Abstract
Background
A peptidyl prolyl cis/trans isomerase, Pin1, regulates insulin signal transduction. Pin1 reduces responses to insulin stimulation by binding CRTC2 (CREB-regulated transcriptional co-activator 2) and PPARγ (peroxisome prolifereator- activated receptor γ), but conversely enhances insulin signaling by binding IRS-1 (insulin receptor substrate-1), Akt kinase, and Smad3. Therefore, it is still unclear whether Pin1 inhibits or enhances adipose cell differentiation.
Methodology/Principal Findings
Pin1−/− and wild-type mice were fed with high fat diets and adipose tissue weight was measured. Compared to wild-type mice, Pin1−/− mice had lower adipose tissue weight, while the weight of other tissues was similar. Mouse embryo fibroblasts (MEFs), prepared from both groups of mice, were induced to differentiate into adipose cells by stimulation with insulin. However, the rate of differentiation of MEFs from Pin1−/− mice was less than that of MEFs from wild-type mice. The rate of insulin-induced MEF cell differentiation in Pin1−/− mice was restored by increasing expression of Pin1. We found that Pin1 binds to phosphoThr172- and phosphoSer271-Pro sites in CREB suppress the activity in COS-7 cells.
Conclusion and Significance
Pin1 enhanced the uptake of triglycerides and the differentiation of MEF cells into adipose cells in response to insulin stimulation. Results of this study suggest that Pin1 down-regulation could be a potential approach in obesity-related dysfunctions, such as high blood pressure, diabetes, non-alcoholic steatohepatitis.
Introduction
A peptidyl prolyl cis/trans isomerase, Pin1, binds to phosphorylated Ser/Thr-Pro motifs in a variety of proteins and catalyzes cis/trans isomerization of peptidyl prolyl bonds. When we created Pin1−/− mice, the first phenotype we noted was that these mice weighed lower than wild-type mice [1], [2]. We hypothesized that a delay in cell cycle progression in Pin1−/− mice causes this loss in body weight.
It is known that Pin1 regulates the activities of regulatory molecules involved in insulin signaling, such as PPARγ [3], CRTC2 [4], IRS-1 [5], Akt [6], and Smad3 [7]. Pin1 binds phosphorylated Ser84-Pro of PPARγ to reduce its transcriptional activity [3]. Pin1 also binds phosphorylated Ser136-Pro of CRTC2 and decreases nuclear CRTC-CREB complexes to inhibit the transcriptional activity of CREB [4]. On the other hand, Pin1 binds phosphorylated Ser434-Pro of IRS1 to directly upregulate insulin signal transduction [5]. IRS-1 is critical for adipose cell differentiation [8]. Pin1 stabilizes Akt kinase, a signaling protein that is down-stream of IRS-1 in the insulin signal transduction cascade [6]. Smad3 is a protein that is activated in response to TGFβ. Smad3 interferes with interactions between C/EBPα and PPARγ and inhibits PPARγ activity [9]. Pin1 accelerates degradation of Smad3 and suppresses TGFβ signaling [7], thereby upregulating insulin signaling.
Immature adipose cells cannot store sufficient triglycerides, and we hypothesized that Pin1 promotes triglyceride storage by enhancing the differentiation of immature cells to mature adipose cells. In this report, we demonstrate the biological role of Pin1 in regulating cellular fat storage in response to insulin signaling.
Results
The adipose tissue measured by ComputerTomography is lower in Pin1−/− mice
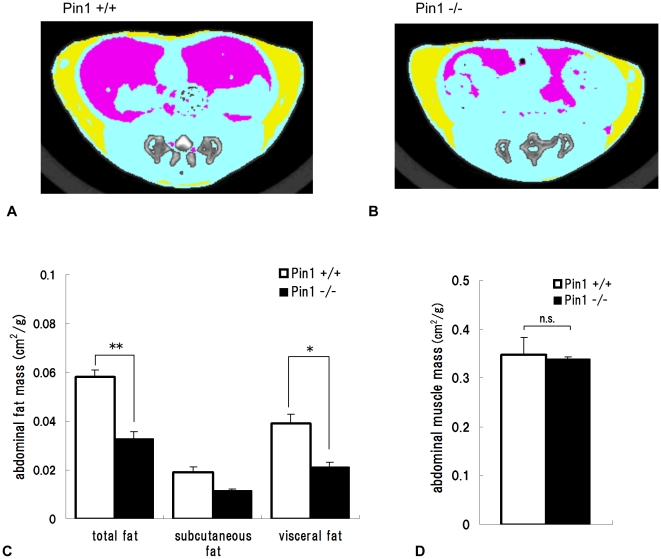
The abdomens of 16 week old male wild-type and Pin1−/− mice were scanned by computer tomography and the images were colored as follows: yellow; subcutaneous fat, pink; visceral fat, blue; muscle (Fig. 1A,B). Quantities of subcutaneous and visceral adipose tissues were determined from the colored areas (Fig. 1C). These results show that the quantity of adipose tissue in Pin1−/− mice was less than that of wild-type mice. The differences in total and visceral fat between wild-type and Pin1−/− mice were significant (Fig. 1C). On the other hand, muscle mass in Pin1−/− and wild-type mice was similar (Fig. 1D).
Figure 1. Computed Tomography analysis of wild-type and Pin1−/− mice.
The abdomens of 16 week-old male wild-type (A) and Pin1−/− (B) mice were scanned (yellow; subcutaneous fat, pink; visceral fat, blue; muscle). Quantities of subcutaneous, visceral, and total adipose tissues (**p<0.01) (C), and the other tissues, mainly muscle (D), were measured from the images. The areas were measured from the computer tomography images (cm2) and they were adjusted for body weights of mice (±SEM, n = 3).
The lack of Pin1 leads to lower fat accumulation in high fat diet mice
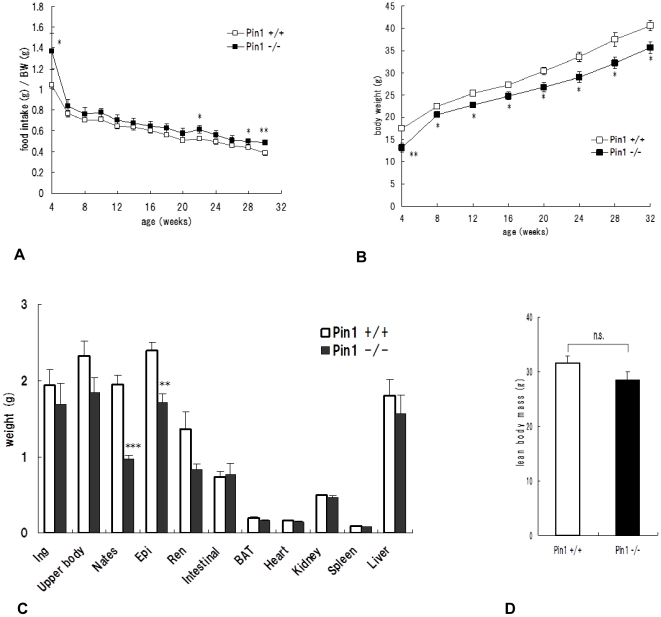
High fat diets were fed to the mice from 4 to 28 weeks old. Food intake and weight of the mice were monitored. Interestingly, although the intake of food by Pin1−/− mice was greater than that of wild-type mice (Fig. 2A), increases in body weight were lower in knockout animals (Fig. 2B). Mice were then sacrificed and the weights of organs and fat tissues were measured. The weight of each fat tissue, such as buttock fat tissue, which represents subcutaneous fat, and genital fat tissue, which represents visceral fat, from Pin1−/− mice weighed lower than those from wild-type mice (Fig. 2C). On the other hand, the weights of organs, such as heart, kidney, liver, spleen, and brown fat tissues were similar (data not shown). Furthermore, the total weight of removed fat tissues was not different between Pin1−/− and wild-type mice (Fig. 2D).
Figure 2. Relationship between quantity of fat and Pin1 expression.
High fat diets were fed to wild-type (n = 8) and Pin1−/− (n = 5) mice between the ages of 4–28 weeks and the amount of food intake (A) and body weight (B) were recorded. Weights of subcutaneous and genital fat tissues were measured (C). The total weight of all fat tissues removed was also measured (D). The amount of food intake per mouse weight was monitored. Student t-test *p<0.05, **p<0.01, ***p<0.001.
Area sizes of mouse adipose cells
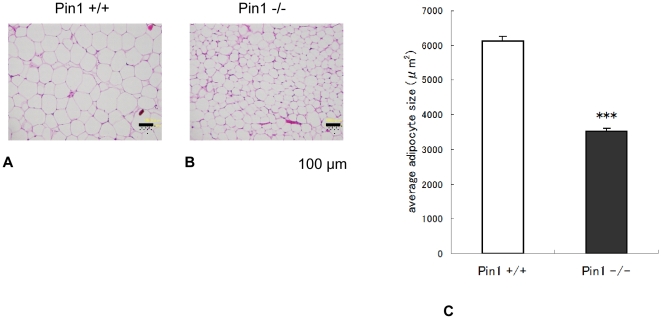
Pathological analysis of genital fat tissue revealed that adipose cells from Pin1−/− and wild-type mice are similar (data not shown). However, adipose cells from inguinal fat tissue of Pin1−/− mice were smaller than those from wild-type mice (Fig. 3A, B). We measured the area of cells quantitatively with Image J analysis soft. The average sizes of the adipose cells of wild and Pin1−/− mice were 6129.6±136.0 µm2 and 3516.6±87.0 µm2 (±SEM) respectively (Fig. 3C) and the difference was statistically significant, p<0.001.
Figure 3. Pathological analysis of adipose tissues from wild-type and Pin1−/− mice.
Paraffin-embedded sections of inguinal fat tissue from wild-type (A) and Pin1−/− (B) mice were stained with hematoxylin and eosin. The size of cells was analyzed quantitatively with Image J analysis soft. The average sizes of the adipose cells of wild and Pin1−/− mice were 6129.6±136.0 µm2 and 3516.6±87.0 µm2 (±SEM) respectively (Figure 3C). Student t-test p<0.001.
The inhibition of Pin1 affects adipose-like differentiation in fibroblasts
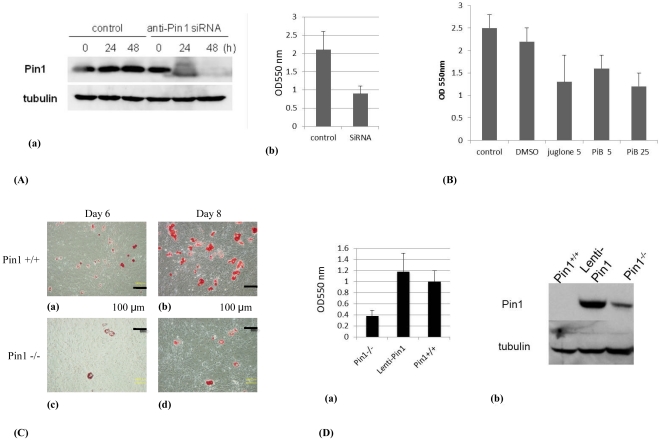
NIH3T3-L1 cells become differentiated into adipose cells containing fat droplets upon stimulation with insulin. The fat droplets were stained red by Oil Red O solution and the rate of differentiation was determined semi-quantitatively. siRNA against Pin1 [4], [10] decreased the Pin1 level in NIH 3T3-L1 cells in 24 hours (Fig. 4A-a) and inhibited their differentiation into adipose cells (Fig. 4A-b). The inhibitory effect of Pin1 depletion was further confirmed by using two Pin1 inhibitors, juglone and PiB (diethyl-1,3,6,8- tetrahydro- 1,3,6,8- tetraoxobenzol - phenanthroline- 2,7-diacetate). Juglone is the most well known inhibitor of Pin1 even if it has the drawback that it blocks transcription directly [11]. Therefore we also used the other Pin1 inhibitor PiB [12]–[14]. 5 µM of juglone inhibited the differentiation as strong as 25 µM of PiB (Fig. 4B). We then generate MEFs from wild-type and Pin1−/− mice that were induced to differentiate into adipose cells by insulin treatment and the fat droplets stained with Oil Red O were observed under a microscope. The wild-type MEFs differentiated into adipose cells that can be clearly observed after 6 days (Fig. 4C-a). 8 days later, differentiation of the cells had progressed further with the number of cells staining red increasing (Fig. 4C-b). Pin1−/− MEFs did not differentiate as much as did wild-type MEFs after 6 days of insulin treatment (Fig. 4C-c), and even after 8 days, the number of stained cells was much less (Fig. 4C-d). Wild-type MEFs, Pin1−/− MEFs-treated with lenti-Pin1 (Lentiviral vector expressing Pin1), and mock-transfected (Lentiviral vector without insert) Pin1−/− MEFs were stimulated with insulin for 8 days, after which the rate of differentiation of MEFs was determined by measuring the absorbance of red color derived from Oil Red O-stained fat droplets. The intensity of red staining in mock-transfected Pin1−/− MEFs was significantly lower than that of wild-type MEFs. However, transfection of Pin1 cDNA into Pin1−/− MEFs increased the intensity of red staining to similar, or slightly higher, levels compared to wild-type MEFs (Fig. 4D-a). Levels of Pin1 expressed in MEFs transfected with lenti-Pin1 were higher than those in wild-type MEFs (Fig. 4D-b).
Figure 4. Differentiation of fibroblasts into adipose cells.
(A) NIH3T3L1 cells were transfected with 20 µg mock or SiRNA-Pin1 plasmids, cultured for 24 and 48 hours, and the expression levels of Pin1 were examined with western blot analysis (a). The NIH3T3-L1 cells were incubated in DMEM medium, containing; 0.5 mM 3-isobutyl-1-methylxanthine, 1 µM dexamethasone, and 1.7 µM insulin for 48 hours, treated with 4% paraformaldehyde and 60% 2-propanol and then stained with Oil Red O. The amount of Oil Red O extracted from cells was determined by measuring the absorbance at 550 nm (b). (B) NIH3T3-L1 cells were incubated in the same medium with juglone (5 µM) or PiB (5, 25 µM), and the fat was measured with oil red O assay. (C) Wild-type (a, b) and Pin1−/− (c, d) MEFs were stimulated in the same way as NIH3T3 cells for 6 (a, c) and 8 days (b, d), stained with Oil Red O, and analyzed by microscopy. (D) Pin1−/− MEF, Lenti-Pin1 (Pin1−/− MEF-infected with lentiviral Pin1 cDNA), and wild-type MEF were also examined like (C) (a). Pin1 levels of the Pin1−/− MEFs, Lenti-Pin1−/−, and wild-type MEFs were analyzed with western blot (b).
Pin1 interacts with CREB suppressing its transcriptional activity
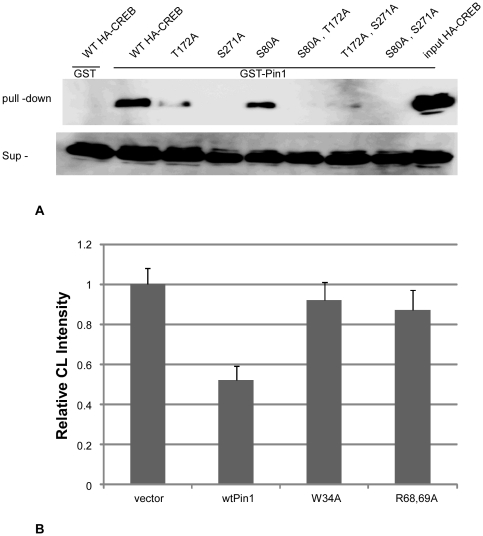
Finally, whether Pin1 directly binds to and regulates CREB in MEFs was examined. Pin1 bound CREB directly after stimulation of MEFs with 1 or 10 µM forskolin. There are 3 Ser/Thr-Pro sites in CREB: Ser80, Thr172, and Ser271. Pin1 did not bind to CREB with Ala mutation at Thr172 or Ser271 but bound to CREB with Ala mutation at Ser80 (Fig. 5A). These results suggest that Pin1 directly binds to CREB at phosphoThr172-Pro and phosphoSer271-Pro. Transcriptional assays showed that Pin1 suppresses the transcriptional activity of CREB in COS-7 cells. The Pin1 with the mutation at WW motif or the substrate binding pocket lost the suppressive function (Fig. 5B).
Figure 5. Association of Pin1 and CREB.
(A) Lysates of COS-7 cells, transfected with HA-CREB (wild-type; T172A; S271A; S80A; S80A/T172A; T172A/S271A; S80A/S271A) and stimulated with 10 µM forskolin for 6 hr, were pulled down with GST-Sepharose (control), GST-Pin1-Sepharose, and input (upper panel). CREB levels in the supernatant are shown in the lower panel. (B) 106 of COS-7 were co-transfected with pCRE-Luc and CMV-Pin1 (wt; wild type, W34A; mutation at WW domain, R68,69A; prolyl isomerase mutant). The cells were treated with 100 µM of forskolin (CST) for 6 hours and luciferase activities were determined using the Dual-Luciferase Reporter Assay System (Promega).
Discussion
Our computer tomography images suggested that Pin1−/− mice have less fat tissue than wild-type mice. In order to verify this, high-fat diets were fed to these mice, after which various fat tissues were removed and weighed. These results clearly showed that there were significantly less subcutaneous and visceral fat tissues in Pin1−/− mice, suggesting they lose the ability to store lipid in these tissues. In fact, adipose cells from inguinal fat tissue of Pin1−/− mice were smaller than those of wild-type mice. It has been reported that the average diameter of a mature adipose cell is approximately 60 µm. However, once adipose cells aggressively uptake triglycerides, cell size increases (diameter: 100 µm [15]). In our studies, adipose cell area sizes of wild-type and Pin1−/− mice were measured. The area size is more reliable than the diameter because the cell shape is not round. The average adipose cell area sizes of wild-type and Pin1−/− mice clearly show that Pin1 enhances maturation of adipose cells and promotes uptake of lipid.
A fibroblast cell line, NIH3T3L1 differentiates into adipose cells upon insulin treatment. We examined the effect of Pin1 on the differentiation by reducing the expression level of Pin1. SiRNA caused knockdown of Pin1 and inhibited the NIH3T3L1 cell differentiation into adipose cells. In the same way, Pin1 inhibitors, juglone and PiB also blocked the differentiation. Also MEFs can be differentiated into adipose cells by the treatment of the cells with insulin [8]. Results from insulin treatment showed that Pin1−/− MEF differentiated less than wild-type MEFs. However, the rate of differentiation of Pin1−/− MEF into adipose cells was restored to levels similar to that of wild-type MEFs by Pin1 forced expression. These results show that it is possible to regulate fibroblast differentiation into adipose cells by regulating the expression, or activity, of Pin1.
Pin1 is known to bind to CRTC, thereby suppressing CREB activity [4]. We hypothesized that in conditions where CREB is overexpressed, Pin1 might directly bind to CREB and activate it. As expected, Pin1 directly bound CREB at phosphoThr172-Pro and at phosphoSer271-Pro sites. However, as previously reported in HepG2 cells [4], Pin1 suppressed the transcriptional activity of CREB in COS-7 cells. These results suggest that suppression of CREB activity by Pin1, whether by direct or indirect mechanisms, may be a factor underlying enhanced differentiation of fibroblasts to adipose cells in response to insulin stimulation. Further studies are required in order to more fully elucidate mechanisms by which Pin1 enhances differentiation of fibroblasts into adipose cells.
One of the most important functions of adipose tissues is to store triglycerides that can be used when energy is required. Another critical function of adipose tissue is to produce a variety of adipokines that control whole body metabolism [16]. Thus, results of this study suggest that Pin1 down-regulation could be a potential approach in obesity-related dysfunctions, such as high blood pressure, diabetes, non-alcoholic steatohepatitis. Future studies could help for discovering an inhibitor against Pin1 with less toxicity to do animal study and clinical application in future [17]–[19].
Materials and Methods
Animal studies
Our study was approved by the Tohoku University Animal Use and Care Committee; approval ID 76-19-84. High fat diets, 20–25 g/week of HFD-60 (Oriental Kobo), were fed to 4-week-old wild-type (n = 8) and Pin1−/− mice (n = 5) [1], with food intake and body weight being measured weekly. High fat diet experiments were continued for 28 weeks.
Cells
NIH3T3-L1 cells (JCRB9014) were provided by the Japanese Human Science Foundation. MEFs were prepared as previously reported [1], [10]. COS7 and HEK293 were cultured as written in the previous report [20]. We did not use previously unpublished cell lines in this manuscript.
Statistical analysis
Values are reported as means ± SEM. Statistical analysis of differences between mean values was carried out by Student's t tests. Values of p<0.05 were considered to be statistically significant.
Computer Tomography Analysis
The abdomens of 16 week-old male wild-type and Pin1−/− mice were scanned by Computer Tomography (LCT-100 Lite (Aloka)), and the images were colored as follows: yellow; subcutaneous fat, pink; visceral fat, blue; muscle. Computer Tomography scans were performed at 1.5 mm intervals×20 slices. The areas were measured from the Computer Tomography images (cm2) and they were adjusted for body weights of mice (±SEM, n = 3).
SiRNA plasmid against Pin1
pSilencer™ (Applied Biosystems) was digested with ApaI and EcoRI and annealed oligos (Pin1: 5′GGGCCCGCCGAGTGTACTACTTCAATTCAAGAGATTGAAGTAGTACACCTCGGCTTTTTTGATTC3′ , control: 5′GGCCCTCGTATGTTGTGTGGAATTTTCAAGAGAAATTCCACACAACATACGATTTTTTGATTC3′ ). Transfection of the plasmid (20 µg) was conducted by using Lipofectamine 2000 (Invitrogen) as previously described [20]. After 24 and 48 hours, expression level of Pin1 was examined by western blot.
Lentiviral- Pin1 cDNA
HEK293 cells were transfected with the packaging plasmids (pCMV-VSVG-RSV-REV, pCAG-HIV-gp), which were kindly provided by Hiroyuki Miyoshi, and Pin1 expression vector (pCDH- CMV-Pin1-EF1-copGFP). The supernatant of the culture medium was passed through 0.45 µM filters (Millipore) and used as lentiviral Pin1 cDNA (4 µg/ml). The lentiviral Pin1 was added into the medium to increase Pin1 expression.
Differentiation of Cells
NIH3T3-L1 (control and Pin1-knockdown) and MEFs (wild-type and Pin1−/−) were incubated in DMEM medium, containing 0.5 mM 3-isobutyl- 1-methylxanthine (Nakalai), 1 µM dexamethasone (Sigma) and 1.7 µM insulin (Wako) for 48 hours. Cells were cultured in insulin-containing medium for 48 hours. Juglone (Sigma) [21] and PiB [12] were solved in ethanol and DMSO respectively (10 mM) and added into the medium to be 5 or 25 µM.
Histopathology
For histopathological analysis, paraffin-embedded sections were stained with hematoxylin and eosin (Wako). The area of 200 adipose cells in the three different points were measured by using Image J analysis soft [22] and the average areas of the wild and Pin1−/− mouse adipose cells were statistically compared.
Oil Red O Staining for Cultured Cells
NIH3T3-L1 and MEFs were treated with 4% paraformaldehyde and 60% 2-propanol and then stained with Oil Red O (Sigma). The stained cells were observed with a BZ-8100 microscope (Keyence) and the quantity of Oil Red O- extracted from cells was determined by measuring absorbance at 550 nm with a DTX 880 Multimode Detector (Beckman Coulter).
Western Blot Analysis
Pin1 was detected using rabbit anti-Pin1 antibodies (Calbiochem), followed by anti-rabbit IgG HRP-linked antibodies (Cell Signaling Technology). α-Tubulin was used as a loading control. Chemiluminescence was detected with an LAS-3000 Image Analyzer (Fuji Film).
Pull-down Assay
Lysates from COS-7 cells transfected with HA-CREB (wild-type; T172A; S271A; S80A; S80A/T172A; T172A/S271A; S80A/S271A) were incubated with 30 µg of GST-Pin1 for 30 min at 4°C. Glutathione-sepharose 4B beads (GE Healthcare) were added to the mixture and incubated at 4°C with gentle rocking for a further 1 hr. The beads were precipitated by centrifugation at 3,000× g in a microcentrifuge and washed three times with protein buffer. Following the final wash, the beads were resuspended in 25 µl SDS-PAGE sample buffer containing 50 mM DTT. Wild-type and mutant HA-CREBs were blotted onto membranes and were identified with anti-HA antibody (Genscript).
Transcriptional Assay of CREB
106 of COS-7 cells were co-transfected with pCRE-Luc and CMV-Pin1 (wt; wild type, W34A; mutation at WW domain, R68,69A; prolyl isomerase mutant) (0.25 µg/well), with an internal reporter, pRL-TK. The cells were treated with 100 µM of forskolin (CST) for 6 hours and luciferase activities were determined using the Dual-Luciferase Reporter Assay System (Promega).
Acknowledgments
We thank Yoshimasa Bamba and Ichie Saitoh for technical help, Masafumi Hidaka for data analysis, Hiroyuki Miyoshi for providing lentiviral system, Takumi Oka for valuable comments.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan, Kiban S ID 20228006(Dr. Uchida). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Fujimori F, Takahashi K, Uchida C, Uchida T. Mice lacking Pin1 develop normally, but are defective in entering cell cycle from G0 arrest. Biochem Biophys Res Commun. 1999;265:658–663. doi: 10.1006/bbrc.1999.1736. [DOI] [PubMed] [Google Scholar]
- 2.Liou YC, Ryo A, Huang HK, Lu PJ, Bronson R, et al. Loss of Pin1 function in the mouse resembles the cyclin D1- null phenotypes. Proc Natl Aca Sci USA. 2002;99:1335–1340. doi: 10.1073/pnas.032404099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fujimoto Y, Shiraki T, Horiuchi Y, Waku T, Shigenaga A, et al. Proline cis/trans-Isomerase Pin1 Regulates Peroxisome Proliferator-activated Receptor γ Activity through the Direct Binding to the Activation Function-1 Domain. J Biol Chem. 2010;285:3126–3132. doi: 10.1074/jbc.M109.055095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakatsu Y, Sakoda H, Kushiyama A, Ono H, Fujishiro M, et al. Pin1 associates with and induces translocation of CRTC2 to the cytosol, thereby suppressing CRE transcriptional activity. J Biol Chem. 2010;285:33018–33027. doi: 10.1074/jbc.M110.137836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakatsu Y, Sakoda H, Kushiyama A, Zhang J, Ono H, et al. Peptidyl-prolyl cis/trans isomerase NIMA-interacting 1 associates with IRS-1 and enhances insulin actions and adipogenesis. J Biol Chem. 2011;286:20812–20822. doi: 10.1074/jbc.M110.206904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liao Y, Wei Y, Zhou X, Yang JY, Dai C, et al. Peptidyl-prolyl cis/trans isomerase Pin1 is critical for the regulation of PKB/Akt stability and activation phosphorylation. Oncogene. 2009;28:2436–2445. doi: 10.1038/onc.2009.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakano A, Koinuma D, Miyazawa K, Uchida T, Saitoh M, et al. Pin1 downregulates TGF-β signaling by inducing degradation of Smad proteins. J Biol Chem. 2009;284:6109–6115. doi: 10.1074/jbc.M804659200. [DOI] [PubMed] [Google Scholar]
- 8.Miki H, Yamauchi T, Suzuki R, Komeda K, Tsuchida A, et al. Essential role of insulin receptor substrate 1 (IRS-1) and IRS-2 in adipocyte differentiation. Mol Cell Biol. 2001;21:2521–2522. doi: 10.1128/MCB.21.7.2521-2532.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choy L, Derynck R. Transforming Growth Factor-β Inhibits Adipocyte Differentiation by Smad3 Interacting with CCAAT/Enhancer-binding Protein (C/EBP) and Repressing C/EBP Transactivation Function. J Biol Chem. 2003;278:9609–9619. doi: 10.1074/jbc.M212259200. [DOI] [PubMed] [Google Scholar]
- 10.Ryo A, Uemura H, Ishiguro H, Saitoh T, Yamaguchi A, et al. Stable Suppression of Tumorigenicity by Pin1-Targeted RNA Interference in Prostate Cancer. Clin Cancer Res. 2005;11:7523–7531. doi: 10.1158/1078-0432.CCR-05-0457. [DOI] [PubMed] [Google Scholar]
- 11.Chao SH, Greenleaf AL, Price DH. Juglone, an inhibitor of the peptidyl-prolyl isomerase Pin1, also directly blocks transcription. Nucleic Acids Res. 2001;29:767–773. doi: 10.1093/nar/29.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uchida T, Takamiya M, Takahashi M, Miyashita H, Ikeda H, et al. Pin1 and Par14 peptidyl prolyl isomerase inhibitors block cell proliferation. Chem Biol. 2003;10:15–24. doi: 10.1016/s1074-5521(02)00310-1. [DOI] [PubMed] [Google Scholar]
- 13.Rustighi A, Tiberi L, Soldano A, Napoli M, Nuciforo P, et al. The prolyl-isomerase Pin1 is a Notch1 target that enhances Notch1 activation in cancer. Nature Cell Biol. 2009;11:133–142. doi: 10.1038/ncb1822. [DOI] [PubMed] [Google Scholar]
- 14.Moretto-Zita M, Jin H, Shen Z, Zao T, Briggs SP, et al. Phosphorylation stabilizes Nanog by promoting its interaction with Pin1. Proc Natl Aca Sci USA. 2010;10:13312–12217. doi: 10.1073/pnas.1005847107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jernås M, Palming J, Sjöholm K, Jennische E, Svensson PA, et al. Separation of human adipocytes by size: hypertrophic fat cells display distinct gene expression. FASEB J. 2006;20:1540–1542. doi: 10.1096/fj.05-5678fje. [DOI] [PubMed] [Google Scholar]
- 16.La DC, Dhillo B, Ya H, Szmitk PE, Verma S. Adipokines: molecular links between obesity and atheroslcerosis. Am J Physiol Heart Circ Physiol. 2005;288:2031–2041. doi: 10.1152/ajpheart.01058.2004. [DOI] [PubMed] [Google Scholar]
- 17.Mori T, Itami S, Yanagi T, Tatara Y, Takamiya M, et al. Use of a Real-time Fluorescence Monitoring System for High-Throughput Screening for Prolyl Isomerase Inhibitors. J Biomol Screen. 2009;14:419–424. doi: 10.1177/1087057109333979. [DOI] [PubMed] [Google Scholar]
- 18.Tatara Y, Lin YC, Bamba Y, Mori T, Uchida T. Dipentamethylene thiuram monosulfide is a novel inhibitor of Pin1, Biochem Biophys Res Commun. 2009;384:394–398. doi: 10.1016/j.bbrc.2009.04.144. [DOI] [PubMed] [Google Scholar]
- 19.Mori T, Hidaka M, Lin Y, Yoshizawa I, Okabe T, et al. A dual inhibitor against prolyl isomerase Pin1 and cyclophilin discovered by a novel real-time fluorescence detection method. Biochem Biophys Res Commun. 2011;406:439–443. doi: 10.1016/j.bbrc.2011.02.066. [DOI] [PubMed] [Google Scholar]
- 20.Akiyama H, Shin R, Uchida C, Kitamoto T, Uchida T. Prolyl isomerase Pin1 facilitates production of Alzheimer's amyloid beta from beta-cleaved amyloid precursor protein. Biochem Biophys Res Commun. 2005;336:521–529. doi: 10.1016/j.bbrc.2005.08.130. [DOI] [PubMed] [Google Scholar]
- 21.Nechama M, Uchida T, Yosef-Levi IM, Silver J, Naveh-Many T. The peptidyl-prolyl isomerase Pin1 determines parathyroid hormone mRNA levels and stability in secondary hyperparathyroidism. J Clin Invest. 2009;119:3103–3114. doi: 10.1172/JCI39522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abramoff MD, Magalhaes PJ, Ram SJ. Image Processing with Image J. Biophotonics International. 2004;11:36–42. [Google Scholar]