Abstract
Kurtoxin is a 63-amino acid polypeptide isolated from the venom of the South African scorpion Parabuthus transvaalicus. It is the first and only peptide ligand known to interact with Cav3 (T-type) voltage-gated Ca2+ channels with high affinity and to modify the voltage-dependent gating of these channels. Here we describe the nuclear magnetic resonance (NMR) solution structure of kurtoxin determined using two- and three-dimensional NMR spectroscopy with dynamical simulated annealing calculations. The molecular structure of the toxin was highly similar to those of scorpion α-toxins and contained an α-helix, three β-strands, and several turns stabilized by four disulfide bonds. This so-called “cysteine-stabilized α-helix and β-sheet (CSαβ)” motif is found in a number of functionally varied small proteins. A detailed comparison of the backbone structure of kurtoxin with those of the scorpion α-toxins revealed that three regions [first long loop (Asp8–Ile15), β-hairpin loop (Gly39–Leu42), and C-terminal segment (Arg57–Ala63)] in kurtoxin significantly differ from the corresponding regions in scorpion α-toxins, suggesting that these regions may be important for interacting with Cav3 (T-type) Ca2+ channels. In addition, the surface profile of kurtoxin shows a larger and more focused electropositive patch along with a larger hydrophobic surface compared to those seen on scorpion α-toxins. These distinct surface properties of kurtoxin could explain its binding to Cav3 (T-type) voltage-gated Ca2+ channels.
Voltage-gated ion channels are expressed by nearly all cells and play a crucial role in regulating membrane potential and a variety of cellular functions. These channels are comprised of two principle domains: a central pore domain formed by two segments, S5 and S6, and four surrounding voltage-sensing domains, each composed of segments S1–S4.1−4 Venomous animals (spiders, scorpions, and cone snails, among others) produce a broad array of polypeptide toxins, many of which bind to voltage-gated Na+, K+, or Ca2+ channels,5−8 and have proven to be valuable pharmacological tools for evaluating specific channel characteristics.
Although the origins of the venomous peptide toxins that interact with voltage-gated ion channels are diverse, their modes of action fall into two major categories, pore blockade and gating modification, based on the domain with which they interact and their mechanisms of action. Pore blockers bind to the external vestibule of the channel and physically obstruct the movement of ions by occluding the ion-conducting pore.9 The three-dimensional structures of many pore-blocking toxins, including the μ-conotoxins for the Na+ channel, charybdotoxin for the K+ channel, and ω-conotoxins for the Ca2+ channel, have all been determined, allowing investigation of the structure–function relationships of the pore-forming domains of the channels.10−15 Gating modifiers, on the other hand, bind to the voltage-sensing domains of voltage-gated ion channels and modify the energetics of either activation or inactivation.16−21 Established gating modifiers include the α- and β-scorpion toxins, sea anemone toxins, and δ-conotoxins for Na+ channels;22−28 hanatoxin (HaTx), SGTx1, GxTx-1E, and VSTx for K+ channels;29−33 and ω-agatoxin IVA (ω-Aga IVA) and ω-grammotoxin SIA (GrTx) for Ca2+ channels.34−37
Studies of the structure and function of gating modifiers have advanced our understanding of the molecular structures and gating mechanisms of voltage-gated ion channels. Studies employing HaTx, SGTx1, GxTx-1E, VSTx, GrTx, and ω-Aga IVA have revealed that, within voltage-gated K+ and Ca2+ channels, these proteins bind to structurally conserved motifs composed of hydrophobic and acidic residues within the C-terminal end of S3 and the N-terminal end of S4.17−19,38−42 The X-ray structures of voltage-gated K+ channels (i.e., KvAP and Kv1.2 channels) show that these regions of S3 and S4 form a helix–turn–helix motif termed the voltage sensor paddle.4,43,44 Gating modifier toxins that partition into the membrane interact with the voltage sensor paddle at the protein–lipid interface.42,45
Cav3 (T-type) voltage-gated Ca2+ channels can be differentiated from other types of Ca2+ channels on the basis of their activation at lower voltages, faster inactivation, slower deactivation, and smaller Ba2+ conductances.46 Their unique gating properties allow Cav3 (T-type) Ca2+ channels to trigger low-threshold spikes that can lead to burst firing and oscillatory behavior and can contribute to standing calcium currents near the resting membrane potential in a variety of cell types.47−50 Although these characteristics imply Cav3 (T-type) channels could play important roles in many tissues,51,52 progress in understanding their subunit composition and physiological functions has been hindered by a scarcity of ligands that interact with these channels.53,54
A gating modifier kurtoxin, isolated from the venom of the scorpion Parabuthus transvaalicus, is the first peptide ligand known to act on Cav3 (T-type) voltage-gated Ca2+ channels.55,56 Here, we describe the solution structure of kurtoxin determined using proton two-dimensional (2D) and heteronuclear three-dimensional (3D) NMR spectroscopy with dynamical simulated annealing calculations. The structure of kurtoxin closely resembles those of scorpion α-toxins (Figure 1) targeting Na+ channels and shows the unique structural characteristic of gating modifiers, electropositive and hydrophobic patches on the surface of the molecule.39,57,58 Detailed inspection of the structure of kurtoxin offers the possibility of understanding the molecular basis of its Cav3 (T-type) Ca2+ channel selectivity and could facilitate clarification of the gating mechanism of voltage-gated ion channels.
Figure 1.
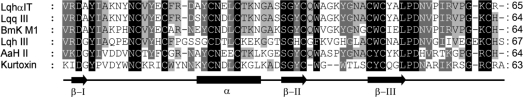
Amino acid sequences and alignment of kurtoxin and five scorpion α-toxins. LqhαIT and Lqq III are highly active in insects. Lqh III and Bmk M1 are α-like toxins. AaH II is highly active in mammals. These sequences were aligned using ClustalX. Highly conserved residues are shaded in black or gray. The secondary structure elements of kurtoxin are shown as arrows (β-strand), bars (α-helix), and lines (connecting loops).
Experimental Procedures
Sample Preparation
Functional kurtoxin was obtained using a bacterial expression system.59 The recombinant kurtoxin was expressed as an inclusion body, solubilized in a denaturing solution, and then refolded in a refolding solution. The crude folded kurtoxin was purified by preparative RP-HPLC, after which the purity of the recombinant protein was confirmed by analytical RP-HPLC and MALDI-TOF MS measurements.
CD Spectral Analysis
CD spectra were recorded on a JASCO J-750 spectropolarimeter in a solution containing 0.01 M sodium phosphate in H2O and 0, 10, 15, 20, 25, or 30% CH3CN at pH 7.0. Measurements were taken at 20 °C using a quartz cell with a 1 mm path length. The spectra were expressed as molecular ellipticity [θ] in degrees square centimeter per decimole.
NMR Spectroscopy
NMR measurements were taken on a Bruker AVANCE 600 spectrometer equipped with an xyz gradient triple-resonance probe. The samples used for proton 2D NMR experiments were 1 mM kurtoxin dissolved in water containing 25% CD3CN at pH 4.0 (uncorrected for the isotope effect). All proton 2D NMR spectra were recorded in a phase-sensitive mode using time-proportional phase incrementation (TPPI) for quadrature detection in the t1 dimension at 278, 288, and 298 K. TOCSY spectra were recorded using a MLEV-17 pulse scheme60 with isotropic mixing times of 60 and 90 ms. NOESY spectra60−62 were recorded with mixing times of 60, 100, and 150 ms. Suppression of the solvent resonance in both the NOESY and TOCSY measurements was achieved using the WATERGATE scheme.63 E-COSY64 spectra were recorded to obtain the constraints for stereospecific assignments.
The following triple-resonance 3D NMR spectra were recorded using 1 mM 13C- and 15N-labeled or 1 mM 15N-labeled kurtoxin in 25% CD3CN at 278 and 288 K. Uniformly 13C- and 15N-enriched kurtoxin was used to record 3D HNCACB, CBCA(CO)NH, HNCA, HN(CO)CA, HNCO, and HN(CA)CO spectra.65−68 Uniformly 15N-enriched kurtoxin was used to record 2D 1H–15N HSQC69 and 3D 15N TOCSY-HSQC spectra with 90 ms mixing times and a 3D 15N NOESY-HSQC spectrum with a 120 ms mixing time. The 3JHN–Hα values were obtained from the 3D HNHA70 and 2D DQF-COSY spectra. Slowly exchanging backbone amide protons were identified by analysis of TOCSY spectra recorded in 75% D2O and 25% CD3CN on time scales of 30 min and 3.5 h and then every 3 h up to 25 h. 1H chemical shifts were referenced to DSS at 0 ppm, and 13C and 15N chemical shifts were calculated from the 1H frequency. All spectra were processed using AZARA version 2.5 (provided by W. Boucher) or XWIN-NMR and were analyzed using ANSIG version 3.371 on a Silicon Graphics Octane2 workstation or on a Linux workstation.
NMR Experimental Restraints and Structure Calculations
The backbone NH–CαH coupling constants were converted to backbone torsion angle ϕ constraints according to the following rules: for a 3JNH–CαH of <5.5 Hz, the ϕ angle was constrained in the range of −65 ± 25°; for a 3JNH–CαH of >8.0 Hz, it was constrained in the range of −120 ± 40°.72,73 Backbone dihedral constraints were not applied to 3JNH–CαH values between 5.5 and 8.0 Hz. The range of the χ1 side chain torsion angle constraints and the stereospecific assignment of the prochiral β-methylene protons were obtained using the 3Jαβ coupling constants combined with the intraresidue NH–CβH NOEs.74 The 3Jαβ coupling constants were determined from the E-COSY spectrum in D2O. For the t2g3, g2g3, and g2t3 conformations around the Cα–Cβ bonds, the χ1 side chain torsion angle was constrained in the ranges of −60 ± 30°, 60 ± 30°, and 180 ± 30°, respectively.75
Quantitative determination of the cross-peak intensities was based on the counting levels. Observed NOE data were classified into four distance ranges (1.8–2.7, 1.8–3.5, 1.8–5.0, and 1.8–6.0 Å) that corresponded to strong, medium, weak, and very weak NOE values, respectively. Pseudoatoms were used for the methyl protons or the nonstereospecifically assigned methylene protons.76 Correcting factors for the use of pseudoatoms were added to the distance constraints, and 0.5 Å was added to the distance constraints involving methyl protons.77 For each disulfide bond, we used three distance constraints, S(i)–S(j), S(i)–Cβ(j), and S(j)–Cβ(i), whose target values were set to 2.02 ± 0.02, 2.99 ± 0.5, and 2.99 ± 0.5 Å, respectively.78 The hydrogen bond acceptors for the slowly exchanged amide protons were identified by analyzing the preliminarily calculated structures.79,80 The distance restraints on the hydrogen bonds were added as target values of 1.8–2.3 Å for NH(i)–O(j) and 2.8–3.3 Å for N(i)–O(j) bonds.
All calculations were conducted on an SGI Octane2 workstation using the X-PLOR version 3.851.81 The three-dimensional structures were calculated on the basis of the experimentally derived distance and torsion angle constraints using a dynamically simulated annealing protocol starting from a template structure with randomized backbone ϕ and ψ torsion angles. The final 20 structures with the lowest energy and smallest Lennard-Jones van der Waals energy were chosen. The convergence of the calculated structures was evaluated in terms of the structural parameters. There were root-mean-square deviations (rmsds) from the experimental distances and dihedral constraints, from the energetic statistics (FNOE, Ftor, Frepel, and EL-J), and from the idealized geometry. The structures were analyzed using the PROCHECK_NMR,82 PROMOTIF,83 MOLMOL,84 and MolProbity.85,86 The distributions of the backbone dihedral angles in the final converged structure were evaluated by representation of the Ramachandran dihedral pattern, which indicated the deviations from the allowed ϕ and ψ angle limits. The degrees of angular variation among the converged structures were further assessed using an angular order parameter.87 The solvent-accessible surface areas for the side chains of the amino acid residues were calculated with a solvent radius of 1.4 Å. Structural figures were generated using MOLMOL and INSIGHT II 2000 (Accelrys Inc.).
Results and Discussion
NMR Sample Preparation
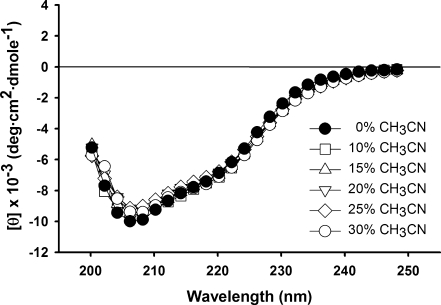
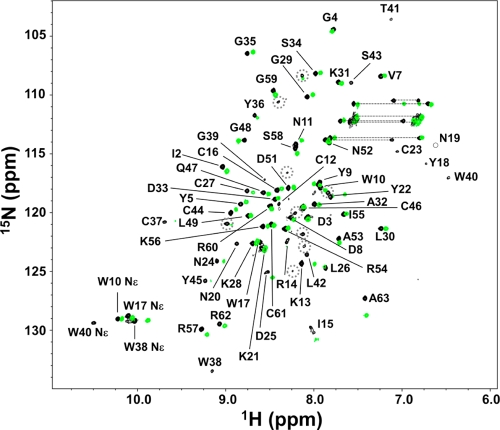
Kurtoxin was insoluble in aqueous solution, even at a concentration of <1 mM. We therefore tested whether kurtoxin could be solubilized using 0–30% acetonitrile (CH3CN) without disrupting the protein’s intrinsic structure. Figure 2 shows that the CD spectra for kurtoxin recorded in the absence or presence of 10, 15, 20, 25, and 30% CH3CN are nearly identical in the far-UV region (200–250 nm), indicating that the backbone structure of kurtoxin is affected little by the addition of CH3CN. Moreover, we obtained high-quality NMR spectra with 1 mM kurtoxin in the presence of 25% deuterated acetonitrile (CD3CN), strongly suggesting that the addition of CD3CN effectively prevented the aggregation of kurtoxin (Figure 3). In the 1H–15N HSQC spectra recorded in pure water, several hydrophobic peaks, including Tyr17, Tyr18, Trp38, Gly39, Trp40, and Leu42, could not be detected, most likely because of the line broadening in the absence of CD3CN (Figure 3). This appeared to be due to the self-aggregation of kurtoxin molecules. On the other hand, in the 1H–15N HSQC spectra recorded in the presence of 25% CD3CN, all of the backbone 1H–15N cross peaks were identifiable, and the intensities of nearly all the peaks were increased without large chemical shift variations (Figure S1 of the Supporting Information).
Figure 2.
CD spectra of 0.05 mM kurtoxin in H2O containing 0, 10, 15, 20, 25, or 30% CH3CN [0.01 M sodium phosphate (pH 7.0)] at 20 °C.
Figure 3.
1H–15N HSQC spectra of kurtoxin in the presence (black) and absence (green) of 25% CD3CN at 288 K. The resonance assignments are indicated by the one-letter amino acid codes and residue numbers. The dotted circles indicate additional and unassigned peaks.
Addition of CH3CN can weaken the hydrophobic interactions between surface hydrophobic regions without altering the three-dimensional structure of the proteins.88 Nonetheless, CH3CN can influence chemical shift variations by changing the polarity of the accessible atom environments; of those, the chemical shifts located in the flexible N- and C-terminal regions (Ala63 of kurtoxin in Figure S1 of the Supporting Information) and loops are most sensitive.89 Comparison of the CD and 1H–15N HSQC spectra recorded in the presence and absence of 25% CD3CN indicates that CD3CN prevented the hydrophobic-related aggregation of kurtoxin without disrupting its overall topology. Linear kurtoxin was also refolded in a redox solution containing 30% CH3CN,59 suggesting the nativelike conformation of kurtoxin is stabilized in an aqueous solution containing CH3CN.
A small number of additional minor peaks were observed in NMR spectra, including 1H–15N HSQC spectra in the presence and absence of CD3CN (Figure 3). The intensity ratio of the minor peaks is <5–10% compared to that of the major peaks according to relevant NMR signals. Unfortunately, we failed to assign these minor peaks because the signals were very weak and it was hard to sequentially connect these minor peaks. In a recent study, two stable conformations of BmκαTx11, a kurtoxin-homologous scorpion α-toxin, were identified by using NMR spectroscopy.90 We assume that the minor peaks of kurtoxin also come from a possible minor conformation similar to BmκαTx11.
NMR Spectroscopy
Sequence-specific assignments for the backbone atoms of kurtoxin were obtained from an analysis of heteronuclear 3D NMR spectra [HNCACB, CBCA(CO)NNH, HNCA, and HN(CO)CA] recorded in 25% CD3CN at pH 4.0 and 288 and 278 K using a uniformly 15N- and 13C-labeled protein. All backbone Cα atoms were assigned except those of Cys12, Arg14, Ile15, Asp25, Leu26, Ala32, and Trp40. The backbone and side chain protons were obtained from 2D NMR spectra (DQF-COSY, TOCSY, and NOESY) and 3D 15N NOESY-HSQC and 3D 15N TOCSY-HSQC spectra recorded in 25% CD3CN at pH 4.0 and 288 K. The assignments of all backbone and side chain protons were complete except for those of the Hζ atoms of Lys13 and Lys21, Hδ2 of Asn19 and Asn20, and Hδ and Hε of Tyr22. For all Pro residues (Pro6 and Pro50), strong sequential dα,δ and no dα,α were observed in the NOESY spectra, indicating the toxin’s proline residues are all in the trans configuration.
Identification of Secondary Structure Elements
As summarized in Figure S2 of the Supporting Information, the pattern of observed NOEs and chemical shift index (CSI) values for Hα was ultimately interpreted in terms of the secondary structure of the molecule. The weak 3JHNα coupling constants, strong dNN NOE peaks and dαN(i,i+3) and dαβ(i,i+3) NOE correlations, and the CSI value of −1 all indicate an α-helical conformation for residues Lys21–Leu30. The extent of the β-strands and their relative orientations within the β-sheet structure were determined using standard criteria: large 3JHNα coupling constants (Ile2, Asp3, Try36, Cys37, Tyr45, Cys46, and Gln47), strong sequential dαN, interstrand NH–NH and NH–CαH connectivities, and slowly exchanging amide protons (Ile2, Gly4, Gly35, Tyr36, Cys44, Tyr45, Cys46, Gln47, and Leu49). Kurtoxin contains three β-strands comprised of residues Ile2–Gly4, Ser34–Cys37, and Cys44–Leu49, which are arranged in an antiparallel fashion with several turns (Figure S3 of the Supporting Information). Our criteria allowed discrimination of the peripheral and central strands within the β-sheet.
Structure Calculations
The structure of kurtoxin was determined from a total of 894 NMR experimental constraints, including 856 experimental distance constraints and 38 dihedral angle constraints, which correspond to an average of 14.2 constraints per residue. Of the 861 distance constraints, there were 311 intraresidue and 492 interresidue NOE distance constraints, 42 hydrogen bond constraints determined from hydrogen–deuterium exchange-out experiments, and 12 disulfide bond constraints. The 42 distance constraints related to hydrogen bonds were as follows: I2(HN)–L49(CO), G4(HN)–C46(CO), D25(HN)–K21(CO), C27(HN)–C23(CO), K28(HN)–N24(CO), G29(HN)–L26(CO), L30(HN)–C27(CO), K31(HN)–K28(CO), A32(HN)–C27(CO), G35(HN)–D33(CO), Y36(HN)–Y45(CO), W38(HN)–S43(CO), C44(HN)–I15(CO), Y45(HN)–Y36(CO), C46(HN)–G4(CO), Q47(HN)–S34(CO), L49(HN)–I2(CO), A53(HN)–P50(CO), K56(HN)–Y5(CO), R62(HN)–W10(CO), and A63(HN)–C61(CO). The disulfide bond pattern of kurtoxin was determined to be Cys12–Cys61, Cys16–Cys37, Cys23–Cys44, and Cys27–Cys46, based on sequential cleavage with proteases and MALDI-TOF MS measurements.59
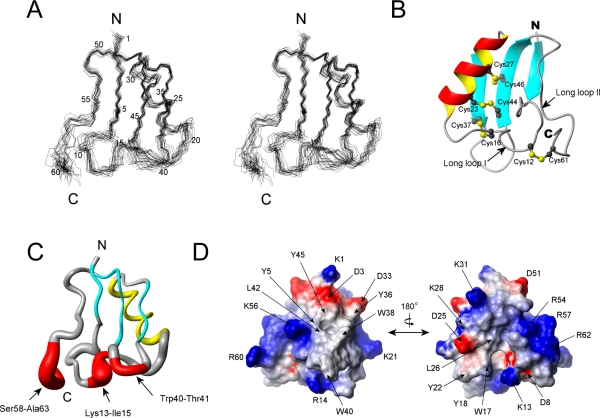
We conducted the simulated annealing calculations starting with 100 random kurtoxin structures. From those, we selected 20 final structures (Figure 4A) that were in good agreement with the NMR experimental constraints (NOE distance and torsion angle violations of <0.2 Å and <2°, respectively). Statistics for the converged structures were evaluated in terms of the structural parameters (Table 1). The deviations from the idealized covalent geometry were very small, and the Lennard-Jones van der Waals energy was large and negative (−212.55 ± 19.40), indicating there were no distortions or nonbonded bad contacts in the converged structures. The atomic rmsd about the mean coordinate positions was 0.87 ± 0.14 Å for the backbone atoms (N, Cα, and C) and 1.54 ± 0.19 Å for all heavy atoms. Ramachandran analysis showed that 98.7% of all residues fell within allowed regions.
Figure 4.
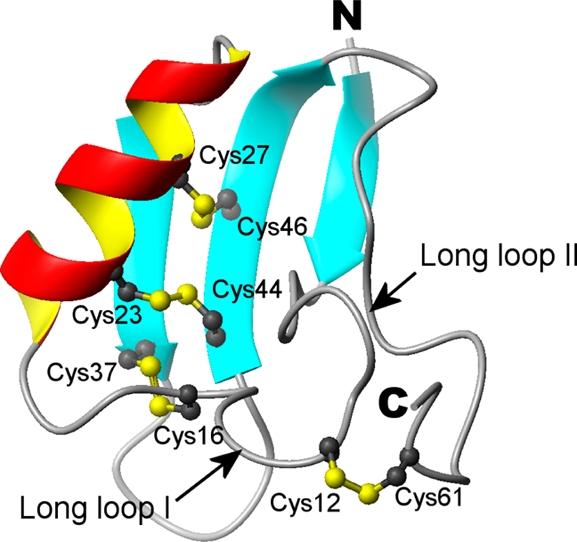
Solution structure of kurtoxin. (A) Stereopairs of backbone heavy atoms (N, Cα, and C) for the 20 converged structures of kurtoxin. These are the results of the best-fit superposition of the backbone heavy atoms of the molecule. N and C indicate N- and C-terminal positions, respectively. (B) Ribbon structure of kurtoxin. Schematic diagram of kurtoxin illustrating the location of the β-strands (cyan), α-helix (red and yellow), and disulfide bonds (numbered ball and stick). Long loop I and long loop II correspond to residues Tyr5–Asn20 and Pro50–Ala63, respectively. (C) Tubular representation of kurtoxin illustrating its motional properties. The diameter of the tube is proportional to the atomic rmsds of the backbone atoms: red for residues whose backbone rmsds are >1.0, cyan for those with β-strands, and yellow for those with an α-helix. (D) Surface profile of kurtoxin. The surface hydrophobic patches and charged residues are indicated. The molecular surface of kurtoxin is shown in color according to the electrostatic potential: red for negatively charged amino acids, blue for positively charged amino acids, and white for uncharged or hydrophobic amino acids. The left and right figures are rotated 180° with respect to one another about a vertical axis. These figures were generated using MOLMOL.
Table 1. Structural Statistics for the 20 Lowest-Energy Structures of Kurtoxina.
| rmsd from experimental distance constraints (Å)b (856) | 0.0115 ± 0.0012 |
| rmsd fom experimental dihedral constraints (deg)b (38) | 0.1555 ± 0.0012 |
| energetic statistics (kcal/mol)c | |
| FNOE | 5.7261 ± 1.2582 |
| Ftor | 0.9845 ± 0.0964 |
| Frepel | 5.3839 ± 1.3992 |
| EL-J | –212.55 ± 19.4000 |
| rmsd from idealized geometry | |
| bonds (Å) | 0.0017 ± 0.0001 |
| angles (deg) | 0.5010 ± 0.0065 |
| impropers (deg) | 0.3391 ± 0.0060 |
| Ramachandran analysisd (%) | |
| most favored regions | 56.0 |
| additionally allowed regions | 36.1 |
| generously allowed regions | 6.5 |
| disallowed regions | 1.3 |
| average rmsd (Å) | |
| backbone (N, Cα, C) | 0.87 ± 0.14 |
| all heavy atoms | 1.54 ± 0.19 |
| MolProbity analysise | |
| Clash score | 94.39 ± 9.94 |
| MolProbity score | 4.37 ± 0.08 |
None of these 20 structures exhibited distance violations of >0.2 Å or dihedral angle violations of >2°.
The number of each experimental constraint used in the calculations is given in parentheses.
FNOE, Ftor, and Frepel are the energies related to the NOE violations, the torsion angle violations, and the van der Waals repulsion term, respectively. The values of the force constants used for these terms are the standard values as depicted in the X-PLOR 3.1 manual. EL-J is the Lennard-Jones van der Waals energy calculated with the CHARMm empirical energy function.102EL-J was not used in the dynamical simulated annealing calculations.
PROCHECK_NMR was used to assess the stereochemical quality of the structures.
The MolProbity webserver was used to evaluate the determined kurtoxin ensemble structures.
Structure Description
The molecular structure of kurtoxin has a compact core consisting of an α-helix and a triple-stranded antiparallel β-sheet stabilized by four disulfide bridges. The 2.5-turn α-helix is composed of residues extending from Lys21 to Leu30 and is linked to the central strand of the β-sheet by two disulfide bridges (Cys23–Cys44 and Cys27–Cys46) (Figure 4B). The three β-strands are formed by residues Ile2–Gly4 (β-strand I), Ser34–Cys37 (β-strand II), and Cys44–Leu49 (β-strand III), with residues Ile2, Gly48, and Leu49 involved in a β-bulge conformation. The last two residues of β-strand III (Gly48 and Leu49) form a classical β-bulge and interact with the first residue of β-strand I (Ile2). As a result, the ϕ angle of Gly48 is positive (105.6°), which causes a distortion in the β-sheet. Two long loops (loop I, Tyr5–Asn20; loop II, Pro50–Ala63) extend from the core (Figure 4B). Long loop I includes two type IV β-turns (Asp8–Asn11 and Asn11–Arg14) and adopts the positive ϕ angles (58.9° and 89.9°, respectively) of Asn11 and Arg14 in the average structure. Long loop I is connected to β-strand II by a disulfide bond (Cys16–Cys37) and to the C-terminus by a disulfide bond (Cys12–Cys61) and a hydrogen bond [R62(HN)–W10(CO)]. Long loop II starts with a well-defined type I β-turn structure (residues 50–53) and extends to the C-terminus. It is stabilized through hydrogen bonding [N11(HD21)–S58(CO), K56(HN)–Y5(CO), and R62(HN)–W10(CO)] and formation of a disulfide bridge (Cys12–Cys61) to long loop I. The short loop regions (Lys13–Ile15 and Trp40–Thr41) and the six C-terminal residues (Ser58–Ala63) are less defined in the final 20 structures than the other regions in kurtoxin (Figure 4C). This may reflect a lack of medium- and long-range NOE constraints due to the inherent flexibility of these regions.
Two surface hydrophobic patches were observed in the kurtoxin structure. The major patch is composed of the solvent-exposed side chains of Tyr5, Tyr36, Trp38, Trp40, Leu42, and Tyr45 (Figure 4D), which, except for Tyr5, are located in the hairpin structure (strands II and III). Their side chains are well stacked, creating a compact hydrophobic patch on the surface of the molecule. The minor hydrophobic patch is situated on the opposite side of the protein and is centered around Trp17, Tyr18, Tyr22, and Leu26 (Figure 4D). Interestingly, all line-broadened residues (including Tyr17, Tyr18, Trp38, Gly39, Trp40, and Leu42) in pure water are located within either the major or minor surface hydrophobic patch in kurtoxin, strongly suggesting that the aggregation of kurtoxin results from these hydrophobic surface properties of kurtoxin. All of the charged residues except Asp8 are highly exposed on the surface of kurtoxin. Several charged residues, including Asp3, Lys21, and Lys56, surround the major surface hydrophobic patch, while Lys13, Arg14, and Asp25 are situated near the minor hydrophobic patch (Figure 4D).
Structural Comparison of Kurtoxin with Scorpion α-Toxins
The amino acid sequence of kurtoxin is homologous with those of the scorpion α-toxins targeting Na+ channels (Figure 1), which is consistent with the finding that kurtoxin can also interact with voltage-gated Na+ channels.55 The three-dimensional structures of the scorpion α-toxins have been determined by 1H 2D NMR spectroscopy and X-ray crystallography.91−101 Their overall folds are remarkably similar to that of kurtoxin and consist of an α-helix and three β-strands stabilized by four disulfide bridges, which has been termed the CSαβ (cysteine-stabilized α-helix and β-sheet) motif.102 In addition, like kurtoxin, the α-helices of the scorpion α-toxins contain 2.5–3 helical turns and are connected to the central β-stand by two disulfide bridges.
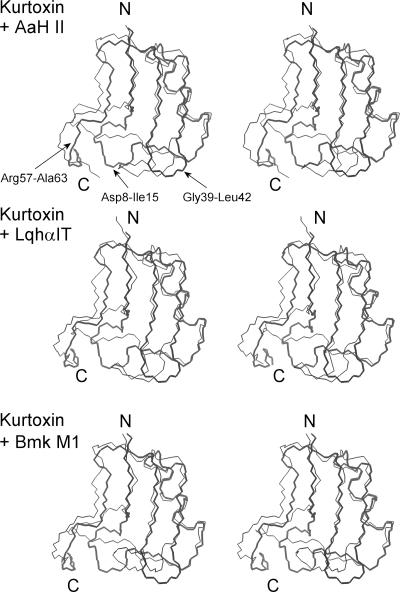
Figure 5 shows the geometric average backbone structure from the 20 NMR kurtoxin models superimposed on the backbones of AaH II (antimammal α-toxin), LqhαIT (anti-insect α-toxin), and BmK M1 (α-like toxin). Comparison of the kurtoxin backbone with those of the scorpion α-toxins clearly highlights three regions of structural difference: the first long loop region (Asp8–Ile15), the β-hairpin loop (Gly39–Leu42), and the C-terminal segment (Arg57–Ala63). The structural differences among these regions are strongly correlated with a marked difference in sequence (Figure 1). Among the eight residues extending from Asp8 to Ile15, only two (Ans11 and Cys12) are conserved in the amino acid sequences of these toxins. The 9th and 10th residues are variably polar or hydrophobic in the scorpion α-toxins, with a non-proline cis peptide bond in BMK M1.99 On the other hand, they are bulky hydrophobic residues (Tyr9 and Trp10) with a common trans peptide bond in kurtoxin. In addition, the structurally well-defined hydrophobic residues Val13 and Tyr14 found in LqhαIT and BmK M1 are replaced with disordered positively charged residues Lys13 and Arg14, respectively, in kurtoxin.
Figure 5.
Stereopairs showing the superposition of the kurtoxin structure on scorpion α-toxin structures. The backbone (C, Cα, and N) atoms of kurtoxin are superimposed on those of the scorpion α-toxins. The top panel shows the superposition of the backbone of kurtoxin on that of the anti-mammal α-toxin AaH II (PDB entry 1PTX). The middle panel shows the superposition on the backbone of the anti-insect α-toxin LqhαIT (PDB entry 1LQH). The bottom panel shows the superposition on the backbone of the α-like toxin Bmk M1 (PDB entry 1DJT). The backbone structures of kurtoxin and scorpion α-toxins are shown as thick and thin lines, respectively. N and C indicate the N- and C-terminal positions, respectively. Labeling shows the kurtoxin residues in regions of structural difference between the two backbones. The backbone rmsd values are 3.13, 2.98, and 2.62 Å for AaH II, LqhαIT, and Bmk M1, respectively.
The sequence of the hairpin loop (Gly39–Leu42) also differs between kurtoxin and the scorpion α-toxins. The length of the loop in kurtoxin (four residues) is shorter than in other toxins (approximately seven residues). In addition, whereas the loop is disordered and involved in the formation of the major hydrophobic patch in kurtoxin, it protrudes from the CSαβ core and turns toward the C-terminal segments in the scorpion α-toxins (Figure 5). Because the C-terminal segments are disordered in both kurtoxin and scorpion α-toxins, it is difficult to assess structural differences in that region. However, site-directed mutagenesis studies and functional assays of scorpion α-toxins have shown that there is a functional site composed of the five-residue reverse turn (Asp8–Cys12) and the C-terminal segment, and that the conserved hydrophobic surface may be involved in maintaining the stability of the protein and its biological activity.103−107 Taken together, these findings indicate that the core region of kurtoxin (i.e., the CSαβ motif) is well-defined and superimposes well on those of the scorpion α-toxins, but the Asp8–Ile15, Gly39–Leu42, and C-terminal segments of kurtoxin are structurally different from those of the scorpion α-toxins, suggesting it is these regions that are responsible for the functional differences between kurtoxin and scorpion α-toxins.
Comparison of the Surface Profiles of Kurtoxin and Scorpion α-Toxins
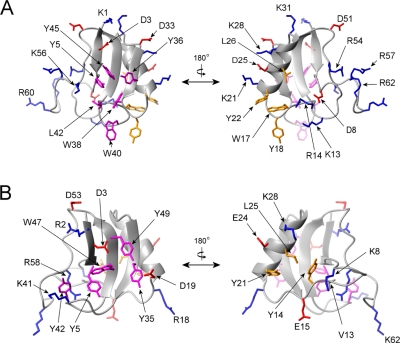
Kurtoxin contains five negatively charged and 11 positively charged residues in its amino acid sequence (Figure 1), and all of these charged residues except Asp8 are highly exposed on the water-accessible surface of the molecule. The side chain oxygen of Asp8 in AaH II forms a hydrogen bond with the amide proton of Val10, and the side chain of Gln8 in Lqh III forms a hydrogen bond with the oxygen of Val13.100 Asp8 of kurtoxin is directed toward Lys13 and Arg14 (Figure 6A). Although there are no experimental data for the hydrogen bond interactions between Asp8 and any other residues in kurtoxin, some side chain oxygens of Asp8 in 20 ensemble structures are close enough to form hydrogen bonds with Lys13 and/or Arg14 in the determined kurtoxin structures. Along the α-helix, the positive and negative charges align toward the solvent-accessible region of the molecule in both scorpion α-toxins and kurtoxin (Figure 6), suggesting that this feature may be involved in ion channel binding and determining selectivity.100 Kurtoxin is highly basic, as compared to the α-scorpion toxins; the net charge of kurtoxin is +6, while the others have net charges ranging from −2 to +3. As shown in Figure 1, the two hydrophobic residues (Val13 and Tyr14) conserved in all scorpion α-toxins except AaH II are replaced with two positively charged residues (Lys13 and Arg14, respectively) in kurtoxin. Figure 6B shows that the side chains of Val13 and Tyr14 in Lqq III (an anti-insect α-toxin) are largely buried in the molecular core. By contrast, Lys13 and Arg14 in kurtoxin are exposed to solvent (Figure 6A) and form a local electropositive surface (Figure 4D). In addition, a large electropositive patch (surface area of 660 Å2) is formed by the five positively charged C-terminal residues (Arg54, Lys56, Arg57, Arg60, and Arg62). This is in contrast to the C-terminal structure of Lqq III, which contains only two positively charged residues (Arg58 and Lys62). The water-exposed, positively charged residues of kurtoxin form a distinctive large electropositive surface, which is located around the five-residue reverse turn and C-terminal segment and is the proposed Na+ channel binding site in scorpion α-toxins.104
Figure 6.
Ribbon diagrams and heavy atom side chains of kurtoxin (A) and Lqq III (B). The surface hydrophobic patches and charged residues are indicated: red for the negatively charged amino acids are colored, blue for the positively charged amino acids, purple for the major surface hydrophobic amino acids, and orange for the minor surface hydrophobic amino acids. The left and right figures are rotated 180° relative to one another about a vertical axis.
A surface hydrophobic patch is a conserved feature of all scorpion α-toxins and is involved in mediating their interaction with Na+ channels.108,109 The orthogonal arrangement of the aromatic side chains in the surface hydrophobic patch, termed a “herringbone” structure, is found in all scorpion α-toxins and has been identified as the lowest-energy configuration of solvent-exposed aromatic rings.110 There are two hydrophobic patches in scorpion α-toxins, a major patch commonly composed of five residues (Tyr5, Tyr35, Tyr42, Trp47, and Tyr49) (Figure 6B) with a surface area of ∼280 Å2 and a minor one with a surface area of ∼210 Å2. Kurtoxin exhibits a larger hydrophobic surface than the scorpion α-toxins. The major hydrophobic patch of kurtoxin consists of the six solvent-exposed side chains of Tyr5, Tyr36, Trp38, Trp40, Leu42, and Tyr45 and has a surface area of 600 Å2 (Figure 6A). The side chains are well-packed on each other, creating a compact hydrophobic patch on the protein surface. The minor hydrophobic patch (surface area of 500 Å2) is centered on Trp17, Tyr18, and Tyr22 and also includes the δ-methyls of Leu26 (Figure 6A). Overall, it appears that kurtoxin shows a distinct surface profile, composed of both positive and hydrophobic residues, compared to other scorpion α-toxins.
Kurtoxin Binding Site on Cav3 (T-type) Ca2+ Channels
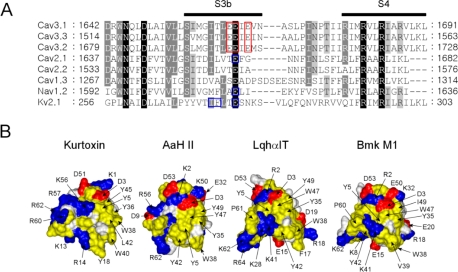
Voltage-gated ion channels consist of a central ion conduction pore (segments S5 and S6) surrounded by voltage sensors (segments S1–S4), which form “voltage sensor paddles” that move in response to changes in membrane voltage.4,43 The overall structure of the voltage sensor paddles includes hydrophobic, cationic, and helix–turn–helix structures formed by the S3b–S4 segment, and it has been suggested that it is the voltage sensor paddles that are recognized by gating modifier toxins.17−19,38−40,42,45 Kurtoxin has been identified as the first high-affinity (Kd = 15 nM) gating modifier of Cav3.1 (α1G T-type) Ca2+ channels, and also the first to show cross-reactivity with voltage-gated Na+ channels.55 This is similar to HaTx and GrTx, which exhibit cross-reactivity with the Kv2.1 K+ channel and P/Q-type Ca2+ channel, modifying the energetics of their gating.38 It has been suggested that hydrophobic and negatively charged residues (Ile273, Phe274, and Glu277) in the Kv2.1 channel form the binding site for HaTx and GrTx.38,111 Glu1613 in the rat brain IIA Na+ channel is equivalent to Glu277 in the Kv2.1 channel, and mutation of Glu1613 has a large effect on the affinity of scorpion α-toxins for Na+ channels.17 In addition, Glu1658, situated at the end of S3, within repeat IV of the P/Q-type Ca2+ channel, contributes to the binding of ω-Aga IVA.19 These findings prompt us to speculate that kurtoxin may bind to the S3b–S4 motif in domain IV of both Cav3 (T-type) Ca2+ channels and Na+ channels, a region that has some conservation between the two channels and that corresponds to the HaTx/GrTx binding site on voltage-gated K+ and Ca2+ channels.55 The region conserved in Cav3 (T-type) Ca2+ channels contains three glutamate residues [Glu1661, Glu1662, and Glu1664 for Cav3.1 (Figure 7A)]. Notably, the region in the Cav3 (T-type) Ca2+ channel makes a larger negative patch than in Na+, K+, or other types of Ca2+ channels, and this negative domain is conserved in all Cav3 (T-type) Ca2+ channel subtypes. As mentioned, kurtoxin is highly electropositive because of the presence of an electropositive patch formed by Lys13, Arg14, and five positively charged residues in the C-terminal segment (Arg54, Lys56, Arg57, Arg60, and Arg62). We therefore speculate that the positively charged surface of kurtoxin is an important determinant of its binding to the conserved negative domain in Cav3 (T-type) Ca2+ channels. Figure 7B shows a comparison of the surface profiles of kurtoxin and three scorpion α-toxins. Kurtoxin exhibits surface characteristics arising from a surface hydrophobic patch in combination with a large electropositive patch. By contrast, scorpion α-toxins show a rather small surface hydrophobic patch with a mixed charged surface. These distinct surface profiles may explain why only kurtoxin is able to interact with the binding domain on Cav3 (T-type) Ca2+ channels, which is composed of electronegative and hydrophobic residues. We are currently in the process of preparing alanine mutants of kurtoxin and the Cav3.1 channel to examine the molecular basis of the interaction between the toxin and channel.
Figure 7.
Comparison of the amino acid sequences of the indicated voltage-gated ion channels and the surface profiles of kurtoxin and scorpion α-toxins. (A) Comparison of the amino acid sequences of the domain IV S3–S4 linker in different voltage-gated ion channels (Cav, voltage-gated calcium channels; Nav, voltage-gated sodium channels; and Kv2.1, voltage-gated potassium channels). These sequences were aligned using ClustalX. Highly conserved residues are shaded in black or gray. The red rectangular boxes highlight the proposed kurtoxin binding site on Cav3 (T-type) Ca2+ channels. The blue rectangular boxes highlight the sequence involved in the binding sites of gating modifiers (Ile273, Phe274, and Glu277 in K+ channels; Glu1613 in Na+ channels; and Glu1658 in P/Q-type Ca2+ channels). (B) Surface profiles of kurtoxin, AaH II, LqqhαIT, and Bmk M1: yellow for hydrophobic residues (Ala, Cys, Gly, Leu, Ile, Phe, Pro, Trp, Tyr and Val), blue and red for basic (Arg and Lys) and acidic (Asp and Glu) residues, respectively, and white for other residues. The surface hydrophobic patch residues and charged residues are indicated.
Conclusion
We investigated the three-dimensional structure of the first peptide toxin known to inhibit Cav3 (T-type) voltage-gated Ca2+ channels and suggest that its unique surface properties are likely responsible for its binding selectivity. Interestingly, kurtoxin can interact with high affinity with native neuronal high-threshold L-type, N-type, and P-type Ca2+ channels in central and peripheral neurons, producing complex gating modifications specific to each channel type.56 When the channels are expressed in Xenopus oocytes, however, kurtoxin interacts only with the α-subunit of Cav3.1 (α1G T-type) voltage-gated Ca2+ channels; it does not interact with any other type of Ca2+ channel.55 At present, the mechanism by which kurtoxin interacts with Ca2+ channels remains unknown. The structural studies of kurtoxin reported here provide clues about the molecular mechanism by which Cav3 (T-type) Ca2+ channel activity is regulated by selective ligands and could contribute to the development of highly specific Cav3 (T-type) Ca2+ channel inhibitors.
Acknowledgments
We are grateful to Dr. Chul-Seung Park for critical reading of the manuscript.
Glossary
Abbreviations
- CD
circular dichroism
- DSS
4,4-dimethyl-4-silapentane-1-sulfonic acid
- DQF-COSY
double-quantum-filtered correlation spectroscopy
- E-COSY
exclusive COSY
- HSQC
heteronuclear single-quantum coherence
- MALDI-TOF MS
matrix-assisted laser desorption ionization time-of-flight mass spectrometry
- NMR
nuclear magnetic resonance
- NOE
nuclear Overhauser effect
- NOESY
nuclear Overhauser effect spectroscopy
- PDB
Protein Data Bank
- RP-HPLC
reverse phase high-performance liquid chromatography
- TOCSY
total correlated spectroscopy.
Supporting Information Available
Figures S1–S3. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Accession Codes
The coordinates for kurtoxin have been deposited in the Protein Data Bank as entry 1T1T.
This research was supported by grants from the Next-Generation BioGreen 21 Program (PJ008158); the Rural Development Administration, Republic of Korea; a National Research Foundation of Korea grant funded by the Korean Government (MEST) (NRF-C1ABA001-2011-0018559); the Brain Research Center of the 21st Century Frontier Research Program (M103KV010005-06K2201-00610); and the BioImaging Research Center at the Gwangju Institute of Science and Technology and Basic Research Projects in High-tech Industrial Technology funded by the Gwangju Institute of Science and Technology in 2011.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Doyle D. A.; Morais Cabral J.; Pfuetzner R. A.; Kuo A.; Gulbis J. M.; Cohen S. L.; Chait B. T.; MacKinnon R. (1998) The structure of the potassium channel: Molecular basis of K+ conduction and selectivity. Science 280, 69–77. [DOI] [PubMed] [Google Scholar]
- Kubo Y.; Baldwin T. J.; Jan Y. N.; Jan L. Y. (1993) Primary structure and functional expression of a mouse inward rectifier potassium channel. Nature 362, 127–133. [DOI] [PubMed] [Google Scholar]
- Lu Z.; Klem A. M.; Ramu Y. (2001) Ion conduction pore is conserved among potassium channels. Nature 413, 809–813. [DOI] [PubMed] [Google Scholar]
- Jiang Y.; Lee A.; Chen J.; Ruta V.; Cadene M.; Chait B. T.; MacKinnon R. (2003) X-ray structure of a voltage-dependent K+ channel. Nature 423, 33–41. [DOI] [PubMed] [Google Scholar]
- Possani L. D.; Merino E.; Corona M.; Bolivar F.; Becerril B. (2000) Peptides and genes coding for scorpion toxins that affect ion-channels. Biochimie 82, 861–868. [DOI] [PubMed] [Google Scholar]
- Rash L. D.; Hodgson W. C. (2002) Pharmacology and biochemistry of spider venoms. Toxicon 40, 225–254. [DOI] [PubMed] [Google Scholar]
- Terlau H.; Olivera B. M. (2004) Conus venoms: A rich source of novel ion channel-targeted peptides. Physiol. Rev. 84, 41–68. [DOI] [PubMed] [Google Scholar]
- Norton R. S.; McDonough S. I. (2008) Peptides targeting voltage-gated calcium channels. Curr. Pharm. Des. 14, 2480–2491. [DOI] [PubMed] [Google Scholar]
- French R. J.; Dudley S. C. Jr. (1999) Pore-blocking toxins as probes of voltage-dependent channels. Methods Enzymol. 294, 575–605. [DOI] [PubMed] [Google Scholar]
- Bontems F.; Roumestand C.; Gilquin B.; Menez A.; Toma F. (1991) Refined structure of charybdotoxin: Common motifs in scorpion toxins and insect defensins. Science 254, 1521–1523. [DOI] [PubMed] [Google Scholar]
- Davis J. H.; Bradley E. K.; Miljanich G. P.; Nadasdi L.; Ramachandran J.; Basus V. J. (1993) Solution structure of ω-conotoxin GVIA using 2-D NMR spectroscopy and relaxation matrix analysis. Biochemistry 32, 7396–7405. [DOI] [PubMed] [Google Scholar]
- Farr-Jones S.; Miljanich G. P.; Nadasdi L.; Ramachandran J.; Basus V. J. (1995) Solution structure of ω-conotoxin MVIIC, a high affinity ligand of P-type calcium channels, using 1H NMR spectroscopy and complete relaxation matrix analysis. J. Mol. Biol. 248, 106–124. [DOI] [PubMed] [Google Scholar]
- Hill J. M.; Alewood P. F.; Craik D. J. (1996) Three-dimensional solution structure of mu-conotoxin GIIIB, a specific blocker of skeletal muscle sodium channels. Biochemistry 35, 8824–8835. [DOI] [PubMed] [Google Scholar]
- Kohno T.; Kim J. I.; Kobayashi K.; Kodera Y.; Maeda T.; Sato K. (1995) Three-dimensional structure in solution of the calcium channel blocker ω-conotoxin MVIIA. Biochemistry 34, 10256–10265. [DOI] [PubMed] [Google Scholar]
- Ott K. H.; Becker S.; Gordon R. D.; Ruterjans H. (1991) Solution structure of μ-conotoxin GIIIA analysed by 2D-NMR and distance geometry calculations. FEBS Lett. 278, 160–166. [DOI] [PubMed] [Google Scholar]
- Li-Smerin Y.; Swartz K. J. (2000) Localization and molecular determinants of the Hanatoxin receptors on the voltage-sensing domains of a K+ channel. J. Gen. Physiol. 115, 673–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J. C.; Qu Y.; Tanada T. N.; Scheuer T.; Catterall W. A. (1996) Molecular determinants of high affinity binding of α-scorpion toxin and sea anemone toxin in the S3-S4 extracellular loop in domain IV of the Na+ channel α subunit. J. Biol. Chem. 271, 15950–15962. [DOI] [PubMed] [Google Scholar]
- Swartz K. J.; MacKinnon R. (1997) Hanatoxin modifies the gating of a voltage-dependent K+ channel through multiple binding sites. Neuron 18, 665–673. [DOI] [PubMed] [Google Scholar]
- Winterfield J. R.; Swartz K. J. (2000) A hot spot for the interaction of gating modifier toxins with voltage-dependent ion channels. J. Gen. Physiol. 116, 637–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W. A.; Cestele S.; Yarov-Yarovoy V.; Yu F. H.; Konoki K.; Scheuer T. (2007) Voltage-gated ion channels and gating modifier toxins. Toxicon 49, 124–141. [DOI] [PubMed] [Google Scholar]
- McDonough S. I. (2007) Gating modifier toxins of voltage-gated calcium channels. Toxicon 49, 202–212. [DOI] [PubMed] [Google Scholar]
- Cahalan M. D. (1975) Modification of sodium channel gating in frog myelinated nerve fibres by Centruroides sculpturatus scorpion venom. J. Physiol. 244, 511–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cestele S.; Ben Khalifa R. B.; Pelhate M.; Rochat H.; Gordon D. (1995) α-Scorpion toxins binding on rat brain and insect sodium channels reveal divergent allosteric modulations by brevetoxin and veratridine. J. Biol. Chem. 270, 15153–15161. [DOI] [PubMed] [Google Scholar]
- Fainzilber M.; Kofman O.; Zlotkin E.; Gordon D. (1994) A new neurotoxin receptor site on sodium channels is identified by a conotoxin that affects sodium channel inactivation in molluscs and acts as an antagonist in rat brain. J. Biol. Chem. 269, 2574–2580. [PubMed] [Google Scholar]
- Meves H.; Simard J. M.; Watt D. D. (1986) Interactions of scorpion toxins with the sodium channel. Ann. N.Y. Acad. Sci. 479, 113–132. [DOI] [PubMed] [Google Scholar]
- Norton R. S. (1991) Structure and structure-function relationships of sea anemone proteins that interact with the sodium channel. Toxicon 29, 1051–1084. [DOI] [PubMed] [Google Scholar]
- Shon K. J.; Hasson A.; Spira M. E.; Cruz L. J.; Gray W. R.; Olivera B. M. (1994) δ-Conotoxin GmVIA, a novel peptide from the venom of Conus gloriamaris. Biochemistry 33, 11420–11425. [DOI] [PubMed] [Google Scholar]
- Strichartz G. R.; Wang G. K. (1986) Rapid voltage-dependent dissociation of scorpion α-toxins coupled to Na channel inactivation in amphibian myelinated nerves. J. Gen. Physiol. 88, 413–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvin L.; De E.; Cosette P.; Gagnon J.; Molle G.; Lange C. (1999) Isolation, amino acid sequence and functional assays of SGTx1. The first toxin purified from the venom of the spider scodra griseipes. Eur. J. Biochem. 265, 572–579. [DOI] [PubMed] [Google Scholar]
- Ruta V.; Jiang Y.; Lee A.; Chen J.; MacKinnon R. (2003) Functional analysis of an archaebacterial voltage-dependent K+ channel. Nature 422, 180–185. [DOI] [PubMed] [Google Scholar]
- Swartz K. J.; MacKinnon R. (1995) An inhibitor of the Kv2.1 potassium channel isolated from the venom of a Chilean tarantula. Neuron 15, 941–949. [DOI] [PubMed] [Google Scholar]
- Herrington J. (2007) Gating modifier peptides as probes of pancreatic β-cell physiology. Toxicon 49, 231–238. [DOI] [PubMed] [Google Scholar]
- Herrington J.; Zhou Y. P.; Bugianesi R. M.; Dulski P. M.; Feng Y.; Warren V. A.; Smith M. M.; Kohler M. G.; Garsky V. M.; Sanchez M.; Wagner M.; Raphaelli K.; Banerjee P.; Ahaghotu C.; Wunderler D.; Priest B. T.; Mehl J. T.; Garcia M. L.; McManus O. B.; Kaczorowski G. J.; Slaughter R. S. (2006) Blockers of the delayed-rectifier potassium current in pancreatic β-cells enhance glucose-dependent insulin secretion. Diabetes 55, 1034–1042. [DOI] [PubMed] [Google Scholar]
- Lampe R. A.; Defeo P. A.; Davison M. D.; Young J.; Herman J. L.; Spreen R. C.; Horn M. B.; Mangano T. J.; Keith R. A. (1993) Isolation and pharmacological characterization of ω-grammotoxin SIA, a novel peptide inhibitor of neuronal voltage-sensitive calcium channel responses. Mol. Pharmacol. 44, 451–460. [PubMed] [Google Scholar]
- McDonough S. I.; Lampe R. A.; Keith R. A.; Bean B. P. (1997) Voltage-dependent inhibition of N- and P-type calcium channels by the peptide toxin ω-grammotoxin-SIA. Mol. Pharmacol. 52, 1095–1104. [DOI] [PubMed] [Google Scholar]
- Mintz I. M.; Adams M. E.; Bean B. P. (1992) P-type calcium channels in rat central and peripheral neurons. Neuron 9, 85–95. [DOI] [PubMed] [Google Scholar]
- Mintz I. M.; Venema V. J.; Swiderek K. M.; Lee T. D.; Bean B. P.; Adams M. E. (1992) P-type calcium channels blocked by the spider toxin ω-Aga-IVA. Nature 355, 827–829. [DOI] [PubMed] [Google Scholar]
- Li-Smerin Y.; Swartz K. J. (1998) Gating modifier toxins reveal a conserved structural motif in voltage-gated Ca2+ and K+ channels. Proc. Natl. Acad. Sci. U.S.A. 95, 8585–8589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. M.; Roh S. H.; Kim S.; Lee C. W.; Kim J. I.; Swartz K. J. (2004) Molecular surface of tarantula toxins interacting with voltage sensors in K(v) channels. J. Gen. Physiol. 123, 455–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz K. J. (2007) Tarantula toxins interacting with voltage sensors in potassium channels. Toxicon 49, 213–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milescu M.; Bosmans F.; Lee S.; Alabi A. A.; Kim J. I.; Swartz K. J. (2009) Interactions between lipids and voltage sensor paddles detected with tarantula toxins. Nat. Struct. Mol. Biol. 16, 1080–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alabi A. A.; Bahamonde M. I.; Jung H. J.; Kim J. I.; Swartz K. J. (2007) Portability of paddle motif function and pharmacology in voltage sensors. Nature 450, 370–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y.; Ruta V.; Chen J.; Lee A.; MacKinnon R. (2003) The principle of gating charge movement in a voltage-dependent K+ channel. Nature 423, 42–48. [DOI] [PubMed] [Google Scholar]
- Long S. B.; Tao X.; Campbell E. B.; MacKinnon R. (2007) Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment. Nature 450, 376–382. [DOI] [PubMed] [Google Scholar]
- Milescu M.; Vobecky J.; Roh S. H.; Kim S. H.; Jung H. J.; Kim J. I.; Swartz K. J. (2007) Tarantula toxins interact with voltage sensors within lipid membranes. J. Gen. Physiol. 130, 497–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Reyes E. (2003) Molecular physiology of low-voltage-activated t-type calcium channels. Physiol. Rev. 83, 117–161. [DOI] [PubMed] [Google Scholar]
- Chen C. F.; Corbley M. J.; Roberts T. M.; Hess P. (1988) Voltage-sensitive calcium channels in normal and transformed 3T3 fibroblasts. Science 239, 1024–1026. [DOI] [PubMed] [Google Scholar]
- Cohen C. J.; McCarthy R. T.; Barrett P. Q.; Rasmussen H. (1988) Ca channels in adrenal glomerulosa cells: K+ and angiotensin II increase T-type Ca channel current. Proc. Natl. Acad. Sci. U.S.A. 85, 2412–2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huguenard J. R. (1996) Low-threshold calcium currents in central nervous system neurons. Annu. Rev. Physiol. 58, 329–348. [DOI] [PubMed] [Google Scholar]
- Steriade M.; Llinas R. R. (1988) The functional states of the thalamus and the associated neuronal interplay. Physiol. Rev. 68, 649–742. [DOI] [PubMed] [Google Scholar]
- Iftinca M. C.; Zamponi G. W. (2009) Regulation of neuronal T-type calcium channels. Trends Pharmacol. Sci. 30, 32–40. [DOI] [PubMed] [Google Scholar]
- Nelson M. T.; Todorovic S. M.; Perez-Reyes E. (2006) The role of T-type calcium channels in epilepsy and pain. Curr. Pharm. Des. 12, 2189–2197. [DOI] [PubMed] [Google Scholar]
- Lory P.; Chemin J. (2007) Towards the discovery of novel T-type calcium channel blockers. Expert Opin. Ther. Targets 11, 717–722. [DOI] [PubMed] [Google Scholar]
- Heady T. N.; Gomora J. C.; Macdonald T. L.; Perez-Reyes E. (2001) Molecular pharmacology of T-type Ca2+ channels. Jpn. J. Pharmacol. 85, 339–350. [DOI] [PubMed] [Google Scholar]
- Chuang R. S.; Jaffe H.; Cribbs L.; Perez-Reyes E.; Swartz K. J. (1998) Inhibition of T-type voltage-gated calcium channels by a new scorpion toxin. Nat. Neurosci. 1, 668–674. [DOI] [PubMed] [Google Scholar]
- Sidach S. S.; Mintz I. M. (2002) Kurtoxin, a gating modifier of neuronal high- and low-threshold ca channels. J. Neurosci. 22, 2023–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. W.; Kim S.; Roh S. H.; Endoh H.; Kodera Y.; Maeda T.; Kohno T.; Wang J. M.; Swartz K. J.; Kim J. I. (2004) Solution structure and functional characterization of SGTx1, a modifier of Kv2.1 channel gating. Biochemistry 43, 890–897. [DOI] [PubMed] [Google Scholar]
- Takahashi H.; Kim J. I.; Min H. J.; Sato K.; Swartz K. J.; Shimada I. (2000) Solution structure of hanatoxin1, a gating modifier of voltage-dependent K+ channels: Common surface features of gating modifier toxins. J. Mol. Biol. 297, 771–780. [DOI] [PubMed] [Google Scholar]
- Lee C. W.; Eu Y. J.; Min H. J.; Cho E. M.; Lee J. H.; Kim H. H.; Nah S. Y.; Swartz K. J.; Kim J. I. (2011) Expression and characterization of recombinant kurtoxin, an inhibitor of T-type voltage-gated calcium channels. Biochem. Biophys. Res. Commun. 416, 277–282. [DOI] [PubMed] [Google Scholar]
- Bax A.; Davis D. G. (1985) MLEV-17-based two-dimensional homonuclear magnetization transfer spectroscopy. J. Magn. Reson. 65, 355–360. [Google Scholar]
- Jeener J.; Meier B. H.; Bachmann P.; Ernst R. R. (1979) Investigation of exchange processes by two-dimensional NMR spectroscopy. J. Chem. Phys. 71, 4546–4553. [Google Scholar]
- Macura S.; Huang Y.; Suter D.; Ernst R. R. (1981) Two-Dimensional Chemical Exchange and Cross-Relaxation Spectroscopy of Coupled Nuclear Spins. J. Magn. Reson. 43, 259–281. [Google Scholar]
- Piotto M.; Saudek V.; Sklenar V. (1992) Gradient-tailored excitation for single-quantum NMR spectroscopy of aqueous solutions. J. Biomol. NMR 2, 661–665. [DOI] [PubMed] [Google Scholar]
- Griesinger C.; Sørensen O. W.; Ernst R. R. (1987) Practical Aspects of the E-Cosy Technique: Measurement of Scalar Spin Spin Coupling-Constants in Peptides. J. Magn. Reson. 75, 474–492. [Google Scholar]
- Grzesiek S.; Bax A. (1992) Correlating backbone amide and side chain resonances in larger proteins by multiple relayed triple resonance NMR. J. Am. Chem. Soc. 114, 6291–6293. [Google Scholar]
- Kay L. E.; Ikura M.; Tschudin R.; Bax A. (1990) Three-dimensional triple-resonance NMR spectroscopy of isotopically enriched proteins. J. Magn. Reson. 89, 496–514. [DOI] [PubMed] [Google Scholar]
- Muhandiram D. R.; Kay L. E. (1994) Gradient-enhanced triple-resonance three-dimensional NMR experiments with improved sensitivity. J. Magn. Reson., Ser. B 103, 203–216. [Google Scholar]
- Wittekind M.; Mueller L. (1993) HNCACB, a high-sensititity 3D NMR experiment to correlate amide-proton and nitrogen resonances with the α- and β-carbon resonaces in proteins. J. Magn. Reson. 101, 201–205. [Google Scholar]
- Bodenhausen G.; Ruben D. J. (1980) Natural abundance nitrogen-15 NMR by enhanced heteronuclear spectroscopy. Chem. Phys. Lett. 69, 185–189. [Google Scholar]
- Vuister G. W.; Bax A. (1993) Quantitative J Correlation: A New Approach for Measuring Homonuclear 3-Bond J(H(N)H(α) Coupling-Constants in N-15-Enriched Proteins. J. Am. Chem. Soc. 115, 7772–7777. [Google Scholar]
- Kraulis P. J.; Domaille P. J.; Campbell-Burk S. L.; Van Aken T.; Laue E. D. (1994) Solution structure and dynamics of ras p21.GDP determined by heteronuclear three- and four-dimensional NMR spectroscopy. Biochemistry 33, 3515–3531. [DOI] [PubMed] [Google Scholar]
- Kline A. D.; Braun W.; Wuthrich K. (1988) Determination of the complete three-dimensional structure of the α-amylase inhibitor Tendamistat in aqueous solution by nuclear magnetic resonance and distance geometry. J. Mol. Biol. 204, 675–724. [DOI] [PubMed] [Google Scholar]
- Pardi A.; Billeter M.; Wuthrich K. (1984) Calibration of the angular dependence of the amide proton-C α proton coupling constants, 3JHNα, in a globular protein. Use of 3JHNα for identification of helical secondary structure. J. Mol. Biol. 180, 741–751. [DOI] [PubMed] [Google Scholar]
- Hyberts S. G.; Marki W.; Wagner G. (1987) Stereospecific assignments of side-chain protons and characterization of torsion angles in Eglin c. Eur. J. Biochem. 164, 625–635. [DOI] [PubMed] [Google Scholar]
- Wagner G.; Braun W.; Havel T. F.; Schaumann T.; Go N.; Wuthrich K. (1987) Protein structures in solution by nuclear magnetic resonance and distance geometry. The polypeptide fold of the basic pancreatic trypsin inhibitor determined using two different algorithms, DISGEO and DISMAN. J. Mol. Biol. 196, 611–639. [DOI] [PubMed] [Google Scholar]
- Wuthrich K.; Billeter M.; Braun W. (1983) Pseudo-structures for the 20 common amino acids for use in studies of protein conformations by measurements of intramolecular proton-proton distance constraints with nuclear magnetic resonance. J. Mol. Biol. 169, 949–961. [DOI] [PubMed] [Google Scholar]
- Clore G. M.; Gronenborn A. M.; Nilges M.; Ryan C. A. (1987) Three-dimensional structure of potato carboxypeptidase inhibitor in solution. A study using nuclear magnetic resonance, distance geometry, and restrained molecular dynamics. Biochemistry 26, 8012–8023. [DOI] [PubMed] [Google Scholar]
- Nilges M.; Gronenborn A. M.; Brunger A. T.; Clore G. M. (1988) Determination of three-dimensional structures of proteins by simulated annealing with interproton distance restraints. Application to crambin, potato carboxypeptidase inhibitor and barley serine proteinase inhibitor 2. Protein Eng. 2, 27–38. [DOI] [PubMed] [Google Scholar]
- Fletcher J. I.; Chapman B. E.; Mackay J. P.; Howden M. E.; King G. F. (1997) The structure of versutoxin (δ-atracotoxin-Hv1) provides insights into the binding of site 3 neurotoxins to the voltage-gated sodium channel. Structure 5, 1525–1535. [DOI] [PubMed] [Google Scholar]
- Fletcher J. I.; Smith R.; O’Donoghue S. I.; Nilges M.; Connor M.; Howden M. E.; Christie M. J.; King G. F. (1997) The structure of a novel insecticidal neurotoxin, ω-atracotoxin-HV1, from the venom of an Australian funnel web spider. Nat. Struct. Biol. 4, 559–566. [DOI] [PubMed] [Google Scholar]
- Brunger A. T. (1992) X-PLOR Manual, version 3.1, Yale University, New Haven, CT. [Google Scholar]
- Laskowski R. A.; Rullmannn J. A.; MacArthur M. W.; Kaptein R.; Thornton J. M. (1996) AQUA and PROCHECK-NMR: Programs for checking the quality of protein structures solved by NMR. J. Biomol. NMR 8, 477–486. [DOI] [PubMed] [Google Scholar]
- Hutchinson E. G.; Thornton J. M. (1996) PROMOTIF: A program to identify and analyze structural motifs in proteins. Protein Sci. 5, 212–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koradi R.; Billeter M.; Wuthrich K. (1996) MOLMOL: A program for display and analysis of macromolecular structures. J. Mol. Graphics 14, 51–55, 29–32. [DOI] [PubMed] [Google Scholar]
- Chen V. B.; Arendall W. B. III; Headd J. J.; Keedy D. A.; Immormino R. M.; Kapral G. J.; Murray L. W.; Richardson J. S.; Richardson D. C. (2010) MolProbity: All-atom structure validation for macromolecular crystallography. Acta Crystallogr. D66, 12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis I. W.; Leaver-Fay A.; Chen V. B.; Block J. N.; Kapral G. J.; Wang X.; Murray L. W.; Arendall W. B. III; Snoeyink J.; Richardson J. S.; Richardson D. C. (2007) MolProbity: All-atom contacts and structure validation for proteins and nucleic acids. Nucleic Acids Res. 35, W375–W383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyberts S. G.; Goldberg M. S.; Havel T. F.; Wagner G. (1992) The solution structure of eglin c based on measurements of many NOEs and coupling constants and its comparison with X-ray structures. Protein Sci. 1, 736–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitke J. L.; Stern L. J.; Klibanov A. M. (1998) Comparison of X-ray crystal structures of an acyl-enzyme intermediate of subtilisin Carlsberg formed in anhydrous acetonitrile and in water. Proc. Natl. Acad. Sci. U.S.A. 95, 12918–12923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morellet N.; Bouaziz S.; Petitjean P.; Roques B. P. (2003) NMR structure of the HIV-1 regulatory protein VPR. J. Mol. Biol. 327, 215–227. [DOI] [PubMed] [Google Scholar]
- Zhu J.; Tong X.; Cao C.; Wu G.; Zhang N.; Wu H. (2010) Solution structure of BmKαTx11, a toxin from the venom of the Chinese scorpion Buthus martensii Karsch. Biochem. Biophys. Res. Commun. 391, 627–633. [DOI] [PubMed] [Google Scholar]
- Zhao B.; Carson M.; Ealick S. E.; Bugg C. E. (1992) Structure of scorpion toxin variant-3 at 1.2 Å resolution. J. Mol. Biol. 227, 239–252. [DOI] [PubMed] [Google Scholar]
- Housset D.; Habersetzer-Rochat C.; Astier J. P.; Fontecilla-Camps J. C. (1994) Crystal structure of toxin II from the scorpion Androctonus australis Hector refined at 1.3 Å resolution. J. Mol. Biol. 238, 88–103. [DOI] [PubMed] [Google Scholar]
- Lebreton F.; Delepierre M.; Ramirez A. N.; Balderas C.; Possani L. D. (1994) Primary and NMR three-dimensional structure determination of a novel crustacean toxin from the venom of the scorpion Centruroides limpidus limpidus Karsch. Biochemistry 33, 11135–11149. [DOI] [PubMed] [Google Scholar]
- Lee W.; Moore C. H.; Watt D. D.; Krishna N. R. (1994) Solution structure of the variant-3 neurotoxin from Centruroides sculpturatus Ewing. Eur. J. Biochem. 219, 89–95. [DOI] [PubMed] [Google Scholar]
- Jablonsky M. J.; Watt D. D.; Krishna N. R. (1995) Solution structure of an Old World-like neurotoxin from the venom of the New World scorpion Centruroides sculpturatus Ewing. J. Mol. Biol. 248, 449–458. [DOI] [PubMed] [Google Scholar]
- Li H. M.; Wang D. C.; Zeng Z. H.; Jin L.; Hu R. Q. (1996) Crystal structure of an acidic neurotoxin from scorpion Buthus martensii Karsch at 1.85 Å resolution. J. Mol. Biol. 261, 415–431. [DOI] [PubMed] [Google Scholar]
- Landon C.; Sodano P.; Cornet B.; Bonmatin J. M.; Kopeyan C.; Rochat H.; Vovelle F.; Ptak M. (1997) Refined solution structure of the anti-mammal and anti-insect LqqIII scorpion toxin: Comparison with other scorpion toxins. Proteins 28, 360–374. [DOI] [PubMed] [Google Scholar]
- Tugarinov V.; Kustanovich I.; Zilberberg N.; Gurevitz M.; Anglister J. (1997) Solution structures of a highly insecticidal recombinant scorpion α-toxin and a mutant with increased activity. Biochemistry 36, 2414–2424. [DOI] [PubMed] [Google Scholar]
- He X. L.; Li H. M.; Zeng Z. H.; Liu X. Q.; Wang M.; Wang D. C. (1999) Crystal structures of two α-like scorpion toxins: Non-proline cis peptide bonds and implications for new binding site selectivity on the sodium channel. J. Mol. Biol. 292, 125–135. [DOI] [PubMed] [Google Scholar]
- Krimm I.; Gilles N.; Sautiere P.; Stankiewicz M.; Pelhate M.; Gordon D.; Lancelin J. M. (1999) NMR structures and activity of a novel α-like toxin from the scorpion Leiurus quinquestriatus hebraeus. J. Mol. Biol. 285, 1749–1763. [DOI] [PubMed] [Google Scholar]
- Jablonsky M. J.; Jackson P. L.; Krishna N. R. (2001) Solution structure of an insect-specific neurotoxin from the New World scorpion Centruroides sculpturatus Ewing. Biochemistry 40, 8273–8282. [DOI] [PubMed] [Google Scholar]
- Cornet B.; Bonmatin J. M.; Hetru C.; Hoffmann J. A.; Ptak M.; Vovelle F. (1995) Refined three-dimensional solution structure of insect defensin A. Structure 3, 435–448. [DOI] [PubMed] [Google Scholar]
- Kahn R.; Karbat I.; Ilan N.; Cohen L.; Sokolov S.; Catterall W. A.; Gordon D.; Gurevitz M. (2009) Molecular Requirements for Recognition of Brain Voltage-gated Sodium Channels by Scorpion α-Toxins. J. Biol. Chem. 284, 20684–20691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberberg N.; Froy O.; Loret E.; Cestele S.; Arad D.; Gordon D.; Gurevitz M. (1997) Identification of structural elements of a scorpion α-neurotoxin important for receptor site recognition. J. Biol. Chem. 272, 14810–14816. [DOI] [PubMed] [Google Scholar]
- Sun Y. M.; Bosmans F.; Zhu R. H.; Goudet C.; Xiong Y. M.; Tytgat J.; Wang D. C. (2003) Importance of the conserved aromatic residues in the scorpion α-like toxin BmK M1: The hydrophobic surface region revisited. J. Biol. Chem. 278, 24125–24131. [DOI] [PubMed] [Google Scholar]
- Wang C. G.; Gilles N.; Hamon A.; Le Gall F.; Stankiewicz M.; Pelhate M.; Xiong Y. M.; Wang D. C.; Chi C. W. (2003) Exploration of the functional site of a scorpion α-like toxin by site-directed mutagenesis. Biochemistry 42, 4699–4708. [DOI] [PubMed] [Google Scholar]
- Ye X.; Bosmans F.; Li C.; Zhang Y.; Wang D. C.; Tytgat J. (2005) Structural basis for the voltage-gated Na+ channel selectivity of the scorpion α-like toxin BmK M1. J. Mol. Biol. 353, 788–803. [DOI] [PubMed] [Google Scholar]
- Fontecilla-Camps J. C.; Habersetzer-Rochat C.; Rochat H. (1988) Orthorhombic crystals and three-dimensional structure of the potent toxin II from the scorpion Androctonus australis Hector. Proc. Natl. Acad. Sci. U.S.A. 85, 7443–7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. M.; Zhao T.; Jin L.; Wang M.; Zhang Y.; Wang D. C. (1999) A series of bioactivity-variant neurotoxins from scorpion Buthus martensii Karsch: Purification, crystallization and crystallographic analysis. Acta Crystallogr. D55, 341–344. [DOI] [PubMed] [Google Scholar]
- Burley S. K.; Petsko G. A. (1985) Aromatic-aromatic interaction: A mechanism of protein structure stabilization. Science 229, 23–28. [DOI] [PubMed] [Google Scholar]
- Swartz K. J.; MacKinnon R. (1997) Mapping the receptor site for hanatoxin, a gating modifier of voltage-dependent K+ channels. Neuron 18, 675–682. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.