Abstract
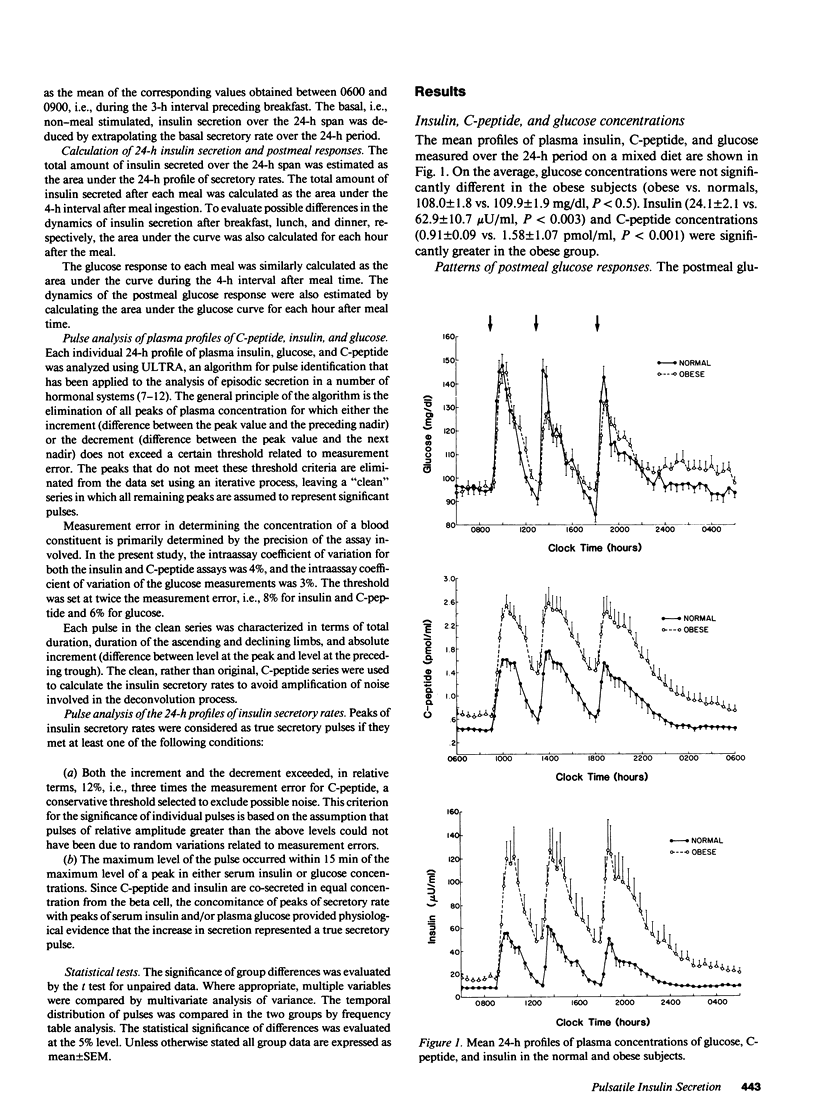
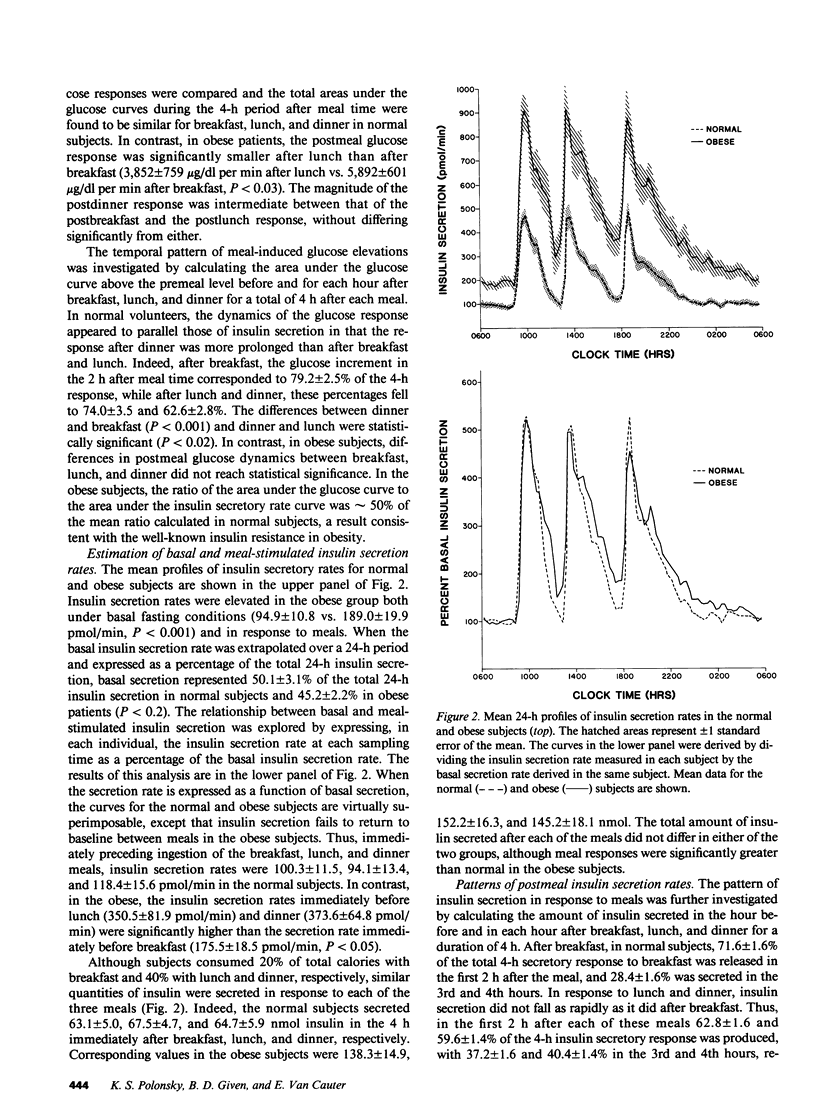
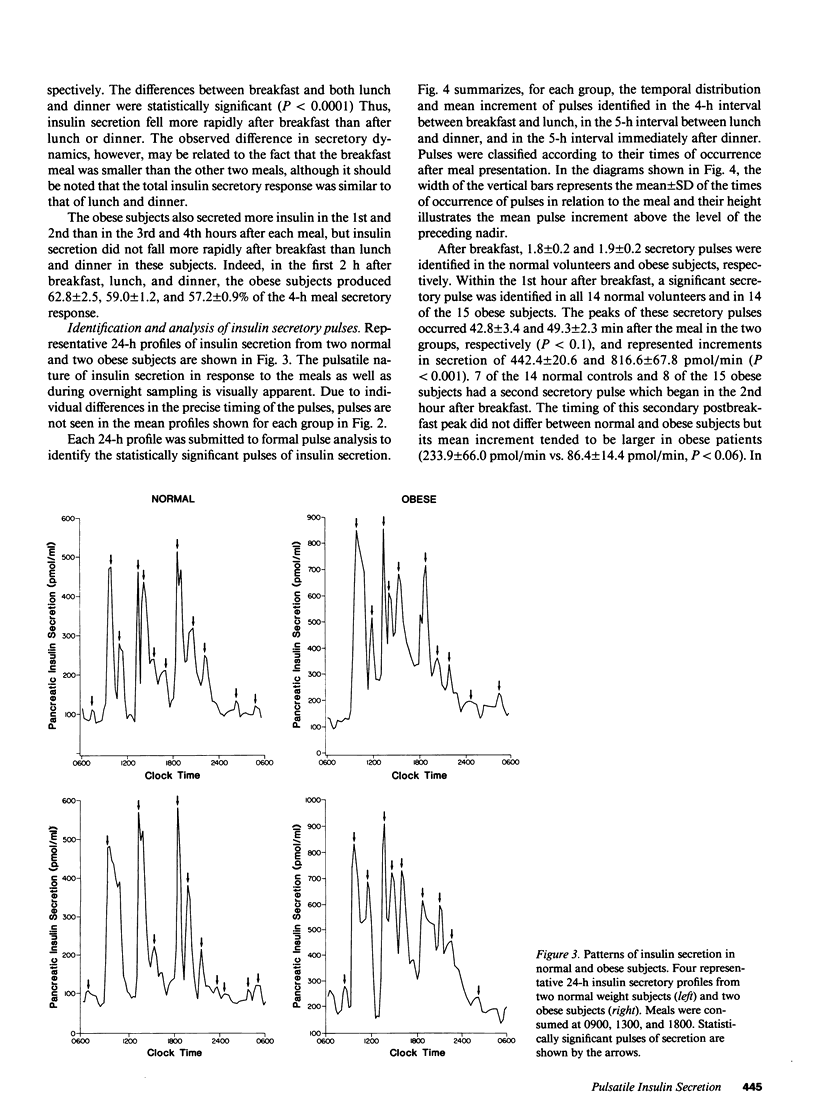
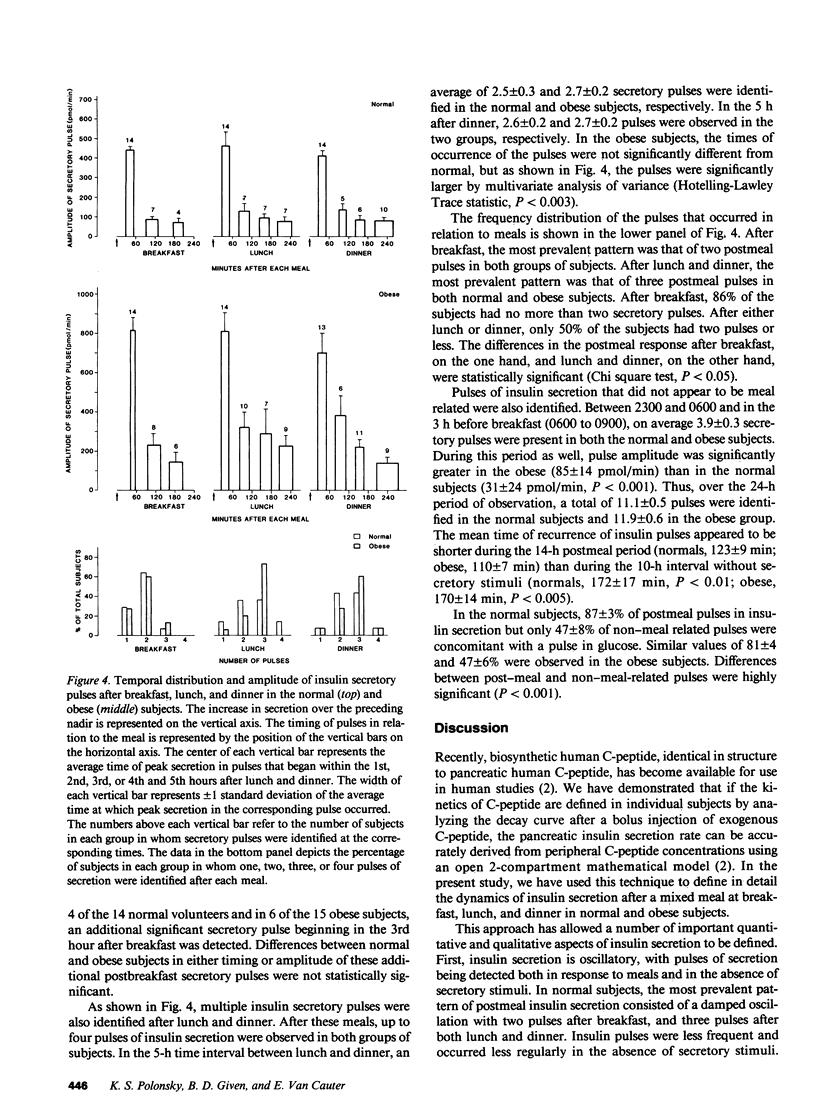
The pattern of endogenous insulin secretion over a 24-h period, which included three mixed meals, was evaluated in 14 normal volunteers and 15 obese subjects. Insulin secretory rates were calculated from plasma C-peptide levels using individually derived C-peptide kinetic parameters and a validated open two-compartment model of peripheral C-peptide kinetics. Insulin secretion rates were consistently elevated in the obese subjects under basal conditions (11.6 +/- 1.2 vs. 5.4 +/- 0.5 nmol/h) and in the 4 h after breakfast (139 +/- 15 vs. 63 +/- 5 nmol/4 h, P less than 0.001), lunch (152 +/- 16 vs. 67 +/- 5 nmol/4 h, P less than 0.001), and dinner (145 +/- 18 vs. 65 +/- 6 nmol/4 h, P less than 0.001). In the normal subjects, basal insulin secretion represented 50 +/- 2.1% of total 24-h insulin production, insulin secretion returned to baseline between meals, and equal quantities of insulin were secreted in the 4 h after breakfast, lunch, and dinner, despite the fact that subjects consumed half the number of calories at breakfast compared to lunch and dinner. Overall glucose responses were also similar after the three meals. In contrast, the pattern of insulin secretion in obese subjects was largely normal, albeit set at a higher level. However, the insulin secretion rate after meals did not return to baseline, and the secretion rate immediately before lunch (350.5 +/- 81.9 pmol/min) and dinner (373.6 +/- 64.8 pmol/min) was considerably higher than the secretion rate immediately before breakfast (175.5 +/- 18.5 pmol/min). In these overweight subjects, the glucose response after lunch was lower than after dinner. Analysis of individual 24-h insulin secretory profiles in the normal subjects revealed that insulin secretion was pulsatile. On average 11.1 +/- 0.5 pulses were produced in each 24-h period. The most prevalent temporal distribution of postmeal secretory pulses was two pulses after breakfast and three pulses after both lunch and dinner. Insulin secretion was also pulsatile during the period without meal stimuli: 3.9 +/- 0.3 pulses occurred during the period of overnight sampling and in the 3-h period before ingestion of the breakfast meal. In the obese subjects, the number and timing of secretory pulses was similar to those of normal volunteers, although the amplitude of the pulses was significantly greater. In both groups of subjects, greater than 80% of insulin pulses were concomitant with a pulse in glucose concentration in the postmeal period. The concomitancy rate was significantly lower in the interval without the meal stimuli, averaging 47% in both groups. Thus in obesity, although hypersecretion of insulin can be documented, the temporal pattern of secretion i s largely unaltered, which suggests that the functioning beta cell mass is enhance, but normal regulatory mechanisms influencing secretion are still operative.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Eaton R. P., Allen R. C., Schade D. S., Erickson K. M., Standefer J. Prehepatic insulin production in man: kinetic analysis using peripheral connecting peptide behavior. J Clin Endocrinol Metab. 1980 Sep;51(3):520–528. doi: 10.1210/jcem-51-3-520. [DOI] [PubMed] [Google Scholar]
- Faber O. K., Binder C., Markussen J., Heding L. G., Naithani V. K., Kuzuya H., Blix P., Horwitz D. L., Rubenstein A. H. Characterization of seven C-peptide antisera. Diabetes. 1978;27 (Suppl 1):170–177. doi: 10.2337/diab.27.1.s170. [DOI] [PubMed] [Google Scholar]
- Golstein J., Van Cauter E., Désir D., Noël P., Spire J. P., Refetoff S., Copinschi G. Effects of "jet lag" on hormonal patterns. IV. Time shifts increase growth hormone release. J Clin Endocrinol Metab. 1983 Mar;56(3):433–440. doi: 10.1210/jcem-56-3-433. [DOI] [PubMed] [Google Scholar]
- Goodner C. J., Walike B. C., Koerker D. J., Ensinck J. W., Brown A. C., Chideckel E. W., Palmer J., Kalnasy L. Insulin, glucagon, and glucose exhibit synchronous, sustained oscillations in fasting monkeys. Science. 1977 Jan 14;195(4274):177–179. doi: 10.1126/science.401543. [DOI] [PubMed] [Google Scholar]
- Hansen B. C., Jen K. C., Belbez Pek S., Wolfe R. A. Rapid oscillations in plasma insulin, glucagon, and glucose in obese and normal weight humans. J Clin Endocrinol Metab. 1982 Apr;54(4):785–792. doi: 10.1210/jcem-54-4-785. [DOI] [PubMed] [Google Scholar]
- Jaspan J. B., Lever E., Polonsky K. S., Van Cauter E. In vivo pulsatility of pancreatic islet peptides. Am J Physiol. 1986 Aug;251(2 Pt 1):E215–E226. doi: 10.1152/ajpendo.1986.251.2.E215. [DOI] [PubMed] [Google Scholar]
- Lang D. A., Matthews D. R., Peto J., Turner R. C. Cyclic oscillations of basal plasma glucose and insulin concentrations in human beings. N Engl J Med. 1979 Nov 8;301(19):1023–1027. doi: 10.1056/NEJM197911083011903. [DOI] [PubMed] [Google Scholar]
- Linkowski P., Mendlewicz J., Leclercq R., Brasseur M., Hubain P., Golstein J., Copinschi G., Van Cauter E. The 24-hour profile of adrenocorticotropin and cortisol in major depressive illness. J Clin Endocrinol Metab. 1985 Sep;61(3):429–438. doi: 10.1210/jcem-61-3-429. [DOI] [PubMed] [Google Scholar]
- Nathan D. M., Lou P., Avruch J. Intensive conventional and insulin pump therapies in adult type I diabetes. A crossover study. Ann Intern Med. 1982 Jul;97(1):31–36. doi: 10.7326/0003-4819-97-1-31. [DOI] [PubMed] [Google Scholar]
- Polonsky K. S., Licinio-Paixao J., Given B. D., Pugh W., Rue P., Galloway J., Karrison T., Frank B. Use of biosynthetic human C-peptide in the measurement of insulin secretion rates in normal volunteers and type I diabetic patients. J Clin Invest. 1986 Jan;77(1):98–105. doi: 10.1172/JCI112308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polonsky K., Frank B., Pugh W., Addis A., Karrison T., Meier P., Tager H., Rubenstein A. The limitations to and valid use of C-peptide as a marker of the secretion of insulin. Diabetes. 1986 Apr;35(4):379–386. doi: 10.2337/diab.35.4.379. [DOI] [PubMed] [Google Scholar]
- Rizza R. A., Gerich J. E., Haymond M. W., Westland R. E., Hall L. D., Clemens A. H., Service F. J. Control of blood sugar in insulin-dependent diabetes: comparison of an artificial endocrine pancreas, continuous subcutaneous insulin infusion, and intensified conventional insulin therapy. N Engl J Med. 1980 Dec 4;303(23):1313–1318. doi: 10.1056/NEJM198012043032301. [DOI] [PubMed] [Google Scholar]
- Robbins D. C., Jaspan J., Vasquez B., Van Cauter E. Biphasic patterns of peripheral insulin and glucose levels after lunch in normal subjects. Diabetes Care. 1987 May-Jun;10(3):293–299. doi: 10.2337/diacare.10.3.293. [DOI] [PubMed] [Google Scholar]
- Santiago J. V., Clemens A. H., Clarke W. L., Kipnis D. M. Closed-loop and open-loop devices for blood glucose control in normal and diabetic subjects. Diabetes. 1979 Jan;28(1):71–84. doi: 10.2337/diab.28.1.71. [DOI] [PubMed] [Google Scholar]
- Schiffrin A. D., Desrosiers M., Aleyassine H., Belmonte M. M. Intensified insulin therapy in the type I diabetic adolescent: a controlled trial. Diabetes Care. 1984 Mar-Apr;7(2):107–113. doi: 10.2337/diacare.7.2.107. [DOI] [PubMed] [Google Scholar]
- Simon C., Brandenberger G., Follenius M. Ultradian oscillations of plasma glucose, insulin, and C-peptide in man during continuous enteral nutrition. J Clin Endocrinol Metab. 1987 Apr;64(4):669–674. doi: 10.1210/jcem-64-4-669. [DOI] [PubMed] [Google Scholar]
- Van Cauter E., L'Hermite M., Copinschi G., Refetoff S., Desir D., Robyn C. Quantitative analysis of spontaneous variations of plasma prolactin in normal man. Am J Physiol. 1981 Nov;241(5):E355–E363. doi: 10.1152/ajpendo.1981.241.5.E355. [DOI] [PubMed] [Google Scholar]


