Abstract
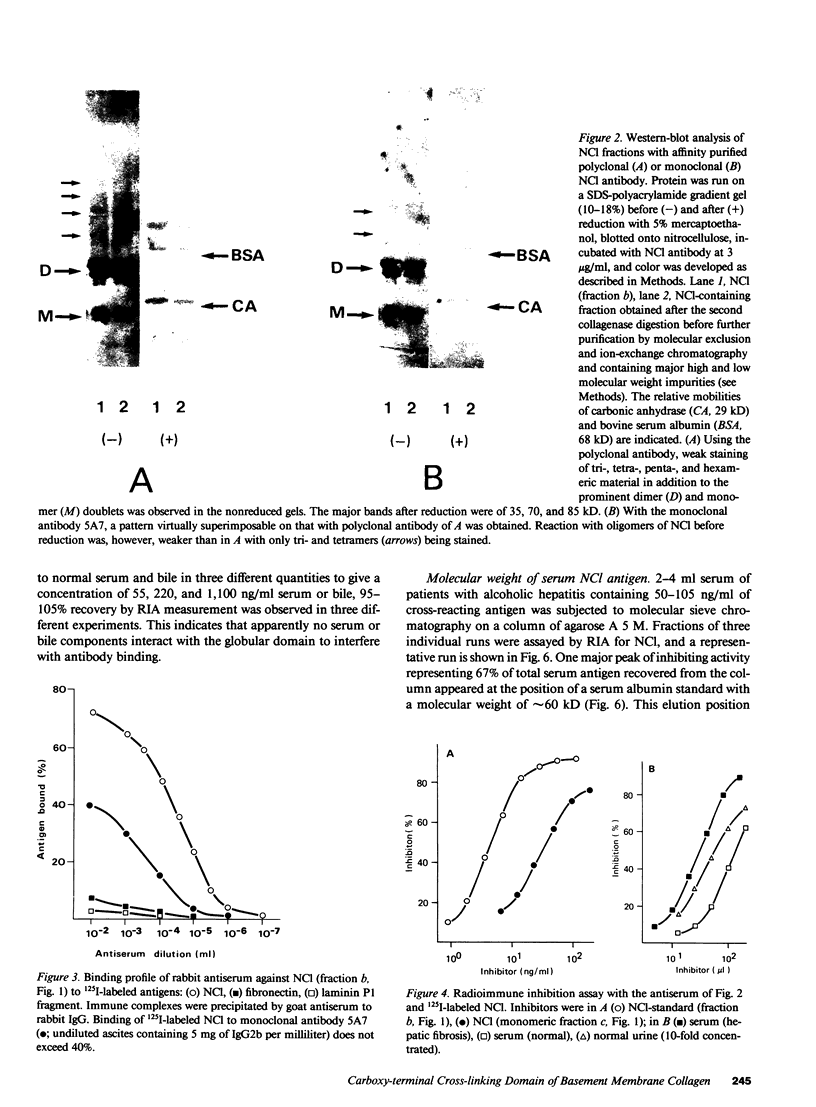
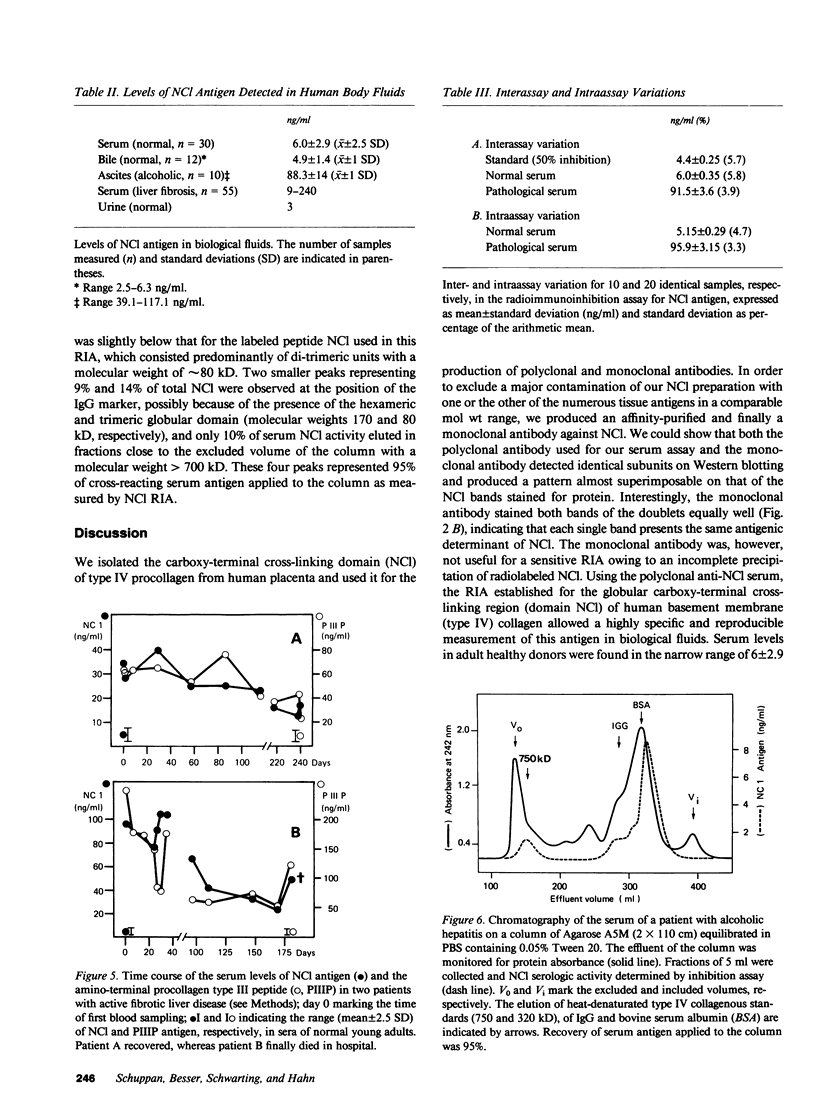
The carboxy-terminal cross-linking domain (NCl) of type IV procollagen was isolated from human placenta and used for the production of polyclonal and monoclonal antibodies. Purity of the antigen and specificity of the antibodies were verified by Western blotting and radioimmunoassays. A radioimmunoassay was developed using rabbit antiserum. Intra- and interassay coefficients of variation were 4.7% and 5.8%, respectively; recovery of NCl added to serum and bile was 95-105%. NCl concentration in sera of healthy volunteers was 6 +/- 2.9 ng/ml (mean +/- 2.5 SD) and was elevated up to 18 ng in sera of patients with autoimmune or metastatic tumor disease and up to 240 ng in sera of patients with fibrogenic liver disease. Substantial amounts of antigen were also found in bile, urine, and ascites. 67% of serum antigens eluted from an agarose A5M column with an apparent molecular weight of 60 kD and 23% with a molecular weight of 90 and 150 kD, well below the molecular weight of type IV procollagen (550 kD). Serum NCl is apparently derived from the degradation of basement membrane collagen. The time course of NCl concentrations in sera of patients with fibrogenic liver disease showed no correlation with the serum concentration of the amino-terminal procollagen type III peptide, a marker of hepatic collagen biosynthesis. A decline of serum NCl levels along with elevated serum procollagen type III peptides apparently indicates bad prognosis in fibrogenic liver disease. The radioimmunoassay for NCl is a useful tool for studying type IV collagen metabolism in conditions causing remodeling or breakdown of basement membranes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barsky S. H., Siegal G. P., Jannotta F., Liotta L. A. Loss of basement membrane components by invasive tumors but not by their benign counterparts. Lab Invest. 1983 Aug;49(2):140–147. [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butkowski R. J., Wieslander J., Wisdom B. J., Barr J. F., Noelken M. E., Hudson B. G. Properties of the globular domain of type IV collagen and its relationship to the Goodpasture antigen. J Biol Chem. 1985 Mar 25;260(6):3739–3747. [PubMed] [Google Scholar]
- Cordell J. L., Falini B., Erber W. N., Ghosh A. K., Abdulaziz Z., MacDonald S., Pulford K. A., Stein H., Mason D. Y. Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP complexes). J Histochem Cytochem. 1984 Feb;32(2):219–229. doi: 10.1177/32.2.6198355. [DOI] [PubMed] [Google Scholar]
- Duncan K. G., Fessler L. I., Bächinger H. P., Fessler J. H. Procollagen IV. Association to tetramers. J Biol Chem. 1983 May 10;258(9):5869–5877. [PubMed] [Google Scholar]
- Dziadek M., Richter H., Schachner M., Timpl R. Monoclonal antibodies used as probes for the structural organization of the central region of fibronectin. FEBS Lett. 1983 May 8;155(2):321–325. doi: 10.1016/0014-5793(82)80629-7. [DOI] [PubMed] [Google Scholar]
- Fessler L. I., Duncan K. G., Fessler J. H., Salo T., Tryggvason K. Characterization of the procollagen IV cleavage products produced by a specific tumor collagenase. J Biol Chem. 1984 Aug 10;259(15):9783–9789. [PubMed] [Google Scholar]
- Hahn E., Wick G., Pencev D., Timpl R. Distribution of basement membrane proteins in normal and fibrotic human liver: collagen type IV, laminin, and fibronectin. Gut. 1980 Jan;21(1):63–71. doi: 10.1136/gut.21.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn K., Wiedemann H., Timpl R., Risteli J., Dieringer H., Voss T., Glanville R. W. Macromolecular structure of basement membrane collagens. FEBS Lett. 1981 Mar 9;125(1):123–128. doi: 10.1016/0014-5793(81)81012-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Martinez-Hernandez A., Amenta P. S. The basement membrane in pathology. Lab Invest. 1983 Jun;48(6):656–677. [PubMed] [Google Scholar]
- Miekka S. I., Ingham K. C., Menache D. Rapid methods for isolation of human plasma fibronectin. Thromb Res. 1982 Jul 1;27(1):1–14. doi: 10.1016/0049-3848(82)90272-9. [DOI] [PubMed] [Google Scholar]
- Oberbäumer I., Wiedemann H., Timpl R., Kühn K. Shape and assembly of type IV procollagen obtained from cell culture. EMBO J. 1982;1(7):805–810. doi: 10.1002/j.1460-2075.1982.tb01251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risteli J., Bächinger H. P., Engel J., Furthmayr H., Timpl R. 7-S collagen: characterization of an unusual basement membrane structure. Eur J Biochem. 1980;108(1):239–250. doi: 10.1111/j.1432-1033.1980.tb04717.x. [DOI] [PubMed] [Google Scholar]
- Risteli J., Draeger K. E., Regitz G., Neubauer H. P. Increase in circulating basement membrane antigens in diabetic rats and effects of insulin treatment. Diabetologia. 1982 Sep;23(3):266–269. doi: 10.1007/BF00252853. [DOI] [PubMed] [Google Scholar]
- Risteli J., Rohde H., Timpl R. Sensitive radioimmunoassays for 7 S collagen and laminin: application to serum and tissue studies of basement membranes. Anal Biochem. 1981 May 15;113(2):372–378. doi: 10.1016/0003-2697(81)90091-9. [DOI] [PubMed] [Google Scholar]
- Risteli L., Timpl R. Isolation and characterization of pepsin fragments of laminin from human placental and renal basement membranes. Biochem J. 1981 Mar 1;193(3):749–755. doi: 10.1042/bj1930749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde H., Bächinger H. P., Timpl R. Characterization of pepsin fragments of laminin in a tumor basement membrane. Evidence for the existence of related proteins. Hoppe Seylers Z Physiol Chem. 1980 Nov;361(11):1651–1660. doi: 10.1515/bchm2.1980.361.2.1651. [DOI] [PubMed] [Google Scholar]
- Rohde H., Langer I., Krieg T., Timpl R. Serum and urine analysis of the aminoterminal procollagen peptide type III by radioimmunoassay with antibody Fab fragments. Coll Relat Res. 1983 Sep;3(5):371–379. doi: 10.1016/s0174-173x(83)80018-1. [DOI] [PubMed] [Google Scholar]
- Rohde H., Vargas L., Hahn E., Kalbfleisch H., Bruguera M., Timpl R. Radioimmunoassay for type III procollagen peptide and its application to human liver disease. Eur J Clin Invest. 1979 Dec;9(6):451–459. doi: 10.1111/j.1365-2362.1979.tb00912.x. [DOI] [PubMed] [Google Scholar]
- Sakashita S., Engvall E., Ruoslahti E. Basement membrane glycoprotein laminin binds to heparin. FEBS Lett. 1980 Jul 28;116(2):243–246. doi: 10.1016/0014-5793(80)80654-5. [DOI] [PubMed] [Google Scholar]
- Sanders E. J. Recent progress towards understanding the roles of the basement membrane in development. Can J Biochem Cell Biol. 1983 Aug;61(8):949–956. doi: 10.1139/o83-121. [DOI] [PubMed] [Google Scholar]
- Schuppan D., Becker J., Boehm H., Hahn E. G. Immunofluorescent localization of type-V collagen as a fibrillar component of the interstitial connective tissue of human oral mucosa, artery and liver. Cell Tissue Res. 1986;243(3):535–543. doi: 10.1007/BF00218060. [DOI] [PubMed] [Google Scholar]
- Schuppan D., Rühlmann T., Hahn E. G. Radioimmunoassay for human type VI collagen and its application to tissue and body fluids. Anal Biochem. 1985 Aug 15;149(1):238–247. doi: 10.1016/0003-2697(85)90501-9. [DOI] [PubMed] [Google Scholar]
- Stanley J. R., Woodley D. T., Katz S. I., Martin G. R. Structure and function of basement membrane. J Invest Dermatol. 1982 Jul;79 (Suppl 1):69s–72s. doi: 10.1111/1523-1747.ep12545830. [DOI] [PubMed] [Google Scholar]
- Terranova V. P., Rohrbach D. H., Martin G. R. Role of laminin in the attachment of PAM 212 (epithelial) cells to basement membrane collagen. Cell. 1980 Dec;22(3):719–726. doi: 10.1016/0092-8674(80)90548-6. [DOI] [PubMed] [Google Scholar]
- Timpl R., Dziadek M., Fujiwara S., Nowack H., Wick G. Nidogen: a new, self-aggregating basement membrane protein. Eur J Biochem. 1983 Dec 15;137(3):455–465. doi: 10.1111/j.1432-1033.1983.tb07849.x. [DOI] [PubMed] [Google Scholar]
- Timpl R., Rohde H., Risteli L., Ott U., Robey P. G., Martin G. R. Laminin. Methods Enzymol. 1982;82(Pt A):831–838. doi: 10.1016/0076-6879(82)82104-6. [DOI] [PubMed] [Google Scholar]
- Timpl R., Wiedemann H., van Delden V., Furthmayr H., Kühn K. A network model for the organization of type IV collagen molecules in basement membranes. Eur J Biochem. 1981 Nov;120(2):203–211. doi: 10.1111/j.1432-1033.1981.tb05690.x. [DOI] [PubMed] [Google Scholar]
- Weber S., Engel J., Wiedemann H., Glanville R. W., Timpl R. Subunit structure and assembly of the globular domain of basement-membrane collagen type IV. Eur J Biochem. 1984 Mar 1;139(2):401–410. doi: 10.1111/j.1432-1033.1984.tb08019.x. [DOI] [PubMed] [Google Scholar]
- Wieslander J., Barr J. F., Butkowski R. J., Edwards S. J., Bygren P., Heinegård D., Hudson B. G. Goodpasture antigen of the glomerular basement membrane: localization to noncollagenous regions of type IV collagen. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3838–3842. doi: 10.1073/pnas.81.12.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieslander J., Bygren P., Heinegård D. Isolation of the specific glomerular basement membrane antigen involved in Goodpasture syndrome. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1544–1548. doi: 10.1073/pnas.81.5.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieslander J., Langeveld J., Butkowski R., Jodlowski M., Noelken M., Hudson B. G. Physical and immunochemical studies of the globular domain of type IV collagen. Cryptic properties of the Goodpasture antigen. J Biol Chem. 1985 Jul 15;260(14):8564–8570. [PubMed] [Google Scholar]
- Yurchenco P. D., Furthmayr H. Self-assembly of basement membrane collagen. Biochemistry. 1984 Apr 10;23(8):1839–1850. doi: 10.1021/bi00303a040. [DOI] [PubMed] [Google Scholar]
- von der Mark H., Oberbäumer I., Timpl R., Kemler R., Wick G. Immunochemical and autoantigenic properties of the globular domain of basement membrane collagen (type IV). Eur J Biochem. 1985 Feb 1;146(3):555–562. doi: 10.1111/j.1432-1033.1985.tb08687.x. [DOI] [PubMed] [Google Scholar]