Abstract
Purpose
Chemotherapy-induced amenorrhea is a serious concern for women undergoing cancer therapy. This prospective randomized trial evaluated the use of gonadotropin-releasing hormone (GnRH) analog triptorelin to preserve ovarian function in women treated with chemotherapy for early-stage breast cancer.
Patients and Methods
Premenopausal women age 44 years or younger were randomly assigned to receive either triptorelin or no triptorelin during (neo)adjuvant chemotherapy and were further stratified by age (< 35, 35 to 39, > 39 years), estrogen receptor status, and chemotherapy regimen. Objectives included the resumption of menses and serial monitoring of follicle-stimulating hormone (FSH) and inhibin A and B levels.
Results
Targeted for 124 patients with a planned 5-year follow-up, the trial was stopped for futility after 49 patients were enrolled (median age, 39 years; range, 21 to 43 years); 47 patients were treated according to assigned groups with four cycles of adriamycin plus cyclophosphamide alone or followed by four cycles of paclitaxel or six cycles of fluorouracil, epirubicin, and cyclophosphamide. Menstruation resumed in 19 (90%) of 21 patients in the control group and in 23 (88%) of 26 in the triptorelin group (P= .36). Menses returned after a median of 5.8 months (range, 1 to 19 months) after completion of chemotherapy in the triptorelin versus 5.0 months (range, 0 to 28 months) in the control arm (P= .58). Two patients (age 26 and 35 years at random assignment) in the control group had spontaneous pregnancies with term deliveries. FSH and inhibin B levels correlated with menstrual status.
Conclusion
When stratified for age, estrogen receptor status, and treatment regimen, amenorrhea rates on triptorelin were comparable to those seen in the control group.
INTRODUCTION
Breast cancer is the most common cancer among women in the United States and still estimated to affect more than 200,000 patients every year.1 Major advances in diagnosis and treatment of breast cancer have reduced mortality, and a majority of patients diagnosed with breast cancer will be long-term survivors. With more women surviving breast cancer, avoidance of treatment-related long-term sequelae has become essential in therapeutic decision making. Incidence of breast cancer is more common among older women; nonetheless, one in 200 women will develop breast cancer before the age of 40 years.1 In young women, chemotherapy is often considered even for smaller tumors without lymph node involvement, which leaves an estimated 20,000 women at risk from lasting effects from chemotherapy. Although rare, serious long-term toxicities related to chemotherapy include lasting myelosuppression, neuropathy, heart failure, leukemia, and other secondary cancers.2 Younger women of childbearing age are confronted with the risk of compromising their fertility by therapy-induced temporary or permanent amenorrhea. The amenorrhea risk is strongly influenced by patient age and type and duration of chemotherapeutic regimen. Reported rates of amenorrhea have varied significantly, ranging from 10% to over 90%.3–9 The wide range in reported amenorrhea rates result at least in part from variations in experimental design and are correlated with duration of follow-up of the studies. Studies often only assess amenorrhea for the first 12 months, which frequently only includes 6 months of follow-up after completion of chemotherapy. More recent reports have suggested that shorter duration of chemotherapy and use of less gonadotoxic agents such as epirubicin or doxorubicin and fluorouracil result in lower rates of amenorrhea.10 Women younger than age 40 years are more likely to retain their menstrual cycles than those older than 40, with a rapid rise in chemotherapy-induced amenorrhea with each year after 40.6 Hence, the lack of age stratification in previous studies may have further confounded the wide range of amenorrhea reported.
Temporary ovarian suppression using GnRH analogs during adjuvant chemotherapy has been proposed to prevent premature ovarian failure after cytotoxic therapy. Several phase II studies have reported a high rate of preserved menstruation in treated patients.11–16 Other studies have suggested that the benefit may be less pronounced, particularly when assessing effects of chemotherapy-induced amenorrhea in younger patients receiving contemporary therapy. Furthermore, use of tamoxifen was not routinely taken in consideration.
Given the conflicting data on the true incidence of amenorrhea induced by contemporary chemotherapy, we performed a randomized control study evaluating the effects of temporary ovarian suppression with triptorelin, a GnRH agonist, during (neo)adjuvant chemotherapy in patients with breast cancer stratified for age, type of regimen, and tamoxifen use.
PATIENTS AND METHODS
Eligibility Criteria
This trial randomly assigned premenopausal patients with newly diagnosed early-stage breast cancer (stages I to III) and planned adjuvant or neoadjuvant chemotherapy in a one-to-one fashion by computerized treatment assignment to receive or not receive triptorelin during chemotherapy. Eligible patients were younger than 45 years of age with a follicle-stimulating hormone (FSH) level less than 40 mIU/mL and at least two menstrual periods in the preceding 6 months. Patients were further stratified by age (< 35, 35 to 39, or 40 to 44 years) and prespecified (neo) adjuvant chemotherapy regimens (four cycles of doxorubicin plus cyclophosphamide [AC], four cycles of doxorubicin plus cyclophosphamide followed by four cycles of a taxane [AC→T], or six cycles with fluorouracil plus epirubicin [FEC] or doxorubicin plus cyclophosphamide [FAC]). Women with estrogen receptor (ER) –positive tumors were offered tamoxifen for 5 years. The trial was initiated after approval was granted by the protocol review ccommittee and institutional review board at Moffitt Cancer Center/University of South Florida and the National Cancer Institute. Patients were enrolled and treated from July 2003 to January 2007 according to federal and institutional guidelines and good clinical practice.
Notable exclusion criteria were as follows: pregnancy or lactation, prior chemotherapy, or bilateral oophorectomy or ovarian irradiation before enrollment; history of other cancers; personal or familial (first-degree relative) premature ovarian failure; and plan to undergo an oophorectomy/hysterectomy within 2 years. Oral contraceptives were not permitted.
Study Objectives
The primary objective of the study was to determine the protective effect of chemical ovarian suppression achieved by short-term use of GnRH analog on the preservation of ovarian function in women treated with chemotherapy as measured by the number of patients with uninterrupted or restored menses during a follow-up period of at least 2 years after the last cycle of chemotherapy. Menses were reported by cycle length and duration of bleeding in study-specific patient diaries. Maintained or uninterrupted menses were defined as the continuation of regular menses 21 to 35 days apart with at least 2 days of bleeding. Resumed menses required resumptions of at least three menses in 6 months and FSH level of less than 40 mIU/mL. Secondary objectives included the effects of triptorelin administered during chemotherapy on serial serum FSH, inhibin A, and inhibin B. The reporting time of amenorrhea referred to the interval from the completion of the last chemotherapy cycle. Patients with disease progression requiring further chemotherapy/ovarian ablation were censored for the return of menses or not at time of recurrence or removal from study. Menstrual follow-up was also stopped for the two pregnant patients.
GnRH Agonist
Triptorelin (Trelstar Depot [3.75 mg], Debio Recherche Pharmaceutique, Martigny, Switzerland; packaged and distributed by Pharmacia & Upjohn, Kalamazoo, MI) was administered every 28 to 30 days by intramuscular injection starting no sooner than 4 weeks and at least 7 days before the first cycle of chemotherapy and continued throughout chemotherapy duration.
Statistical Analysis
The sample size/power calculation was based on testing the primary hypothesis: effect of chemical ovarian suppression achieved by short-term use of GnRH analog on the preservation of ovarian function, with amenorrhea as a surrogate marker. The trial was designed to enroll 124 evaluable patients to detect a 20% difference in amenorrhea rate, from 10% in the triptorelin arm to 30% in the control arm, with 80% power and a two-sided 5% significance level. Interim statistical review after 49 patients were enrolled demonstrated futility in demonstrating a benefit of ovarian preservation with triptorelin over the control arm. The amenorrhea rates at each time point were compared by a Pearson χ2 test. Logistic regression was performed to study the effect of chemotherapy and age and possible interactions on the probability of ovarian failure. Log-rank statistics was used to compare time to return of menses between the treatment groups and among the age groups. All tests were two sided. P values less than .05 were considered statistically significant. Analyses were performed by using SAS version 9.2 software (SAS Institute, Cary, NC).
RESULTS
Patient Characteristics
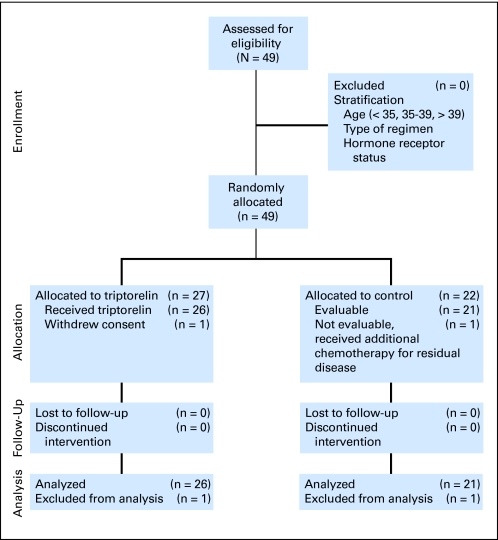
This trial enrolled 49 patients (median age, 39 years; range, 21 to 44 years) between July 2003 and January 2007. Because of computerized randomization and stratification in age, treatment, and hormone receptor status, there was an imbalance in patient number between the triptorelin and control arms at closure of the trial (Fig 1). The demographics and characteristics of the 49 randomly assigned patients are listed in Table 1. Two patients, one in each arm, were not evaluable. One patient in the triptorelin arm withdrew consent before first treatment. The second patient, in the control arm, was found to have residual disease after neoadjuvant chemotherapy and was treated with additional chemotherapy not permitted by protocol. The planned follow-up time was 5 years but was stopped early because no difference between the two groups was seen. Median follow-up time was 18 months (range, 5 to 43 months) from completion of last chemotherapy and 22 months after initiation of chemotherapy. All five patients who did not resume their menses underwent follow-up for at least 24 months.
Fig 1.
CONSORT diagram.
Table 1.
Patient Demographics and Clinical Characteristics
| Characteristic | Triptorelin Arm (n = 27) |
Control Arm (n = 22) |
P* | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Female sex | 27 | 100 | 22 | 100 | |
| Age, years | .99 | ||||
| Median | 39 | 38 | |||
| Range | 21-44 | 26-44 | |||
| < 35 | 7 | 26 | 7 | 32 | |
| 35-39 | 12 | 44 | 8 | 36 | |
| 40-44 | 8 | 30 | 7 | 32 | |
| Race | .19 | ||||
| White | 25 | 93 | 19 | 86 | |
| Black | 2 | 7 | 1 | 5 | |
| Asian | 0 | 0 | 2 | 9 | |
| Ethnicity | .76 | ||||
| Hispanic | 24 | 89 | 20 | 91 | |
| Non-Hispanic | 3 | 11 | 2 | 9 | |
| Estrogen receptor status | .92 | ||||
| Positive | 20 | 74 | 16 | 73 | |
| Negative | 7 | 26 | 6 | 27 | |
| Progesterone receptor status | .14 | ||||
| Positive | 14 | 52 | 16 | 73 | |
| Negative | 13 | 48 | 6 | 27 | |
| Chemotherapy regimen | .86 | ||||
| AC (four cycles) | 12 | 44 | 11 | 50 | |
| AC (four cycles) followed by taxane (four cycles) | 8 | 30 | 5 | 23 | |
| FEC/FAC (six cycles) | 7 | 26 | 6 | 27 | |
| Tamoxifen | .25 | ||||
| Yes | 20 | 74 | 16 | 73 | |
| No | 7 | 26 | 6 | 27 | |
Abbreviations: AC, doxorubicin plus cyclophosphamide; FAC, fluorouracil plus doxorubicin plus cyclophosphamide; FEC, fluorouracil plus epirubicin plus cyclophosphamide.
P value from Pearson χ2 or Fisher's exact test.
Resumption of Menses
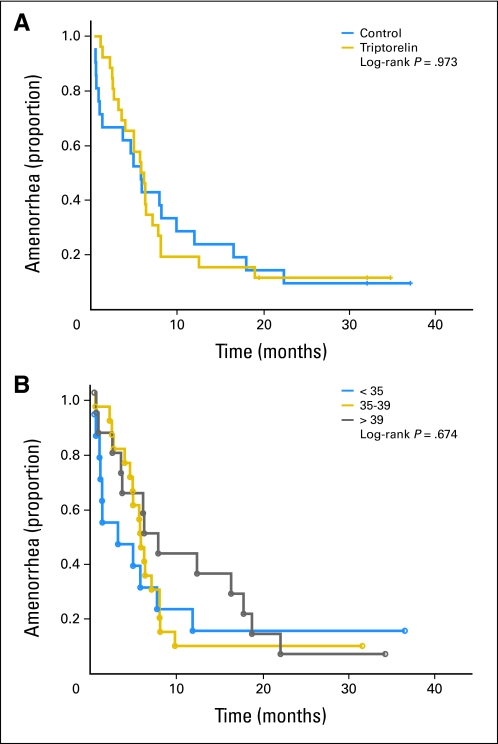
Menses were maintained throughout chemotherapy and study follow-up in six patients, one in the triptorelin arm and five in the control arm (Table 2). Menses did not resume in five patients, two in the control arm with 24 and 39 months of follow-up and three in the triptorelin arm with 32, 32, and 34 months follow-up, respectively. All five patients were receiving tamoxifen (Table 2). Menses returned after a median of 5.8 months (range, 1 to 19 months) after completion of chemotherapy in the triptorelin arm versus a median of 5.0 months (range, 0 to 28 months) in the control arm (P= .58; Table 2; Figs 2A, 2B), including the six patients with uninterrupted menses. Two spontaneous pregnancies occurred in patients in the control group, one in a 26-year-old woman and one in a 35 year-old woman; both pregnancies were unassisted and resulted in normal-term deliveries. Neither pregnancies nor miscarriages were reported in the triptorelin group.
Table 2.
Effects of Triptorelin on Menstrual Cycle
| Effect | Triptorelin Arm (n = 27) |
Control Arm (n = 22) |
P* | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Evaluable patients | 26 | 96 | 21 | 95 | .51 |
| Interval to resumption of menses, months† | .58 | ||||
| Median | 4.96 | 5.82 | |||
| Range | 0.43–28.0 | 1.15–19.0 | |||
| Menses | .16 | ||||
| Maintained throughout‡ | 1 | 4 | 5 | 24 | |
| Resumed | 22 | 85 | 14 | 67 | |
| Not resumed | 3 | 12 | 2 | 10 | |
| Patient who have not resumed menses | |||||
| Age, years | 39, 39, 42 | 38, 32 | |||
| Regimen | AC, AC, FEC | AC, AC→T | |||
| Tamoxifen | Yes (3) | Yes (2) | |||
| Menses at interval follow-up, months | |||||
| 6 | 44 | 55 | .18 | ||
| 12 | 74 | 68 | .22 | ||
| 18 | 78 | 73 | .24 | ||
| 24 | 85 | 86 | .32 | ||
| 30 | 88 | 90 | .36 | ||
| Pregnancies | 0 | 2 (spontaneous) | .20 | ||
Abbreviations: AC, doxorubicin plus cyclophosphamide; AC→T, doxorubicin plus cyclophosphamide followed by taxane; FEC, fluorouracil plus epirubicin plus cyclophosphamide.
P value from Fisher's exact test.
Interval from end of chemotherapy to start of first menses; includes six patients who maintained menses.
In triptorelin arm, patient age 29 years; in control arm, patients ages 26, 32, 32, 38, and 41 years, respectively.
Fig 2.
Resumption of menses by (A) treatment and (B) age.
Correlative Studies
Serum inhibin A and B were collected in parallel with FSH levels at each follow-up to determine whether circulating inhibins would be an early indicator of amenorrhea. FSH levels corresponded to menstruation status (Table 3) and did not differ between the intervention groups. Mean baseline FSH levels were 4.3 mIU/mL in the control group and 5.2 mIU/mL (P= .3) in the arm treated with triptorelin. At least 18 months after completion of chemotherapy, mean FSH levels were 19.0 mIU/mL in the control arm and 15.7 mIU/mL (P= .46) in the triptorelin arm. Mean FSH levels 18 months after completion of chemotherapy were significantly higher compared with baseline FSH levels (all patients: 4.7 v17.2 mIU/mL; P< .001; control: 4.4 v19.1 mIU/mL; P< .001; triptorelin: 5.2 v15.7 mIU/mL; P< .001) in those women who resumed or maintained menses. FSH levels remained less than 10 mIU/mL in women younger than 35 years of age with resumed or maintained menses in both groups. Both patients with subsequent pregnancies had FSH levels less than 10 mIU/mL.
Table 3.
Mean Serum FSH Levels (mIU/mL) in Women With Resumed or Maintained Menses
| Age (years) | Baseline | Interval From Chemotherapy (months) |
Pregnancy | ||
|---|---|---|---|---|---|
| 0–6 | 6–18 | > 18 | |||
| Control | |||||
| < 35 | 3.9 | 14.2 | 7.8 | 1.8 | 1 |
| 35-39 | 4.8 | 56.7 | 13.4 | 15.4 | 1 |
| > 39 | 4.5 | 49.5 | 24.7 | 23.5 | |
| Treatment | |||||
| < 35 | 3.9 | 19 | 5.5 | 7 | |
| 35-39 | 4.9 | 22.5 | 11.3 | 15.2 | |
| > 39 | 6.4 | 35.7 | 13.3 | 20.3 | |
Abbreviation: FSH, follicle-stimulating hormone.
Inhibin B downregulates FSH biosynthesis, inhibits FSH secretion, and has been proposed as an alternative marker of premature ovarian failure.17 Timed inhibin B levels are used in infertility assessments to predict ovarian reserve. Levels less than 45 pg/mL are associated with impaired ovulation and may correlate with decreased success of in vitro fertilization, lower pregnancy rates, and increased risk of miscarriage.18,19 The coefficient of variation for the inhibin B assay used in this study ranges from 4.29% to 6.82%.20 Our data showed varying levels of inhibin B (Table 4) and inverse correlation with FSH levels.
Table 4.
Mean Inhibin B Levels (pg/mL) in Women With Resumed or Maintained Menses
| Age (years) | Baseline | Interval From End of Chemotherapy (months) |
Pregnancy | ||
|---|---|---|---|---|---|
| 0–6 | 6–18 | > 18 | |||
| Control | |||||
| < 35 | 11.1 | 19.9 | 39.3 | 6.5 | 1 |
| 35-39 | 67.0 | 6.5 | 16.6 | 11.8 | 1 |
| > 39 | 71.8 | 8.6 | 14.7 | 9.4 | |
| Treatment | |||||
| < 35 | 66.2 | 7.1 | 42.8 | 9.9 | |
| 35-39 | 63.5 | 22.2 | 23.3 | 19.8 | |
| > 39 | 23.8 | 25.2 | 13.2 | 12.9 | |
DISCUSSION
This study tested the potential benefits of using a GnRH agonist to prevent chemotherapy-induced amenorrhea. In addition, we sought to define incidence of amenorrhea when patients were stratified for age, chemotherapy regimen, and tamoxifen use. Previous reports have suggested a considerable reduction in amenorrhea rates with temporary ovarian suppression using GnRH analogs during chemotherapy.11–16 Our results show that amenorrhea rates between the control and triptorelin groups did not differ when treating women age 44 years or younger with contemporary chemotherapeutic agents for early-stage breast cancer. All patients received four to six cycles of chemotherapy with an anthracycline-based regimen alone or followed by a taxane.
Interim analysis after enrollment of 49 patients showed a 90% resumption of menses in the control group and 88% in the triptorelin group when observed for a median of 18 months after completion of chemotherapy. Therefore, this study did not meet its primary end point of a benefit of GnRH agonists over no intervention. The study had originally planned to enroll a total of 124 patients with an expected amenorrhea rate of 10% in the triptorelin arm, based on historical data, and 30% in the control arm. However, the observed amenorrhea rate in this randomized, age- and regimen-stratified study was comparable in both arms (10% and 12%). All but one of the patients without resumption of menses were observed for at least 36 months from the onset of chemotherapy.
Defining chemotherapy-induced amenorrhea is complicated by the fact that natural menopause also occurs in this patient population over the time of follow-up, and treatment-induced amenorrhea is not distinguishable from natural menopause unless menses are observed to resume after treatment completion. Szwarc et al21 discuss a parametric model for time to amenorrhea and time to recovery of menses, which accounts for the presence of censoring and possibility that treatment may induce natural menopause. To limit bias of our observation, we performed a proportional hazards model to test if the treatment (triptorelin) had any impact on time to menses. In comparing the triptorelin with the control group, a hazard ratio of 0.76 (95% CI, 0.40 to 1.46) demonstrates that no impact was observed.
Four nonrandomized studies have shown 67% to 90% of premenopausal patients resumed menstruation if given a GnRH analog during chemotherapy.11–13,16 Median age was 37 years; in our study, it was 39 years. In those studies, the chemotherapy regimens included were primarily FEC; cyclophosphamide, methotrexate, and fluorouracil (CMF); cyclophosphamide, epirubicin, and fluorouracil (CEF); and AC. Using similar anthracycline-based treatments, our data are consistent with these findings; 88% of women receiving triptorelin resumed menstruation. However, in contrast to our study, these early trials were nonrandomized phase II studies and did not assess incidence of amenorrhea stratified by contemporary chemotherapy, age, or tamoxifen use in a control arm. Two studies with a randomized control arm have recently been presented but have not yet been published. In addition, a large, multi-institutional study SWOG (Southwest Oncology Group) 0230 (POEMS [Prevention of Early Menopause]) in women with hormone receptor–negative breast cancer is ongoing. Results of the multicenter Italian trial by GIM (Gruppo Italiano Mammella) were presented at the 46th Annual Meeting of the American Society of Clinical Oncology (ASCO) in 2010; 281 patients younger than age 45 years undergoing chemotherapy were randomly assigned to receive triptorelin or not. Patients were treated with CEF or EC, AC, FEC or EC followed by taxane, an anthracycline followed by CMF, or CMF alone. The primary end point was early menopause, defined as postmenopausal levels of both FSH and estradiol and no menstrual activity 1 year after end of chemotherapy. With a median age of 39 years and median number of chemotherapy cycles of six, menses resumption and/or premenopausal estradiol levels were observed in 77 (58%) of 133 patients in the control arm versus 114 (77%) of 148 patients in the triptorelin arm (P= .006).22 This study had a shorter follow-up time (ie, 12 months) than our study, with 18 months.
In contrast, results of the multicenter German study ZORO (Zoladex Rescue of Ovarian Function) were recently published in Journal of Clinical Oncology. Sixty evaluable patients younger than age 46 years with hormone receptor–negative breast cancer treated with AC with or without a taxane were randomly assigned to receive goserelin or not. The primary objective was reappearance of ovarian function, defined as two consecutive menstrual periods within 21 to 35 days at 6 months after end of chemotherapy. Return of menses occurred after a median of 6.1 months in the control arm versus 6.8 months in the goserelin arm (P= .3). By 6 months, 21 women (70%) in the goserelin arm and 17 (56.7%) in the control arm had resumed their menses (P= .42). Two women, one in each arm, became pregnant.23,24 Preliminary data from the OPTION (Ovarian Protection Trial In Estrogen Negative) trial were presented at the 46th Annual Meeting of ASCO. This trial tested the effects of goserelin during chemotherapy in 227 premenopausal patients with breast cancer. At the time of presentation, 140 had adequate data on menstruation and 1 year of follow-up. The primary end point was cessation or no resumption of menses within 12 months of chemotherapy initiation. Women were stratified into two age groups: younger than 40 years (n = 87) and older than 40 years (n = 53) at diagnosis. In the group age younger than 40 years at 12-month follow-up, 34.7% of women treated with goserelin continued to have no resumption of menses compared with 15.8% in the chemotherapy-only group (P≤ .05).25
Results of ZORO and OPTION were similar to those of our study and support our findings that GnRH agonists do not preserve ovarian function as measured by resumption of menses. These data are also comparable to those reported by Fornier et al5 and Sukumvanich et al.9 Fornier et al reported that 141 (85%) of 166 patients treated with contemporary chemotherapy (AC plus paclitaxel) maintained or resumed menstruation. Sukumvanich et al pointed to the importance of age and chemotherapy regimen in assessing chemotherapy-induced amenorrhea. In that study, less than 5% of women age 20 to 34 years had prolonged chemotherapy-induced amenorrhea compared with 11% of women age 35 to 39 and 40% of women age older than 39 years. Women receiving AC or AC plus paclitaxel were more likely to resume menses than those treated with AC plus docetaxel or CMF.
Our data show that in patients with a median age of 39 years, triptorelin does not prevent chemotherapy-induced amenorrhea. In this study, amenorrhea was an indirect measure of fertility. Although preservation of ovarian function is important to retain fecundity, emerging data suggest that chemotherapy-induced amenorrhea in premenopausal women may actually convey a survival advantage and that prolonged amenorrhea improves overall survival from breast cancer.
A large adjuvant study (NSABP [National Surgical Adjuvant Breast and Bowel Project] B-30) designed to compare differences in adjuvant chemotherapy (AC→T, AT, ATC) in node-positive breast cancer showed that in 2,343 premenopausal women, those with prolonged amenorrhea had better disease-free survival (relative risk, 0.70; P< .001) and overall survival (relative risk, 0.76; P= .04). Adjustment for treatment, ER status, age, lymph nodes, tumor size, and hormonal therapy showed consistent results among all subgroups.26 In other studies, ovarian ablation or suppression has also been correlated with better overall outcome in premenopausal women with breast cancer.3,27 On the basis of these data, it seems that ovarian preservation may adversely affect survival.
In summary, the observed incidence of chemotherapy-induced amenorrhea in our study was 10% in the control arm and 12% in the arm treated with triptorelin, with no statistical difference. These results suggest that use of GnRH agonists in premenopausal patients treated with contemporary (neo)adjuvant chemotherapy does not offer a benefit in preserving ovarian function compared with patients not treated with GnRH agonists, and it should not be recommended. The rate of amenorrhea in these patients was lower than expected based on historical, nonrandomized, non–age- or –regimen-stratified studies, with often shorter follow-up. With recent data suggesting a survival benefit in patients who become amenorrheic for at least 6 months or longer, means to preserve ovarian function in the early period after breast cancer diagnosis should be reevaluated. Instead, a number of alternative techniques for fertility preservation could be considered in these patients before administration of chemotherapy, such as in vitro fertilization with embryo cryopreservation or oocyte or ovarian cryopreservation.10,28–32
Acknowledgment
We thank the patients who enrolled onto this trial as well as the staff at the University of South Florida and Moffitt Cancer Center for support in data collection and patient care.
Footnotes
See accompanying article on page 479; listen to the podcast by Dr Partridge at www.jco.org/podcasts
Supported by National Cancer Institute Award No. U10CA081920.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00090844.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: None Research Funding: Pamela N. Munster, Pfizer Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Pamela N. Munster, Margaret Gross-King, Ping Xu
Collection and assembly of data: Pamela N. Munster, Roohi Ismail-Khan, Charles E. Cox, W. Bradford Carter, Margaret Gross-King, Ping Xu, Mensura Lacevic, Susan E. Minton
Data analysis and interpretation: Pamela N. Munster, Amy P. Moore, Roohi Ismail-Khan, Ping Xu, Mensura Lacevic, Susan E. Minton
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Shapiro CL, Recht A. Side effects of adjuvant treatment of breast cancer. N Engl J Med. 2001;344:1997–2008. doi: 10.1056/NEJM200106283442607. [DOI] [PubMed] [Google Scholar]
- 3.Goldhirsch A, Gelber RD, Castiglione M. The magnitude of endocrine effects of adjuvant chemotherapy for premenopausal breast cancer patients: The International Breast Cancer Study Group. Ann Oncol. 1990;1:183–188. doi: 10.1093/oxfordjournals.annonc.a057718. [DOI] [PubMed] [Google Scholar]
- 4.Bines J, Oleske DM, Cobleigh MA. Ovarian function in premenopausal women treated with adjuvant chemotherapy for breast cancer. J Clin Oncol. 1996;14:1718–1729. doi: 10.1200/JCO.1996.14.5.1718. [DOI] [PubMed] [Google Scholar]
- 5.Fornier MN, Modi S, Panageas KS, et al. Incidence of chemotherapy-induced, long-term amenorrhea in patients with breast carcinoma age 40 years and younger after adjuvant anthracycline and taxane. Cancer. 2005;104:1575–1579. doi: 10.1002/cncr.21385. [DOI] [PubMed] [Google Scholar]
- 6.Petrek JA, Naughton MJ, Case LD, et al. Incidence, time course, and determinants of menstrual bleeding after breast cancer treatment: A prospective study. J Clin Oncol. 2006;24:1045–1051. doi: 10.1200/JCO.2005.03.3969. [DOI] [PubMed] [Google Scholar]
- 7.Tham YL, Sexton K, Weiss H, et al. The rates of chemotherapy-induced amenorrhea in patients treated with adjuvant doxorubicin and cyclophosphamide followed by a taxane. Am J Clin Oncol. 2007;30:126–132. doi: 10.1097/01.coc.0000251398.57630.4f. [DOI] [PubMed] [Google Scholar]
- 8.Davis AL, Klitus M, Mintzer DM. Chemotherapy-induced amenorrhea from adjuvant breast cancer treatment: The effect of the addition of taxanes. Clin Breast Cancer. 2005;6:421–424. doi: 10.3816/CBC.2005.n.046. [DOI] [PubMed] [Google Scholar]
- 9.Sukumvanich P, Case LD, Van Zee K, et al. Incidence and time course of bleeding after long-term amenorrhea after breast cancer treatment: A prospective study. Cancer. 2010;116:3102–3111. doi: 10.1002/cncr.25106. [DOI] [PubMed] [Google Scholar]
- 10.Levine J, Canada A, Stern CJ. Fertility preservation in adolescents and young adults with cancer. J Clin Oncol. 2010;28:1–11. doi: 10.1200/JCO.2009.22.8312. [DOI] [PubMed] [Google Scholar]
- 11.Fox KR, Sicallia J, Moore H. Preventing chemotherapy-related amenorrhea using leuprolide during adjuvant chemotherapy for early-stage breast cancer. Proc Am Soc Clin Oncol. 2003:22. (abstr 50) [Google Scholar]
- 12.Urruticoechea A, Arnedos M, Walsh G, et al. Ovarian protection with goserelin during adjuvant chemotherapy for pre-menopausal women with early breast cancer (EBC) Breast Cancer Res Treat. 2008;110:411–416. doi: 10.1007/s10549-007-9745-y. [DOI] [PubMed] [Google Scholar]
- 13.Del Mastro L, Catzeddu T, Boni L, et al. Prevention of chemotherapy-induced menopause by temporary ovarian suppression with goserelin in young, early breast cancer patients. Ann Oncol. 2006;17:74–78. doi: 10.1093/annonc/mdj029. [DOI] [PubMed] [Google Scholar]
- 14.Blumenfeld Z, Avivi I, Linn S, et al. Prevention of irreversible chemotherapy-induced ovarian damage in young women with lymphoma by a gonadotrophin-releasing hormone agonist in parallel to chemotherapy. Hum Reprod. 1996;11:1620–1626. doi: 10.1093/oxfordjournals.humrep.a019457. [DOI] [PubMed] [Google Scholar]
- 15.Blumenfeld Z, Benaroush M, Zuckerman T. Spontaneous pregnancy and normal delivery after repeated autologous bone marrow transplantation and GnRH agonist treatment. Hum Reprod. 2007;22:2346. doi: 10.1093/humrep/dem066. [DOI] [PubMed] [Google Scholar]
- 16.Recchia F, Saggio G, Amiconi G, et al. Gonadotropin-releasing hormone analogues added to adjuvant chemotherapy protect ovarian function and improve clinical outcomes in young women with early breast carcinoma. Cancer. 2006;106:514–523. doi: 10.1002/cncr.21646. [DOI] [PubMed] [Google Scholar]
- 17.Blumenfeld Z. Preservation of fertility and ovarian function and minimalization of chemotherapy associated gonadotoxicity and premature ovarian failure: The role of inhibin-A and -B as markers. Mol Cell Endocrinol. 2002;187:93–105. doi: 10.1016/s0303-7207(01)00712-2. [DOI] [PubMed] [Google Scholar]
- 18.Sowers M, McConnell D, Gast K, et al. Anti-Mullerian hormone and inhibin B variability during normal menstrual cycles. Fertil Steril. 2010;94:1482–1486. doi: 10.1016/j.fertnstert.2009.07.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Penarrubia J, Peralta S, Fabregues F, et al. Day-5 inhibin B serum concentrations and antral follicle count as predictors of ovarian response and live birth in assisted reproduction cycles stimulated with gonadotropin after pituitary suppression. Fertil Steril. 2010;94:2590–2595. doi: 10.1016/j.fertnstert.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 20.Brea, CA: 2010. Beckman Coulter: Inhibin B Gen II ELISA. [Google Scholar]
- 21.Szwarc SE, Bonetti M. Modelling menstrual status during and after adjuvant treatment for breast cancer. Stat Med. 2006;25:3534–3547. doi: 10.1002/sim.2445. [DOI] [PubMed] [Google Scholar]
- 22.Del Mastro L, Boni L, Michelotti A, et al. Role of luteinizing hormone-releasing hormone analog (LHRHa) triptorelin (T) in preserving ovarian function during chemotherapy for early breast cancer patients: Results of a multicenter phase III trial of Gruppo Italiano Mammella (GIM) group. J Clin Oncol. 2010;28(suppl):74s. abstr 528. [Google Scholar]
- 23.Gerber B, von Minckwitz G, Stehle H, et al. Effect of luteinizing hormone-releasing hormone agonist on ovarian function after modern adjuvant breast cancer chemotherapy: The GBG 37 ZORO study. J Clin Oncol. 2011;29:2334–2341. doi: 10.1200/JCO.2010.32.5704. [DOI] [PubMed] [Google Scholar]
- 24.Gerber B, Stehle H, Ricardo F, et al. ZORO: A prospective randomized multicenter study to prevent chemotherapy-induced ovarian failure with the GnRH-agonist goserelin in young hormone-insensitive breast cancer patients receiving anthracycline containing (neo-) adjuvant chemotherapy (GBG 37) J Clin Oncol. 2009;27(suppl):13s. abstr 526. [Google Scholar]
- 25.Leonard RC, Adamson D, Anderson R, et al. The OPTION trial of adjuvant ovarian protection by goserelin in adjuvant chemotherapy for early breast cancer. J Clin Oncol. 2010;28(suppl):89s. abstr 590. [Google Scholar]
- 26.Swain SM, Jeong JH, Geyer CE, Jr, et al. Longer therapy, iatrogenic amenorrhea, and survival in early breast cancer. N Engl J Med. 2010;362:2053–2065. doi: 10.1056/NEJMoa0909638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Early Breast Cancer Trialists' Collaborative Group: Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 28.Lobo RA. Potential options for preservation of fertility in women. N Engl J Med. 2005;353:64–73. doi: 10.1056/NEJMra043475. [DOI] [PubMed] [Google Scholar]
- 29.Goldman M, O'Hair K. Women's health, breast health: A review of the gynecologic effects of breast cancer. Obstet Gynecol Surv. 2009;64:469–480. doi: 10.1097/OGX.0b013e3181a713f1. quiz 499. [DOI] [PubMed] [Google Scholar]
- 30.Sonmezer M, Oktay K. Fertility preservation in female patients. Hum Reprod Update. 2004;10:251–266. doi: 10.1093/humupd/dmh021. [DOI] [PubMed] [Google Scholar]
- 31.Lee SJ, Schover LR, Partridge AH, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24:2917–2931. doi: 10.1200/JCO.2006.06.5888. [DOI] [PubMed] [Google Scholar]
- 32.Oktay K, Sonmezer M. Ovarian tissue banking for cancer patients: Fertility preservation, not just ovarian cryopreservation. Hum Reprod. 2004;19:477–480. doi: 10.1093/humrep/deh152. [DOI] [PubMed] [Google Scholar]