Abstract
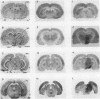
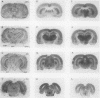
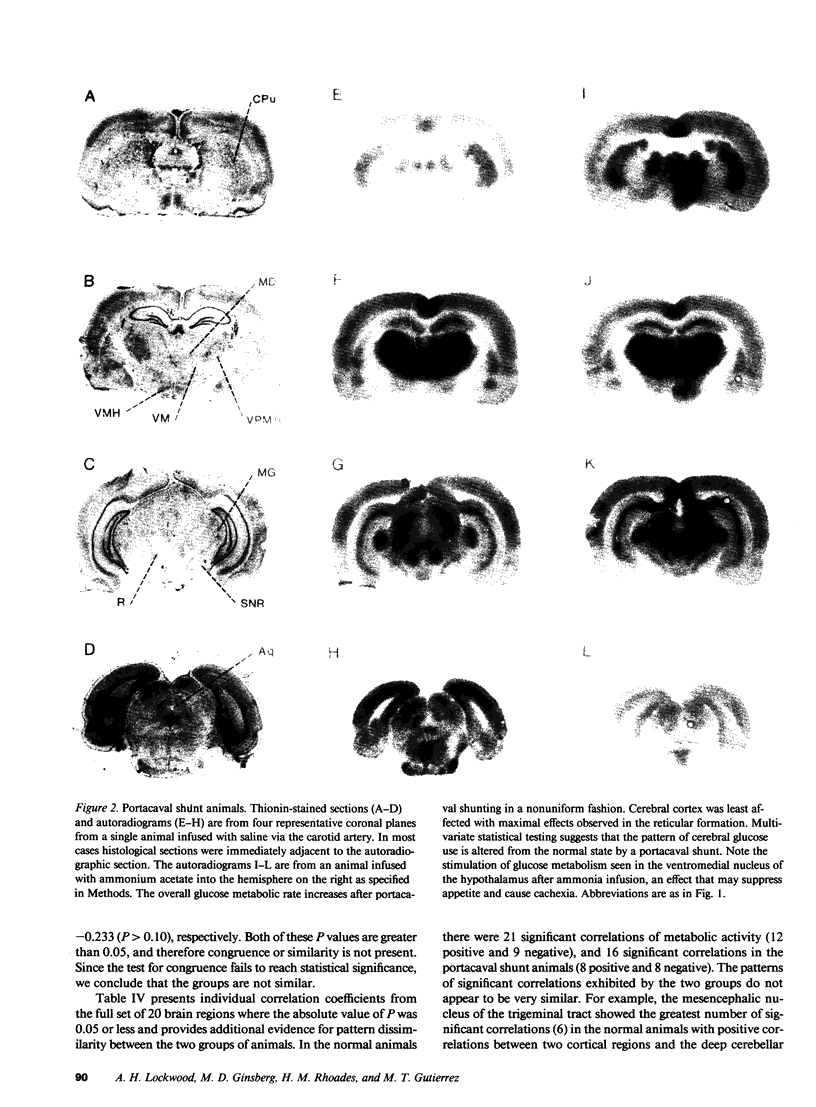
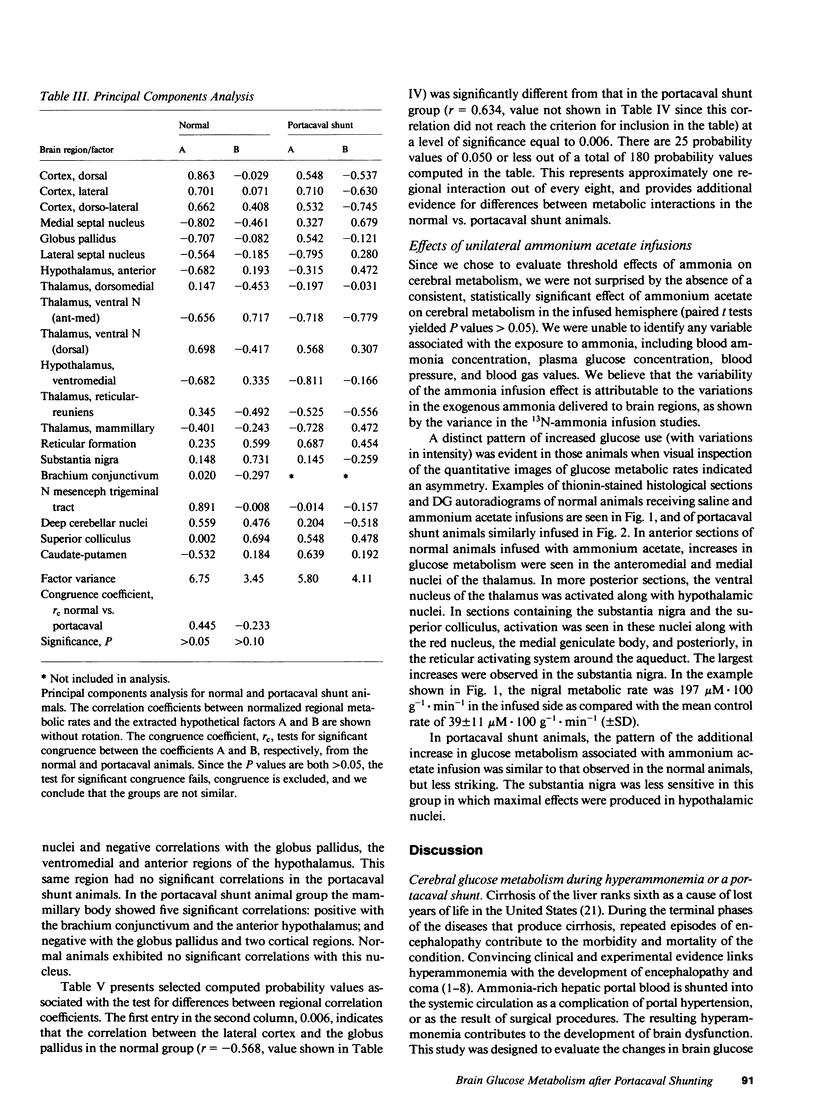
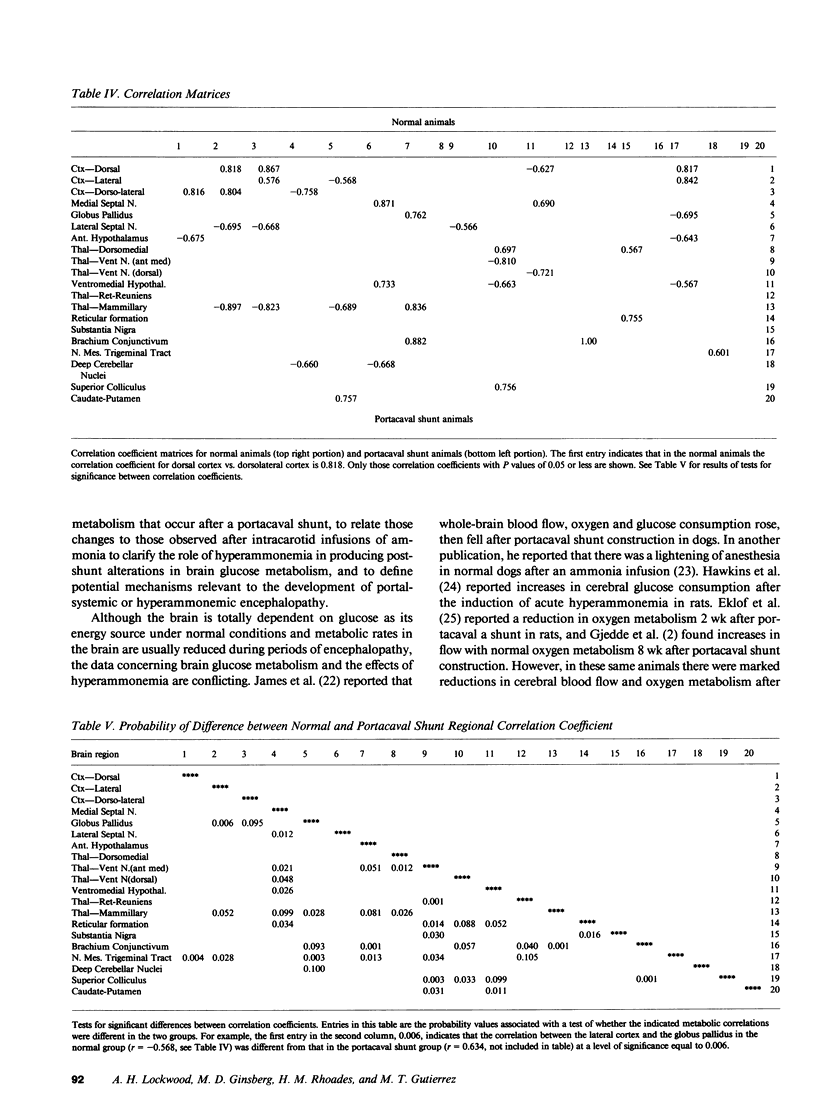
The regional cerebral metabolic rate for glucose was measured in normal and portacaval shunted rats and the effects of unilateral carotid infusions of "threshold" amounts of ammonia were assessed. 8 wk after shunting the glucose metabolic rate was increased in all 20 brain regions sampled. Effects on subcortical and phylogenetically older regions of the brain were most pronounced with a 74% increase observed in the reticular formation at the collicular level. Increases in the cerebral cortex ranged from 12 to 18%. Unilateral infusions of ammonia did not affect behavior but altered the electroencephalogram and selectively increased the glucose metabolic rate in the thalamus, hypothalamus, and substantia nigra in half of the animals, a pattern similar to that seen after a portacaval shunt, suggesting hyperammonemia as the cause of postshunt increases in glucose metabolism. Visual inspection of autoradiograms, computed correlation coefficients relating interregional metabolism, and principal component analysis suggest that normal cerebral metabolic and functional interrelationships are altered by shunting. Ammonia stimulation of the hypothalamic satiety centers may suppress appetite and lead to cachexia. Reductions in the ammonia detoxification capacity of skeletal muscle may increase the probability of developing future episodes of hyperammonemia, perpetuating the process. Direct effects of ammonia on specific brain centers such as the dorsomedial hypothalamus and reticular activating system may combine with global disruptions of cerebral metabolic-functional relationships to produce the protean manifestations of portal-systemic encephalopathy.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANAND B. K., BROBECK J. R. Hypothalamic control of food intake in rats and cats. Yale J Biol Med. 1951 Nov;24(2):123–140. [PMC free article] [PubMed] [Google Scholar]
- Bianchi Porro G., Maiolo A. T., Della Porta P. Cerebral blood flow and metabolism in hepatic cirrhosis before and after portacaval shunt operation. Gut. 1969 Nov;10(11):894–897. doi: 10.1136/gut.10.11.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz N. F., Duffy T. E. Local cerebral glucose metabolism in rats with chronic portacaval shunts. J Cereb Blood Flow Metab. 1983 Sep;3(3):311–320. doi: 10.1038/jcbfm.1983.46. [DOI] [PubMed] [Google Scholar]
- Eklöf B., Holmin T., Jóhannsson H., Siesjö B. K. Cerebral blood flow and cerebral metabolic rate for oxygen in rats with porta-caval anastomosis. Acta Physiol Scand. 1974 Feb;90(2):337–344. doi: 10.1111/j.1748-1716.1974.tb05595.x. [DOI] [PubMed] [Google Scholar]
- Fischer J. E., Rosen H. M., Ebeid A. M., James J. H., Keane J. M., Soeters P. B. The effect of normalization of plasma amino acids on hepatic encephalopathy in man. Surgery. 1976 Jul;80(1):77–91. [PubMed] [Google Scholar]
- Folbergrová J., Passonneau J. V., Lowry O. H., Schulz D. W. Glycogen, ammonia and related metabolities in the brain during seizures evoked by methionine sulphoximine. J Neurochem. 1969 Feb;16(2):191–203. doi: 10.1111/j.1471-4159.1969.tb05937.x. [DOI] [PubMed] [Google Scholar]
- Gjedde A., Lockwood A. H., Duffy T. E., Plum F. Cerebral blood flow and metabolism in chronically hyperammonemic rats: effect of an acute ammonia challenge. Ann Neurol. 1978 Apr;3(4):325–330. doi: 10.1002/ana.410030409. [DOI] [PubMed] [Google Scholar]
- Goochee C., Rasband W., Sokoloff L. Computerized densitometry and color coding of [14C] deoxyglucose autoradiographs. Ann Neurol. 1980 Apr;7(4):359–370. doi: 10.1002/ana.410070414. [DOI] [PubMed] [Google Scholar]
- Hawkins R. A., Miller A. L., Nielsen R. C., Veech R. L. The acute action of ammonia on rat brain metabolism in vivo. Biochem J. 1973 Aug;134(4):1001–1008. doi: 10.1042/bj1341001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindfelt B., Plum F., Duffy T. E. Effect of acute ammonia intoxication on cerebral metabolism in rats with portacaval shunts. J Clin Invest. 1977 Mar;59(3):386–396. doi: 10.1172/JCI108651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James I. M., MacDonell L., Xanalatos C. The effect of acute portacaval shunting in dogs on cerebral and peripheral blood flow and metabolism. Clin Sci. 1972 Jun;42(6):769–774. doi: 10.1042/cs0420769. [DOI] [PubMed] [Google Scholar]
- James I. M., MacDonnell L., Xanalatos C. Effect of ammonium salts on brain metabolism. J Neurol Neurosurg Psychiatry. 1974 Aug;37(8):948–953. doi: 10.1136/jnnp.37.8.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones E. A., Schafer D. F., Ferenci P., Pappas S. C. The neurobiology of hepatic encephalopathy. Hepatology. 1984 Nov-Dec;4(6):1235–1242. doi: 10.1002/hep.1840040624. [DOI] [PubMed] [Google Scholar]
- LEE S. H., FISHER B. Portacaval shunt in the rat. Surgery. 1961 Oct;50:668–672. [PubMed] [Google Scholar]
- Lockwood A. H., Bolomey L., Napoleon F. Blood-brain barrier to ammonia in humans. J Cereb Blood Flow Metab. 1984 Dec;4(4):516–522. doi: 10.1038/jcbfm.1984.76. [DOI] [PubMed] [Google Scholar]
- Lockwood A. H., Finn R. D., Campbell J. A., Richman T. B. Factors that affect the uptake of ammonia by the brain: the blood-brain pH gradient. Brain Res. 1980 Jan 13;181(2):259–266. doi: 10.1016/0006-8993(80)90611-3. [DOI] [PubMed] [Google Scholar]
- Lockwood A. H., McDonald J. M., Reiman R. E., Gelbard A. S., Laughlin J. S., Duffy T. E., Plum F. The dynamics of ammonia metabolism in man. Effects of liver disease and hyperammonemia. J Clin Invest. 1979 Mar;63(3):449–460. doi: 10.1172/JCI109322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux H. D. Ammonium and chloride extrusion: hyperpolarizing synaptic inhibition in spinal motoneurons. Science. 1971 Aug 6;173(3996):555–557. doi: 10.1126/science.173.3996.555. [DOI] [PubMed] [Google Scholar]
- MORGANE P. J. Electrophysiological studies of feeding and satiety centers in the rat. Am J Physiol. 1961 Nov;201:838–844. doi: 10.1152/ajplegacy.1961.201.5.838. [DOI] [PubMed] [Google Scholar]
- Mans A. M., Biebuyck J. F., Davis D. W., Bryan R. M., Hawkins R. A. Regional cerebral glucose utilization in rats with portacaval anastomosis. J Neurochem. 1983 Apr;40(4):986–991. doi: 10.1111/j.1471-4159.1983.tb08082.x. [DOI] [PubMed] [Google Scholar]
- McCandless D. W., Schenker S. Effect of acute ammonia intoxication on energy stores in the cerebral reticular activating system. Exp Brain Res. 1981;44(3):325–330. doi: 10.1007/BF00236570. [DOI] [PubMed] [Google Scholar]
- Morgan M. Y., Jakobovits A. W., James I. M., Sherlock S. Successful use of bromocriptine in the treatment of chronic hepatic encephalopathy. Gastroenterology. 1980 Apr;78(4):663–670. [PubMed] [Google Scholar]
- Noda K., Chikamori K. Effect of ammonia via prepyriform cortex on regulation of food intake in the rat. Am J Physiol. 1976 Oct;231(4):1263–1266. doi: 10.1152/ajplegacy.1976.231.4.1263. [DOI] [PubMed] [Google Scholar]
- POSNER J. B., PLUM F. The toxic effects of carbon dioxide and acetazolamide in hepatic encephalopathy. J Clin Invest. 1960 Aug;39:1246–1258. doi: 10.1172/JCI104140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savaki H. E., Davidsen L., Smith C., Sokoloff L. Measurement of free glucose turnover in brain. J Neurochem. 1980 Aug;35(2):495–502. doi: 10.1111/j.1471-4159.1980.tb06293.x. [DOI] [PubMed] [Google Scholar]
- Sokoloff L., Reivich M., Kennedy C., Des Rosiers M. H., Patlak C. S., Pettigrew K. D., Sakurada O., Shinohara M. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem. 1977 May;28(5):897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- Walker C. O., Schenker S. Pathogenesis of hepatic encephalopathy--with special reference to the role of ammonia. Am J Clin Nutr. 1970 May;23(5):619–632. doi: 10.1093/ajcn/23.5.619. [DOI] [PubMed] [Google Scholar]