Abstract
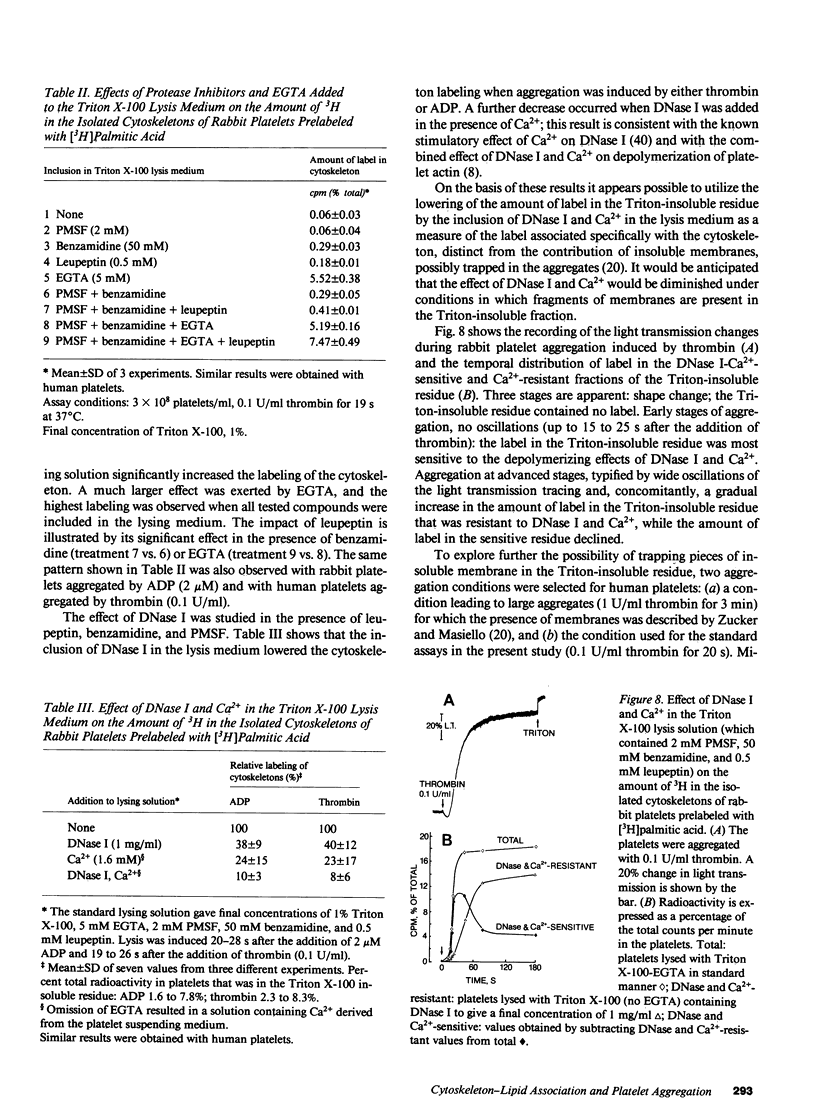
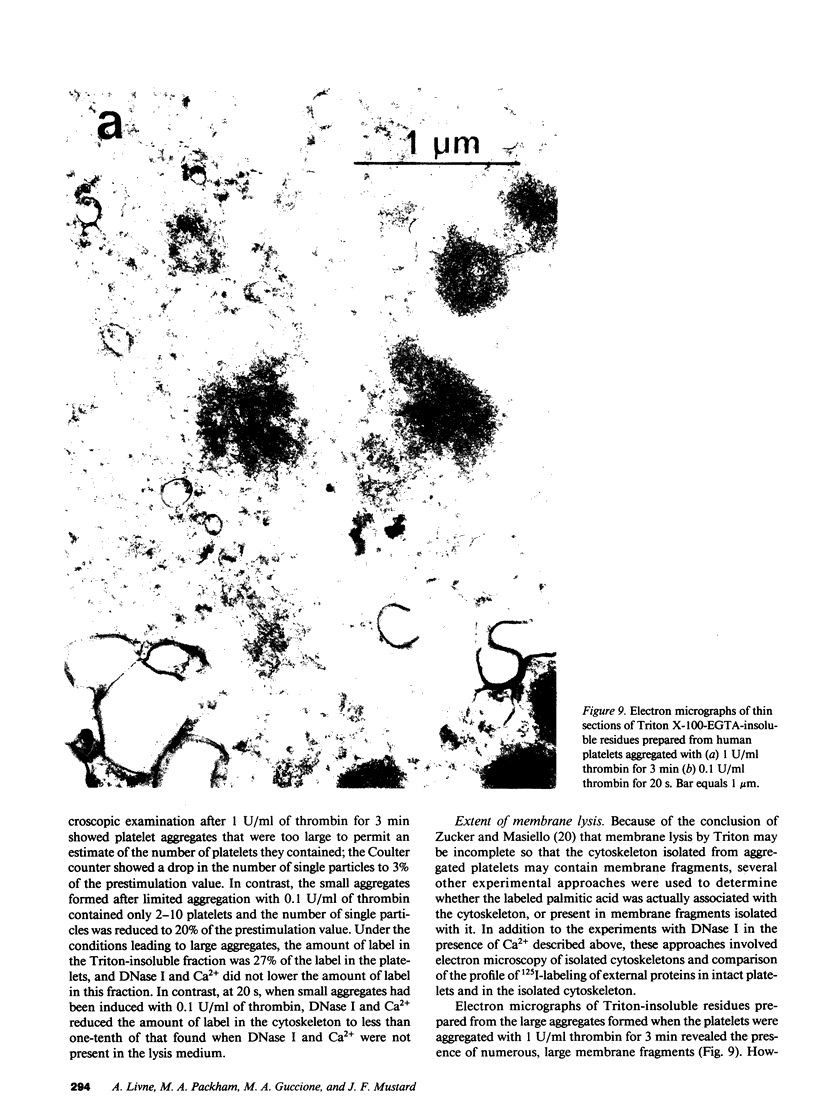
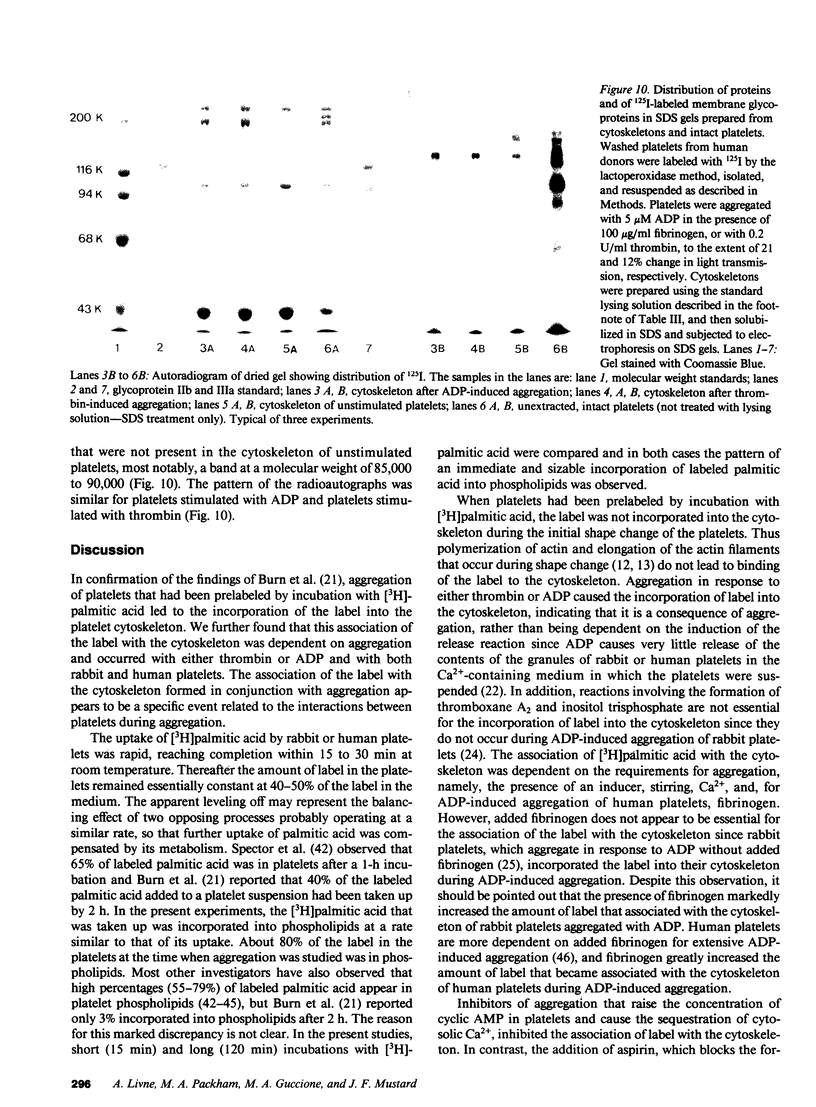
To investigate the association of lipid with the cytoskeleton of platelets during aggregation, rabbit and human platelets were isolated and labeled with [3H]palmitic acid; lipid extraction showed approximately 80% in phospholipid. Limited aggregation was induced with ADP or thrombin, and the cytoskeleton was isolated after lysis with 1% Triton X-100, 5 mM EGTA. Cytoskeleton from unactivated platelets had approximately 0.03% of the total label in the platelets, but after aggregation with ADP (2 microM) or thrombin (0.1 U/ml) for 20-30 s, 1.5-8% of the label was with the cytoskeleton. Fibrinogen enhanced aggregation and the association of label with the cytoskeleton; incorporation of label increased exponentially as aggregation proceeded, decreased exponentially during deaggregation, and appeared to be related to the number of sites of contact. Inhibitors that increase cyclic AMP inhibited aggregation and cytoskeletal labeling, but aspirin had no effect. Some experiments were done with DNase I and Ca2+ in the Triton X-100 lysis medium to cause actin depolymerization, under conditions in which the Ca2+-dependent protease activity was inhibited. This greatly reduced the association of label with the cytoskeleton at early time points, but when aggregation had proceeded further, a large proportion of the label was not dissociated by this treatment. These findings, electron microscopy, and the enrichment of the cytoskeleton of aggregated platelets with only some of the membrane proteins that were labeled by the 125I-lactoperoxidase method, indicated that with limited aggregation, the 3H-labeled lipid was mainly associated with the cytoskeleton and not with trapped membrane fragments resulting from incomplete lysis. Since the pattern of cytoskeleton labeling ([3H]palmitate) and the selective association of some membrane proteins with the cytoskeleton/lipid complex was the same with ADP and thrombin, the reactions must be dependent on aggregation and not on events associated with the release of granule contents.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agam G., Livne A. Platelet-platelet recognition during aggregation: distinct mechanisms determined by the release reaction. Thromb Haemost. 1984 Apr 30;51(2):145–149. [PubMed] [Google Scholar]
- Anderson R. A., Marchesi V. T. Regulation of the association of membrane skeletal protein 4.1 with glycophorin by a polyphosphoinositide. Nature. 1985 Nov 21;318(6043):295–298. doi: 10.1038/318295a0. [DOI] [PubMed] [Google Scholar]
- Andreoli V. M. Platelet lipids. II. In vitro incorporation of 1-14-C palmitic acid into lipid fractions of rabbit platelets. Eur J Pharmacol. 1968 Nov;4(4):404–410. doi: 10.1016/0014-2999(68)90026-5. [DOI] [PubMed] [Google Scholar]
- Ardlie N. G., Packham M. A., Mustard J. F. Adenosine diphosphate-induced platelet aggregation in suspensions of washed rabbit platelets. Br J Haematol. 1970 Jul;19(1):7–17. doi: 10.1111/j.1365-2141.1970.tb01596.x. [DOI] [PubMed] [Google Scholar]
- Ardlie N. G., Perry D. W., Packham M. A., Mustard J. F. Influence of apyrase on stability of suspensions of washed rabbit platelets. Proc Soc Exp Biol Med. 1971 Apr;136(4):1021–1023. doi: 10.3181/00379727-136-35419. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Begg D. A., Rodewald R., Rebhun L. I. The visualization of actin filament polarity in thin sections. Evidence for the uniform polarity of membrane-associated filaments. J Cell Biol. 1978 Dec;79(3):846–852. doi: 10.1083/jcb.79.3.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blikstad I., Markey F., Carlsson L., Persson T., Lindberg U. Selective assay of monomeric and filamentous actin in cell extracts, using inhibition of deoxyribonuclease I. Cell. 1978 Nov;15(3):935–943. doi: 10.1016/0092-8674(78)90277-5. [DOI] [PubMed] [Google Scholar]
- Burn P., Rotman A., Meyer R. K., Burger M. M. Diacylglycerol in large alpha-actinin/actin complexes and in the cytoskeleton of activated platelets. Nature. 1985 Apr 4;314(6010):469–472. doi: 10.1038/314469a0. [DOI] [PubMed] [Google Scholar]
- Deykin D., Desser R. K. The incorporation of acetate and palmitate into lipids by human platelets. J Clin Invest. 1968 Jul;47(7):1590–1602. doi: 10.1172/JCI105851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J. E. Identification of actin-binding protein as the protein linking the membrane skeleton to glycoproteins on platelet plasma membranes. J Biol Chem. 1985 Oct 5;260(22):11970–11977. [PubMed] [Google Scholar]
- Fox J. E. Linkage of a membrane skeleton to integral membrane glycoproteins in human platelets. Identification of one of the glycoproteins as glycoprotein Ib. J Clin Invest. 1985 Oct;76(4):1673–1683. doi: 10.1172/JCI112153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J. E., Phillips D. R. Polymerization and organization of actin filaments within platelets. Semin Hematol. 1983 Oct;20(4):243–260. [PubMed] [Google Scholar]
- Fox J. E., Phillips D. R. Role of phosphorylation in mediating the association of myosin with the cytoskeletal structures of human platelets. J Biol Chem. 1982 Apr 25;257(8):4120–4126. [PubMed] [Google Scholar]
- Kinlough-Rathbone R. L., Mustard J. F., Perry D. W., Dejana E., Cazenave J. P., Packham M. A., Harfenist E. J. Factors influencing the deaggregation of human and rabbit platelets. Thromb Haemost. 1983 Jun 28;49(3):162–167. [PubMed] [Google Scholar]
- Kometani M., Sato T., Fujii T. Platelet cytoskeletal components involved in shape change and secretion. Thromb Res. 1986 Mar 15;41(6):801–809. doi: 10.1016/0049-3848(86)90378-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Langer B. G., Gonnella P. A., Nachmias V. T. alpha-Actinin and vinculin in normal and thrombasthenic platelets. Blood. 1984 Mar;63(3):606–614. [PubMed] [Google Scholar]
- Lind S. E., Stossel T. P. The microfilament network of the platelet. Prog Hemost Thromb. 1982;6:63–84. [PubMed] [Google Scholar]
- Lind S. E., Yin H. L., Stossel T. P. Human platelets contain gelsolin. A regulator of actin filament length. J Clin Invest. 1982 Jun;69(6):1384–1387. doi: 10.1172/JCI110578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOLNAR J., LORAND L. Studies on apyrases. Arch Biochem Biophys. 1961 May;93:353–363. doi: 10.1016/0003-9861(61)90278-8. [DOI] [PubMed] [Google Scholar]
- Mustard J. F., Packham M. A., Kinlough-Rathbone R. L., Perry D. W., Regoeczi E. Fibrinogen and ADP-induced platelet aggregation. Blood. 1978 Aug;52(2):453–466. [PubMed] [Google Scholar]
- Mustard J. F., Packham M. A. Platelet aggregation and the platelet release reaction in thromboembolism. Can Med Assoc J. 1970 Oct 17;103(8):859–863. [PMC free article] [PubMed] [Google Scholar]
- Nachmias V. T. Platelet and megakaryocyte shape change: triggered alterations in the cytoskeleton. Semin Hematol. 1983 Oct;20(4):261–281. [PubMed] [Google Scholar]
- Okita J. R., Pidard D., Newman P. J., Montgomery R. R., Kunicki T. J. On the association of glycoprotein Ib and actin-binding protein in human platelets. J Cell Biol. 1985 Jan;100(1):317–321. doi: 10.1083/jcb.100.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuma M., Steiner M., Baldini M. Lipid content and in vitro incorporation of free fatty acids into lipids of human platelets: the effect of storage at 4 degrees C. Blood. 1971 Jul;38(1):27–38. [PubMed] [Google Scholar]
- Packham M. A., Guccione M. A., Greenberg J. P., Kinlough-Rathbone R. L., Mustard J. F. Release of 14C-serotonin during initial platelet changes induced by thrombin, collagen, or A23187. Blood. 1977 Nov;50(5):915–926. [PubMed] [Google Scholar]
- Painter R. G., Gaarde W., Ginsberg M. H. Direct evidence for the interaction of platelet surface membrane proteins GPIIb and III with cytoskeletal components: protein crosslinking studies. J Cell Biochem. 1985;27(3):277–290. doi: 10.1002/jcb.240270309. [DOI] [PubMed] [Google Scholar]
- Phillips D. R., Agin P. P. Platelet plasma membrane glycoproteins. Evidence for the presence of nonequivalent disulfide bonds using nonreduced-reduced two-dimensional gel electrophoresis. J Biol Chem. 1977 Mar 25;252(6):2121–2126. [PubMed] [Google Scholar]
- Phillips D. R., Jakábová M. Ca2+-dependent protease in human platelets. Specific cleavage of platelet polypeptides in the presence of added Ca2+. J Biol Chem. 1977 Aug 25;252(16):5602–5605. [PubMed] [Google Scholar]
- Phillips D. R., Jennings L. K., Edwards H. H. Identification of membrane proteins mediating the interaction of human platelets. J Cell Biol. 1980 Jul;86(1):77–86. doi: 10.1083/jcb.86.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pribluda V., Rotman A. Dynamics of membrane-cytoskeleton interactions in activated blood platelets. Biochemistry. 1982 Jun 8;21(12):2825–2832. doi: 10.1021/bi00541a003. [DOI] [PubMed] [Google Scholar]
- Schick P. K., Tuszynski G. P., Vander Voort P. W. Human platelet cytoskeletons: specific content of glycolipids and phospholipids. Blood. 1983 Jan;61(1):163–166. [PubMed] [Google Scholar]
- Solum N. O., Olsen T. M. Glycoprotein Ib in the Triton-insoluble (cytoskeletal) fraction of blood platelets. Biochim Biophys Acta. 1984 Jun 29;799(3):209–220. doi: 10.1016/0304-4165(84)90263-0. [DOI] [PubMed] [Google Scholar]
- Spector A. A., Hoak J. C., Warner E. D., Fry G. L. Utilization of long-chain free fatty acids by human platelets. J Clin Invest. 1970 Aug;49(8):1489–1496. doi: 10.1172/JCI106366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truglia J. A., Stracher A. Purification and characterization of a calcium dependent sulfhydryl protease from human platelets. Biochem Biophys Res Commun. 1981 May 29;100(2):814–822. doi: 10.1016/s0006-291x(81)80247-1. [DOI] [PubMed] [Google Scholar]
- Tuszynski G. P., Daniel J. L., Stewart G. Association of proteins with the platelet cytoskeleton. Semin Hematol. 1985 Oct;22(4):303–312. [PubMed] [Google Scholar]
- Vaskovsky V. E., Kostetsky E. Y. Modified spray for the detection of phospholipids on thin-layer chromatograms. J Lipid Res. 1968 May;9(3):396–396. [PubMed] [Google Scholar]
- Vickers J. D., Kinlough-Rathbone R. L., Mustard J. F. Accumulation of the inositol phosphates in thrombin-stimulated, washed rabbit platelets in the presence of lithium. Biochem J. 1984 Dec 1;224(2):399–405. doi: 10.1042/bj2240399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers J. D., Kinlough-Rathbone R. L., Mustard J. F. The decrease in phosphatidylinositol 4,5-bisphosphate in ADP-stimulated washed rabbit platelets is not primarily due to phospholipase C activation. Biochem J. 1986 Jul 15;237(2):327–332. doi: 10.1042/bj2370327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K., Dubyak G., Nachmias V. T. Rapid effects of phorbol ester on platelet shape change, cytoskeleton and calcium transient. FEBS Lett. 1986 Oct 6;206(2):273–278. doi: 10.1016/0014-5793(86)80995-4. [DOI] [PubMed] [Google Scholar]
- Zucker M. B., Masiello N. C. The Triton X-100-insoluble residue ("cytoskeleton") of aggregated platelets contains increased lipid phosphorus as well as 125I-labeled glycoproteins. Blood. 1983 Apr;61(4):676–683. [PubMed] [Google Scholar]