Abstract
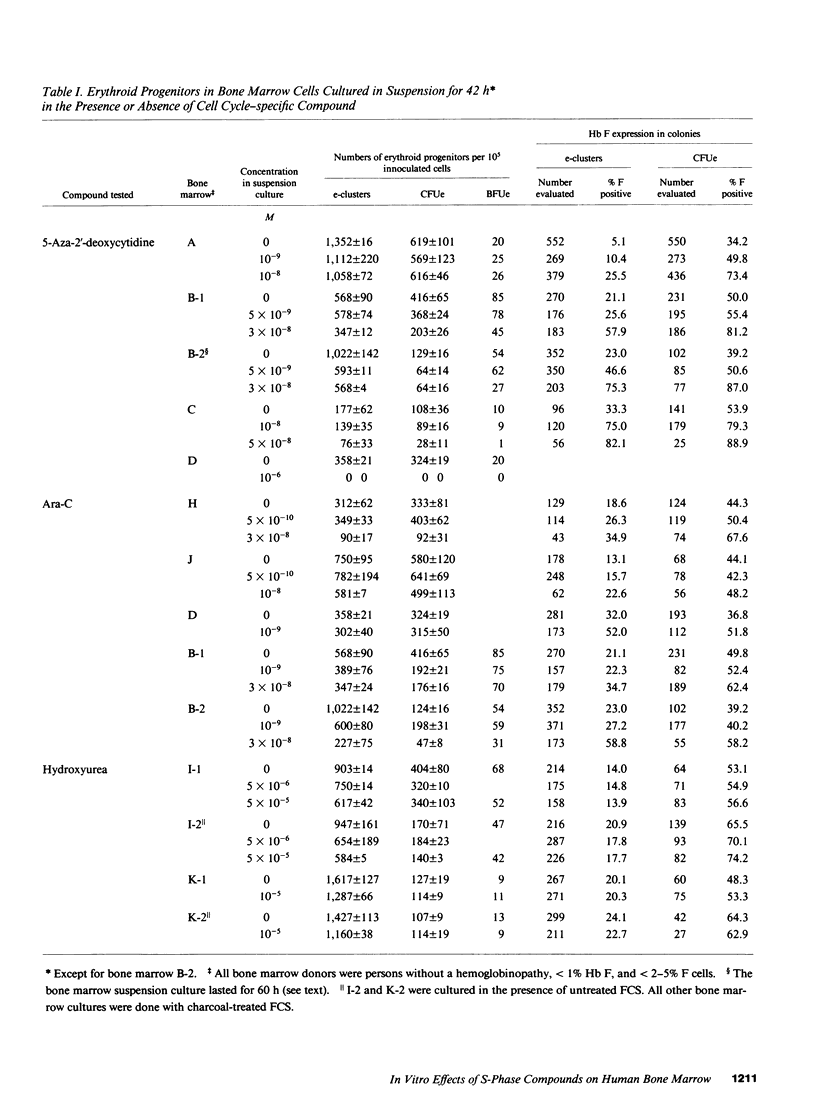
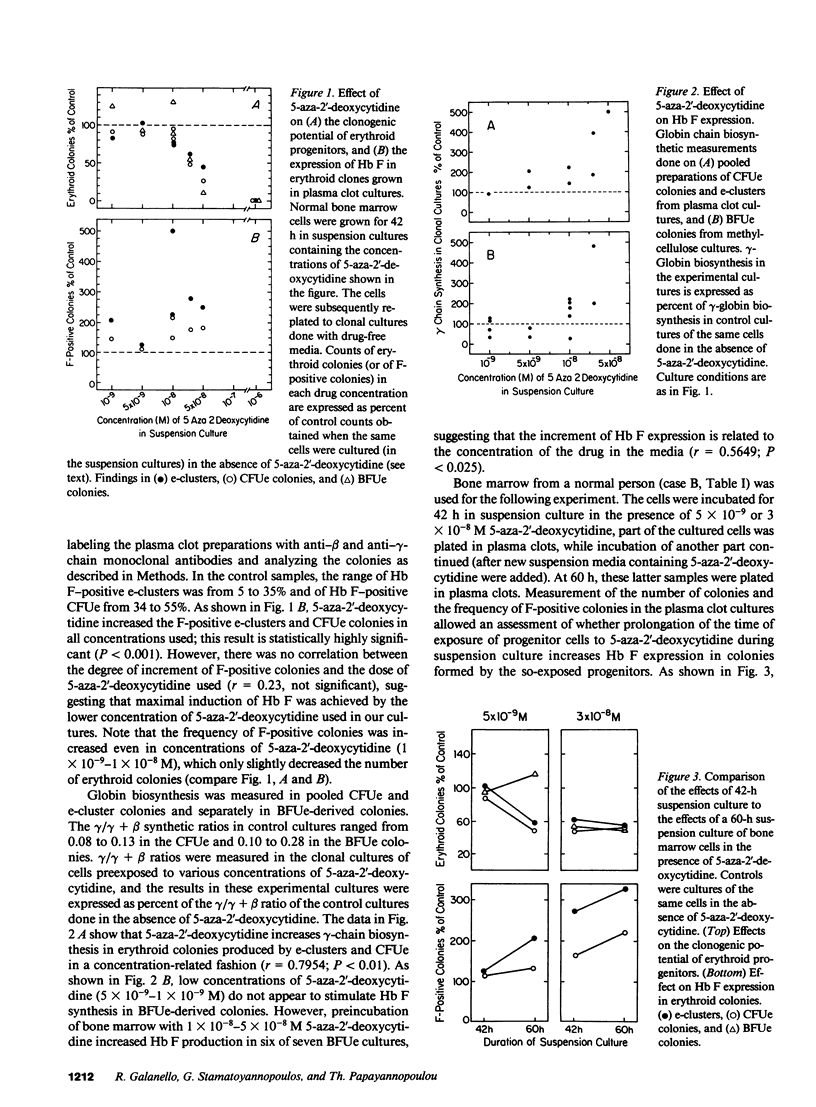
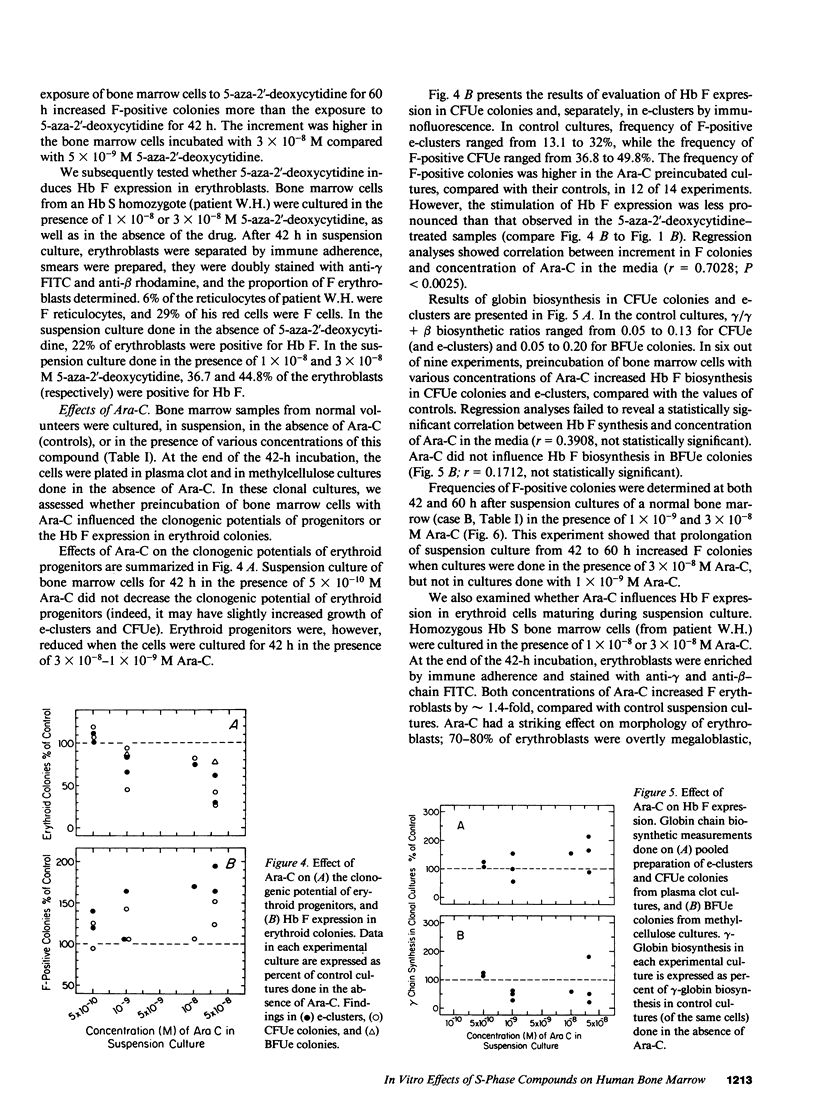
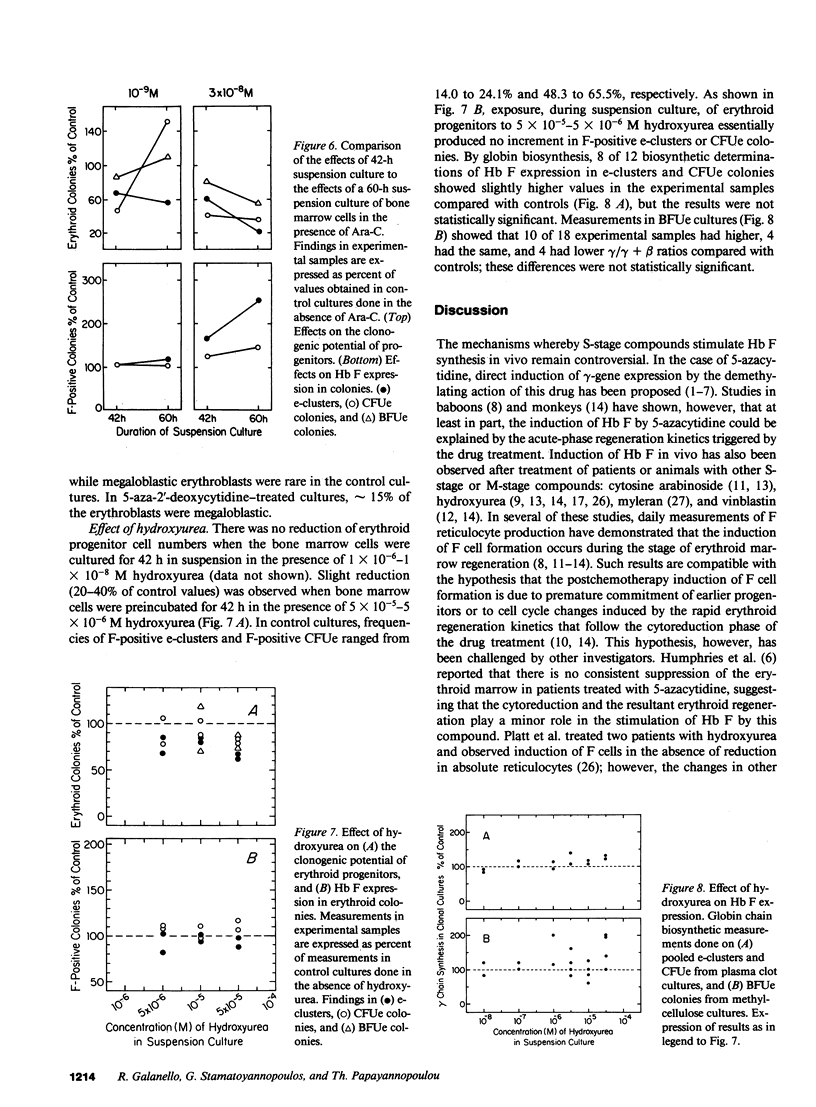
The in vitro effect of S-stage-specific drugs on the fetal hemoglobin (Hb F) potential of erythroid precursors and progenitors was tested by exposing bone marrow cells to 5-aza-2'-deoxycytidine, Ara-C, or hydroxyurea in suspension cultures and reculturing the cells in drug-free clonal cultures. Analysis of Hb F in the erythroblasts present at the end of suspension cultures and in the erythroid colonies formed from treated progenitors showed that 1 X 10(-9)-5 X 10(-8) M 5-aza-2'-deoxycytidine produced a concentration-related increase in the proportion of Hb F-positive erythroblasts, of Hb F-positive erythroid CFU (CFUe) colonies, and at the higher doses used, an increased Hb F expression in erythroid burst-forming unit (BFUe)-derived colonies. Preincubation of bone marrow cells with Ara-C produced significant megaloblastic changes by the end of the 2-d incubation and increased the proportion of Hb F-positive erythroblasts, CFUe colonies, and e-clusters, but BFUe-derived progeny was unaffected. Hydroxyurea failed to produce significant changes in Hb F at the range of concentrations used. The data raise the possibility of more than one mechanism underlying the stimulation of Hb F by S-stage drugs.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bigbee W. L., Branscomb E. W., Weintraub H. B., Papayannopoulou T., Stamatoyannopoulos G. Cell sorter immunofluorescence detection of human erythrocytes labelled in suspension with antibodies specific for hemoglobin S and C. J Immunol Methods. 1981;45(2):117–127. doi: 10.1016/0022-1759(81)90206-4. [DOI] [PubMed] [Google Scholar]
- Charache S., Dover G., Smith K., Talbot C. C., Jr, Moyer M., Boyer S. Treatment of sickle cell anemia with 5-azacytidine results in increased fetal hemoglobin production and is associated with nonrandom hypomethylation of DNA around the gamma-delta-beta-globin gene complex. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4842–4846. doi: 10.1073/pnas.80.15.4842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper K. D., Kang K., Bush T. L., Hanifin J. M. Direct depletion and purification of monoclonal antibody defined cells from unfractionated human mononuclear leukocytes using antibody coated polystyrene Petri dishes. J Clin Lab Immunol. 1984 Feb;13(2):97–100. [PubMed] [Google Scholar]
- DeSimone J., Heller P., Hall L., Zwiers D. 5-Azacytidine stimulates fetal hemoglobin synthesis in anemic baboons. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4428–4431. doi: 10.1073/pnas.79.14.4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessypris E. N., Krantz S. B. Effect of pure erythropoietin on DNA-synthesis by human marrow day 15 erythroid burst forming units in short-term liquid culture. Br J Haematol. 1984 Feb;56(2):295–306. doi: 10.1111/j.1365-2141.1984.tb03957.x. [DOI] [PubMed] [Google Scholar]
- Dover G. J., Boyer S. H., Zinkham W. H. Production of erythrocytes that contain fetal hemoglobin in anemia. Transient in vivo changes. J Clin Invest. 1979 Feb;63(2):173–176. doi: 10.1172/JCI109286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dover G. J., Charache S., Boyer S. H., Vogelsang G., Moyer M. 5-Azacytidine increases HbF production and reduces anemia in sickle cell disease: dose-response analysis of subcutaneous and oral dosage regimens. Blood. 1985 Sep;66(3):527–532. [PubMed] [Google Scholar]
- Dover G. J., Humphries R. K., Moore J. G., Ley T. J., Young N. S., Charache S., Nienhuis A. W. Hydroxyurea induction of hemoglobin F production in sickle cell disease: relationship between cytotoxicity and F cell production. Blood. 1986 Mar;67(3):735–738. [PubMed] [Google Scholar]
- Gregory C. J., Eaves A. C. Three stages of erythropoietic progenitor cell differentiation distinguished by a number of physical and biologic properties. Blood. 1978 Mar;51(3):527–537. [PubMed] [Google Scholar]
- Humphries R. K., Dover G., Young N. S., Moore J. G., Charache S., Ley T., Nienhuis A. W. 5-Azacytidine acts directly on both erythroid precursors and progenitors to increase production of fetal hemoglobin. J Clin Invest. 1985 Feb;75(2):547–557. doi: 10.1172/JCI111731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikura H., Okazaki T., Mochizuki T., Izumi Y., Tashima M., Sawada H., Uchino H. Effect of antimetabolites and thymidine blockage on the induction of differentiation of HL-60 cells by retinoic acid or 1 alpha,25-dihydroxyvitamin D3. Exp Hematol. 1985 Nov;13(10):981–988. [PubMed] [Google Scholar]
- Letvin N. L., Linch D. C., Beardsley G. P., McIntyre K. W., Miller B. A., Nathan D. G. Influence of cell cycle phase-specific agents on simian fetal hemoglobin synthesis. J Clin Invest. 1985 Jun;75(6):1999–2005. doi: 10.1172/JCI111918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letvin N. L., Linch D. C., Beardsley G. P., McIntyre K. W., Nathan D. G. Augmentation of fetal-hemoglobin production in anemic monkeys by hydroxyurea. N Engl J Med. 1984 Apr 5;310(14):869–873. doi: 10.1056/NEJM198404053101401. [DOI] [PubMed] [Google Scholar]
- Ley T. J., DeSimone J., Noguchi C. T., Turner P. H., Schechter A. N., Heller P., Nienhuis A. W. 5-Azacytidine increases gamma-globin synthesis and reduces the proportion of dense cells in patients with sickle cell anemia. Blood. 1983 Aug;62(2):370–380. [PubMed] [Google Scholar]
- Momparler R. L., Momparler L. F., Samson J. Comparison of the antileukemic activity of 5-AZA-2'-deoxycytidine, 1-beta-D-arabinofuranosylcytosine and 5-azacytidine against L1210 leukemia. Leuk Res. 1984;8(6):1043–1049. doi: 10.1016/0145-2126(84)90059-6. [DOI] [PubMed] [Google Scholar]
- Motoji T., Hoang T., Tritchler D., McCulloch E. A. The effect of 5-azacytidine and its analogues on blast cell renewal in acute myeloblastic leukemia. Blood. 1985 Apr;65(4):894–901. [PubMed] [Google Scholar]
- Nienhuis A. W., Ley T. J., Humphries R. K., Young N. S., Dover G. Pharmacological manipulation of fetal hemoglobin synthesis in patients with severe beta-thalassemia. Ann N Y Acad Sci. 1985;445:198–211. doi: 10.1111/j.1749-6632.1985.tb17189.x. [DOI] [PubMed] [Google Scholar]
- Papayannopoulou T., Brice M., Stamatoyannopoulos G. Hemoglobin F synthesis in vitro: evidence for control at the level of primitive erythroid stem cells. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2923–2927. doi: 10.1073/pnas.74.7.2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulou T., Kalmantis T., Stamatoyannopoulos G. Cellular regulation of hemoglobin switching: evidence for inverse relationship between fetal hemoglobin synthesis and degree of maturity of human erythroid cells. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6420–6424. doi: 10.1073/pnas.76.12.6420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulou T., Torrealba de Ron A., Veith R., Knitter G., Stamatoyannopoulos G. Arabinosylcytosine induces fetal hemoglobin in baboons by perturbing erythroid cell differentiation kinetics. Science. 1984 May 11;224(4649):617–619. doi: 10.1126/science.6200940. [DOI] [PubMed] [Google Scholar]
- Papayannopoulou T., Vichinsky E., Stamatoyannopoulos G. Fetal Hb production during acute erythroid expansion. I. Observations in patients with transient erythroblastopenia and post-phlebotomy. Br J Haematol. 1980 Apr;44(4):535–546. doi: 10.1111/j.1365-2141.1980.tb08707.x. [DOI] [PubMed] [Google Scholar]
- Platt O. S., Orkin S. H., Dover G., Beardsley G. P., Miller B., Nathan D. G. Hydroxyurea enhances fetal hemoglobin production in sickle cell anemia. J Clin Invest. 1984 Aug;74(2):652–656. doi: 10.1172/JCI111464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Righetti P. G., Gianazza E., Gianni A. M., Comi P., Giglioni B., Ottolenghi S., Secchi C., Rossi-Bernardi L. Human globin chain separation by isoelectric focusing. J Biochem Biophys Methods. 1979;1(1):45–57. doi: 10.1016/0165-022x(79)90045-9. [DOI] [PubMed] [Google Scholar]
- Stamatoyannopoulos G., Nute P. E., Papayannopoulou T., McGuire T., Lim G., Bunn H. F., Rucknagel D. Development of a somatic mutation screening system using Hb mutants. IV. Successful detection of red cells containing the human frameshift mutants Hb Wayne and Hb Cranston using monospecific fluorescent antibodies. Am J Hum Genet. 1980 Jul;32(4):484–496. [PMC free article] [PubMed] [Google Scholar]
- Stamatoyannopoulos G., Veith R., Galanello R., Papayannopoulou T. Hb F production in stressed erythropoiesis: observations and kinetic models. Ann N Y Acad Sci. 1985;445:188–197. doi: 10.1111/j.1749-6632.1985.tb17188.x. [DOI] [PubMed] [Google Scholar]
- Torrealba-de Ron A. T., Papayannopoulou T., Knapp M. S., Fu M. F., Knitter G., Stamatoyannopoulos G. Perturbations in the erythroid marrow progenitor cell pools may play a role in the augmentation of HbF by 5-azacytidine. Blood. 1984 Jan;63(1):201–210. [PubMed] [Google Scholar]
- Veith R., Galanello R., Papayannopoulou T., Stamatoyannopoulos G. Stimulation of F-cell production in patients with sickle-cell anemia treated with cytarabine or hydroxyurea. N Engl J Med. 1985 Dec 19;313(25):1571–1575. doi: 10.1056/NEJM198512193132503. [DOI] [PubMed] [Google Scholar]
- Veith R., Papayannopoulou T., Kurachi S., Stamatoyannopoulos G. Treatment of baboon with vinblastine: insights into the mechanisms of pharmacologic stimulation of Hb F in the adult. Blood. 1985 Aug;66(2):456–459. [PubMed] [Google Scholar]
- Yokochi T., Brice M., Rabinovitch P. S., Papayannopoulou T., Stamatoyannopoulos G. Monoclonal antibodies detecting antigenic determinants with restricted expression on erythroid cells: from the erythroid committed progenitor level to the mature erythroblast. Blood. 1984 Jun;63(6):1376–1384. [PubMed] [Google Scholar]



