Abstract
Sleep-disordered breathing (SDB) comprises a diverse set of disorders marked by abnormal respiration during sleep. Clinicians should realize that SDB may present as acute cardiopulmonary failure in susceptible patients. In this review, we discuss three clinical phenotypes of acute cardiopulmonary failure from SDB: acute ventilatory failure, acute congestive heart failure, and sudden death. We review the pathophysiologic mechanisms and recommend general principles for management. Timely recognition of, and therapy for, SDB in the setting of acute cardiopulmonary failure may improve short- and long-term outcomes.
Sleep-disordered breathing (SDB) encompasses a diverse group of disorders characterized by abnormal respiration during sleep. Although commonly associated with obstructive or central sleep apnea syndromes, SDB also complicates diseases of the neuromuscular system (eg, amyotrophic lateral sclerosis), chest wall (eg, kyphoscoliosis and obesity), heart (congestive heart failure [CHF]), and lung (eg, COPD).1 Most forms of SDB are generally viewed as chronic conditions whose adverse effects are expressed gradually over months to years. However, some patients with SDB may present with acute cardiopulmonary failure largely or entirely due to decompensated SDB. Such patients may be given a misdiagnosis if the clinician is not alert to the possibility of SDB as a cause of acute illness, leading to the administration of ineffective and potentially harmful therapies in the acute setting and a missed opportunity for long-term treatment upon hospital discharge. Indeed, without definitive long-term treatment of SDB, such patients are at risk of future episodes of cardiopulmonary failure and death, even when short-term stabilization is achieved.
Unfortunately, multiple factors confound the timely diagnosis of acute cardiopulmonary failure from SDB. SDB is relatively common but it may be subtle in its presentation.2 Furthermore, many affected patients have not yet received a diagnosis at the time of presentation to the ICU. Full montage polysomnography (PSG) is labor intensive and is not routinely available in many ICUs. Finally, the presence of other common comorbidities such as asthma or diastolic heart failure may distract the clinician from considering latent SDB. Because of these issues and because successful therapy for obstructive sleep apnea (OSA) requires specific interventions, a high index of suspicion is necessary.
We believe that physicians should routinely consider decompensated SDB in the differential diagnosis of acute cardiopulmonary failure. To inform timely diagnosis and therapy, this review presents an overview of the acute cardiopulmonary manifestations of SDB, focusing on acutely decompensated OSA, the obesity hypoventilation syndrome (OHS), and the so-called “overlap syndrome” of patients with both COPD and OSA. An exhaustive review of these disorders is beyond the scope of this article. Instead, we describe three clinical phenotypes of acute cardiopulmonary failure that may be seen in patients with these disorders, including acute ventilatory failure, acute CHF, and sudden death.
OSA: a Major Cause of SDB
Population-based studies have shown that the prevalence of moderate or severe OSA (defined as apnea-hypopnea index [AHI] ≥15) is 7% to 14% in men and 2% to 7% in women.3 Still, the vast majority of affected individuals in the community have probably not been given this diagnosis.4,5 Typical clinical features should prompt suspicion for OSA, including unrefreshing sleep, excessive daytime somnolence, witnessed apneas or choking during sleep, loud snoring, morning headaches, difficulty concentrating, and depressed mood or energy.
When the history is not available or is inconclusive, clinicians may need to base a presumptive clinical diagnosis on the presence of known or putative risk factors, including older age, increased BMI, increased waist and neck circumference, crowded oropharynx, high Mallampati score, short mandibular length, and alcohol or sedative use.2-3 Prolonged bedside observation may reveal the presence of excessive daytime somnolence or obstructive respiratory events, and nursing reports of intermittent and cyclic nocturnal desaturation should prompt consideration of SDB. Laboratory data are occasionally helpful: polycythemia is infrequent but it suggests longstanding hypoxemia in this context. The presence of an elevated bicarbonate level may implicate OHS or other causes of chronic ventilatory failure, and an arterial blood gas measurement should be obtained.6
An increasing body of evidence links OSA to hypertension, coronary artery disease, CHF, stroke, impaired glucose metabolism, arrhythmia, and neurocognitive abnormalities.7 Furthermore, several large, prospective, longitudinal, population-based studies have shown that OSA is associated with an increased risk of all-cause mortality, particularly in cases of severe OSA and in middle-aged men.8-13
Recent research has elucidated the mechanisms by which OSA leads to adverse health effects. OSA is characterized by repeated cycles of upper airway obstruction, apnea, and arousal.14 This causes repetitive cycles of intermittent hypoxemia and sympathetic activation, with numerous deleterious consequences, including adverse mechanical cardiopulmonary interactions, endothelial dysfunction, inflammation, and impaired glucose metabolism.7 Accordingly, OSA may be particularly dangerous when superimposed upon chronic diseases such as CHF and COPD,15 underscoring the importance of identifying untreated OSA in the setting of decompensated cardiopulmonary disease.
The Role of Obesity in SDB
The relationship between obesity and SDB deserves further consideration before we address the broader problem of acute cardiopulmonary failure in SDB. Obesity is a major risk factor for OSA, and BMI and other measures of adiposity such as an increased neck circumference and a high waist to hip ratio correlate with its severity as measured by the AHI.16,17 Alarmingly, there has been a dramatic rise in severe obesity (BMI ≥40 kg/m2) over the past 2 decades.18
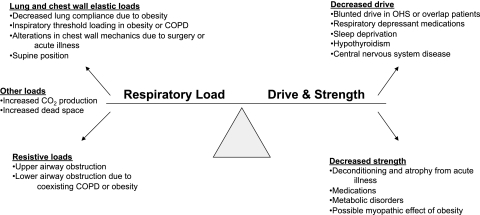
Figure 1.
Mechanisms of ventilatory failure. OHS = obesity hypoventilation syndrome. (Adapted with permission from Schmidt and Hall22)
However, uncomplicated OSA is not the only form of SDB in obese patients. Obese patients display a spectrum of abnormal breathing during sleep, and some obese patients develop reduced daytime ventilation.19 These latter patients are said to have OHS, defined by the presence of awake hypercapnia (room air resting Paco2 ≥45 mm Hg at sea level) and SDB in patients with BMI ≥30 kg/m2 (after other causes of hypercapnia have been excluded).17 Although OSA is not necessary for the diagnosis of OHS, it is present in 90% of all patients with OHS.20 In obese patients with chronic hypoventilation, daytime hypercapnia is associated with severity of OSA, higher BMI, and degree of restrictive chest wall mechanics.21 In summary, breathing during sleep may have a variable relationship with obesity, with three main groups of patients: obese patients with normal breathing during sleep, obese patients with OSA but with normal daytime ventilation, and obese patients with nighttime and daytime hypoventilation (OHS), most of whom have concurrent moderate to severe OSA.
Phenotypes of Acute Cardiopulmonary Failure in SDB
There are few prospective studies that have systematically addressed the problem of acute cardiopulmonary failure from SDB. Most evidence implicating SDB in acute cardiac or respiratory failure comes from laboratory experiments, retrospective and descriptive studies, case reports, and extrapolation from related clinical studies. From these, we can identify three main phenotypes, which we discuss following. These syndromes may occur in isolation but may develop more often in patients with comorbid cardiopulmonary disease.
Acute Ventilatory Failure
Characterized by inadequate alveolar ventilation, this form of respiratory failure is marked by acute or acute-on-chronic respiratory acidosis. Specific causes are diverse and often multifactorial and result from an imbalance among respiratory drive, strength, and load. Patients with SDB are susceptible to ventilatory failure from a variety of insults (Figure 1).22 Although many such patients are readily given a diagnosis of acute ventilatory failure after routine evaluation, patients who present with the more subtle manifestations of decompensated SDB, such as lethargy and confusion, may be given a misdiagnosis if SDB is not considered.
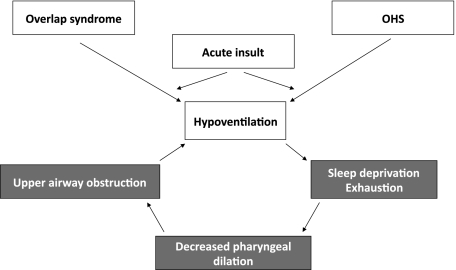
Figure 2.
Proposed “vicious cycle” of acute ventilatory failure in patients with obstructive sleep apnea (OSA). Diverse acute insults may occur (eg, pulmonary embolism, infection, or abdominal surgery). Because obese patients or patients with overlap syndrome have substantial chronic respiratory loads, they develop hypoventilation with a lower degree of acute insult. OSA compounds this hypoventilation because some patients with OSA have a blunted ventilatory response to hypercapnia. As respiratory failure progresses to somnolence and exhaustion, upper airway tone is compromised, further loading the respiratory system, and reinforcing the deleterious effects of the acute load. See Figure 1 legend for expansion of abbreviations.
Ventilatory Failure Related to OSA:
In patients with OSA, acute ventilatory failure may develop when illness increases ventilatory load or when medications acutely attenuate respiratory drive or airway patency. The latter phenomenon is an important consideration in postoperative patients with OSA.23 Anesthetic agents may promote hypoventilation by affecting upper airway patency while simultaneously causing respiratory depression, effects that may persist over several days.24 Similarly, medical patients with isolated OSA may develop acute ventilatory failure in the setting of acute neuromuscular weakness, new toxic or metabolic encephalopathy, pharmacologically induced CNS depression, acute sleep deprivation, or other disorders that affect airway patency or respiratory drive.25,26
Ventilatory Failure in Patients With OSA and COPD:
The phrase “overlap syndrome” has been used to describe the coexistence of OSA and COPD in the same patient. The prevalence of this syndrome among consecutive patients with OSA is between 10% and 15%.6,27,28 The prevalence of COPD in patients with OSA, however, is similar to its prevalence in the general population.29
The overlap syndrome has important pathophysiologic consequences. Patients with the overlap syndrome experience profound nocturnal oxygen desaturation and are more likely to have abnormal daytime blood gas values than are patients with either COPD or OSA alone.30 Correspondingly, right-sided heart failure (cor pulmonale) is common.31 A stark illustration of the synergy between OSA and COPD is evident in a clinical study that found that 86% of overlap patients had pulmonary hypertension, compared with 16% of patients with OSA alone.32 Finally, some data suggest that patients with the overlap syndrome have a blunted CNS response to hypercapnia.33
In the overlap syndrome, the pathophysiologic synergy may cause chronic hypercapnia at a lower BMI than that of patients with OHS, and at a higher FEV1 than that of hypercapnic patients with severe COPD. The cause of this hypoventilation is likely multifactorial and is not entirely explained by measures such as AHI, BMI, or pulmonary function studies.28
Patients with the overlap syndrome are vulnerable to developing acute ventilatory failure. Sleep hypoventilation, particularly during rapid eye movement sleep, is common in patients with COPD who present with hypercapnic respiratory failure.34,35 In addition, patients with the overlap syndrome are at increased risk of death and hospitalization due to COPD compared with patients with only COPD of similar severity.36 These data reinforce the importance of considering OSA when patients present with ventilatory failure otherwise attributed to COPD. This is particularly true given that many COPD exacerbations are not associated with an obvious cause, leaving open the possibility that untreated OSA has a role.36,37
Ventilatory Failure in Patients With OHS:
Similar to patients with the overlap syndrome or with uncomplicated COPD, patients with OHS may present with acute-on-chronic respiratory failure. Hypercapnia has been reported to be present in 10% to 20% of obese ambulatory patients with OSA.16 Moreover, in a case series of obese patients with OSA and respiratory failure, all patients had evidence of acute-on-chronic respiratory acidosis.38 Alarmingly, even though the chronic hypoventilation of OHS is associated with excess morbidity and mortality in obese patients, it is frequently underdiagnosed and undertreated.39,40
The effects of obesity on respiration are complex. Obesity reduces the compliance of the lung and the chest wall, while increasing airways resistance.41 Obese patients have a blunted central response to hypercapnia and may also have relative neuromuscular weakness, with increased work of breathing.1,16,42 Finally, obesity imposes a higher oxygen cost of breathing and increases CO2 production.43,44 In summary, excess body fat imposes substantial mechanical and metabolic loads on the respiratory system and may also affect respiratory drive and strength.
In the context of these chronic pathophysiologic changes, the effects of acute insults are likely magnified. In severely obese patients with OSA (most of whom have OHS), ventilation may be seriously compromised by even small increases in acute airflow obstruction or ventilatory load, or by minor depression of neuromuscular competence. Furthermore, even without new acute insults, patients with OSA may develop acute hypercapnia during sleep if there is insufficient ventilation time between nocturnal apneas to exhale the accumulated CO2.45 Given these observations, it is imperative to suspect OSA when obese patients develop seemingly acute ventilatory failure.
In patients with the overlap syndrome or OHS, acute ventilatory failure likely develops because of a “vicious cycle” (Figure 2).7 At baseline, these patients have both intrathoracic and extrathoracic airflow obstruction. Acute insults (such as infection, pulmonary edema, or pulmonary embolism) may lead to the typical consequences of muscle fatigue, hypoventilation, and sleep deprivation. Crucially, however, in patients with concurrent OSA, sleep deprivation may worsen upper airway obstruction,46 further compromising ventilation. Even as Paco2 rises, some patients with the overlap syndrome or obesity have a blunted ventilatory response, and catastrophic acidosis and hypoxemia may result from seemingly small insults.
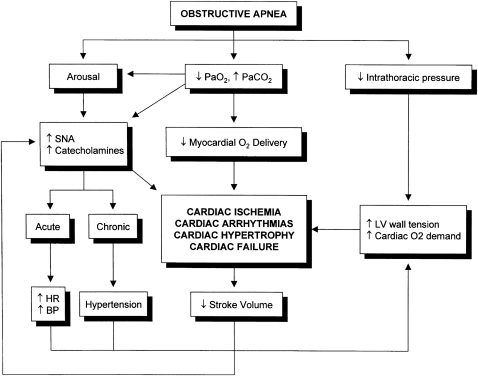
Figure 3.
Mechanisms of cardiac dysfunction in OSA. HR = heart rate; LV =left ventricle; SNA =sympathetic nervous system activity. See Figure 2 legend for expansion of other abbreviations. (Reprinted with permission from the American Thoracic Society.50)
Acute CHF
Heart failure is associated with multiple patterns of abnormal breathing during sleep. One distinctive pattern, Cheyne-Stokes respiration with central sleep apnea, occurs mostly in patients with severe chronic heart failure and hypocapnia and is associated with a worse prognosis.47 The management of this condition is controversial and is beyond the scope of this review. We instead focus on the role of OSA in acutely decompensated heart failure.
An association between OSA and heart failure has been shown in multiple clinical reports. In a recent prospective study of 395 consecutive patients admitted with acute decompensated heart failure who were able to complete inpatient sleep studies, Khayat et al48 found that SDB was present in 298 patients and that OSA was the predominant form of SDB (226 out of 298 patients, or 75%). In the Sleep Heart Health Study, one of the largest, prospective, longitudinal, community-based studies of OSA, 4,422 patients who were free of heart disease at the time of baseline PSG were followed for a median of 8.7 years.49 The investigators found that OSA was significantly associated with incident heart failure in multivariate analysis.
Multiple phenomena may converge to cause acute heart failure in OSA (Figure 3).50 During apnea events, patients with OSA generate substantial negative intrathoracic pressure as respiratory efforts continue against a partially or totally obstructed upper airway. This causes a dramatic increase in afterload, culminating in decreased left ventricular (LV) stroke volume.51 One group estimated that, for each 10 cm H2O fall in esophageal pressure below −10 cm H2O, LV stroke volume falls by 6.3 mL.52 Patients with OSA may develop intrathoracic pressures as low as −70 cm H2O.
These effects on LV afterload and myocardial work may be particularly important in patients with coronary artery disease. Scharf et al53 examined the effect of large negative intrathoracic pressure swings in patients undergoing radionuclide ventriculography. During a Mueller maneuver, 64% of patients with coronary artery disease developed regional wall motion abnormalities, whereas patients without coronary artery disease maintained normal wall motion.
In addition to these mechanical interactions, nocturnal myocardial ischemia may contribute to episodes of acute heart failure in patients with OSA. Intermittent hypoxia and repeated surges in sympathetic activity during arousal increase myocardial oxygen demand and compromise myocardial oxygen supply.13 Susceptible patients are, therefore, vulnerable to acute ischemic LV dysfunction.14,54 Indeed, patients with nocturnal onset of myocardial ischemia have a high likelihood of having OSA, suggesting that acute neurohormonal and hemodynamic abnormalities of OSA may be a trigger for myocardial infarction.55
Nocturnal cycles of apnea, hypoxemia, and arousal may also cause acute CHF by inducing arrhythmias. Significant evidence shows an association between OSA and atrial fibrillation.56,57 Moreover, in patients younger than 65 years, obesity and nocturnal oxygen desaturation are significantly associated with incident atrial fibrillation.56 Therefore, atrial fibrillation represents another mechanism through which OSA can lead to acute heart failure.
Interestingly, just as OSA may cause acute CHF, decompensated heart failure may worsen OSA. Heart failure leads to fluid retention. When affected patients sleep in the supine or recumbent positions, fluid is displaced from the legs into the thorax and neck. Several investigators have shown that such rostral fluid displacement causes extrathoracic airflow obstruction by worsening pharyngeal collapsibility.58 In this regard, patients with comorbid OSA and CHF may also develop a vicious cycle, analogous to the one discussed previously for patients with the overlap syndrome or OHS. Indeed, the Sleep Heart Health Study investigators recently reported that incident cardiovascular disease was independently associated with worsening of SDB over 5 years.59 The mutually reinforcing interactions between OSA, heart failure, fluid retention, and worsening pharyngeal obstruction may underlie many episodes of acute CHF.
The phenomena outlined above are likely directly relevant in OSA patients with established CHF. However, a clinical syndrome of acute pulmonary edema has been reported in OSA, without LV dysfunction. In an animal model of obstruction, Fletcher et al60 showed that pulmonary edema developed after as little as 8 h, despite normal pulmonary capillary wedge pressure and cardiac function. Similarly, multiple reports describe a syndrome of acute pulmonary edema in patients with OSA but normal LV function.61,62 The mechanism of this pulmonary edema is unclear. It may be related to the mechanical interactions and other phenomena outlined above, or it may reflect the development of so-called “negative pressure edema” as respiratory effort persists against an obstructed upper airway. However, regardless of LV function, clinicians should suspect untreated OSA when obese patients present with acute pulmonary edema or CHF.
Acute Cor Pulmonale:
OSA has received much attention as a cause of pulmonary hypertension, but its role in isolated severe acute right-sided heart failure is probably limited. Although it has been reported that pulmonary hypertension can be present in 20% to 40% of patients with isolated OSA, the degree is usually mild.14,63 Factors such as age, BMI, lung function, and comorbid left-sided heart disease are more important than AHI as determinants of severe pulmonary hypertension in patients with OSA.64,65 Accordingly, when confronted with acute cor pulmonale in patients with OSA, clinicians should search for other causes of pulmonary hypertension, in addition to OSA, including coexisting diastolic dysfunction, OHS, COPD, chronic thromboembolic disease, and others. Indeed, severe pulmonary hypertension should never be assumed to be due to OSA alone.
In patients with acute cor pulmonale, respiratory failure can be particularly challenging to treat. Instituting mechanical ventilation may provoke hemodynamic instability, sometimes with catastrophic consequences. Positive pressure ventilation reduces right ventricular stroke volume (and consequently cardiac output) by reducing venous return.66,67 This effect may be poorly tolerated in some patients with right-sided heart failure. In addition, positive pressure ventilation has complex effects on pulmonary vascular resistance, reducing it in circumstances in which hypoxic pulmonary vasoconstriction is alleviated68 but increasing it when lung hyperinflation occurs.69 These effects are likely to be less prominent with noninvasive ventilation (NIV) than with conventional mechanical ventilation, providing a further rationale for the use of NIV in these circumstances. Still, patients should be monitored closely, and if endotracheal intubation is anticipated, preparations should be made to support the circulation simultaneously. Volume boluses should be used cautiously, and the use of inotropes and vasopressors is often required.70
Sudden Death
Sudden death is an extreme manifestation of acute cardiopulmonary failure from OSA.71 The association between OSA and sudden cardiac death is most apparent in outpatient studies. Doherty et al72 found that sudden unexpected death was more likely in untreated OSA patients than in patients receiving continuous positive airway pressure (CPAP) therapy. Another seminal report found that OSA patients were much more likely to experience sudden death during the nighttime hours.73 Intensivists may encounter this phenomenon when patients are brought to the ICU after being resuscitated from out-of-hospital cardiac arrest. However, these data also suggest that patients with OSA may be at risk of sudden, in-hospital cardiac arrest, particularly if left untreated.
Multiple phenomena may cause sudden death in OSA patients. OSA is strongly associated with arrhythmias including malignant ventricular rhythms, which could cause sudden death.74,75 Such arrhythmias would be particularly dangerous in patients with significant concurrent heart or lung disease. Sudden death may also result from extreme hypoxemia, particularly in morbidly obese patients with OSA. Obesity reduces the functional residual capacity, meaning that the lungs contain much less oxygen at end-expiration. With apnea, oxygen reserve is limited, and obese patients are well known to desaturate quickly. However, when the functional residual capacity is further reduced by acute illness, altered chest wall mechanics, or atelectasis, obstructive respiratory events will cause abrupt and extreme oxygen desaturation.76,77 Rigorous clinical data are limited, but it is reasonable to infer that such periods of extreme hypoxemia may precipitate sudden death in susceptible patients, possibly by provoking arrhythmias or cardiac dysfunction.
Finally, sudden death may result from failure of the homeostatic response to pharyngeal closure. Normally, upper airway obstruction from OSA is resolved by either arousal or arousal-independent reflexive opening of the upper airway. However, if these mechanisms are attenuated, hypoxemia and hypoventilation will worsen, causing cerebral and myocardial ischemia. As evidence of this, Khoo et al78 reported the case of a 37-year-old with OSA and recurrent, nocturnal, in-hospital cardiac arrest. When the patient was studied with PSG, a 3-min apnea during which the oxygen saturation dropped from 100% to 30% was observed. Cyanosis and diffuse electroencephalographic slowing occurred. This apnea was only terminated when the technician awoke the patient. This intriguing finding suggests that some episodes of “sudden” cardiac arrest among hospitalized patients may reflect untreated OSA, particularly if acute sleep deprivation or another condition affecting the arousal mechanism and threshold (such as administration of sedatives and narcotics even at judicious doses) has developed during the course of hospitalization.
Critical Care Considerations
Suspicion of SDB as a cause of acute cardiopulmonary failure should prompt several considerations (Table 1). First, when decompensated SDB is suspected in patients with established or impending ventilatory failure, NIV should be instituted immediately, as long as no contraindications are present. In this setting, failure of NIV should be recognized promptly (Table 2). Rapid recognition and treatment of OSA or OHS may interrupt the vicious cycle of ventilatory failure and avert further deterioration, but the effective use of NIV requires both a frequent bedside presence by trained personnel as well as careful attention to detail (see Table 2).
Table 1.
—Critical Care Considerations in the Patient With Suspected Sleep-Disordered Breathing
| Recommendation | Rationale |
| Consider comorbid OSA, OHS, or the overlap syndrome in patients with ventilatory failure. | Many patients with SDB have not received a diagnosis at the time of acute cardiopulmonary failure. |
| Failure to diagnose SDB may lead to increased morbidity or mortality. | |
| Look for and treat OSA in CHF. | NIV may improve left ventricular ejection fraction and outcomes. |
| Consider OSA in patients who survive cardiac arrest. | Untreated OSA may represent a potentially reversible cause of sudden death. |
| Consider early empiric NIV for ventilatory failure in appropriate candidates. | SDB is treated directly and complications of endotracheal intubation and sedation are avoided. |
| Consider extubation to NIV as a liberation strategy when patients require endotracheal intubation. | NIV may reduce postextubation respiratory failure. |
| Some obese patients are capable of spontaneous breathing even though they do not meet the traditional success criteria on spontaneous breathing trials. | |
| Consider performing spontaneous breathing trials on higher CPAP or PEEP levels. | Higher levels of end-expiratory pressure may be necessary to offset increased chest wall elastic load even when lung compliance is acceptable. |
| Use sedation and analgesia judiciously. | Opiates and benzodiazepines promote pharyngeal collapsibility, blunt respiratory drive, and impair the arousal mechanism. |
| Limit sleep disruption at night. | SDB is worsened by sleep deprivation. |
| Arrange close follow-up with sleep specialist. | Chronic SDB should be formally diagnosed and treated. |
CHF = congestive heart failure; CPAP = continuous positive airway pressure; NIV = noninvasive ventilation; OHS = obesity hypoventilation syndrome; OSA = obstructive sleep apnea; PEEP = positive end-expiratory pressure; SDB = sleep-disordered breathing.
Table 2.
—Ensuring Effective Noninvasive Ventilation
| Assess for conditions that may reduce efficacy or increase harm from NIV, including hemodynamic instability, multiple organ failure, abdominal distension, agitation, excessive secretions, or upper GI bleeding. |
| Ensure appropriate mask fit. Most critically ill patients require oronasal or full-face masks. |
| Monitor and minimize leak. |
| Set initial bi-level PAP settings at 10/5 cm H2O to promote tolerance, but reassess frequently. Most patients require higher inspiratory and expiratory pressures to treat OSA and to achieve adequate ventilation. |
| Titrate IPAP to achieve effective minute ventilation (tidal volume of 6-8 mL/kg ideal body weight). |
| Administer supplemental oxygen to achieve saturation between 90% and 93%. (In a subgroup of patients with COPD and OHS excessive oxygen supplementation can worsen hypoventilation).79-82 |
| Reassess patients at least every 30 min during the initial phase of NIV. |
| Monitor closely for NIV failure. Potential signs include hemodynamic instability, agitation, combativeness, persistent snoring or apneas, or lack of improvement in hypercapnia or hypoxia. |
IPAP = inspiratory positive airway pressure; PAP = positive airway pressure. See Table 1 legend for expansion of other abbreviations.
CPAP has been compared with bi-level positive airway pressure (PAP) in several randomized controlled trials of patients presenting with acute cardiogenic pulmonary edema. These studies reported no difference between these two modalities in terms of time to resolution of hypercapnia or need for endotracheal intubation and mechanical ventilation.83-86 However, CPAP and bi-level PAP therapy have not been systematically compared in patients with noncardiogenic acute hypercapnic respiratory failure. We believe bi-level PAP should be considered first-line therapy for noninvasive ventilatory support in patients with hypoventilation and SDB. This mode provides inspiratory positive airway pressure (IPAP) and expiratory positive airway pressure (EPAP), each of which are set independently. EPAP maintains upper airway patency, whereas the difference between the IPAP and the EPAP supports ventilation. Therefore, increasing the level of IPAP over EPAP will lead to larger tidal volumes and increased ventilation. In obese patients, the EPAP typically needs to be set at a higher level than that for patients requiring NIV due to neuromuscular disease or acute exacerbation of COPD.
IPAP and EPAP settings should be tailored to each patient. A reasonable starting point is to set EPAP at 5 cm H2O and IPAP at 10 cm H2O if there is no prior PSG to guide the clinician. Effective treatment should result in adequate tidal volumes (typically, 6-8 mL/kg ideal body weight) and a reduction in the work of breathing. Arterial blood gases may improve as well, but in our experience do not always do so acutely, even in patients who ultimately are successfully treated with NIV, and, therefore, should not be interpreted in isolation. Higher settings are frequently required, however. In studies that have reported significant improvement in hypercapnia and hypoxia with bi-level PAP therapy, the IPAP was set at least 8 to 10 cm H2O above EPAP in order to achieve adequate ventilation. Furthermore, patients with OHS in chronic steady state typically require an IPAP of 16 to 20 cm H2O and an EPAP of 8 to 10 cm H2O.20,87 Therefore, clinicians should note that higher pressures may be needed to adequately splint open the upper airway and to ventilate the patient. In general, however, patients have difficulty tolerating IPAP above 20 cm H2O.
In addition to selecting appropriate NIV settings and titrating them carefully, clinicians should appreciate the need for proper equipment and support staff. Clinicians should ensure that the mask fits appropriately in order to minimize air leak and optimize patient comfort. Furthermore, the benefits of having an attentive and experienced respiratory therapist available cannot be overstated.
When instituting NIV, the clinician should be mindful of its effects on left- and right-ventricular function.88 NIV improves LV function by reducing LV afterload and preload. Indeed, NIV (specifically, CPAP alone) is a class IIa recommendation by the European Society of Cardiology for the treatment of respiratory failure due to pulmonary edema,89 although caution is advised in patients with cardiogenic shock and right ventricular failure, for the reasons discussed previously, or in patients with myocardial ischemia or arrhythmias.88
If endotracheal intubation is required, some patients with OSA and/or OHS may benefit from NIV immediately after extubation. A recent trial found that this strategy led to a significant reduction in the risk of postextubation respiratory failure in severely obese patients.90 Although this study did not report the severity of OSA, it supports the view that the routine application of NIV after extubation may be beneficial in obese patients with severe OSA or OHS.
Sedation and analgesia should be used judiciously in patients with SDB and acute cardiopulmonary failure. When used overzealously to facilitate invasive mechanical ventilation, opiates and sedatives may hinder liberation. As discussed above, many sedatives and analgesics promote pharyngeal collapse, and their effects may persist for several nights after they are discontinued. Accordingly, some authors recommend limiting opiate doses in patients with OSA, and considering alternative or adjunctive agents such as ketamine or dexmedetomidine.24
Finally, long-term treatment with NIV may improve morbidity and mortality in appropriate patients with hypoventilation or CHF.7,8,36,40,58,91-93 Nocturnal positive pressure ventilation should almost always be continued after the resolution of critical illness, and prompt follow-up with a sleep specialist should be arranged. It may be useful to obtain a formal inpatient sleep study prior to hospital discharge in order to confirm the diagnosis of SDB and prescribe appropriate therapy. Furthermore, many such patients will likely benefit from additional in-laboratory PSG and PAP titration with or without supplemental oxygen 1 to 3 months after hospital discharge, when therapy can be titrated in a more stable clinical state.94 However, implementation of PAP therapy should not be delayed while awaiting PSG.
Conclusion
Commonly thought of as a chronic problem, SDB may manifest as acute ventilatory failure, CHF, and sudden death. Clinicians should routinely suspect decompensated SDB in patients presenting with theses syndromes. If the role of SDB in acute critical illness is not recognized, clinicians may miss an important opportunity to impact long-term symptoms and outcomes.
Acknowledgments
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Mokhlesi has served as a consultant to Philips/Respironics. Dr Carr and Dr Gehlbach have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or in the preparation of the manuscript. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health.
Abbreviations
- AHI
apnea-hypopnea index
- CHF
congestive heart failure
- CPAP
continuous positive airway pressure
- EPAP
expiratory positive airway pressure
- IPAP
inspiratory positive airway pressure
- LV
left ventricle
- NIV
noninvasive ventilation
- OHS
obesity hypoventilation syndrome
- OSA
obstructive sleep apnea
- PAP
positive airway pressure
- PSG
polysomnography
- SDB
sleep-disordered breathing
Footnotes
Funding/Support: This study was funded by the National Heart, Lung, and Blood Institute [K23HL088020 to Dr Gehlbach].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.Casey KR, Cantillo KO, Brown LK. Sleep-related hypoventilation/hypoxemic syndromes. Chest. 2007;131(6):1936–1948. doi: 10.1378/chest.06-2334. [DOI] [PubMed] [Google Scholar]
- 2.Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):136–143. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee W, Nagubadi S, Kryger MH, Mokhlesi B. Epidemiology of obstructive sleep apnea: a population-based perspective. Expert Rev Respir Med. 2008;2(3):349–364. doi: 10.1586/17476348.2.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kapur V, Strohl KP, Redline S, Iber C, O’Connor G, Nieto J. Underdiagnosis of sleep apnea syndrome in U.S. communities. Sleep Breath. 2002;6(2):49–54. doi: 10.1007/s11325-002-0049-5. [DOI] [PubMed] [Google Scholar]
- 5.Young T, Evans L, Finn L, Palta M. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep. 1997;20(9):705–706. doi: 10.1093/sleep/20.9.705. [DOI] [PubMed] [Google Scholar]
- 6.Mokhlesi B, Tulaimat A, Faibussowitsch I, Wang Y, Evans AT. Obesity hypoventilation syndrome: prevalence and predictors in patients with obstructive sleep apnea. Sleep Breath. 2007;11(2):117–124. doi: 10.1007/s11325-006-0092-8. [DOI] [PubMed] [Google Scholar]
- 7.Bradley TD, Floras JS. Obstructive sleep apnoea and its cardiovascular consequences. Lancet. 2009;373(9657):82–93. doi: 10.1016/S0140-6736(08)61622-0. [DOI] [PubMed] [Google Scholar]
- 8.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365(9464):1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 9.Marshall NS, Wong KK, Liu PY, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea as an independent risk factor for all-cause mortality: the Busselton Health Study. Sleep. 2008;31(8):1079–1085. [PMC free article] [PubMed] [Google Scholar]
- 10.Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009;6(8):e1000132. doi: 10.1371/journal.pmed.1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31(8):1071–1078. [PMC free article] [PubMed] [Google Scholar]
- 12.Gooneratne NS, Richards KC, Joffe M, et al. Sleep disordered breathing with excessive daytime sleepiness is a risk factor for mortality in older adults. Sleep. 2011;34(4):435–442. doi: 10.1093/sleep/34.4.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mokhlesi B, Pamidi S, Yaggi HK. Sleep disordered breathing and subjective sleepiness in the elderly: a deadly combination? Sleep. 2011;34(4):413–415. doi: 10.1093/sleep/34.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Selim B, Won C, Yaggi HK. Cardiovascular consequences of sleep apnea. Clin Chest Med. 2010;31(2):203–220. doi: 10.1016/j.ccm.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 15.Lavie P, Herer P, Lavie L. Mortality risk factors in sleep apnoea: a matched case-control study. J Sleep Res. 2007;16(1):128–134. doi: 10.1111/j.1365-2869.2007.00578.x. [DOI] [PubMed] [Google Scholar]
- 16.Mokhlesi B. Obesity hypoventilation syndrome: a state-of-the-art review. Respir Care. 2010;55(10):1347–1365. [PubMed] [Google Scholar]
- 17.Piper AJ, Grunstein RR. Obesity hypoventilation syndrome: mechanisms and management. Am J Respir Crit Care Med. 2011;183(3):292–298. doi: 10.1164/rccm.201008-1280CI. [DOI] [PubMed] [Google Scholar]
- 18.Sturm R. Increases in morbid obesity in the USA: 2000-2005. Public Health. 2007;121(7):492–496. doi: 10.1016/j.puhe.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berger KI, Ayappa I, Chatr-Amontri B, et al. Obesity hypoventilation syndrome as a spectrum of respiratory disturbances during sleep. Chest. 2001;120(4):1231–1238. doi: 10.1378/chest.120.4.1231. [DOI] [PubMed] [Google Scholar]
- 20.Mokhlesi B, Tulaimat A. Recent advances in obesity hypoventilation syndrome. Chest. 2007;132(4):1322–1336. doi: 10.1378/chest.07-0027. [DOI] [PubMed] [Google Scholar]
- 21.Kaw R, Hernandez AV, Walker E, Aboussouan L, Mokhlesi B. Determinants of hypercapnia in obese patients with obstructive sleep apnea: a systematic review and metaanalysis of cohort studies. Chest. 2009;136(3):787–796. doi: 10.1378/chest.09-0615. [DOI] [PubMed] [Google Scholar]
- 22.Schmidt GA, Hall JB. Acute or chronic respiratory failure. Assessment and management of patients with COPD in the emergency setting. JAMA. 1989;261:3444–3453. doi: 10.1001/jama.261.23.3444. [DOI] [PubMed] [Google Scholar]
- 23.Memtsoudis S, Liu SS, Ma Y, et al. Perioperative pulmonary outcomes in patients with sleep apnea after noncardiac surgery. Anesth Analg. 2011;112(1):113–121. doi: 10.1213/ANE.0b013e3182009abf. [DOI] [PubMed] [Google Scholar]
- 24.Adesanya AO, Lee W, Greilich NB, Joshi GP. Perioperative management of obstructive sleep apnea. Chest. 2010;138(6):1489–1498. doi: 10.1378/chest.10-1108. [DOI] [PubMed] [Google Scholar]
- 25.Cooper KR, Phillips BA. Effect of short-term sleep loss on breathing. J Appl Physiol. 1982;53(4):855–858. doi: 10.1152/jappl.1982.53.4.855. [DOI] [PubMed] [Google Scholar]
- 26.Persson HE, Svanborg E. Sleep deprivation worsens obstructive sleep apnea. Comparison between diurnal and nocturnal polysomnography. Chest. 1996;109(3):645–650. doi: 10.1378/chest.109.3.645. [DOI] [PubMed] [Google Scholar]
- 27.Chaouat A, Bugnet AS, Kadaoui N, et al. Severe pulmonary hypertension and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172(2):189–194. doi: 10.1164/rccm.200401-006OC. [DOI] [PubMed] [Google Scholar]
- 28.Resta O, Foschino Barbaro MP, Brindicci C, Nocerino MC, Caratozzolo G, Carbonara M. Hypercapnia in overlap syndrome: possible determinant factors. Sleep Breath. 2002;6(1):11–18. doi: 10.1007/s11325-002-0011-6. [DOI] [PubMed] [Google Scholar]
- 29.Bednarek M, Plywaczewski R, Jonczak L, Zielinski J. There is no relationship between chronic obstructive pulmonary disease and obstructive sleep apnea syndrome: a population study. Respiration. 2005;72(2):142–149. doi: 10.1159/000084044. [DOI] [PubMed] [Google Scholar]
- 30.Owens RL, Malhotra A. Sleep-disordered breathing and COPD: the overlap syndrome. Respir Care. 2010;55(10):1333–1344. [PMC free article] [PubMed] [Google Scholar]
- 31.Weitzenblum E, Chaouat A, Kessler R, Oswald M, Apprill M, Krieger J. Daytime hypoventilation in obstructive sleep apnoea syndrome. Sleep Med Rev. 1999;3(1):79–93. doi: 10.1016/s1087-0792(99)90015-1. [DOI] [PubMed] [Google Scholar]
- 32.Hawryłkiewicz I, Sliwiński P, Górecka D, Pływaczewski R, Zieliński J. Pulmonary haemodynamics in patients with OSAS or an overlap syndrome. Monaldi Arch Chest Dis. 2004;61(3):148–152. doi: 10.4081/monaldi.2004.693. [DOI] [PubMed] [Google Scholar]
- 33.Radwan L, Maszczyk Z, Koziorowski A, et al. Control of breathing in obstructive sleep apnoea and in patients with the overlap syndrome. Eur Respir J. 1995;8(4):542–545. [PubMed] [Google Scholar]
- 34.O’Donoghue FJ, Catcheside PG, Ellis EE, et al. Australian trial of Noninvasive Ventilation in Chronic Airflow Limitation investigators Sleep hypoventilation in hypercapnic chronic obstructive pulmonary disease: prevalence and associated factors. Eur Respir J. 2003;21(6):977–984. doi: 10.1183/09031936.03.00066802. [DOI] [PubMed] [Google Scholar]
- 35.Rizzi M, Palma P, Andreoli A, et al. Prevalence and clinical feature of the “overlap syndrome,” obstructive sleep apnea (OSA) and chronic obstructive pulmonary disease (COPD), in OSA population. Sleep Breath. 1997;2(3):68–72. doi: 10.1007/BF03038868. [DOI] [PubMed] [Google Scholar]
- 36.Marin JM, Soriano JB, Carrizo SJ, Boldova A, Celli BR. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea: the overlap syndrome. Am J Respir Crit Care Med. 2010;182(3):325–331. doi: 10.1164/rccm.200912-1869OC. [DOI] [PubMed] [Google Scholar]
- 37.Afessa B, Morales IJ, Scanlon PD, Peters SG. Prognostic factors, clinical course, and hospital outcome of patients with chronic obstructive pulmonary disease admitted to an intensive care unit for acute respiratory failure. Crit Care Med. 2002;30(7):1610–1615. doi: 10.1097/00003246-200207000-00035. [DOI] [PubMed] [Google Scholar]
- 38.BaHammam A, Syed S, Al-Mughairy A. Sleep-related breathing disorders in obese patients presenting with acute respiratory failure. Respir Med. 2005;99(6):718–725. doi: 10.1016/j.rmed.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 39.Nowbar S, Burkart KM, Gonzales R, et al. Obesity-associated hypoventilation in hospitalized patients: prevalence, effects, and outcome. Am J Med. 2004;116(1):1–7. doi: 10.1016/j.amjmed.2003.08.022. [DOI] [PubMed] [Google Scholar]
- 40.Pérez de Llano LA, Golpe R, Ortiz Piquer M, et al. Short-term and long-term effects of nasal intermittent positive pressure ventilation in patients with obesity-hypoventilation syndrome. Chest. 2005;128(2):587–594. doi: 10.1378/chest.128.2.587. [DOI] [PubMed] [Google Scholar]
- 41.Behazin N, Jones SB, Cohen RI, Loring SH. Respiratory restriction and elevated pleural and esophageal pressures in morbid obesity. J Appl Physiol. 2010;108(1):212–218. doi: 10.1152/japplphysiol.91356.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steier J, Jolley CJ, Seymour J, Roughton M, Polkey MI, Moxham J. Neural respiratory drive in obesity. Thorax. 2009;64(8):719–725. doi: 10.1136/thx.2008.109728. [DOI] [PubMed] [Google Scholar]
- 43.Gilbert R, Sipple JH, Auchincloss JH., Jr Respiratory control and work of breathing in obese subjects. J Appl Physiol. 1961;16:21–26. doi: 10.1152/jappl.1961.16.1.21. [DOI] [PubMed] [Google Scholar]
- 44.Kress JP, Pohlman AS, Alverdy J, Hall JB. The impact of morbid obesity on oxygen cost of breathing (Vo2RESP) at rest. Am J Respir Crit Care Med. 1999;160(3):883–886. doi: 10.1164/ajrccm.160.3.9902058. [DOI] [PubMed] [Google Scholar]
- 45.Ayappa I, Berger KI, Norman RG, Oppenheimer BW, Rapoport DM, Goldring RM. Hypercapnia and ventilatory periodicity in obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2002;166(8):1112–1115. doi: 10.1164/rccm.200203-212OC. [DOI] [PubMed] [Google Scholar]
- 46.Leiter JC, Knuth SL, Bartlett D., Jr The effect of sleep deprivation on activity of the genioglossus muscle. Am Rev Respir Dis. 1985;132(6):1242–1245. doi: 10.1164/arrd.1985.132.6.1242. [DOI] [PubMed] [Google Scholar]
- 47.Yumino D, Bradley TD. Central sleep apnea and Cheyne-Stokes respiration. Proc Am Thorac Soc. 2008;5(2):226–236. doi: 10.1513/pats.200708-129MG. [DOI] [PubMed] [Google Scholar]
- 48.Khayat RN, Jarjoura D, Patt B, Yamokoski T, Abraham WT. In-hospital testing for sleep-disordered breathing in hospitalized patients with decompensated heart failure: report of prevalence and patient characteristics. J Card Fail. 2009;15(9):739–746. doi: 10.1016/j.cardfail.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gottlieb DJ, Yenokyan G, Newman AB, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: The Sleep Heart Health Study. Circulation. 2010;122(4):352–360. doi: 10.1161/CIRCULATIONAHA.109.901801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leung RS, Bradley TD. Sleep apnea and cardiovascular disease. Am J Respir Crit Care Med. 2001;164(12):2147–2165. doi: 10.1164/ajrccm.164.12.2107045. [DOI] [PubMed] [Google Scholar]
- 51.Buda AJ, Pinsky MR, Ingels NB, Jr, Daughters GT, II, Stinson EB, Alderman EL. Effect of intrathoracic pressure on left ventricular performance. N Engl J Med. 1979;301(9):453–459. doi: 10.1056/NEJM197908303010901. [DOI] [PubMed] [Google Scholar]
- 52.Tolle FA, Judy WV, Yu PL, Markand ON. Reduced stroke volume related to pleural pressure in obstructive sleep apnea. J Appl Physiol. 1983;55(6):1718–1724. doi: 10.1152/jappl.1983.55.6.1718. [DOI] [PubMed] [Google Scholar]
- 53.Scharf SM, Bianco JA, Tow DE, Brown R. The effects of large negative intrathoracic pressure on left ventricular function in patients with coronary artery disease. Circulation. 1981;63(4):871–875. doi: 10.1161/01.cir.63.4.871. [DOI] [PubMed] [Google Scholar]
- 54.Hamilton GS, Meredith IT, Walker AM, Solin P. Obstructive sleep apnea leads to transient uncoupling of coronary blood flow and myocardial work in humans. Sleep. 2009;32(2):263–270. doi: 10.1093/sleep/32.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kuniyoshi FH, Garcia-Touchard A, Gami AS, et al. Day-night variation of acute myocardial infarction in obstructive sleep apnea. J Am Coll Cardiol. 2008;52(5):343–346. doi: 10.1016/j.jacc.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gami AS, Hodge DO, Herges RM, et al. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol. 2007;49(5):565–571. doi: 10.1016/j.jacc.2006.08.060. [DOI] [PubMed] [Google Scholar]
- 57.Gami AS, Pressman G, Caples SM, et al. Association of atrial fibrillation and obstructive sleep apnea. Circulation. 2004;110(4):364–367. doi: 10.1161/01.CIR.0000136587.68725.8E. [DOI] [PubMed] [Google Scholar]
- 58.Kasai T, Bradley TD. Obstructive sleep apnea and heart failure: pathophysiologic and therapeutic implications. J Am Coll Cardiol. 2011;57(2):119–127. doi: 10.1016/j.jacc.2010.08.627. [DOI] [PubMed] [Google Scholar]
- 59.Chami HA, Resnick HE, Quan SF, Gottlieb DJ. Association of incident cardiovascular disease with progression of sleep-disordered breathing. Circulation. 2011;123(12):1280–1286. doi: 10.1161/CIRCULATIONAHA.110.974022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fletcher EC, Proctor M, Yu J, et al. Pulmonary edema develops after recurrent obstructive apneas. Am J Respir Crit Care Med. 1999;160(5 Pt 1):1688–1696. doi: 10.1164/ajrccm.160.5.9810003. [DOI] [PubMed] [Google Scholar]
- 61.Chan HS, Chiu HF, Tse LK, Woo KS. Obstructive sleep apnea presenting with nocturnal angina, heart failure, and near-miss sudden death. Chest. 1991;99(4):1023–1025. doi: 10.1378/chest.99.4.1023. [DOI] [PubMed] [Google Scholar]
- 62.Chaudhary BA, Ferguson DS, Speir WA., Jr Pulmonary edema as a presenting feature of sleep apnea syndrome. Chest. 1982;82(1):122–124. doi: 10.1378/chest.82.1.122. [DOI] [PubMed] [Google Scholar]
- 63.Weitzenblum E, Krieger J, Apprill M, et al. Daytime pulmonary hypertension in patients with obstructive sleep apnea syndrome. Am Rev Respir Dis. 1988;138(2):345–349. doi: 10.1164/ajrccm/138.2.345. [DOI] [PubMed] [Google Scholar]
- 64.Atwood CW, Jr, McCrory D, Garcia JG, Abman SH, Ahearn GS. American College of Chest Physicians Pulmonary artery hypertension and sleep-disordered breathing: ACCP evidence-based clinical practice guidelines. Chest. 2004;126(suppl 1):72S–77S. doi: 10.1378/chest.126.1_suppl.72S. [DOI] [PubMed] [Google Scholar]
- 65.O’Hearn DJ, Gold AR, Gold MS, Diggs P, Scharf SM. Lower extremity edema and pulmonary hypertension in morbidly obese patients with obstructive sleep apnea. Sleep Breath. 2009;13(1):25–34. doi: 10.1007/s11325-008-0200-z. [DOI] [PubMed] [Google Scholar]
- 66.Pinsky MR. Determinants of pulmonary arterial flow variation during respiration. J Appl Physiol. 1984;56(5):1237–1245. doi: 10.1152/jappl.1984.56.5.1237. [DOI] [PubMed] [Google Scholar]
- 67.Pinsky MR. Instantaneous venous return curves in an intact canine preparation. J Appl Physiol. 1984;56(3):765–771. doi: 10.1152/jappl.1984.56.3.765. [DOI] [PubMed] [Google Scholar]
- 68.Dawson CA, Grimm DJ, Linehan JH. Lung inflation and longitudinal distribution of pulmonary vascular resistance during hypoxia. J Appl Physiol. 1979;47(3):532–536. doi: 10.1152/jappl.1979.47.3.532. [DOI] [PubMed] [Google Scholar]
- 69.Vieillard-Baron A, Loubieres Y, Schmitt JM, Page B, Dubourg O, Jardin F. Cyclic changes in right ventricular output impedance during mechanical ventilation. J Appl Physiol. 1999;87(5):1644–1650. doi: 10.1152/jappl.1999.87.5.1644. [DOI] [PubMed] [Google Scholar]
- 70.Zamanian RT, Haddad F, Doyle RL, Weinacker AB. Management strategies for patients with pulmonary hypertension in the intensive care unit. Crit Care Med. 2007;35(9):2037–2050. doi: 10.1097/01.ccm.0000280433.74246.9e. [DOI] [PubMed] [Google Scholar]
- 71.Fletcher EC, Shah A, Qian W, Miller CC., III “Near miss” death in obstructive sleep apnea: a critical care syndrome. Crit Care Med. 1991;19(9):1158–1164. doi: 10.1097/00003246-199109000-00011. [DOI] [PubMed] [Google Scholar]
- 72.Doherty LS, Kiely JL, Swan V, McNicholas WT. Long-term effects of nasal continuous positive airway pressure therapy on cardiovascular outcomes in sleep apnea syndrome. Chest. 2005;127(6):2076–2084. doi: 10.1378/chest.127.6.2076. [DOI] [PubMed] [Google Scholar]
- 73.Gami AS, Howard DE, Olson EJ, Somers VK. Day-night pattern of sudden death in obstructive sleep apnea. N Engl J Med. 2005;352(12):1206–1214. doi: 10.1056/NEJMoa041832. [DOI] [PubMed] [Google Scholar]
- 74.Mehra R, Benjamin EJ, Shahar E, et al. Sleep Heart Health Study Association of nocturnal arrhythmias with sleep-disordered breathing: The Sleep Heart Health Study. Am J Respir Crit Care Med. 2006;173(8):910–916. doi: 10.1164/rccm.200509-1442OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Monahan K, Storfer-Isser A, Mehra R, et al. Triggering of nocturnal arrhythmias by sleep-disordered breathing events. J Am Coll Cardiol. 2009;54(19):1797–1804. doi: 10.1016/j.jacc.2009.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gallagher SF, Haines KL, Osterlund LG, Mullen M, Downs JB. Postoperative hypoxemia: common, undetected, and unsuspected after bariatric surgery. J Surg Res. 2010;159(2):622–626. doi: 10.1016/j.jss.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 77.Sands SA, Edwards BA, Kelly VJ, et al. Mechanism underlying accelerated arterial oxygen desaturation during recurrent apnea. Am J Respir Crit Care Med. 2010;182(7):961–969. doi: 10.1164/rccm.201003-0477OC. [DOI] [PubMed] [Google Scholar]
- 78.Khoo SM, Mukherjee JJ, Phua J, Shi DX. Obstructive sleep apnea presenting as recurrent cardiopulmonary arrest. Sleep Breath. 2009;13(1):89–92. doi: 10.1007/s11325-008-0209-3. [DOI] [PubMed] [Google Scholar]
- 79.Dick CR, Liu Z, Sassoon CS, Berry RB, Mahutte CK. O2-induced change in ventilation and ventilatory drive in COPD. Am J Respir Crit Care Med. 1997;155(2):609–614. doi: 10.1164/ajrccm.155.2.9032202. [DOI] [PubMed] [Google Scholar]
- 80.Mokhlesi B, Tulaimat A, Parthasarathy S. Oxygen for obesity hypoventilation syndrome: a double-edged sword? Chest. 2011;139(5):975–977. doi: 10.1378/chest.10-2858. [DOI] [PubMed] [Google Scholar]
- 81.Robinson TD, Freiberg DB, Regnis JA, Young IH. The role of hypoventilation and ventilation-perfusion redistribution in oxygen-induced hypercapnia during acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;161(5):1524–1529. doi: 10.1164/ajrccm.161.5.9904119. [DOI] [PubMed] [Google Scholar]
- 82.Wijesinghe M, Williams M, Perrin K, Weatherall M, Beasley R. The effect of supplemental oxygen on hypercapnia in subjects with obesity-associated hypoventilation: a randomized, crossover, clinical study. Chest. 2011;139(5):1018–1024. doi: 10.1378/chest.10-1280. [DOI] [PubMed] [Google Scholar]
- 83.Gray A, Goodacre S, Newby DE, Masson M, Sampson F, Nicholl J. 3CPO Trialists Noninvasive ventilation in acute cardiogenic pulmonary edema. N Engl J Med. 2008;359(2):142–151. doi: 10.1056/NEJMoa0707992. [DOI] [PubMed] [Google Scholar]
- 84.Mehta S, Nava S. Mask ventilation and cardiogenic pulmonary edema: “another brick in the wall”. Intensive Care Med. 2005;31(6):757–759. doi: 10.1007/s00134-005-2650-0. [DOI] [PubMed] [Google Scholar]
- 85.Bellone A, Vettorello M, Monari A, Cortellaro F, Coen D. Noninvasive pressure support ventilation vs. continuous positive airway pressure in acute hypercapnic pulmonary edema. Intensive Care Med. 2005;31(6):807–811. doi: 10.1007/s00134-005-2649-6. [DOI] [PubMed] [Google Scholar]
- 86.Park M, Sangean MC, Volpe MdeS, et al. Randomized, prospective trial of oxygen, continuous positive airway pressure, and bilevel positive airway pressure by face mask in acute cardiogenic pulmonary edema. Crit Care Med. 2004;32(12):2407–2415. doi: 10.1097/01.ccm.0000147770.20400.10. [DOI] [PubMed] [Google Scholar]
- 87.Piper AJ, Wang D, Yee BJ, Barnes DJ, Grunstein RR. Randomised trial of CPAP vs bilevel support in the treatment of obesity hypoventilation syndrome without severe nocturnal desaturation. Thorax. 2008;63(5):395–401. doi: 10.1136/thx.2007.081315. [DOI] [PubMed] [Google Scholar]
- 88.Pinsky MR. Cardiovascular issues in respiratory care. Chest. 2005;128(5) suppl 2:592S–597S. doi: 10.1378/chest.128.5_suppl_2.592S. [DOI] [PubMed] [Google Scholar]
- 89.Dickstein K, Cohen-Solal A, Filippatos G, et al. ESC Committee for Practice Guidelines (CPG) ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM) [published correction appears in Eur J Heart Fail. 2010;12(4):416] Eur J Heart Fail. 2008;10(10):933–989. doi: 10.1016/j.ejheart.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 90.El-Solh AA, Aquilina A, Pineda L, Dhanvantri V, Grant B, Bouquin P. Noninvasive ventilation for prevention of post-extubation respiratory failure in obese patients. Eur Respir J. 2006;28(3):588–595. doi: 10.1183/09031936.06.00150705. [DOI] [PubMed] [Google Scholar]
- 91.Budweiser S, Hitzl AP, Jörres RA, Schmidbauer K, Heinemann F, Pfeifer M. Health-related quality of life and long-term prognosis in chronic hypercapnic respiratory failure: a prospective survival analysis. Respir Res. 2007;8:92. doi: 10.1186/1465-9921-8-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Budweiser S, Riedl SG, Jörres RA, Heinemann F, Pfeifer M. Mortality and prognostic factors in patients with obesity-hypoventilation syndrome undergoing noninvasive ventilation. J Intern Med. 2007;261(4):375–383. doi: 10.1111/j.1365-2796.2007.01765.x. [DOI] [PubMed] [Google Scholar]
- 93.Khayat RN, Abraham WT, Patt B, Pu M, Jarjoura D. In-hospital treatment of obstructive sleep apnea during decompensation of heart failure. Chest. 2009;136(4):991–997. doi: 10.1378/chest.09-0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nader NZ, Steinel JA, Auckley DH. Newly identified obstructive sleep apnea in hospitalized patients: analysis of an evaluation and treatment strategy. J Clin Sleep Med. 2006;2(4):431–437. [PubMed] [Google Scholar]