deletions, typically causing chronic, progressive external opthalmoplegia and myopathy. In this PD family, polymerase-γ gene mutations co-segregate with PD.
Further, age-dependent accumulation of somatic mtDNA changes have been reported in substantia niagral neurons in patients and mouse models of PD [133]. These findings indicate that both germ-line and somatic mtDNA defects are involved in PD pathogenesis.
3. Abnormal mitochondrial dynamics in PD
Several recent studies have found abnormal mitochondrial fission and fusion, suggesting that mitochondria play a large role in PD [134–136]. Although mitochondrial dysfunction is well established in PD, the involvement of Drp1 (a fission protein responsible for mitochondrial fragmentation) and mitochondrial fission and fusion imbalance in unclear. Dagda et al [134] studied the connection between PTEN-induced kinase 1 (PINK1) knocking down and mitochondrial dysfunction in PD. They found mutations in PINK1 associated with familial PD. Although overexpressed PINK1 is neuroprotective, less is known about neuronal responses to loss of PINK1 function. Stable knockdown of PINK1 induced mitochondrial fragmentation and autophagy in SH-SY5Y cells both of which were reversed by the reintroduction of an RNA interference (RNAi)-resistant plasmid for PINK1. Moreover, stable or transient overexpression of wild-type PINK1 increased oxidant production played an essential role in triggering mitochondrial fragmentation and autophagy in PINK1 shRNA lines. Autophagy/mitophagy served a protective role in limiting cell death, and overexpressing parkin further enhanced this protective mitophagic response. Furthermore, PINK1 and parkin may cooperate through different mechanisms to maintain mitochondrial homeostasis.
Poole et al [135] studied genetic alterations affecting abnormal mitochondrial dynamics on the PINK1 and parkin mutant phenotypes in fruit flies. They found that heterozygous loss-of-function mutations of Drp1 are lethal in PINK1 or parkin. Conversely, increased Drp1 expression suppresses the flight muscle degeneration and mitochondrial morphological alterations that result from mutations in PINK1 and by heterozygous loss-of-function mutations affecting the mitochondrial fusion-promoting factors Opa1 and Mfn2. This study suggests that the PINK1/Parkin pathway promotes mitochondrial fission and that the loss of mitochondrial and tissue integrity in PINK1 and parkin mutants derives from reduced mitochondrial fission.
Deng et al [136] explored interactions between pink1/parkin and the mitochondrial fusion/fission. Muscle-specific knockdown of the fly homologue of Mfn (Marf) or Opa1, or overexpression of Drp1, results in significant mitochondrial fragmentation. Mfn-knockdown flies also displayed altered cristae morphology. Interestingly, knockdown of Mfn rescued the phenotypes from muscle degeneration, cell death, and mitochondrial abnormalities in pink1 or parkin mutants. These results suggest that pink1 and parkin are likely not core components of the Drp1-mediated mitochondrial fission machinery. Modification of fusion and fission may provide a novel therapeutic strategy for PD
Overall, evidence suggests that mitochondrial dysfunction is involved in the pathogenesis of PD.
4. Mutant proteins association with PD
Recently, several groups found mutant α-synuclein in mitochondrial membranes, in mice with PD [137–139]. Figure 5 summarizes the localization of mutant proteins from PD in mitochondrial membranes.
Figure 5. Localization of mutant proteins of PD in the mitochondria in neurons affected by PD.
In PD neurons, mutant proteins of α-synuclein, parkin, PINK1, and DJ1 are associated with mitochondria and cause mitochondrial dysfunction. Complex I activity is inhibited in PD neurons.
Devi and colleagues [137] investigated the connection between α-synuclein and mitochondria. They found that 32 amino acids of N-terminal human α-synuclein contain cryptic mitochondrial targeting signals, and it is important for targeting α-synuclein into mitochondria. Mitochondria in PD-affected regions, including the substantia nigra and striatum, but not in the cerebellum from PD subjects showed a significant accumulation of α-synuclein and decreased complex I activity [137].
Recent cellular and biochemical studies of animal models of LRRK2 suggest that LRRK2 plays a role in synaptic vesicle functions, including neurite outgrowth. LRRK2 resides diffusely throughout the cytoplasm and is associated with the outer membrane of mitochondria [140]. Kinase activity may be the link between LRRK2 and its role in PD pathogenesis, and disease-causing mutations in LRRK2 gene affects cell viability due to mitochondria-dependent cell death [118].
Recent evidence suggests that DJ-1 is localized to the mitochondrial matrix and the mitochondrial intermembrane space, in addition to its cytoplasmic pool [141]. Interestingly, a quantitative proteomic study of the substantia nigra of mice treated with MPTP revealed a significant increase in the protein DJ-1 in mitochondrial fraction of the substantia nigra. Together, this evidence suggests that DJ-1 may play an important role in neuroprotection against oxidative damage caused by mitochondrial toxins.
Overall, as discussed above, mitochondrial abnormalities are overwhelmingly involved in the progression of PD and treating mitochondria with mitochondria-targeted molecules is an ideal option to treat PD.
Mitochondrial dysfunction in amyotrophic lateral sclerosis
ALS is a progressive, invariably fatal neurological disease that attacks the neurons responsible for controlling voluntary muscles. In ALS, both the upper and lower motor neurons degenerate or die, ceasing to send messages to muscles [1]. Unable to function, the muscles gradually weaken, twitch, and waste away. Eventually, the brain loses its ability to control voluntary movement [117, 142, 143]. Unlike AD, ALS affects far fewer numbers of humans, 1 to 2 persons per 100,000. ALS affects persons from all ethnic groups, but mainly aged individuals are affected. Mutations superoxide dismutase gene 1 cause ALS [117, 142, 143].
Similar to AD and PD, ALS is characterized by mitochondrial dysfunction and oxidative stress. Evidences in support of the involvement of mitochondrial dysfunction in ALS are: 1) Mitochondrial dysfunction in postmortem brain and mutant SOD1 transgenic mice, 2) mutant SOD1 is associated with mitochondria dysfunction and 3) abnormal mitochondrial dynamics and ALS.
1. Mitochondrial dysfunction and ALS
Histo-pathological studies revealed that mitochondria may be targets of toxicity in ALS since vacuolated and dilated mitochondria with disorganized cristae and membranes in motor neurons have been observed in patients with ALS [144–150]. Mitochondrial defects have been reported in the spinal cords and muscle biopsies of patients with ALS; such defects range from impaired mitochondrial respiration to increased levels of uncoupling proteins [151, 152].
2. Mutant SOD1 and mitochondrial association
Recently, several lines of transgenic mice have been generated for SOD1 to mimic ALS features, and all of these transgenic mice exhibited mitochondrial abnormalities [146]. A small proportion of the cytosolic SOD1 mitochondria, in postmortem brain samples from ALS rodent models and in patients with ALS. In these samples, mutant SOD1 was present in fractions enriched for mitochondria that were derived from affected tissues, but not from unaffected tissues [153–156]. Mitochondrial localization of SOD1 has been confirmed by electron microscopy in both isolated mitochondria [155] and motor neurons in situ [157]. SOD1 mutants that cause disease at the lowest accumulated levels (hSOD1G85R and hSOD1G127X) have the highest relative proportions that are mitochondrially associated [155]. Mutant SOD1 has been reported in the intermembrane space and the matrix, as well as both in the inner and outer membranes of the spinal cord and brain mitochondria [153–157]. Figure 6 summarizes the SOD1 localization of mitochondrial membranes, matrix and intermembrane space.
Figure 6. Localization of mutant proteins of SOD1 in the mitochondria in neurons affected by ALS.
In ALS, mutant SOD1 is localized to inner, outer mitochondrial membranes, intermembrane space and matrix, and induce free radical production and oxidative damage. Impairment of Complex II and IV are associated with ALS.
3. Abnormal mitochondrial dynamics and ALS
Recently, Magrané et al. [158] have generated motor neuronal cell lines expressing wild-type or mutant SOD1 containing a cleavable intermembrane space (IMS) targeting signal to directly investigate the pathogenic role of mutant SOD1 in mitochondria. They demonstrated that mitochondrial-targeted SOD1 localizes to the IMS, where it is enzymatically active. The mutant IMS-targeted SOD1 causes neuronal toxicity under metabolic and oxidative stress conditions. They found that neurite mitochondrial fragmentation and impaired mitochondrial dynamics in motor neurons expressing IMS mutant SOD1
Overall, mitochondrial dysfunction is largely involved in patients with ALS and therapeutic approach targeting mitochondrial may be an important option to treat patients with ALS [159].
Therapeutic approaches to treating the mitochondria of aging and neurodegenerative diseases
Overwhelming evidence suggests that mitochondrial dysfunction and oxidative stress are involved in aging and neurodegenerative diseases. It is possible to treat these mitochondrial pathogenic events using molecules that target ROS and boost mitochondrial ATP, decrease free radical production and oxidative damage, and boost overall mitochondrial function, and ultimately increases synaptic branching of neurons.
Recently, several groups treated mouse models of neurodegenerative diseases with antioxidants-enriched diet, including blue berry juice, grape juice, vitamin C, E, beta carotene, coenzyme Q10 [160–164], polyphenols and other herbal products [reviews of 165, 166, 167, 168, 5] and IGF insulin signaling drugs [169]. Transgenic mice fed with antioxidants-enriched diet have shown beneficial effects, including extended lifespan, decreased toxic proteins and improved phenotypic behavior [164–169]. Some of these antioxidants are in clinical trials of patients with AD, PD, HD and ALS, and the outcome of these ongoing trials is much waited.
Further, given the huge involvement of mitochondrial dysfunction in aging and neurodegenerative diseases, it is reasonable to treat patients with neurodegenerative diseases or to supplement their diet with antioxidants. Recent antioxidant intake studies in patients with AD, HD and PD did not show strong beneficial effects and gave mixed results. Some epidemiologic studies suggest that the increased intake of the antioxidant vitamins vitamin E, vitamin C, and beta carotene might reduce the risk of developing AD or PD [22, 170]. Other studies did not show beneficial effects [170]. Available antioxidant approaches are not effective in treating neurodegenerative diseases because naturally occurring antioxidants, such as vitamins E and C, do not cross the blood-brain barrier and so cannot reach the relevant sites of free radical generation. To overcome these problems and to better assess whether antioxidant approaches may be valuable therapeutic treatments, improved delivery of antioxidants to the brains of patients with neurodegenerative diseases is needed.
In the last decade, considerable progress has been made in developing mitochondria-targeted antioxidants. To increase the delivery of antioxidants into mitochondria, several antioxidants have been developed: Olesoxime, the triphenylphosphonium-based antioxidants (MitoQ, MitoVitE, and MitoPBN), the cell-permeable, small peptide-based antioxidant SS31, and mitochondrial permeability transition pore inhibitors such as Dimebon [1, 171–174]. The application of these mitochondria-targeted agents to neurodegenerative diseases is at its early stages and is focused on animal models of AD, PD and ALS. Recent work in our lab using MitoQ, SS31 and resveratrol in N2a cells and primary neurons from AβPP transgenic mice have shown beneficial effects. In N2a cells treated with MitoQ, SS31, and resveratrol, and then incubated with Aβ compared to N2a cells incubated with Aβ abnormal expression of peroxiredoxins and mitochondrial structural genes were prevented and mitochondrial function was normal; intact mitochondria were present and neurite outgrowth was significantly increased. In primary neurons from amyloid-β precursor protein transgenic mice that were treated with MitoQ and SS31, neurite outgrowth was significantly increased. These findings suggest that MitoQ and SS31 prevent Aβ toxicity in AD neurons, which warrants the study of MitoQ and SS31 as potential drugs to treat patients with AD. However, further research is still needed to test the efficacies of mitochondria-targeted molecules using animal models of aging and neurodegenerative diseases.
Concluding remarks and future directions
Tremendous progress has been made in understanding relationships between aging and age-related neurodegenerative diseases. It is now clear that mitochondrial abnormalities play a large role in age-related neurodegenerative diseases, such as AD, PD, HD and ALS. Age-dependent accumulation of mitochondrial abnormalities and mutant proteins lead to both structural and functional changes in neuronal function and to cell death. Through studies that are elucidating the role of mitochondria in disease onset and development, investigators have begun focusing research efforts on developing therapies, such as molecules that target and protect mitochondria and neurons from the toxicity of aging and mutant proteins. Recent evidence from studies investigating antioxidants (e.g., SS31 and MitoQ) that targets mitochondria indicate that they protect neurons against mutant proteins, mitochondrial structural, and functional abnormalities and that they enhance neurite outgrowth and neuronal connectivity. However, these antioxidants needs to be studied in mouse models of neurodegenerative diseases and patients with neurodegenerative diseases.
Acknowledgments
The research presented in this chapter was supported by grants from American Federation for Aging Research, National Institutes of Health (AG020872 and AG025061, RR00163), Alzheimer Association (IIRG grant 09-92429), Vertex Pharmaceuticals and Medivation Inc.
Abbreviations
- Aβ
amyloid beta
- AβPP
amyloid beta precursor protein
- AD
Alzheimer’s disease
- ALS
amyotrophic lateral sclerosis
- ATP
adenosine triphosphate
- CR
caloric restricted
- COX1
cytochrome oxidase 1
- Drp1
dynamin related protein 1
- ETC
electron transport chain
- H2O2
hydrogen peroxide
- Mfn1
mitochondrial fusin 1
- mtDNA
mitochondrial DNA
- O2*-
superoxide radical
- Opa1
optic atrophy 1
- PD
Parkinson’s disease
- ROS
reactive oxygen species
- SOD1
superoxide dismutase 1
- SS31 peptide
Szeto-Schiller 31 peptide
- TCA
tricarboxylic acid
Footnotes
Conflict of interest
Authors declare that they do not have any conflict of interest.
References
- 1.Reddy PH. Mitochondrial medicine for aging and neurodegenerative diseases. Neuromolecular Med. 2008;10:291–315. doi: 10.1007/s12017-008-8044-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beal MF. Mitochondria take center stage in aging and neurodegeneration. Ann Neurol. 2005;58:495–505. doi: 10.1002/ana.20624. [DOI] [PubMed] [Google Scholar]
- 3.Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 4.Reddy PH. Amyloid beta, mitochondrial structural and functional dynamics in Alzheimer’s disease. Exp Neurol. 2009;218:286–292. doi: 10.1016/j.expneurol.2009.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swerdlow RH. Mitochondrial Medicine and the Neurodegenerative Mitochondriopathies. Pharmaceuticals. 2009;2:150–167. doi: 10.3390/ph2030150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beers M, Jones TV. Merck Manual of Health and Aging. Merck. 2004 [Google Scholar]
- 7.Fontana L, Partridge L, Longo VD. Extending healthy life span--from yeast to humans. Science. 2010 Apr 16;328(5976):321–6. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Austad SN. Why We Age: What Science Is Discovering about the Body’s Journey through Life. JohnWiley & Sons; New York: 1997a. [Google Scholar]
- 9.Strehler BL. Time, Cells, and Aging. Demetriades Brothers; Larnaca: 1999. [Google Scholar]
- 10.Beckman KB, Ames BN. The free radical theory of aging matures. Physiol Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- 11.de Carvalho CV, Payao SL, Smith MA. DNA methylation, ageing and ribosomal genes activity. Biogerontology. 2000;1:357–361. doi: 10.1023/a:1026542618182. [DOI] [PubMed] [Google Scholar]
- 12.Mattson MP. Emerging neuroprotective strategies for Alzheimer’s disease: dietary restriction, telomerase activation, and stem cell therapy. Exp Gerontol. 2000;35:489–502. doi: 10.1016/s0531-5565(00)00115-7. [DOI] [PubMed] [Google Scholar]
- 13.Vijg J. Somatic mutations and aging: a re-evaluation. Mutat Res. 2000;447:117–135. doi: 10.1016/s0027-5107(99)00202-x. [DOI] [PubMed] [Google Scholar]
- 14.Perry G, Nunomura A, Hirai K, et al. Is oxidative damage the fundamental pathogenic mechanism of Alzheimer’s and other neurodegenerative diseases? Free Radic Biol Med. 2002;1:1475–1479. doi: 10.1016/s0891-5849(02)01113-9. [DOI] [PubMed] [Google Scholar]
- 15.Harman D. Aging: minimizing free radical damage. J Anti-Aging Med. 1999;2:15–36. [Google Scholar]
- 16.Cadenas E, Davies KJ. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med. 2000;29:222–230. doi: 10.1016/s0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- 17.Biersalski HK. Free radical theory of aging. Curr Opin Clin Nutr Metab Care. 2002;5:5–10. doi: 10.1097/00075197-200201000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Bishop NA, Lu T, Yankner BA. Neural mechanisms of ageing and cognitive decline. Nature. 2010;464:529–35. doi: 10.1038/nature08983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sahin E, Depinho RA. Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature. 2010 Mar 25;464(7288):520–8. doi: 10.1038/nature08982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swerdlow RH, Khan SM. A “mitochondrial cascade hypothesis” for sporadic Alzheimer’s disease. Med Hypotheses. 2004;63:8–20. doi: 10.1016/j.mehy.2003.12.045. [DOI] [PubMed] [Google Scholar]
- 21.Reddy PH. Amyloid precursor protein-mediated free radicals and oxidative damage: implications for the development and progression of Alzheimer’s disease. J Neurochem. 2006;96:1–13. doi: 10.1111/j.1471-4159.2005.03530.x. [DOI] [PubMed] [Google Scholar]
- 22.Reddy PH. Mitochondrial dysfunction in aging and Alzheimer’s disease: strategies to protect neurons. Antioxidants & Redox Signaling. 2007;9:1647–1658. doi: 10.1089/ars.2007.1754. [DOI] [PubMed] [Google Scholar]
- 23.DiMauro S, Schon EA. Mitochondrial disorders in the nervous system. Annu Rev Neurosci. 2008;31:91–123. doi: 10.1146/annurev.neuro.30.051606.094302. [DOI] [PubMed] [Google Scholar]
- 24.Reddy PH, Beal MF. Amyloid beta, mitochondrial dysfunction and synaptic damage: implications for cognitive decline in aging and Alzheimer’s disease. Trends Mol Med. 2008;14:45–53. doi: 10.1016/j.molmed.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swerdlow RH, Khan SM. The Alzheimer’s disease mitochondrial cascade hypothesis: an update. Exp Neurol. 2009;218:308–15. doi: 10.1016/j.expneurol.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swerdlow RH, Burns JM, Khan SM. The Alzheimer’s disease mitochondrial cascade hypothesis. J Alzheimers Dis. 2010;20 (Suppl 2):S265–79. doi: 10.3233/JAD-2010-100339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reddy PH, Beal MF. Are mitochondria critical in the pathogenesis of Alzheimer’s disease? Brain Res Brain Res Rev. 2005;49:618–632. doi: 10.1016/j.brainresrev.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 28.Anderson S, Bankier AT, Barrell BG, de Bruijn MH, Coulson AR, Drouin J, Eperon IC, Nierlich DP, Roe BA, Sanger F, Schreier PH, Smith AJ, Staden R, Young IG. Sequence and organization of the human mitochondrial genome. Nature. 1981;290:457–65. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- 29.Cooper JM, Mann VM, Schapira AH. Analyses of mitochondrial respiratory chain function and mitochondrial DNA deletion in human skeletal muscle: effect of ageing. J Neurol Sci. 1992;113:91–98. doi: 10.1016/0022-510x(92)90270-u. [DOI] [PubMed] [Google Scholar]
- 30.Mao P, Gallagher P, Nedungadi S, Manczak M, Kohama S, Ferguson B, Park BS, Reddy PH. Mitochondrial DNA deletions in Rhesus monkeys: Implications to aging. Submitted to Journal of Alzheimer’s Disease. 2009 [Google Scholar]
- 31.Trifunovic A, Wredenberg A, Falkenberg M, et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature. 2004;429:417–423. doi: 10.1038/nature02517. [DOI] [PubMed] [Google Scholar]
- 32.Kujoth GC, Hiona A, Pugh TD, et al. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science. 2005;309:481–448. doi: 10.1126/science.1112125. [DOI] [PubMed] [Google Scholar]
- 33.Lu T, Pan Y, Kao SY, Li C, Kohane I, Chan J, Yankner BA. Gene regulation and DNA damage in the ageing human brain. Nature. 2004;429:883–891. doi: 10.1038/nature02661. [DOI] [PubMed] [Google Scholar]
- 34.Wang AL, Lukas TJ, Yuan M, Neufeld AH. Age-related increase in mitochondrial DNA damage and loss of DNA repair capacity in the neural retina. Neurobiol Aging. 2008 Dec 10; doi: 10.1016/j.neurobiolaging.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 35.Hoeijmakers JH. DNA damage, aging, and cancer. N Engl J Med. 2009;361:1475–1485. doi: 10.1056/NEJMra0804615. [DOI] [PubMed] [Google Scholar]
- 36.Schriner SE, Linford NJ, Martin GM, et al. Extension of murine lifespan by overexpression of catalase targeted to mitochondria. Science. 2005;308:1909–1911. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- 37.Swerdlow RH, Parks JK, Miller SW, Tuttle JB, Trimmer PA, Sheehan JP, Bennett JP, Davis RE, Parker WD. Origin and functional consequences of the complex I defect in Parkinson’s disease. Ann Neurol. 1996;40:663–670. doi: 10.1002/ana.410400417. [DOI] [PubMed] [Google Scholar]
- 38.Swerdlow RH, Parks JK, Cassarino DS, Maguire DJ, Maguire RS, Bennett JP, Parker WD. Cybrids in Alzheimer’s disease: A cellular model of the disease? Neurology. 1997;49:918–925. doi: 10.1212/wnl.49.4.918. [DOI] [PubMed] [Google Scholar]
- 39.Swerdlow RH, Miller SW, Parks JK, Sheehan JP, Cassarino DS, Maguire DJ, Maguire RS, Bennett JP, Juel VC, Phillips LH, Trimmer PA, Pattee G, Tuttle JB, Davis RE, Parker WD. Mitochondria in sporadic amyotrophic lateral sclerosis. Experimental Neurology. 1998;153:135–142. doi: 10.1006/exnr.1998.6866. [DOI] [PubMed] [Google Scholar]
- 40.Reddy PH, McWeeney S. Mapping cellular transcriptosomes in autopsied Alzheimer’s disease subjects and relevant animal models. Neurobiol Aging. 2006;27:1060–1077. doi: 10.1016/j.neurobiolaging.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 41.Alzheimer Association. Report: 2010 Alzheimer Disease Facts and Figures. [Google Scholar]
- 42.Reddy PH, Mani G, Park BS, Jacques J, Murdoch G, Whetsell W, Jr, Kaye J, Manczak M. Differential loss of synaptic proteins in Alzheimer’s disease: implications for synaptic dysfunction. J Alzheimers Dis. 2005;7:103–17. doi: 10.3233/jad-2005-7203. discussion 173–180. [DOI] [PubMed] [Google Scholar]
- 43.Harold D, Abraham R, Hollingworth P, Sims R, Gerrish A, Hamshere ML, Pahwa JS, Moskvina V, Dowzell K, Williams A, Jones N, Thomas C, Stretton A, Morgan AR, Lovestone S, Powell J, Proitsi P, Lupton MK, Brayne C, Rubinsztein DC, Gill M, Lawlor B, Lynch A, Morgan K, Brown KS, Passmore PA, Craig D, McGuinness B, Todd S, Holmes C, Mann D, Smith AD, Love S, Kehoe PG, Hardy J, Mead S, Fox N, Rossor M, Collinge J, Maier W, Jessen F, Schürmann B, van den Bussche H, Heuser I, Kornhuber J, Wiltfang J, Dichgans M, Frölich L, Hampel H, Hüll M, Rujescu D, Goate AM, Kauwe JS, Cruchaga C, Nowotny P, Morris JC, Mayo K, Sleegers K, Bettens K, Engelborghs S, De Deyn PP, Van Broeckhoven C, Livingston G, Bass NJ, Gurling H, McQuillin A, Gwilliam R, Deloukas P, Al-Chalabi A, Shaw CE, Tsolaki M, Singleton AB, Guerreiro R, Mühleisen TW, Nöthen MM, Moebus S, Jöckel KH, Klopp N, Wichmann HE, Carrasquillo MM, Pankratz VS, Younkin SG, Holmans PA, O’Donovan M, Owen MJ, Williams J. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat Genet. 2009;41:1088–93. doi: 10.1038/ng.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lambert JC, Heath S, Even G, Campion D, Sleegers K, Hiltunen M, Combarros O, Zelenika D, Bullido MJ, Tavernier B, Letenneur L, Bettens K, Berr C, Pasquier F, Fiévet N, Barberger-Gateau P, Engelborghs S, De Deyn P, Mateo I, Franck A, Helisalmi S, Porcellini E, Hanon O, de Pancorbo MM, Lendon C, Dufouil C, Jaillard C, Leveillard T, Alvarez V, Bosco P, Mancuso M, Panza F, Nacmias B, Bossù P, Piccardi P, Annoni G, Seripa D, Galimberti D, Hannequin D, Licastro F, Soininen H, Ritchie K, Blanché H, Dartigues JF, Tzourio C, Gut I, Van Broeckhoven C, Alperovitch A, Lathrop M, Amouyel P European Alzheimer’s Disease Initiative Investigators. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nat Genet. 2009;41(10):1094–9. doi: 10.1038/ng.439. [DOI] [PubMed] [Google Scholar]
- 45.Reddy PH, Manczak M, Mao P, Calkins MJ, Reddy AP, Shirendeb U. Amyloid-beta and mitochondria in aging and Alzheimer’s disease: implications for synaptic damage and cognitive decline. J Alzheimers Dis. 2010;20 (Suppl 2):S499–512. doi: 10.3233/JAD-2010-100504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Du H, Guo L, Yan S, Sosunov AA, McKhann GM, Yan SS. Early deficits in synaptic mitochondria in an Alzheimer’s disease mouse model. Proc Natl Acad Sci U S A. 2010;107(43):18670–5. doi: 10.1073/pnas.1006586107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nunomura A, Perry G, Aliev G, Hirai K, Takeda A, Balraj EK, Jones PK, Ghanbari H, Wataya T, Shimohama S, Chiba S, Atwood CS, Petersen RB, Smith MA. Oxidative damage is the earliest event in Alzheimer disease. J Neuropathol Exp Neurol. 2001;60:759–767. doi: 10.1093/jnen/60.8.759. [DOI] [PubMed] [Google Scholar]
- 48.Lin MT, Simon DK, Ahn CH, Kim LM, Beal MF. High aggregate burden of somatic mtDNA point mutations in aging and Alzheimer’s disease brain. Hum Mol Genet. 2002;11:133–145. doi: 10.1093/hmg/11.2.133. [DOI] [PubMed] [Google Scholar]
- 49.Coskun PE, Beal MF, Wallace DC. Alzheimer’s brains harbor somatic mtDNA control-region mutations that suppress mitochondrial transcription and replication. Proc Natl Acad Sci U S A. 2004;101:10726– 10731. doi: 10.1073/pnas.0403649101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Coskun PE, Wyrembak J, Derbereva O, Melkonian G, Doran E, Lott IT, Head E, Cotman CW, Wallace DC. Systemic mitochondrial dysfunction and the etiology of Alzheimer’s disease and down syndrome dementia. J Alzheimers Dis. 2010;20 (Suppl 2):S293–310. doi: 10.3233/JAD-2010-100351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lakatos A, Derbeneva O, Younes D, Keator D, Bakken T, Lvova M, Brandon M, Guffanti G, Reglodi D, Saykin A, Weiner M, Macciardi F, Schork N, Wallace DC, Potkin SG. Alzheimer’s Disease Neuroimaging Initiative. Association between mitochondrial DNA variations and Alzheimer’s disease in the ADNI cohort. Neurobiol Aging. 2010 Jun 8; doi: 10.1016/j.neurobiolaging.2010.04.031. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Manczak M, Anekonda TS, Henson E, Park BS, Quinn J, Reddy PH. Mitochondria are a direct site of A beta accumulation in Alzheimer’s disease neurons:implications for free radical generation and oxidative damage in disease progression. Hum Mol Genet. 2006;15:1437–49. doi: 10.1093/hmg/ddl066. [DOI] [PubMed] [Google Scholar]
- 53.Yao J, Irwin RW, Zhao L, Nilsen J, Hamilton RT, Brinton RD. Mitochondrial bioenergetic deficit precedes Alzheimer’s pathology in female mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2009;106:14670–5. doi: 10.1073/pnas.0903563106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Caspersen C, Wang N, Yao J, Sosunov A, Chen X, Lustbader JW, Xu HW, Stern D, McKhann G, Yan SD. Mitochondrial Abeta: a potential focal point for neuronal metabolic dysfunction in Alzheimer’s disease. FASEB J. 2005;19:2040–1. doi: 10.1096/fj.05-3735fje. [DOI] [PubMed] [Google Scholar]
- 55.Reddy PH, McWeeney S, Park BS, Manczak M, Gutala RV, Partovi D, Jung Y, Yau V, Searles R, Mori M, Quinn J. Gene expression profiles of transcripts in amyloid precursor protein transgenic mice: upregulation of mitochondrial metabolism and apoptotic genes is an early cellular change in Alzheimer’s disease. Hum Mol Genet. 2004;13:1225–1240. doi: 10.1093/hmg/ddh140. [DOI] [PubMed] [Google Scholar]
- 56.Manczak M, Park BS, Jung Y, Reddy PH. Differential expression of oxidative phosphorylation genes in patients with Alzheimer’s disease: implications for early mitochondrial dysfunction and oxidative damage. Neuromolecular Med. 2004;5:147–162. doi: 10.1385/NMM:5:2:147. [DOI] [PubMed] [Google Scholar]
- 57.Manczak M, Mao P, Calkins MJ, Cornea A, Reddy AP, Murphy MP, Szeto HH, Park B, Reddy PH. Mitochondria-targeted antioxidants protect against amyloid-beta toxicity in Alzheimer’s disease neurons. J Alzheimers Dis Suppl. 2010;2:S609–31. doi: 10.3233/JAD-2010-100564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lustbader JW, Cirilli M, Lin C, et al. ABAD directly links Abeta to mitochondrial toxicity in Alzheimer’s disease. Science. 2004;304:448–452. doi: 10.1126/science.1091230. [DOI] [PubMed] [Google Scholar]
- 59.Crouch PJ, Blake R, Duce JA, Ciccotosto GD, Li QX, Barnham KJ, Curtain CC, Cherny RA, Cappai R, Dyrks T, Masters CL, Trounce IA. Copper-dependent inhibition of human cytochrome c oxidase by a dimeric conformer of amyloid-beta1–42. J Neurosci. 2005;25:672–9. doi: 10.1523/JNEUROSCI.4276-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Devi L, Prabhu BM, Galati DF, Avadhani NG, Anandatheerthavarada HK. Accumulation of amyloid precursor protein in the mitochondrial import channels of human Alzheimer’s disease brain is associated with mitochondrial dysfunction. J Neurosci. 2006;26:9057–9068. doi: 10.1523/JNEUROSCI.1469-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Du H, Guo L, Fang F, Chen D, Sosunov AA, McKhann GM, Yan Y, Wang C, Zhang H, Molkentin JD, Gunn-Moore FJ, Vonsattel JP, Arancio O, Chen JX, Yan SD. Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer’s disease. Nat Med. 2008;14:1097–105. doi: 10.1038/nm.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang X, Su B, Siedlak SL, Moreira PI, Fujioka H, Wang Y, Casadesus G, Zhu X. Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proc Natl Acad Sci U S A. 2008;105:19318–23. doi: 10.1073/pnas.0804871105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhao XL, Wang WA, Tan JX, Huang JK, Zhang X, Zhang BZ, Wang YH, YangCheng HY, Zhu HL, Sun XJ, Huang FD. Expression of beta-amyloid Induced age-dependent presynaptic and axonal changes in Drosophila. J Neurosci. 2010;30:1512–22. doi: 10.1523/JNEUROSCI.3699-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chan DC. Mitochondrial fusion and fission in mammals. Annu Rev Cell Dev Biol. 2006;22:79–99. doi: 10.1146/annurev.cellbio.22.010305.104638. [DOI] [PubMed] [Google Scholar]
- 65.Gibson GE, Sheu KF, Blass JP. Abnormalities of mitochondrial enzymes in Alzheimer disease. J NeuralTransm. 1998;105:855–870. doi: 10.1007/s007020050099. [DOI] [PubMed] [Google Scholar]
- 66.Parker WD, Jr, Filley CM, Parks JK. Cytochrome oxidase deficiency in Alzheimer’s disease. Neurology. 1990;40:1302–303. doi: 10.1212/wnl.40.8.1302. [DOI] [PubMed] [Google Scholar]
- 67.Maurer I, Zierz S, Möller HJ. A selective defect of cytochrome c oxidase is present in brain of Alzheimer disease patients. Neurobiol Aging. 2000;21:455–462. doi: 10.1016/s0197-4580(00)00112-3. [DOI] [PubMed] [Google Scholar]
- 68.Smith MA, Perry G, Richey PL, Sayre LM, Anderson VE, Beal MF, Kowall N. Oxidative damage in Alzheimer’s. Nature. 1996;382:120–121. doi: 10.1038/382120b0. [DOI] [PubMed] [Google Scholar]
- 69.Dragicevic N, Mamcarz M, Zhu Y, Buzzeo R, Tan J, Arendash GW, Bradshaw PC. Mitochondrial amyloid-beta levels are associated with the extent of mitochondrial dysfunction in different brain regionsand the degree of cognitive impairment in Alzheimer’s transgenic mice. J Alzheimers Dis. 2010;20 (Suppl 2):S535–50. doi: 10.3233/JAD-2010-100342. [DOI] [PubMed] [Google Scholar]
- 70.Vonsattel JP, Myers RH, Stevens TJ, Ferrante RJ, Bird ED, Richardson EP., Jr Neuropathological classification of Huntington’s disease. J Neuropathol Exp Neurol. 1985;44:559–577. doi: 10.1097/00005072-198511000-00003. [DOI] [PubMed] [Google Scholar]
- 71.Folstein SE. Huntington’s Disease. Johns Hopkins University Press; 1990. [Google Scholar]
- 72.Montoya A, Price BH, Menear M, Lepage M. Brain imaging and cognitive dysfunctions in Huntington’s disease. J Psychiatry Neurosci. 2006;31:21–29. [PMC free article] [PubMed] [Google Scholar]
- 73.Byers RK, Gilles FH, Fung C. Huntington’s disease in children. Neuropathologic study of four cases. Neurology. 1973;23:561–569. doi: 10.1212/wnl.23.6.561. [DOI] [PubMed] [Google Scholar]
- 74.Spargo E, Everall IP, Lantos PL. Neuronal loss in the hippocampus in Huntington’s disease: a comparison with HIV infection. J Neurol Neurosurg Psychiatry. 1993;56:487–91. doi: 10.1136/jnnp.56.5.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reddy PH, Charles V, Williams M, Miller G, Whetsell WO, Jr, Tagle DA. Transgenic mice expressing mutated full-length HD cDNA: a paradigm for locomotor changes and selective neuronal loss in Huntington’s disease. Philos Trans R Soc Lond B Biol Sci. 1999;354:1035–1045. doi: 10.1098/rstb.1999.0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Reddy PH, Williams M, Tagle DA. Recent advances in understanding the pathogenesis of Huntington’s disease. Trends Neurosci. 1999;22:248–255. doi: 10.1016/s0166-2236(99)01415-0. [DOI] [PubMed] [Google Scholar]
- 77.Reddy PH, Tagle DA. Biology of trinucleotide repeat disorders. In: Mattson Mark P., editor. Genetic Aberrancies and Neurodegenerative Disorders. 3. Jai press Inc; Stanford and Connecticut: 1999. Advances in cell aging and gerontology. [Google Scholar]
- 78.Reddy PH, Williams M, Charles V, Garrett L, Pike-Buchanan L, Whetsell WO, Jr, Miller G, Tagle DA. Behavioural abnormalities and selective neuronal loss in HD transgenic mice expressing mutated full-length HD cDNA. Nat Genet. 1998;20:198–202. doi: 10.1038/2510. [DOI] [PubMed] [Google Scholar]
- 79.Reddy PH, Mao P, Manczak M. Mitochondrial structural and functional dynamics in Huntington’s disease. Brain Res Rev. 2009;61:33–48. doi: 10.1016/j.brainresrev.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li S, Li XJ. Multiple pathways contribute to the pathogenesis of Huntington disease. Mol Neurodegener. 2006;1:19. doi: 10.1186/1750-1326-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bates GP. History of genetic disease: the molecular genetics of Huntington disease - a history. Nat Rev Genet. 2005;6:766–773. doi: 10.1038/nrg1686. [DOI] [PubMed] [Google Scholar]
- 82.Orr HT, Zoghbi HY. Trinucleotide repeat disorders. Ann Rev Neurosci. 2007;30:575–621. doi: 10.1146/annurev.neuro.29.051605.113042. [DOI] [PubMed] [Google Scholar]
- 83.Borrell-Pagès M, Zala D, Humbert S, Saudou F. Huntington’s disease: from huntingtin function and dysfunction to therapeutic strategies. Cell Mol Life Sci. 2006;63:2642–2660. doi: 10.1007/s00018-006-6242-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sayer JA, Manczak M, Akileswaran L, Reddy PH, Coghlan VM. Interaction of the nuclear matrix protein NAKAP with HypA and huntingtin: implications for nuclear toxicity in Huntington’s disease pathogenesis. Neuromolecular Med. 2005;7:297–310. doi: 10.1385/NMM:7:4:297. [DOI] [PubMed] [Google Scholar]
- 85.Rigamonti D, Bauer JH, De-Fraja C, Conti L, Sipione S, Sciorati C, Clementi E, Hackam A, Hayden MR, Li Y, Cooper JK, Ross CA, Govoni S, Vincenz C, Cattaneo E. Wild-type huntingtin protects from apoptosis upstream of caspase-3. J Neurosci. 2000;20:3705–3713. doi: 10.1523/JNEUROSCI.20-10-03705.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jana NR, Zemskov EA, Wang GH, Nukina N. Altered proteasomal function due to the expression of polyglutamine-expanded truncated N-terminal huntingtin induces apoptosis by caspase activation through mitochondrial cytochrome c release. Hum Mol Genet. 2001;10:1049–1059. doi: 10.1093/hmg/10.10.1049. [DOI] [PubMed] [Google Scholar]
- 87.Hermel E, Gafni J, Propp SS, Leavitt BR, Wellington CL, Young JE, Hackam AS, Logvinova AV, Peel AL, Chen SF, Hook V, Singaraja R, Krajewski S, Goldsmith PC, Ellerby HM, Hayden MR, Bredesen DE, Ellerby LM. Specific caspase interactions and amplification are involved in selective neuronal vulnerability in Huntington’s disease. Cell Death Differ. 2004;11:424–438. doi: 10.1038/sj.cdd.4401358. [DOI] [PubMed] [Google Scholar]
- 88.Zhang Y, Leavitt BR, van Raamsdonk JM, Dragatsis I, Goldowitz D, MacDonald ME, Hayden MR, Friedlander RM. Huntingtin inhibits caspase-3 activation. EMBO J. 2006;25:5896–5906. doi: 10.1038/sj.emboj.7601445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee WT, Chang C. Magnetic resonance imaging and spectroscopy in assessing 3-nitropropionic acidinduced brain lesions: an animal model of Huntington’s disease. Prog Neurobiol. 2004;72:87–110. doi: 10.1016/j.pneurobio.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 90.Fernandes HB, Baimbridge KG, Church J, Hayden MR, Raymond LA. Mitochondrial sensitivity and altered calcium handling underlie enhanced NMDA induced apoptosis in YAC128 model of Huntington’s disease. J Neurosci. 2007;27:13614–13623. doi: 10.1523/JNEUROSCI.3455-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Panov AV, Gutekunst CA, Leavitt BR, Hayden MR, Burke JR, Strittmatter WJ, Greenamyre JT. Early mitochondrial calcium defects in Huntington’s disease are a direct effect of polyglutamines. Nat Neurosci. 2002;5:731–736. doi: 10.1038/nn884. [DOI] [PubMed] [Google Scholar]
- 92.Panov AV, Lund S, Greenamyre JT. Ca2+-induced permeability transition in humanlymphoblastoid cell mitochondria from normal and Huntington’s disease individuals. Mol Cell Biochem. 2005;269:143–52. doi: 10.1007/s11010-005-3454-9. [DOI] [PubMed] [Google Scholar]
- 93.Oliveira JM, Chen S, Almeida S, Riley R, Gonçalves J, Oliveira CR, Hayden MR, Nicholls DG, Ellerby LM, Rego AC. Mitochondrial-dependent Ca2+ handling in Huntington’s disease striatal cells: effect of histone deacetylase inhibitors. J Neurosci. 2006;26:11174–11186. doi: 10.1523/JNEUROSCI.3004-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Milakovic T, Quintanilla RA, Johnson GV. Mutant huntingtin expression induces mitochondrial calcium handling defects in clonal striatal cells: functional consequences. J Biol Chem. 2006;281:34785–34795. doi: 10.1074/jbc.M603845200. [DOI] [PubMed] [Google Scholar]
- 95.Oliveira JM, Jekabsons MB, Chen S, Lin A, Rego AC, Gonçalves J, Ellerby LM, Nicholls DG. Mitochondrial dysfunction in Huntington’s disease: the bioenergetics of isolated and in situ mitochondria from transgenic mice. J Neurochem. 2007;101:241–249. doi: 10.1111/j.1471-4159.2006.04361.x. [DOI] [PubMed] [Google Scholar]
- 96.Gellerich FN, Gizatullina Z, Nguyen HP, Trumbeckaite S, Vielhaber S, Seppet E, Zierz S, Landwehrmeyer B, Riess O, von Hörsten S, Striggow F. Impaired regulation of brain mitochondriaby extramitochondrial Ca2+ in transgenic Huntington disease rats. J Biol Chem. 2008;283:30715–30724. doi: 10.1074/jbc.M709555200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lim D, Fedrizzi L, Tartari M, Zuccato C, Cattaneo E, Brini M, Carafoli E. Calcium homeostasisand mitochondrial dysfunction in striatal neurons of Huntington disease. J Biol Chem. 2008;283:5780–5789. doi: 10.1074/jbc.M704704200. [DOI] [PubMed] [Google Scholar]
- 98.Oliveira JM, Gonçalves J. In situ mitochondrial Ca2+ buffering differences of intact neurons and astrocytes from cortex and striatum. J Biol Chem. 2009;284:5010–5020. doi: 10.1074/jbc.M807459200. [DOI] [PubMed] [Google Scholar]
- 99.Browne SE, Beal MF. The energetics of Huntington’s disease. Neurochem Res. 2004;29:531–546. doi: 10.1023/b:nere.0000014824.04728.dd. [DOI] [PubMed] [Google Scholar]
- 100.Browne SE, Beal MF. Oxidative damage in Huntington’s disease pathogenesis. Antioxid Redox Signal. 2006;8:2061–2073. doi: 10.1089/ars.2006.8.2061. [DOI] [PubMed] [Google Scholar]
- 101.Trushina E, Dyer RB, Badger JD, 2nd, Ure D, Eide L, Tran DD, Vrieze BT, Legendre-Guillemin V, McPherson PS, Mandavilli BS, Van Houten B, Zeitlin S, McNiven M, Aebersold R, Hayden M, Parisi JE, Seeberg E, Dragatsis I, Doyle K, Bender A, Chacko C, McMurray CT. Mutant huntingtin impairs axonal trafficking in mammalian neurons in vivo and in vitro. Mol Cell Biol. 2004;24:8195–209. doi: 10.1128/MCB.24.18.8195-8209.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chang DT, Rintoul GL, Pandipati S, Reynolds IJ. Mutant huntingtin aggregates impair mitochondrial movement and trafficking in cortical neurons. Neurobiol Dis. 2006;22:388–400. doi: 10.1016/j.nbd.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 103.Orr AL, Li S, Wang CE, Li H, Wang J, Rong J, Xu X, Mastroberardino PG, Greenamyre JT, Li XJ. N-terminal mutant huntingtin associates with mitochondria and impairs mitochondrial trafficking. J Neurosci. 2008;28:2783–2792. doi: 10.1523/JNEUROSCI.0106-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Browne SE, Bowling AC, MacGarvey U, Baik MJ, Berger SC, Muqit MM, Bird ED, Beal MF. Oxidative damage and metabolic dysfunction in Huntington’s disease: selective vulnerability of the basal ganglia. Ann Neurol. 1997;41:646–653. doi: 10.1002/ana.410410514. [DOI] [PubMed] [Google Scholar]
- 105.Tabrizin SJ, Cleeter MW, Xuereb J, Taanman JW, Cooper JM, Schapira AH. Biochemical abnormalities and excitotoxicity in Huntington’s disease brain. Ann Neurol. 1999;45:25–32. doi: 10.1002/1531-8249(199901)45:1<25::aid-art6>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 106.Senatorov VV, Charles V, Reddy PH, Tagle DA, Chuang DM. Overexpression and nuclear accumulation of glyceraldehyde-3-phosphate dehydrogenase in a transgenic mouse model of Huntington’s disease. Mol Cell Neurosci. 2003;22:285–97. doi: 10.1016/s1044-7431(02)00013-1. [DOI] [PubMed] [Google Scholar]
- 107.Fukui H, Moraes CT. Extended polyglutamine repeats trigger a feedback loop involving the mitochondrial complex III, the proteasome and huntingtin aggregates. Hum Mol Genet. 2007;16:783–797. doi: 10.1093/hmg/ddm023. [DOI] [PubMed] [Google Scholar]
- 108.Pandey M, Varghese M, Sindhu KM, Sreetama S, Navneet AK, Mohanakumar KP, Usha R. Mitochondrial NAD+-linked State 3 respiration and complex-I activity are compromised in the cerebral cortex of 3-nitropropionic acid-induced rat model of Huntington’s disease. J Neurochem. 2008;104:420–34. doi: 10.1111/j.1471-4159.2007.04996.x. [DOI] [PubMed] [Google Scholar]
- 109.Seong IS, Ivanova E, Lee JM, et al. HD CAG repeat implicates a dominant property of huntingtin in mitochondrial energy metabolism. Hum Mol Genet. 2005;14:2871–80. doi: 10.1093/hmg/ddi319. [DOI] [PubMed] [Google Scholar]
- 110.Acevedo-Torres K, Berrios L, Rosario N, Dufault V, Skatchkov S, Eaton MJ, Torres-Ramos CA, Ayala-Torres S. Mitochondrial DNA damage is a hallmark ofchemically induced and the R6/2 transgenic model of Huntington’s disease. DNA Repair (Amst) 2009;8:126–36. doi: 10.1016/j.dnarep.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chen CM, Wu YR, Cheng ML, Liu JL, Lee YM, Lee PW, Soong BW, Chiu DT. Increased oxidative damage and mitochondrial abnormalities in the peripheral blood of Huntington’s disease patients. Biochem Biophys Res Commun. 2007;359:335–40. doi: 10.1016/j.bbrc.2007.05.093. [DOI] [PubMed] [Google Scholar]
- 112.Banoei MM, Houshmand M, Panahi MS, Shariati P, Rostami M, Manshadi MD, Majidizadeh T. Huntington’s disease and mitochondrial DNA deletions: event or regular mechanism for mutant huntingtin protein and CAG repeats expansion?! Cell Mol Neurobiol. 2007;27:867–75. doi: 10.1007/s10571-007-9206-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ono T, Isobe K, Nakada K, Hayashi JI. Human cells are protected from mitochondrial dysfunction by complementation of DNA products in fused mitochondria. Nat Genet. 2001;28:272–5. doi: 10.1038/90116. [DOI] [PubMed] [Google Scholar]
- 114.Solans A, Zambrano A, Rodriguez M, Barrientos A. Cytotoxicity of a mutant huntingtin fragment in yeastinvolves early alterations in mitochondrial OXPHOS complexes II and III. Hum Mol Genet. 2006;15:3063–81. doi: 10.1093/hmg/ddl248. [DOI] [PubMed] [Google Scholar]
- 115.Gandhi S, Wood NW. Molecular pathogenesis of Parkinson’s disease. Hum Mol Genet. 2005;14(Spec No 2):2749–2755. [PubMed] [Google Scholar]
- 116.Abeliovich A, Beal MF. Parkinsonism genes: culprits and clues. J Neurochem. 2006;99:1062–72. doi: 10.1111/j.1471-4159.2006.04102.x. [DOI] [PubMed] [Google Scholar]
- 117.Martin LJ. Mitochondriopathy in Parkinson disease and amyotrophic lateral sclerosis. J Neuropathol Exp Neurol. 2006;65:1103–1110. doi: 10.1097/01.jnen.0000248541.05552.c4. [DOI] [PubMed] [Google Scholar]
- 118.Thomas B, Beal MF. Parkinson’s disease. Hum Mol Genet. 2007;16(Spec No 2):R183–94. doi: 10.1093/hmg/ddm159. [DOI] [PubMed] [Google Scholar]
- 119.Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219:979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- 120.Polymeropoulos MH, Lavedan C, Leroy E, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 121.Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, Yokochi M, Mizuno Y, Shimizu N. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392:605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- 122.Bonifati V, Rizzu P, van Baren MJ, et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset Parkinsonism. Science. 2003;299:256–259. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- 123.Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, Ali Z, Del Turco D, Bentivoglio AR, Healy DG, Albanese A, Nussbaum R, González-Maldonado R, Deller T, Salvi S, Cortelli P, Gilks WP, Latchman DS, Harvey RJ, Dallapiccola B, Auburger G, Wood NW. Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science. 2004;304:1158–60. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- 124.Strauss KM, Martins LM, Plun-Favreau H, et al. Loss of function mutations in the gene encodingOmi/HtrA2 in Parkinson’s disease. Hum Mol Genet. 2005;14:2099–2111. doi: 10.1093/hmg/ddi215. [DOI] [PubMed] [Google Scholar]
- 125.Zimprich A, Biskup S, Leitner P, et al. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44:601–607. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 126.Leroy E, Boyer R, Auburger G, et al. The ubiquitin pathway in Parkinson’s disease. Nature. 1998;395:451– 452. doi: 10.1038/26652. [DOI] [PubMed] [Google Scholar]
- 127.Schapira AH, Cooper JM, Dexter D, Clark JB, Jenner P, Marsden CD. Mitochondrialcomplex I deficiency in Parkinson’s disease. J Neurochem. 1990;54:823–827. doi: 10.1111/j.1471-4159.1990.tb02325.x. [DOI] [PubMed] [Google Scholar]
- 128.Parker WD, Jr, Boyson SJ, Parks JK. Abnormalities of the electron transport chain in idiopathic Parkinson’s disease. Ann Neurol. 1989:719–23. doi: 10.1002/ana.410260606. [DOI] [PubMed] [Google Scholar]
- 129.Dagda RK, Zhu J, Chu CT. Mitochondrial kinases in Parkinson’s disease: converging insights from neurotoxin and genetic models. Mitochondrion. 2009;9:289–98. doi: 10.1016/j.mito.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Andersen JK. Iron dysregulation and Parkinson’s disease. J Alzheimers Dis. 2006;6:S47–52. doi: 10.3233/jad-2004-6s602. [DOI] [PubMed] [Google Scholar]
- 131.Sian J, Dexter DT, Lees AJ, Daniel S, Agid Y, Javoy-Agid F, Jenner P, Marsden CD. Alterations in glutathione levels in Parkinson’s disease and other neurodegenerative disorders affecting basalganglia. Ann Neurol. 1994;36:348–355. doi: 10.1002/ana.410360305. [DOI] [PubMed] [Google Scholar]
- 132.Simon DK, Pulst SM, Sutton JP, Browne SE, Beal MF, Johns DR. Familial multisystem degeneration with parkinsonism associated with the 11778 mitochondrial DNA mutation. Neurology. 1999;53:1787–93. doi: 10.1212/wnl.53.8.1787. [DOI] [PubMed] [Google Scholar]
- 133.Zhu J, Chu CT. Mitochondrial dysfunction in Parkinson’s disease. J Alzheimers Dis. 2010;2010;20(Suppl 2):S325–34. doi: 10.3233/JAD-2010-100363. [DOI] [PubMed] [Google Scholar]
- 134.Dagda RK, Cherra SJ, 3rd, Kulich SM, Tandon A, Park D, Chu CT. Loss of PINK1 function promotes mitophagy through effects on oxidative stress and mitochondrial fission. J Biol Chem. 2009;284:13843–55. doi: 10.1074/jbc.M808515200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Poole AC, Thomas RE, Andrews LA, McBride HM, Whitworth AJ, Pallanck LJ. The PINK1/Parkin pathway regulates mitochondrial morphology. Proc Natl Acad Sci U S A. 2008;105:1638–43. doi: 10.1073/pnas.0709336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Deng H, Dodson MW, Huang H, Guo M. The Parkinson’s disease genes pink1 and parkin promote mitochondrial fission and/or inhibit fusion in Drosophila. Proc Natl Acad Sci U S A. 2008;105:14503–8. doi: 10.1073/pnas.0803998105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Devi L, Raghavendran V, Prabhu BM, Avadhani NG, Anandatheerthavarada HK. Mitochondrial import and accumulation of alpha-synuclein impair complex I in human dopaminergic neuronal cultures and Parkinson disease brain. J Biol Chem. 2008;283:9089–100. doi: 10.1074/jbc.M710012200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Parihar MS, Parihar A, Fujita M, Hashimoto M, Ghafourifar P. Mitochondrial association of alpha-synuclein causes oxidative stress. Cell Mol Life Sci. 2008;65:1272–84. doi: 10.1007/s00018-008-7589-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Shavali S, Brown-Borg HM, Ebadi M, Porter J. Mitochondrial localization of alpha-synuclein protein in alpha-synuclein overexpressing cells. Neurosci Lett. 2008;439:125–128. doi: 10.1016/j.neulet.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.West AB, Moore DJ, Biskup S, Bugayenko A, Smith WW, Ross CA, Dawson VL, Dawson TM. Parkinson’s disease-associated mutations in leucine-rich repeat kinase 2 augment kinase activity. Proc Natl Acad Sci U S A. 2005;102:16842–7. doi: 10.1073/pnas.0507360102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhang L, Shimoji M, Thomas B, Moore DJ, Yu SW, Marupudi NI, Torp R, Torgner IA, Ottersen OP, Dawson TM, Dawson VL. Mitochondrial localization of the Parkinson’s disease related protein DJ-1: implications for pathogenesis. Hum Mol Genet. 2005;14:2063–73. doi: 10.1093/hmg/ddi211. [DOI] [PubMed] [Google Scholar]
- 142.Hervias I, Beal MF, Manfredi G. Mitochondrial dysfunction and amyotrophic lateral sclerosis. Muscle Nerve. 2006;33:598–608. doi: 10.1002/mus.20489. [DOI] [PubMed] [Google Scholar]
- 143.Wong M, Martin LJ. Skeletal muscle-restricted expression of human SOD1 causes motor neuron degeneration in transgenic mice. Hum Mol Genet. 2010;19:2284–302. doi: 10.1093/hmg/ddq106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hirano A, Donnenfeld H, Sasaki S, Nakano I. Fine structural observations of neurofilamentous changes in amyotrophic lateral sclerosis. J Neuropathol Exp Neurol. 1984;43:461–470. doi: 10.1097/00005072-198409000-00001. [DOI] [PubMed] [Google Scholar]
- 145.Dupuis L. Oxidative stress sensitivity in ALS muscle cells. Exp Neurol. 2009;220(2):219–23. doi: 10.1016/j.expneurol.2009.08.023. [DOI] [PubMed] [Google Scholar]
- 146.Kawamata H, Manfredi G. Mitochondrial dysfunction and intracellular calcium dysregulation in ALS. Mech Ageing Dev. 2010 May 18; doi: 10.1016/j.mad.2010.05.003. [Epub ahead of print] PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Martin LJ. Mitochondrial pathobiology in Parkinson’s disease and amyotrophic lateral sclerosis. J Alzheimers Dis. 2010;20 (Suppl 2):S335–56. doi: 10.3233/JAD-2010-100348. [DOI] [PubMed] [Google Scholar]
- 148.Shi P, Wei Y, Zhang J, Gal J, Zhu H. Mitochondrial dysfunction is a converging point of multiple pathological pathways in amyotrophic lateral sclerosis. J Alzheimers Dis. 2010;20 (Suppl 2):S311–24. doi: 10.3233/JAD-2010-100366. [DOI] [PubMed] [Google Scholar]
- 149.Pedrini S, Sau D, Guareschi S, Bogush M, Brown RH, Jr, Naniche N, Kia A, Trotti D, Pasinelli P. ALS-linked mutant SOD1 damages mitochondria by promoting conformational changes in Bcl-2. Hum Mol Genet. 2010 May 22; doi: 10.1093/hmg/ddq202. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Keeney PM, Bennett JP., Jr ALS spinal neurons show varied and reduced mtDNA gene copy numbers and increased mtDNA gene deletions. Mol Neurodegener. 2010;5:21. doi: 10.1186/1750-1326-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chung MJ, Suh YL. Ultrastructural changes of mitochondria in the skeletal muscle of patients with amyotrophic lateral sclerosis. Ultrastruct Pathol. 2002;26:3–7. doi: 10.1080/01913120252934260. [DOI] [PubMed] [Google Scholar]
- 152.Dupuis L, di Scala F, Rene F, de Tapia M, Oudart H, Pradat PF, Meininger V, Loeffler JP. Up-regulation of mitochondrial uncoupling protein 3 reveals an early muscular metabolic defect in amyotrophic lateral sclerosis. FASEB J. 2003;17:2091–3. doi: 10.1096/fj.02-1182fje. [DOI] [PubMed] [Google Scholar]
- 153.Bergemalm D, Jonsson PA, Graffmo KS, Andersen PM, Brannstrom T, Rehnmark A, Marklund SL. Overloading of stable and exclusion of unstable human superoxide dismutase-1 variants in mitochondria of murine amyotrophic lateral sclerosis models. J Neurosci. 2006;26:4147–4154. doi: 10.1523/JNEUROSCI.5461-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Deng HX, Shi Y, Furukawa Y, Zhai H, et al. Conversion to the amyotrophic lateral sclerosis phenotype is associated with intermolecular linked insoluble aggregates of SOD1 in mitochondria. Proc Natl Acad Sci U S A. 2006;103:7142–7147. doi: 10.1073/pnas.0602046103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Liu J, Lillo C, Jonsson PA, et al. Toxicity of familial ALS-linked SOD1 mutants from selective recruitment to spinal mitochondria. Neuron. 2004;43:5–17. doi: 10.1016/j.neuron.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 156.Vijayvergiya C, Beal MF, Buck J, Manfredi G. Mutant superoxide dismutase 1 forms aggregates in the brain mitochondrial matrix of amyotrophic lateral sclerosis mice. J Neurosci. 2005;25:2463–2470. doi: 10.1523/JNEUROSCI.4385-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Higgins CM, Jung C, Ding H, Xu Z. Mutant Cu, Zn superoxide dismutase that causes motoneuron degeneration is present in mitochondria in the CNS. J Neurosci. 2002;22:RC215. doi: 10.1523/JNEUROSCI.22-06-j0001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Magrané J, Hervias I, Henning MS, Damiano M, Kawamata H, Manfredi G. Mutant SOD1 in neuronal mitochondria causes toxicity and mitochondrial dynamics abnormalities. Hum Mol Genet. 2009;18:4552–64. doi: 10.1093/hmg/ddp421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Martin LJ. The mitochondrial permeability transition pore: a molecular target for amyotrophic lateral sclerosis therapy. Biochim Biophys Acta. 2010;180:186–97. doi: 10.1016/j.bbadis.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Beal MF. Mitochondrial dysfunction and oxidative damage in Alzheimer’s and Parkinson’s diseases and coenzyme Q10 as a potential treatment. J Bioenerg Biomembr. 2004;36:381–6. doi: 10.1023/B:JOBB.0000041772.74810.92. [DOI] [PubMed] [Google Scholar]
- 161.Thomas B, Beal MF. Mitochondrial therapies for Parkinson’s disease. Mov Disord. 2010;25(Suppl 1):S155–60. doi: 10.1002/mds.22781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Cleren C, Yang L, Lorenzo B, Calingasan NY, Schomer A, Sireci A, Wille EJ, Beal MF. Therapeutic effects of coenzyme Q10 (CoQ10) and reduced CoQ10 in the MPTP model of Parkinsonism. J Neurochem. 2008;104:1613–21. doi: 10.1111/j.1471-4159.2007.05097.x. [DOI] [PubMed] [Google Scholar]
- 163.Henchcliffe C, Beal MF. Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis. Nat Clin Pract Neurol. 2008;4:600–9. doi: 10.1038/ncpneuro0924. [DOI] [PubMed] [Google Scholar]
- 164.Yang L, Calingasan NY, Wille EJ, Cormier K, Smith K, Ferrante RJ, Beal MF. Combination therapy with coenzyme Q10 and creatine produces additive neuroprotective effects in models of Parkinson’s and Huntington’s diseases. J Neurochem. 2009;109:1427–39. doi: 10.1111/j.1471-4159.2009.06074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Anekonda TS, Reddy PH. Can Plants Provide a New Generation of Drugs for Treating Alzheimer’s Disease? 2005;50:361–376. doi: 10.1016/j.brainresrev.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 166.Anekonda TS, Reddy PH. Neuronal protection by sirtuins in Alzheimer’s disease. J Neurochem. 2006;96:305–313. doi: 10.1111/j.1471-4159.2005.03492.x. [DOI] [PubMed] [Google Scholar]
- 167.Anekonda TS. Resveratrol--a boon for treating Alzheimer’s disease? Brain Res Rev. 2006;52:316–26. doi: 10.1016/j.brainresrev.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 168.Du H, Yan SS. Unlocking the Door to Neuronal Woes in Alzheimer’s Disease: Aβ and Mitochondrial Permeability Transition Pore. Pharmaceuticals. 2010;3:1936–1948. doi: 10.3390/ph3061936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Cardoso S, Santos R, Correia S, Carvalho C, Zhu X, Lee H-G, Casadesus G, Smith MA, Perry G, Moreira PI. Insulin and Insulin-Sensitizing Drugs in Neurodegeneration: Mitochondria as Therapeutic Targets. Pharmaceuticals 2009. 2009;2:250–286. doi: 10.3390/ph2030250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Praticò D. Oxidative stress hypothesis in Alzheimer’s disease: a reappraisal. Trends Pharmacol Sci. 2008;29:609–615. doi: 10.1016/j.tips.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 171.Bordet T, Berna P, Abitbol JL, Pruss RM. Olesoxime (TRO19622): A Novel Mitochondrial-Targeted Neuroprotective Compound. Pharmaceuticals. 2010;3:345–368. doi: 10.3390/ph3020345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Szeto HH. Development of mitochondria-targeted aromatic-cationic peptides for neurodegenerativediseases. Ann N Y Acad Sci. 2008;1147:112–121. doi: 10.1196/annals.1427.013. [DOI] [PubMed] [Google Scholar]
- 173.Murphy MP, Smith RA. Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu Rev Pharmacol Toxicol. 2007;47:629–656. doi: 10.1146/annurev.pharmtox.47.120505.105110. [DOI] [PubMed] [Google Scholar]
- 174.Doody RS, Gavrilova SI, Sano M, Thomas RG, Aisen PS, Bachurin SO, Seely L, Hung D dimebon investigators. Effect of dimebon on cognition, activities of daily living, behaviour, and global function in patients with mild-to-moderate Alzheimer’s disease: a randomised, double-blind, placebo-controlled study. Lancet. 2008;372:207–15. doi: 10.1016/S0140-6736(08)61074-0. [DOI] [PubMed] [Google Scholar]