Abstract
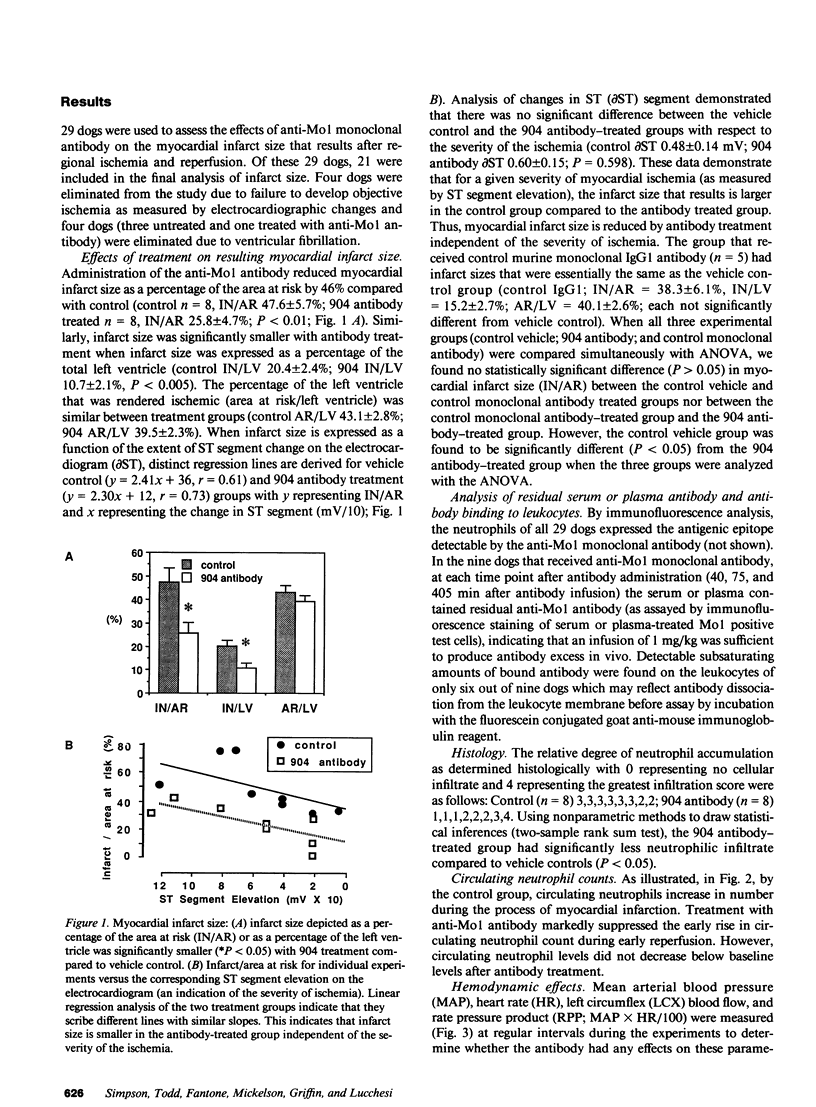
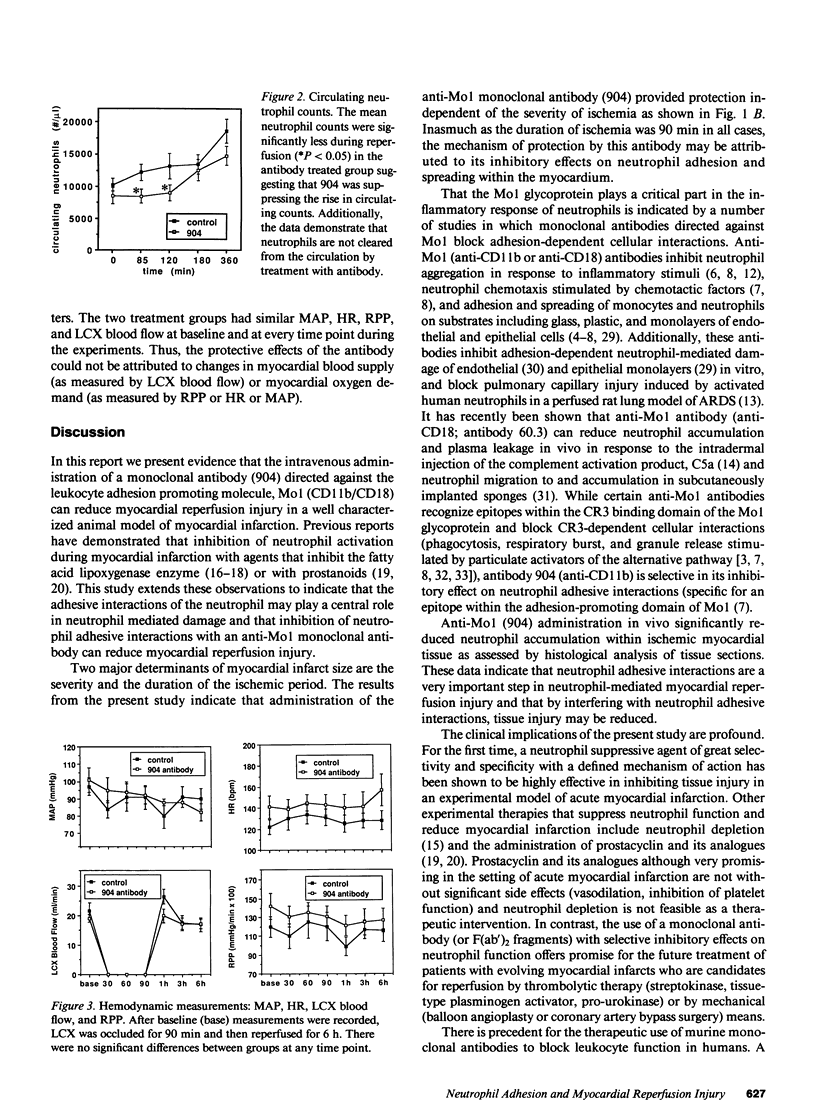
A monoclonal antibody (904) that binds to a leukocyte cell adhesion-promoting glycoprotein, (Mo1; CD11b/CD18) was administered (1 mg/kg, iv.) to open chest anesthetized dogs 45 min after the induction of regional myocardial ischemia. Ischemia was produced by occluding the left circumflex coronary artery (LCX) for 90 min and then reperfusing for 6 h. There was no difference between control and antibody treated groups with respect to arterial blood pressure, heart rate, or LCX blood flow. Administration of antibody produced no observable effect on circulating neutrophil counts, suggesting that antibody-bound neutrophils were not cleared from the circulation. The mean size of myocardial infarct expressed as percentage of the area at risk of infarction that resulted was reduced by 46% with anti-Mo1 treatment (25.8 +/- 4.7%, n = 8) compared to control (47.6 +/- 5.7%, n = 8; P less than 0.01). The area at risk of infarction was similar between groups. Circulating (serum) antibody excess was confirmed in all 8 anti-Mo1 treated dogs by immunofluorescence analysis. Analysis of ST segment elevation on the electrocardiogram as an indicator of the severity of ischemia suggests that the anti-Mo1 reduces infarct size independent of the severity of ischemia. An additional group of dogs (n = 5) was tested with a control monoclonal antibody of the same subtype (murine IgG1) and was found to produce no significant reduction in myocardial infarct size. Accumulation of neutrophils within the myocardium was significantly attenuated with 904 treatment when analyzed by histological methods. These data demonstrate that administration of anti-Mo1 monoclonal antibody after the induction of regional myocardial ischemia results in reduced myocardial reperfusion injury as measured by ultimate infarct size.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. C., Miller L. J., Schmalstieg F. C., Rothlein R., Springer T. A. Contributions of the Mac-1 glycoprotein family to adherence-dependent granulocyte functions: structure-function assessments employing subunit-specific monoclonal antibodies. J Immunol. 1986 Jul 1;137(1):15–27. [PubMed] [Google Scholar]
- Anderson D. C., Springer T. A. Leukocyte adhesion deficiency: an inherited defect in the Mac-1, LFA-1, and p150,95 glycoproteins. Annu Rev Med. 1987;38:175–194. doi: 10.1146/annurev.me.38.020187.001135. [DOI] [PubMed] [Google Scholar]
- Arfors K. E., Lundberg C., Lindbom L., Lundberg K., Beatty P. G., Harlan J. M. A monoclonal antibody to the membrane glycoprotein complex CD18 inhibits polymorphonuclear leukocyte accumulation and plasma leakage in vivo. Blood. 1987 Jan;69(1):338–340. [PubMed] [Google Scholar]
- Arnaout M. A., Hakim R. M., Todd R. F., 3rd, Dana N., Colten H. R. Increased expression of an adhesion-promoting surface glycoprotein in the granulocytopenia of hemodialysis. N Engl J Med. 1985 Feb 21;312(8):457–462. doi: 10.1056/NEJM198502213120801. [DOI] [PubMed] [Google Scholar]
- Arnaout M. A., Todd R. F., 3rd, Dana N., Melamed J., Schlossman S. F., Colten H. R. Inhibition of phagocytosis of complement C3- or immunoglobulin G-coated particles and of C3bi binding by monoclonal antibodies to a monocyte-granulocyte membrane glycoprotein (Mol). J Clin Invest. 1983 Jul;72(1):171–179. doi: 10.1172/JCI110955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednar M., Smith B., Pinto A., Mullane K. M. Nafazatrom-induced salvage of ischemic myocardium in anesthetized dogs is mediated through inhibition of neutrophil function. Circ Res. 1985 Jul;57(1):131–141. doi: 10.1161/01.res.57.1.131. [DOI] [PubMed] [Google Scholar]
- Beller D. I., Springer T. A., Schreiber R. D. Anti-Mac-1 selectively inhibits the mouse and human type three complement receptor. J Exp Med. 1982 Oct 1;156(4):1000–1009. doi: 10.1084/jem.156.4.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson S. B., Bussel J. B., Kimberly R. P., Valinsky J. E., Nachman R. L., Unkeless J. C. Treatment of refractory immune thrombocytopenic purpura with an anti-Fc gamma-receptor antibody. N Engl J Med. 1986 May 8;314(19):1236–1239. doi: 10.1056/NEJM198605083141907. [DOI] [PubMed] [Google Scholar]
- Clarkson S. B., Kimberly R. P., Valinsky J. E., Witmer M. D., Bussel J. B., Nachman R. L., Unkeless J. C. Blockade of clearance of immune complexes by an anti-Fc gamma receptor monoclonal antibody. J Exp Med. 1986 Aug 1;164(2):474–489. doi: 10.1084/jem.164.2.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dana N., Styrt B., Griffin J. D., Todd R. F., 3rd, Klempner M. S., Arnaout M. A. Two functional domains in the phagocyte membrane glycoprotein Mo1 identified with monoclonal antibodies. J Immunol. 1986 Nov 15;137(10):3259–3263. [PubMed] [Google Scholar]
- Dana N., Todd R. F., 3rd, Pitt J., Springer T. A., Arnaout M. A. Deficiency of a surface membrane glycoprotein (Mo1) in man. J Clin Invest. 1984 Jan;73(1):153–159. doi: 10.1172/JCI111186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener A. M., Beatty P. G., Ochs H. D., Harlan J. M. The role of neutrophil membrane glycoprotein 150 (Gp-150) in neutrophil-mediated endothelial cell injury in vitro. J Immunol. 1985 Jul;135(1):537–543. [PubMed] [Google Scholar]
- Fischer A., Griscelli C., Blanche S., Le Deist F., Veber F., Lopez M., Delaage M., Olive D., Mawas C., Janossy G. Prevention of graft failure by an anti-HLFA-1 monoclonal antibody in HLA-mismatched bone-marrow transplantation. Lancet. 1986 Nov 8;2(8515):1058–1061. doi: 10.1016/s0140-6736(86)90465-4. [DOI] [PubMed] [Google Scholar]
- Fishbein M. C., Meerbaum S., Rit J., Lando U., Kanmatsuse K., Mercier J. C., Corday E., Ganz W. Early phase acute myocardial infarct size quantification: validation of the triphenyl tetrazolium chloride tissue enzyme staining technique. Am Heart J. 1981 May;101(5):593–600. doi: 10.1016/0002-8703(81)90226-x. [DOI] [PubMed] [Google Scholar]
- Giger U., Boxer L. A., Simpson P. J., Lucchesi B. R., Todd R. F., 3rd Deficiency of leukocyte surface glycoproteins Mo1, LFA-1, and Leu M5 in a dog with recurrent bacterial infections: an animal model. Blood. 1987 Jun;69(6):1622–1630. [PubMed] [Google Scholar]
- Harlan J. M., Killen P. D., Senecal F. M., Schwartz B. R., Yee E. K., Taylor R. F., Beatty P. G., Price T. H., Ochs H. D. The role of neutrophil membrane glycoprotein GP-150 in neutrophil adherence to endothelium in vitro. Blood. 1985 Jul;66(1):167–178. [PubMed] [Google Scholar]
- Ismail G., Morganroth M. L., Todd R. F., 3rd, Boxer L. A. Prevention of pulmonary injury in isolated perfused rat lungs by activated human neutrophils preincubated with anti-Mo1 monoclonal antibody. Blood. 1987 Apr;69(4):1167–1174. [PubMed] [Google Scholar]
- Letvin N. L., Todd R. F., 3rd, Palley L. S., Schlossman S. F., Griffin J. D. Conservation of myeloid surface antigens on primate granulocytes. Blood. 1983 Feb;61(2):408–410. [PubMed] [Google Scholar]
- Lucchesi B. R., Burmeister W. E., Lomas T. E., Abrams G. D. Ischemic changes in the canine heart as affected by the dimethyl quaternary analog of propranolol, UM-272 (SC-27761). J Pharmacol Exp Ther. 1976 Nov;199(2):310–328. [PubMed] [Google Scholar]
- Mullane K. M., Read N., Salmon J. A., Moncada S. Role of leukocytes in acute myocardial infarction in anesthetized dogs: relationship to myocardial salvage by anti-inflammatory drugs. J Pharmacol Exp Ther. 1984 Feb;228(2):510–522. [PubMed] [Google Scholar]
- Roberts A. J., Cipriano P. R., Alonso D. R., Jacbostein J. G., Combes J. R., Gay W. A., Jr Evaluation of methods for the quantification of experimental myocardial infarction. Circulation. 1978 Jan;57(1):35–41. doi: 10.1161/01.cir.57.1.35. [DOI] [PubMed] [Google Scholar]
- Romaschin A. D., Rebeyka I., Wilson G. J., Mickle D. A. Conjugated dienes in ischemic and reperfused myocardium: an in vivo chemical signature of oxygen free radical mediated injury. J Mol Cell Cardiol. 1987 Mar;19(3):289–302. doi: 10.1016/s0022-2828(87)80596-5. [DOI] [PubMed] [Google Scholar]
- Romson J. L., Hook B. G., Kunkel S. L., Abrams G. D., Schork M. A., Lucchesi B. R. Reduction of the extent of ischemic myocardial injury by neutrophil depletion in the dog. Circulation. 1983 May;67(5):1016–1023. doi: 10.1161/01.cir.67.5.1016. [DOI] [PubMed] [Google Scholar]
- Romson J. L., Hook B. G., Rigot V. H., Schork M. A., Swanson D. P., Lucchesi B. R. The effect of ibuprofen on accumulation of indium-111-labeled platelets and leukocytes in experimental myocardial infarction. Circulation. 1982 Nov;66(5):1002–1011. doi: 10.1161/01.cir.66.5.1002. [DOI] [PubMed] [Google Scholar]
- Simon R. H., DeHart P. D., Todd R. F., 3rd Neutrophil-induced injury of rat pulmonary alveolar epithelial cells. J Clin Invest. 1986 Nov;78(5):1375–1386. doi: 10.1172/JCI112724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson P. J., Mickelson J., Fantone J. C., Gallagher K. P., Lucchesi B. R. Iloprost inhibits neutrophil function in vitro and in vivo and limits experimental infarct size in canine heart. Circ Res. 1987 May;60(5):666–673. doi: 10.1161/01.res.60.5.666. [DOI] [PubMed] [Google Scholar]
- Simpson P. J., Mitsos S. E., Ventura A., Gallagher K. P., Fantone J. C., Abrams G. D., Schork M. A., Lucchesi B. R. Prostacyclin protects ischemic reperfused myocardium in the dog by inhibition of neutrophil activation. Am Heart J. 1987 Jan;113(1):129–137. doi: 10.1016/0002-8703(87)90020-2. [DOI] [PubMed] [Google Scholar]
- Todd R. F., 3rd, Arnaout M. A., Rosin R. E., Crowley C. A., Peters W. A., Babior B. M. Subcellular localization of the large subunit of Mo1 (Mo1 alpha; formerly gp 110), a surface glycoprotein associated with neutrophil adhesion. J Clin Invest. 1984 Oct;74(4):1280–1290. doi: 10.1172/JCI111538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd R. F., 3rd, Nadler L. M., Schlossman S. F. Antigens on human monocytes identified by monoclonal antibodies. J Immunol. 1981 Apr;126(4):1435–1442. [PubMed] [Google Scholar]
- Todd R. F., 3rd, Van Agthoven A., Schlossman S. F., Terhorst C. Structural analysis of differentiation antigens Mo1 and Mo2 on human monocytes. Hybridoma. 1982;1(3):329–337. doi: 10.1089/hyb.1.1982.1.329. [DOI] [PubMed] [Google Scholar]
- Vivaldi M. T., Kloner R. A., Schoen F. J. Triphenyltetrazolium staining of irreversible ischemic injury following coronary artery occlusion in rats. Am J Pathol. 1985 Dec;121(3):522–530. [PMC free article] [PubMed] [Google Scholar]
- Wallis W. J., Beatty P. G., Ochs H. D., Harlan J. M. Human monocyte adherence to cultured vascular endothelium: monoclonal antibody-defined mechanisms. J Immunol. 1985 Oct;135(4):2323–2330. [PubMed] [Google Scholar]
- Wallis W. J., Hickstein D. D., Schwartz B. R., June C. H., Ochs H. D., Beatty P. G., Klebanoff S. J., Harlan J. M. Monoclonal antibody-defined functional epitopes on the adhesion-promoting glycoprotein complex (CDw18) of human neutrophils. Blood. 1986 Apr;67(4):1007–1013. [PubMed] [Google Scholar]
- Wright S. D., Rao P. E., Van Voorhis W. C., Craigmyle L. S., Iida K., Talle M. A., Westberg E. F., Goldstein G., Silverstein S. C. Identification of the C3bi receptor of human monocytes and macrophages by using monoclonal antibodies. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5699–5703. doi: 10.1073/pnas.80.18.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]


