Abstract
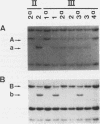
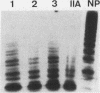
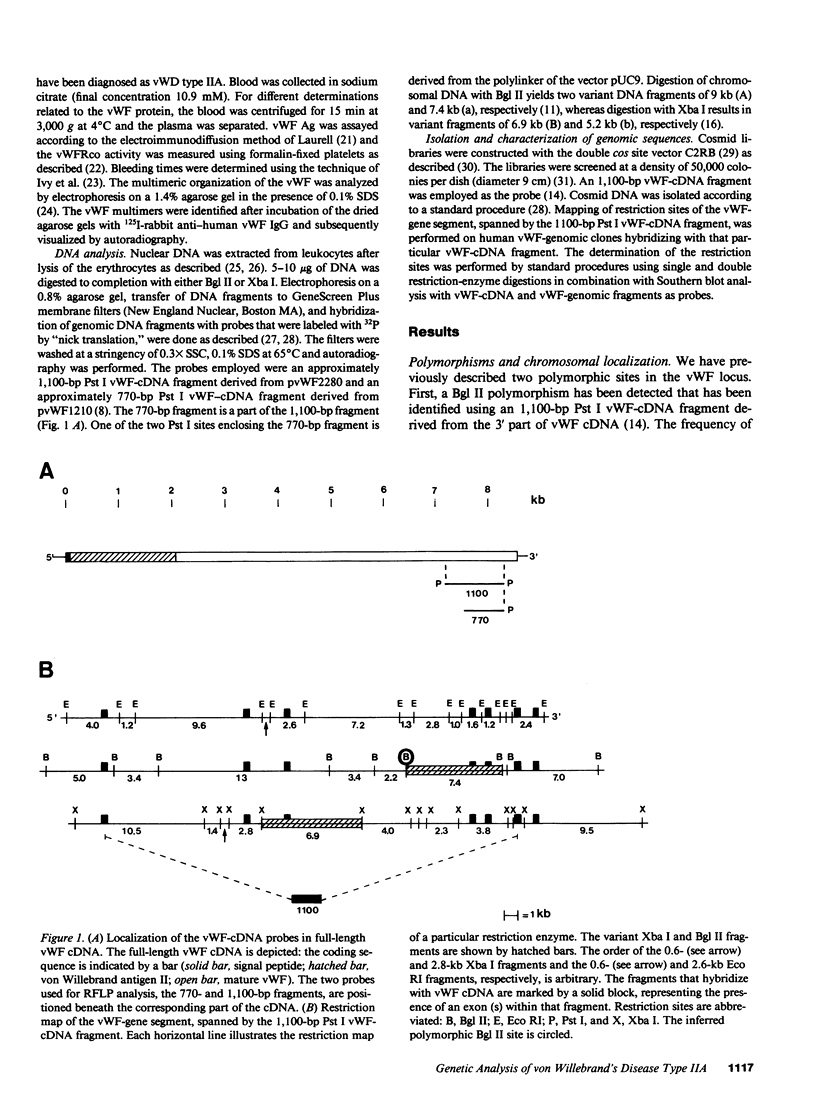
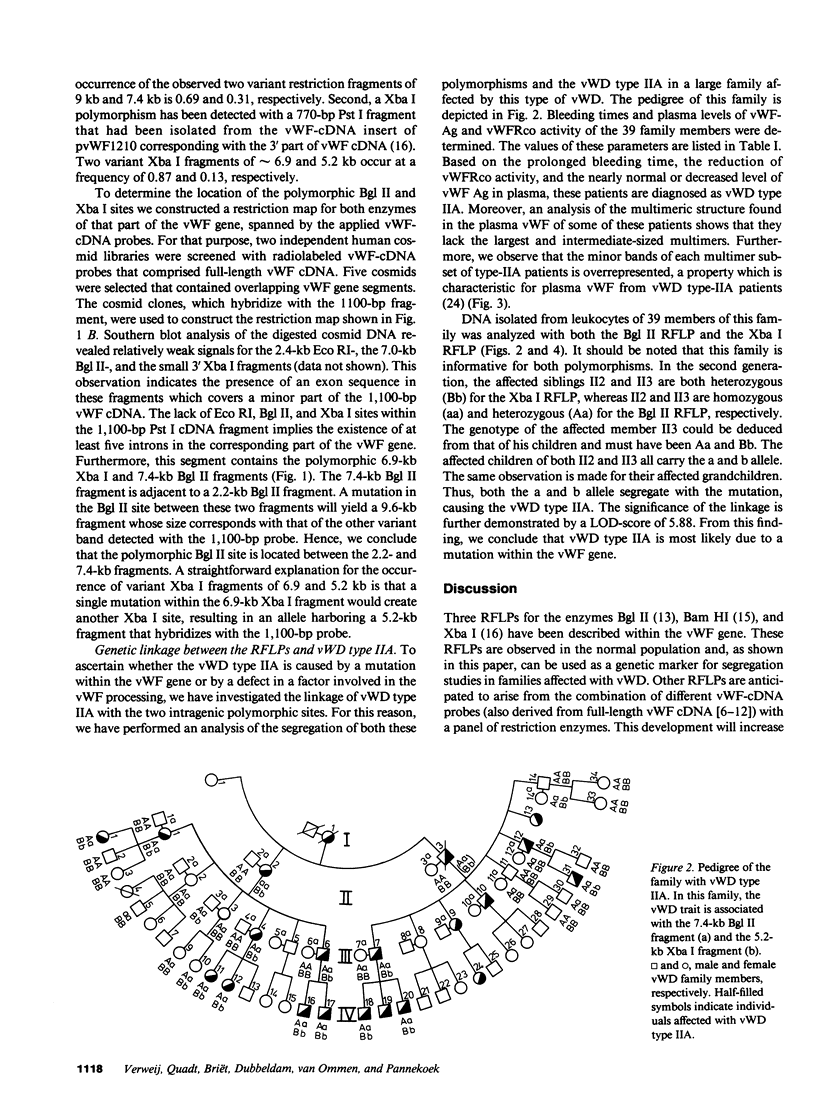
Restriction fragment length polymorphisms (RFLPs), using the enzymes Bgl II and Xba I in conjunction with human von Willebrand factor (vWF) cDNA probes, have been described previously. In the present study we demonstrate the localization of both genetic markers within the vWF gene. The RFLPs were used to study the segregation of alleles associated with von Willebrand's disease (vWD) type IIA in a comprehensive, affected family. Individuals of this family were tested for their bleeding time and their plasma was analyzed for vWF antigen concentration and vWF ristocetin-cofactor activity. Based on these data, the affected members were diagnosed as vWD type-IIA patients; this conclusion was confirmed by the analysis of the multimeric vWF pattern of some of the patients. It was demonstrated that both RFLPs are completely linked with the vWD type-IIA trait. From this finding, we conclude that the defect that causes the vWD type IIA is most likely due to a mutation in the vWF gene and not to a mutation in a gene involved in posttranslational processing of the vWF protein.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baas F., Bikker H., van Ommen G. J., de Vijlder J. J. Unusual scarcity of restriction site polymorphism in the human thyroglobulin gene. A linkage study suggesting autosomal dominance of a defective thyroglobulin allele. Hum Genet. 1984;67(3):301–305. doi: 10.1007/BF00291357. [DOI] [PubMed] [Google Scholar]
- Bates P. F., Swift R. A. Double cos site vectors: simplified cosmid cloning. Gene. 1983 Dec;26(2-3):137–146. doi: 10.1016/0378-1119(83)90183-x. [DOI] [PubMed] [Google Scholar]
- Bonthron D. T., Handin R. I., Kaufman R. J., Wasley L. C., Orr E. C., Mitsock L. M., Ewenstein B., Loscalzo J., Ginsburg D., Orkin S. H. Structure of pre-pro-von Willebrand factor and its expression in heterologous cells. Nature. 1986 Nov 20;324(6094):270–273. doi: 10.1038/324270a0. [DOI] [PubMed] [Google Scholar]
- Botstein D., White R. L., Skolnick M., Davis R. W. Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am J Hum Genet. 1980 May;32(3):314–331. [PMC free article] [PubMed] [Google Scholar]
- Fay P. J., Kawai Y., Wagner D. D., Ginsburg D., Bonthron D., Ohlsson-Wilhelm B. M., Chavin S. I., Abraham G. N., Handin R. I., Orkin S. H. Propolypeptide of von Willebrand factor circulates in blood and is identical to von Willebrand antigen II. Science. 1986 May 23;232(4753):995–998. doi: 10.1126/science.3486471. [DOI] [PubMed] [Google Scholar]
- Fujimura Y., Titani K., Holland L. Z., Russell S. R., Roberts J. R., Elder J. H., Ruggeri Z. M., Zimmerman T. S. von Willebrand factor. A reduced and alkylated 52/48-kDa fragment beginning at amino acid residue 449 contains the domain interacting with platelet glycoprotein Ib. J Biol Chem. 1986 Jan 5;261(1):381–385. [PubMed] [Google Scholar]
- Ginsburg D., Handin R. I., Bonthron D. T., Donlon T. A., Bruns G. A., Latt S. A., Orkin S. H. Human von Willebrand factor (vWF): isolation of complementary DNA (cDNA) clones and chromosomal localization. Science. 1985 Jun 21;228(4706):1401–1406. doi: 10.1126/science.3874428. [DOI] [PubMed] [Google Scholar]
- Gitschier J., Drayna D., Tuddenham E. G., White R. L., Lawn R. M. Genetic mapping and diagnosis of haemophilia A achieved through a BclI polymorphism in the factor VIII gene. 1985 Apr 25-May 1Nature. 314(6013):738–740. doi: 10.1038/314738a0. [DOI] [PubMed] [Google Scholar]
- Gralnick H. R., Coller B. S., Sultan Y. Studies of the human factor VIII/von Willebrand factor protein. III. Qualitative defects in von Willebrand's disease. J Clin Invest. 1975 Oct;56(4):814–827. doi: 10.1172/JCI108160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gralnick H. R., Williams S. B., McKeown L. P., Maisonneuve P., Jenneau C., Sultan Y., Rick M. E. In vitro correction of the abnormal multimeric structure of von Willebrand factor in type IIa von Willebrand's disease. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5968–5972. doi: 10.1073/pnas.82.17.5968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D., Meselson M. Plasmid screening at high colony density. Gene. 1980 Jun;10(1):63–67. doi: 10.1016/0378-1119(80)90144-4. [DOI] [PubMed] [Google Scholar]
- Hoyer L. W., Shainoff J. R. Factor VIII-related protein circulates in normal human plasma as high molecular weight multimers. Blood. 1980 Jun;55(6):1056–1059. [PubMed] [Google Scholar]
- Kan Y. W., Dozy A. M. Polymorphism of DNA sequence adjacent to human beta-globin structural gene: relationship to sickle mutation. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5631–5635. doi: 10.1073/pnas.75.11.5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurell C. B. Quantitative estimation of proteins by electrophoresis in agarose gel containing antibodies. Anal Biochem. 1966 Apr;15(1):45–52. doi: 10.1016/0003-2697(66)90246-6. [DOI] [PubMed] [Google Scholar]
- Lynch D. C., Zimmerman T. S., Collins C. J., Brown M., Morin M. J., Ling E. H., Livingston D. M. Molecular cloning of cDNA for human von Willebrand factor: authentication by a new method. Cell. 1985 May;41(1):49–56. doi: 10.1016/0092-8674(85)90060-1. [DOI] [PubMed] [Google Scholar]
- Myers R. M., Fischer S. G., Maniatis T., Lerman L. S. Modification of the melting properties of duplex DNA by attachment of a GC-rich DNA sequence as determined by denaturing gradient gel electrophoresis. Nucleic Acids Res. 1985 May 10;13(9):3111–3129. doi: 10.1093/nar/13.9.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers R. M., Larin Z., Maniatis T. Detection of single base substitutions by ribonuclease cleavage at mismatches in RNA:DNA duplexes. Science. 1985 Dec 13;230(4731):1242–1246. doi: 10.1126/science.4071043. [DOI] [PubMed] [Google Scholar]
- Nishino K., Lynch D. C. A polymorphism of the human von Willebrand factor (vWf) gene with BamHI. Nucleic Acids Res. 1986 Jun 11;14(11):4697–4697. doi: 10.1093/nar/14.11.4697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkin S. H., Kazazian H. H., Jr, Antonarakis S. E., Goff S. C., Boehm C. D., Sexton J. P., Waber P. G., Giardina P. J. Linkage of beta-thalassaemia mutations and beta-globin gene polymorphisms with DNA polymorphisms in human beta-globin gene cluster. Nature. 1982 Apr 15;296(5858):627–631. doi: 10.1038/296627a0. [DOI] [PubMed] [Google Scholar]
- Quadt R., Verweij C. L., de Vries C. J., Briët E., Pannekoek H. A polymorphic Xba I site within the human von Willebrand factor (vWF) gene identified by a vWF cDNA clone. Nucleic Acids Res. 1986 Sep 11;14(17):7139–7139. doi: 10.1093/nar/14.17.7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggeri Z. M., Zimmerman T. S. Platelets and von Willebrand disease. Semin Hematol. 1985 Jul;22(3):203–218. [PubMed] [Google Scholar]
- Ruggeri Z. M., Zimmerman T. S. The complex multimeric composition of factor VIII/von Willebrand factor. Blood. 1981 Jun;57(6):1140–1143. [PubMed] [Google Scholar]
- Ruggeri Z. M., Zimmerman T. S. Variant von Willebrand's disease: characterization of two subtypes by analysis of multimeric composition of factor VIII/von Willebrand factor in plasma and platelets. J Clin Invest. 1980 Jun;65(6):1318–1325. doi: 10.1172/JCI109795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler J. E., Shelton-Inloes B. B., Sorace J. M., Harlan J. M., Titani K., Davie E. W. Cloning and characterization of two cDNAs coding for human von Willebrand factor. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6394–6398. doi: 10.1073/pnas.82.19.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakariassen K. S., Bolhuis P. A., Sixma J. J. Human blood platelet adhesion to artery subendothelium is mediated by factor VIII-Von Willebrand factor bound to the subendothelium. Nature. 1979 Jun 14;279(5714):636–638. doi: 10.1038/279636a0. [DOI] [PubMed] [Google Scholar]
- Shelton-Inloes B. B., Titani K., Sadler J. E. cDNA sequences for human von Willebrand factor reveal five types of repeated domains and five possible protein sequence polymorphisms. Biochemistry. 1986 Jun 3;25(11):3164–3171. doi: 10.1021/bi00359a014. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Titani K., Kumar S., Takio K., Ericsson L. H., Wade R. D., Ashida K., Walsh K. A., Chopek M. W., Sadler J. E., Fujikawa K. Amino acid sequence of human von Willebrand factor. Biochemistry. 1986 Jun 3;25(11):3171–3184. doi: 10.1021/bi00359a015. [DOI] [PubMed] [Google Scholar]
- Tuddenham E. G., Lane R. S., Rotblat F., Johnson A. J., Snape T. J., Middleton S., Kernoff P. B. Response to infusions of polyelectrolyte fractionated human factor VIII concentrate in human haemophilia A and von Willebrand's disease. Br J Haematol. 1982 Oct;52(2):259–267. doi: 10.1111/j.1365-2141.1982.tb03888.x. [DOI] [PubMed] [Google Scholar]
- Verweij C. L., Diergaarde P. J., Hart M., Pannekoek H. Full-length von Willebrand factor (vWF) cDNA encodes a highly repetitive protein considerably larger than the mature vWF subunit. EMBO J. 1986 Aug;5(8):1839–1847. doi: 10.1002/j.1460-2075.1986.tb04435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verweij C. L., Hofker M., Quadt R., Briet E., Pannekoek H. RFLP for a human von Willebrand factor (vWF) cDNA clone, pvWF1100. Nucleic Acids Res. 1985 Nov 25;13(22):8289–8289. doi: 10.1093/nar/13.22.8289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verweij C. L., de Vries C. J., Distel B., van Zonneveld A. J., van Kessel A. G., van Mourik J. A., Pannekoek H. Construction of cDNA coding for human von Willebrand factor using antibody probes for colony-screening and mapping of the chromosomal gene. Nucleic Acids Res. 1985 Jul 11;13(13):4699–4717. doi: 10.1093/nar/13.13.4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D. D., Mayadas T., Marder V. J. Initial glycosylation and acidic pH in the Golgi apparatus are required for multimerization of von Willebrand factor. J Cell Biol. 1986 Apr;102(4):1320–1324. doi: 10.1083/jcb.102.4.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D. D., Mayadas T., Urban-Pickering M., Lewis B. H., Marder V. J. Inhibition of disulfide bonding of von Willebrand protein by monensin results in small, functionally defective multimers. J Cell Biol. 1985 Jul;101(1):112–120. doi: 10.1083/jcb.101.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weening R. S., Roos D., Loos J. A. Oxygen consumption of phagocytizing cells in human leukocyte and granulocyte preparations: a comparative study. J Lab Clin Med. 1974 Apr;83(4):570–577. [PubMed] [Google Scholar]
- Zimmerman T. S., Dent J. A., Ruggeri Z. M., Nannini L. H. Subunit composition of plasma von Willebrand factor. Cleavage is present in normal individuals, increased in IIA and IIB von Willebrand disease, but minimal in variants with aberrant structure of individual oligomers (types IIC, IID, and IIE). J Clin Invest. 1986 Mar;77(3):947–951. doi: 10.1172/JCI112394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman T. S., Ruggeri Z. M. Von Willebrand's disease. Prog Hemost Thromb. 1982;6:203–236. [PubMed] [Google Scholar]
- van Ommen G. J., Arnberg A. C., Baas F., Brocas H., Sterk A., Tegelaers W. H., Vassart G., de Vijlder J. J. The human thyroglobulin gene contains two 15-17 kb introns near its 3'-end. Nucleic Acids Res. 1983 Apr 25;11(8):2273–2285. doi: 10.1093/nar/11.8.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]