Abstract
Objective
Apathy is a common feature of Parkinson's disease (PD) that can manifest independently of depression, but little is known about its natural progression in medically-managed patients. The present study sought to characterize and compare trajectories of apathy, depression, and motor symptoms in PD over 18 months.
Method
Data from a sample of 186 PD patients (mean disease duration of 8.2 years) followed by the University of Florida Movement Disorders Center were obtained from a clinical research database. Scores on the Unified Parkinson's Disease Rating Scale (motor portion), Apathy Scale, and Beck Depression Inventory at three time-points (baseline, 6 months, 18 months) were analyzed in a structural equation modeling framework.
Results
A multivariate growth model controlling for age, sex, education, and disease duration identified linear worsening of both apathy (slope estimate = 0.73; p <.001) and motor symptoms (slope estimate = 1.51; p <.001), and quadratic changes in depression (slope estimate = 1.18; p = .07). All symptoms were positively correlated. Higher education was associated with lower apathy, depression, and motor severity. Advanced age was associated with greater motor and apathy severity. Female sex and longer disease duration were associated with attenuated motor worsening. Antidepressant use was associated only with depression scores.
Conclusions
These longitudinal results support the differentiation of apathy and depression in PD. Like motor progression, apathy progression may be linked at least partially to dopaminergic neurodegeneration. Empirically-supported treatments for apathy in PD are needed.
Keywords: Apathy, depression, antidepressants, structural equation modeling, neurodegeneration
Parkinson's disease (PD) is an age-related neurodegenerative disorder characterized by the loss of dopamine neurons in the midbrain. While PD was initially recognized as a purely motor disorder producing resting tremor, rigidity, bradykinesia and postural instability, the high prevalence of non-motor symptoms has led to its contemporary conceptualization as a neuropsychiatric disorder (Simuni & Sethi 2008). Non-motor symptoms of PD can include depression, apathy, anxiety, psychosis, and sleep disturbance, among several others.
In PD, one of the major neuropathologic features is loss of dopaminergic input to the striatum, which disrupts cortico-basal ganglia-thalamocortical circuits (Bar-Gad & Bergman, 2001). The motor symptoms of PD result from dysfunction within the presumed motor loop, which comprises primary motor cortex, putamen, lateral globus pallidus interna, and the ventral lateral nucleus of the thalamus (Gibb, 1997). Parallel basal ganglia loops, however, contain cognitive and affective information flow as well (Alexander, DeLong & Strick, 1986; Middleton & Strick, 2000). The neurodegenerative process responsible for PD may also affect these non-motor loops, many of which refine signals originating in dorsolateral prefrontal, anterior cingulate, and orbitofrontal cortices. Dysfunction within these systems, which partially rely on midbrain dopaminergic input, may underlie certain cognitive and affective symptoms of PD (Obeso et al., 2008). However, there may be other neuroanatomical and neurochemical pathways also responsible for dysfunction.
Depression is one of the most common non-motor features of PD, with average prevalence rates in cross-sectional studies in PD reaching 40% in most populations (Brown & Jahanshahi, 1995). The neurobiology of depression in PD is unclear, but one hypothesis involves degeneration of mesolimbic structures of the dopaminergic system, including the ventral tegmental area (VTA; Fibiger, 1984). The neuropatholophysiology of depression in PD is likely more complex, as studies using transcranial sonography have linked PD depression to morphological changes in non-dopaminergic brain regions (Becker et al., 1997; Walter, Skoloudik & Berg, 2009). Further evidence that pathological changes extend beyond those occurring within basal ganglia circuitry is the finding that the relationship between depressive and motor symptoms in PD is not clearly linear (Brown & Jahanshahi, 1995). Several studies have shown that symptoms can worsen and remit over time, with more severe symptoms at baseline predictive of potential depressive symptoms at follow-up (Brown et al., 1988; Ravina et al., 2009; Starkstein et al., 1992).
Apathy refers to a lack of motivation and occurs in up to 60% of patients with PD (Leentjens et al., 2008; Marin, 1991). While apathy is a common symptom of depression, it can also manifest as an independent syndrome in up to 30% of patients with PD (Kirsch-Darrow et al., 2006). In contrast to the more heterogeneous neuropathophysiology implicated in PD depression, apathy has been consistently linked to fronto-striatal circuitry involving the anterior cingulate cortex and the striatum (Hama et al, 2007; Lavretsky et al., 2007). For example, patients with more severe apathy exhibit dopaminergic under-activity in the ventral striatum (Remy et al., 2005). Higher levels of apathy are associated with more severe parkinsonian motor symptoms in both PD and Alzheimer's disease (Oguru, et al., 2010; Pedersen et al., 2010; Starkstein et al., 2009). However, the precise relationship between clinical trajectories of motor and apathy symptoms in PD is unknown (Schrag, Jahanshahi & Quinn, 2001).
To date, the course of these mood and motor symptoms in PD has not been examined longitudinally using a sophisticated modeling technique. The purpose of the present study was to compare the trajectories of motor, apathy, and depressive symptoms in a cohort of patients with PD followed by a single movement disorders center. Given evidence for stronger relationships between motor symptoms and apathy, as compared to depression, we predicted that both motor and apathy symptoms would worsen linearly. In contrast, we predicted non-linear changes in depressive symptoms based on prior studies suggesting a more complex (e.g., non-dopaminergic) neuropathophysiology of PD depression.
Method
Participants
The present sample included 186 individuals with idiopathic Parkinson's disease (PD) being followed by the University of Florida (UF) Center for Movement Disorders and Neurorestoration. Data were obtained from the IRB-approved UF INFORM clinical research database. All patients with a diagnosis of PD who had completed apathy questionnaires during at least three clinical visits (baseline, 6 months, 18 months) were included. Completion of three apathy questionnaires was chosen as our primary inclusion criterion because the routine administration of this questionnaire to all patients in our clinic was initiated more recently than the routine administration of the depression questionnaire. This criterion allowed us to gather the largest sample possible with the least amount of missing data across the three symptom domains of interest. All clinical assessments were conducted at the UF Movement Disorders Clinic, an outpatient tertiary-care center. Patients were excluded for a history of: 1) a Parkinson plus syndrome, 2) neurological surgery, or 3) significant neurologic illness other than PD (e.g., stroke). Participant characteristics at each assessment, including the proportion of participants scoring above psychometric criteria for clinically significant apathy or depression as described in the next session, are shown in Table 1.
Table 1. Patient characteristics at each clinical assessment.
| Baseline | 6-Months | 18-Months | ||||
|---|---|---|---|---|---|---|
| Mean | (SD) | Mean | (SD) | Mean | (SD) | |
| Demographics | ||||||
| Age | 65.9 | (10.6) | 66.4 | (10.5) | 67.3 | (10.6) |
| Education | 14.9 | (3.0) | ||||
| Sex (% Male) | 66.0 | |||||
| PD Duration (years) | 8.2 | (6.1) | ||||
| Mood | ||||||
| AS | 13.0 | (6.9) | 12.9 | (6.8) | 14.0 | (7.3) |
| % with AS ≥ 14 | 43.0 | - | 43.0 | - | 52.5 | - |
| BDI | 9.4 | (7.0) | 8.6 | (6.3) | 9.5 | (6.2) |
| % with BDI ≥ 15 | 17.6 | - | 17.1 | - | 20.3 | - |
| % on an antidepressant | 31.5 | - | 32.4 | - | 34.1 | - |
| Motor | ||||||
| LEDa | 617.5 | (376.4) | 776.1 | (580.8) | 803.6 | (623.4) |
| UPDRS on | 28.6 | (10.8) | 28.6 | (10.5) | 30.7 | (11.5) |
| UPDRS off | 34.3 | (12.9) | ||||
LED data only available for 90 patients
Note. PD=Parkinson's disease; AS=Apathy Scale; BDI=Beck Depression Inventory; LED=Levodopa equivalent dose; UPDRS=Unified Parkinson's Disease Rating Scale (motor portion)
Measures
Parkinson's disease severity was quantified with the Unified Parkinson's Disease Rating Scale, motor portion (UPDRS-III; Fahn, Elton & Committee, 1987). Depressive symptoms were assessed at each occasion with the Beck Depression Inventory (BDI), which comprises 21 items on a 4-point Likert-type scale. Scores on this scale can range from 0 to 63, and a cut-off of 14/15 indicates clinically-significant depression in PD (Visser et al., 2006). Apathy was quantified with the Apathy Scale (AS), which comprises 14 items on a 4-point Likert-type scale. Scores on this scale can range from 0 to 42, and a cut-off of 13/14 is thought to reflect clinically-significant apathy (Starkstein et al., 1992).
Statistical Analysis
Data were analyzed in AMOS 17.0 with a special case of structural equation modeling often referred to as latent growth curve (LGC) modeling. Missing data were handled using full information maximum likelihood parameter estimation.1 Presented models carry the assumption of homogeneity of error variance and dependence of errors within each domain (i.e., motor symptoms, apathy or depression). The effects of different error structure specifications (e.g., complete independence of errors) on model fit were examined. Importantly, specifying errors differently had little effect on parameter estimates or their pattern of significance.
A strength of LGC modeling is that it allows the study of multiple outcomes over time in a multivariate framework. The overall level (i.e., intercept) and amount of change (slope) of the each of the three symptoms (i.e., motor symptoms, apathy, depression) represented the key parameter estimates. Additional information regarding parameter estimation in multivariate LGC is available in the Appendix. Model fit was assessed with the following, commonly-used statistics in this approach: chi square, Akaike information criterion (AIC), root mean square error of approximation (RMSEA), and comparative fit index (CFI). Smaller values of chi square, AIC, and RMSEA (particularly values below .06) indicate better model fit. Values of CFI that are close to 1 indicate better fit. Fit between nested models was compared statistically using the chi square test.
Model building proceeded in three broad stages. First, the trajectories of the three variables of interest were examined separately with unconditional growth models. Due to the lack of previous studies examining the functional form of apathy progression in PD and reports that depression does not change linearly in PD, the potential for a quadratic effect of time was explored separately for all three symptom types. To do this, models that estimated only linear change were statistically compared to those that estimated both linear and quadratic change. In models that included only linear slopes, slope vectors for the three timepoints (i.e., baseline, 6 months, 18 months) were: 0, 0.5, and 1.5. In models that included both linear and quadratic slopes, orthogonalized slope vectors were computed by creating a set of mean-centered linear slope loadings, regressing these centered loadings out of their squared loadings and saving the residuals. Resultant centered, orthogonal slope vectors were: 1.67, -0.17, 0.83 (linear) and 0.21, -0.32, 0.11 (quadratic). In this first stage, six separate unconditional growth models were planned: 1) motor, linear slope only; 2) motor, linear and quadratic slopes; 3) apathy, linear slope only; 4) apathy, linear and quadratic slopes; 5) depression, linear slope only; 6) depression, linear and quadratic slopes. Model fit was statistically compared between models within each domain (i.e., motor symptoms, apathy, depression) in order to characterize the trajectory of each symptom type.
Second, these models were combined into a single, unconditional multivariate model, in which obtained parameter estimates control for all others. For example, the estimate of baseline levels of apathy (i.e., apathy intercept) in the unconditional multivariate model controls for baseline levels of motor symptoms and depression. In this multivariate model, correlations between initial levels of the three symptom types and changes in these symptoms can be estimated. Third, four covariates (i.e., age, sex, education in years, and disease duration in years) were added to the multivariate model. In this conditional multivariate model, covariate effects on both the overall levels of symptoms (intercepts) as well as on symptom change (slopes) were examined.
Results
In the following section, we present results from fitting the proposed LGC models to the data separately for each of the three broad stages described above.
Unconditional latent growth curve models
Raw scores for motor symptoms, apathy, and depression at each time point are shown as dashed lines with errors bars in Figure 1. A model that estimated quadratic change did not significantly improve fit over a model that estimated both linear and quadratic change for both motor symptoms (Δχ2(2)=-0.162, p>.05) and apathy (Δχ2(1)=-2.31, p>.05). Thus, subsequent models included only linear slopes for these variables, which can be interpreted as the constant rates of change over time.
Figure 1.
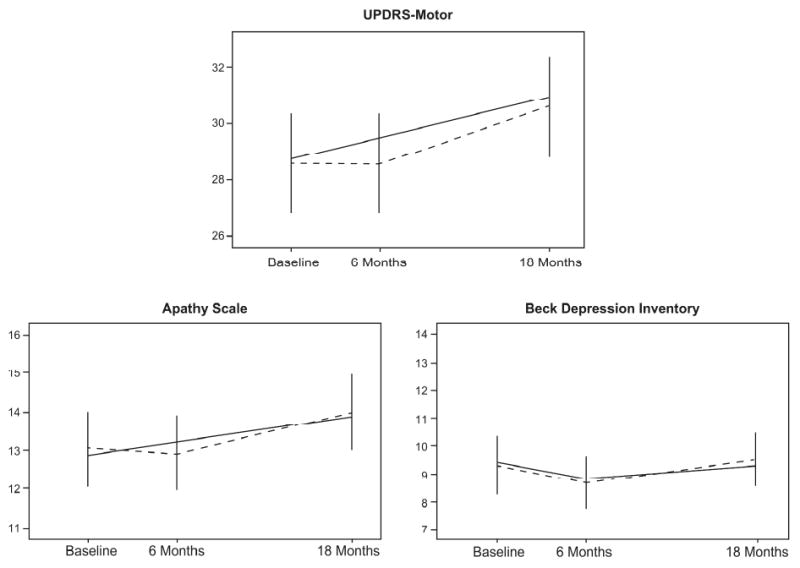
Change trajectories for motor symptoms, apathy, and depression. Dashed lines correspond to mean raw scores at each time-point. Solid lines represent trajectories estimated by the unconditional multivariate model. UPDRS=Unified Parkinson's Disease Rating Scale.
For the depression variable, estimating quadratic change significantly improved fit over the model that estimated only linear change (Δχ2(1)=-5.428, p<.05). However, the fixed effects of the linear and quadratic slopes were not significant, suggesting that there were not significant unique levels of linear and quadratic change in depression over time. A third model estimating only quadratic change did not worsen model fit (Δχ2(1)=0.128, p>.05), and the fixed effect of the quadratic slope in this model was significant (p=.02). For parsimony, the final model estimated only quadratic change for depression, which can be interpreted as the change in the rate of change over time. Fit statistics for the three, best-fitting univariate growth models are shown in Table 2.
Table 2. Fit statistics for univariate growth curve models.
| df | χ2 | p | RMSEA | CFI | |
|---|---|---|---|---|---|
| Motor symptoms | 4 | 1.599 | .809 | .000 | 1.000 |
| Apathy | 4 | 4.655 | .325 | .030 | .998 |
| Depression | 5 | 9.188 | .102 | .067 | .982 |
In summary, the best-fitting, unconditional univariate growth models indicate that motor symptoms and apathy both worsened linearly over the 18-month observation period. In contrast, depressive symptoms improved and then worsened, suggesting quadratic change.
Unconditional multivariate latent growth curve model
The unconditional multivariate model, which estimated baseline levels and change in motor symptoms, apathy and depression but did not include the exogenous variables of age, sex, education and disease duration, is shown schematically in Figure 2. Model-estimated trajectories for each symptom type are shown as solid lines in Figure 1. The unconditional multivariate model provided the following fit statistics: χ2(27)=38.80 (p=.07); model AIC=92.80 (saturated AIC=108.00, independence AIC=1079.34); CFI=.99; RMSEA=.05 (p>.05). Overall, these statistics indicate very good fit to the data. The model explained between 71% and 74% of the variance in motor severity, between 62% and 63% of the variance in measured apathy, but only 48% of the variance in measured depression across the three occasions of measurement. Note that due to an identification error resulting from an inability to estimate individual differences in depression trajectories (i.e., random effect of the BDI quadratic slope), all reported values correspond to a subsequent model in which this parameter was fixed to 0. Consequently, correlations between the BDI slope and the other factors could not be estimated.
Figure 2.
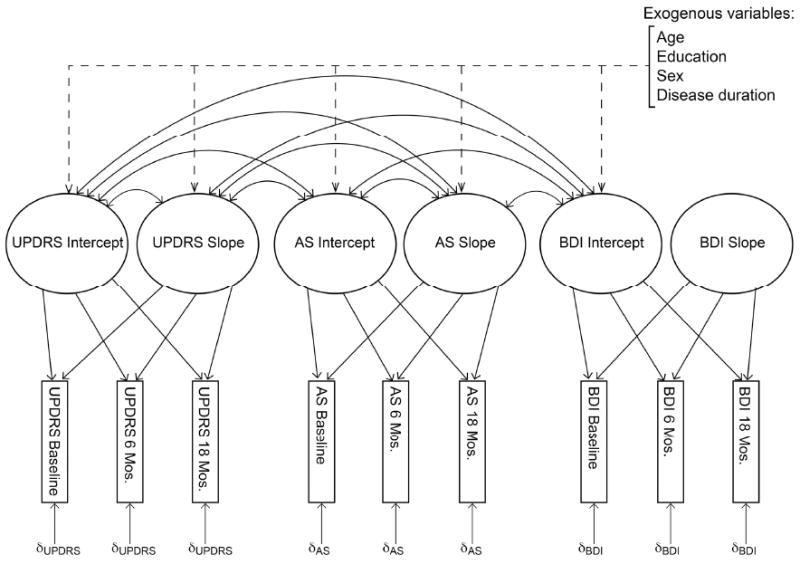
Schematic representation of the multivariate latent growth curve model. Dashed lines represent paths included only in the conditional model. Relationships involving the BDI slope were not estimated due to the model's inability to estimate random variance in the factor. UPDRS=Unified Parkinson's Disease Rating Scale. AS=Apathy Scale. BDI=Beck Depression Inventory.
Mean initial scores (intercepts) on the UPDRS, AS and BDI were 28.7, 12.8, and 9.2, respectively. There were significant individual differences in all three intercepts (p's<.001). Correlations between these intercepts are shown in the upper panel of Table 3. Initial levels of all symptoms were positively associated, indicating that higher levels of one symptom were associated with higher levels of the other two.
Table 3. Correlations (r) between the factors in the unconditional and conditional models.
| UPDRS intercept | UPDRS slope | AS intercept | AS slope | BDI intercept | |
|---|---|---|---|---|---|
| Unconditional Model | |||||
| UPDRS intercept | - | ||||
| UPDRS slope | -.094 | - | |||
| AS intercept | .429** | -.101 | - | ||
| AS slope | -.098 | .340 | -.154 | - | |
| BDI intercept | .376** | -.071 | .655** | .073 | - |
| BDI slope | - | - | - | - | - |
|
| |||||
| Conditional Model | |||||
| UPDRS intercept | - | ||||
| UPDRS slope | -.116 | - | |||
| AS intercept | .391** | -.119 | - | ||
| AS slope | -.150 | .432+ | -.178 | - | |
| BDI intercept | .407** | -.021 | .654** | .060 | - |
| BDI slopea | - | - | - | - | - |
Correlations involving the BDI slope could not be estimated due to an inability to estimate its random variance
Note. UPDRS=Unified Parkinson's Disease Rating Scale (motor portion); AS=Apathy Scale; BDI=Beck Depression Inventory
p<.001
p<.10
The rates of change (slopes) in the UPDRS and AS were 1.5 and 0.7, respectively (p's<.001). That these parameter estimates are positive and significant indicates that, on average, patients experienced significant linear worsening of both motor symptoms and apathy over the 18-month observation period. There were significant individual differences in the rate of change in motor symptoms (p=.001), and there was a trend for individual differences in the rate of change in apathy (p=.06). Thus, while change in these symptom types was linear on average, there were differences in the individual trajectories experienced by different patients.
The change in the rate of change in the BDI was 1.18 (p=.07). The positive effect of the depression slope can be interpreted by examining the trajectory of BDI scores displayed in Figure 1. Patients reported a slight decrease in depressive symptoms at the second assessment and a gradual return to symptom levels similar to those reported at baseline. Thus, while apathy and motor severity worsened linearly over the 18-month observation period, depression improved and then worsened. As noted above, the multivariate model was unable to estimate individual differences in the rate of change in depression, suggesting that there was insufficient variability within the sample in depression trajectories.
As shown in Table 3 (upper panel), non-significant correlations between all three intercepts and the UPDRS and AS slopes suggest that initial levels of any of the three symptoms were not related to changes in motor or apathy symptoms over time. In other words, patients with more motor symptoms or apathy were not more or less likely to experience more extreme worsening over time. A medium-sized correlation between UPDRS and AS slopes suggests an association between motor severity and apathy over time; however, this correlation did not reach significance (p=.16).
In summary, motor symptoms and apathy each worsened significantly and in a linear fashion, even when controlling for the other two symptom types. There was a trend for depressive symptoms to improve and then worsen when controlling for the other two symptom types. A greater baseline level of any one symptom type was associated with greater baseline levels in the other two, but baseline symptom levels were not associated with the amount of change experienced in any of the symptoms over time. There was a trend for an association between the rates of change in motor symptoms and apathy, suggesting that not only are baseline levels of these symptoms correlated, but their trajectories may be coupled.
Conditional multivariate latent growth curve model
Four predictors measured at baseline (i.e., age, sex, education in years, and disease duration in years) were added to the multivariate model, as shown by the dashed lines in Figure 2. Because unconditional (i.e., without predictors) and conditional models are not directly comparable in overall measured variance, a transition model was built that did not allow the four exogenous variables to predict the factors. In the transition model, all predictors were allowed to correlate with one another. This model represents the poorest-fitting model that includes variance related to the exogenous variables, to which the conditional model can be statistically compared. Such a statistical comparison allows for a determination of whether or not accounting for the covariates improves our understanding of the variables of interest (motor symptoms, apathy, depression). The transition model provided the following fit statistics: χ2(63)=126.38 (p<.001); model AIC=208.38 (saturated AIC=208.00, independence AIC=1194.32); CFI=.94; and RMSEA=.07 (p<.05).
In the conditional multivariate model, the exogenous variables were permitted to predict the factors, and these regression paths were estimated. The conditional model provided the following fit criteria: χ2(43)=57.30 (p=.07); model AIC = 179.30 (saturated AIC = 208.00, independence AIC = 1194.32); CFI=.99; and RMSEA=.04 (p=.65). Overall, these criteria indicate excellent fit to the data, and a nested model comparison confirmed that allowing the exogenous variables to predict the factors significantly improved the fit of the model (Δχ2(20)=-69.08, p<.001).
Correlations between the factors in the unconditional model are shown in the lower panel of Table 3. After controlling for the four covariates, levels of the three symptoms were still positively correlated with one another. There was a large, positive correlation between motor and apathy slopes that did not reach significance (p=.08).
Table 4 presents the regression path estimates separately for each of the three symptom types. Note that all parameters were estimated within the single, multivariate model. As shown, older age was significantly associated with worse baseline motor severity (p<.001), and there was a trend for older age to be associated with worse baseline apathy (p=.09). Age was not associated with baseline depressive symptoms or with the rates of change in motor symptoms or apathy. Sex was not associated with baseline levels of any of the variables of interest or with the rates of change in apathy. However, female sex was associated with attenuation of the rate of change in motor symptoms (p=.02). Lower levels of education were associated with higher levels of motor (p=.03), apathy (p<.001) and depressive (p<.001) symptoms. Education was not associated with the rates of change in motor or apathy symptoms. Longer disease duration was associated with greater baseline motor severity (p<.01) but not with baseline levels of apathy or depression. There was a trend for longer disease duration to be associated with an attenuation of the rate of change in motor symptoms (p=.07).
Table 4. Regression paths in the conditional multivariate growth curve model.
| Motor symptoms | Apathy | Depression | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B. | β | SE | B. | β | SE | B. | β | SE | ||||
| Intercept | ||||||||||||
| Age | 0.25 | .27 | 0.07 | ** | 0.08 | .14 | 0.04 | + | -0.05 | -.10 | 0.04 | |
| Education | -0.59 | -.18 | 0.27 | * | -0.58 | -.29 | 0.17 | ** | -0.52 | -.29 | 0.15 | ** |
| Sex | 0.63 | .03 | 1.68 | -0.36 | -.03 | 1.03 | 0.66 | .06 | 0.91 | |||
| PD Duration | 0.00 | .21 | 0.00 | * | 0.00 | -.03 | 0.00 | 0.00 | -.05 | 0.00 | ||
| Slope | ||||||||||||
| Age | 0.05 | .12 | 0.04 | -0.02 | -.10 | 0.02 | ||||||
| Education | -0.02 | -.01 | 0.16 | -0.04 | -.07 | 0.08 | ||||||
| Sex | -2.38 | -.29 | 1.01 | * | -0.10 | -.03 | 0.52 | |||||
| PD Duration | -0.00 | -.23 | 0.00 | + | 0.00 | .20 | 0.00 | |||||
Note. PD=Parkinson's disease
p<.10
p<.05
p<.001
In the conditional multivariate model, the linear slopes of motor severity and apathy were no longer significant, which suggests that the covariates explained an adequate amount of variance in the rates of change in these variables, even though no single covariate was independently associated with the apathy slope. There continued to be a trend for the quadratic slope of depression similar to that in the unconditional model (p=.06), but it should be noted that relationships between the covariates and changes in depression could not be estimated due to the lack of significant individual differences in the depression slope noted above.
The effect of antidepressant use was explored with a dichotomous time-varying covariate indicating the use/non-use of antidepressants at each occasion. Because this variable was centered at the first occasion, its values at the second and third occasions correspond to the initiation, maintenance, or discontinuation of an antidepressant. These variables were allowed to predict corresponding occasions of all three variables of interest (UPDRS, AS, and BDI) and were allowed to correlate with all other predictors. Inclusion of antidepressant use at each occasion yielded the following model fit statistics: χ2(62)=127.71 (p<.001); model AIC=307.71 (saturated AIC=304.00, independence AIC=1383.83); CFI=.95; and RMSEA=.08 (p<.05). Initiation of an antidepressant was associated with an increase in BDI of approximately 1.61 points at time 2 (p<.05). No other regression paths were significant. There was no longer a trend for a BDI slope, suggesting that inclusion of antidepressant regime significantly explained the quadratic change in depressive symptoms.
In summary, accounting for covariates of age, sex, education and disease duration improved the fit of the model. Controlling for these variables, baseline levels of all three symptom types remained correlated. The trend for coupled changes in motor symptoms and apathy also remained. Older age was associated with greater motor and apathy severity, and lower education was associated with greater severity of all three symptom types. Female sex was associated with attenuated motor worsening. Longer disease duration was associated with greater motor severity but attenuated motor worsening. Accounting for antidepressant use eliminated the trend for quadratic change in depressive symptoms, but antidepressant use was unrelated to motor symptoms and apathy.
Discussion
The present study supports linear worsening of both motor and apathy symptoms over 1.5 years in moderate, medically-managed PD. In contrast, depressive symptoms evidenced both improvement and worsening over the study period. The divergent trajectories for apathy and depression, as well as the divergent relationships between these mood states and variables such as antidepressant use and age, provide further evidence for the separability of these neuropsychiatric states in PD. Baseline levels of mood and motor symptoms were highly correlated, likely reflecting global disease severity.
In addition to similarity in the functional forms of motor and apathy progression, there was a medium-sized correlation between their slopes. This finding suggests that motor severity and apathy may increase together over time. While this relationship did not reach significance, detecting correlated change in a multivariate latent growth curve model framework is known to be extremely difficult. It has been suggested that studies using these models often fail to detect correlated change because of low power rather than a lack of relationship between variables (Hertzog, Lindenberger, Ghisletta & Oertzen 2006). Thus, it remains a strong possibility that motor and apathy symptoms of PD travel together over time, perhaps due to analogous dopaminergic neuropathophysiologies involving the dorsal and ventral striatum, respectively.
The observation that patients with PD endorse fewer symptoms of apathy when assessed on versus off their normal dopaminergic medications suggests that apathy may be at least partially dopamine-mediated (Czernecki, et al., 2002). In addition, several case series as well as at least one randomized control trial have suggested that drugs with dopamine-stimulating properties, such as methyphenidate (Hermann et al., 2008; Watanabe et al., 1995), amantadine (Andersson et al., 1992), bupropion (Corcoran, Wong & O'Keane, 2004), and bromocriptine (Powell, al-Adawai, Morgan & Greenword, 1996), improve apathy and related disorders of motivation.
In contrast, the functional form of the depression slope was dissimilar to that of motor severity, which may reflect differences in their underlying neuropathophysiologies. Indeed, substantial evidence exists to suggest that the neuropathophysiology of depression in PD involves non-dopaminergic systems. One hypothesis implicates reduced serotonergic activity, which has been identified in the cerebrospinal fluid of PD patients (Mayeux, Stern, Cote & Williams, 1984). Serotonergic reduction may represent a compensatory mechanism for striatal dopamine depletion, as serotonin is known to inhibit the release of dopamine in the striatum normally (Mayeux, 1990). In studies using transcranial sonography, depression in PD has been associated with morphological alteration of serotonergic projections from the superior central nucleus and the dorsal raphe nucleus, as well as of noradrenergic fibers from the locus coeruleus (Becker et al., 1997; Walter, Skoloudik & Berg, 2009).
In this study, older age was associated with worse motor and apathy symptoms at baseline, although the latter association was at trend. While apathy worsening may result from the underlying neurodegenerative process, it may also reflect “normal” age-related changes in the neural system. A recent longitudinal study reported that apathy worsens over time in healthy older adults (Brodaty, Altendorf, Withall & Sachdev, 2010). The magnitude of apathy worsening identified in that study is not directly comparable to the present results due to different measurement instruments (AS vs. the Apathy Evaluation Scale), different sampling procedures, and age differences. Patients in the present study were four years younger at baseline, on average. Despite this younger age of the present sample, the rate of apathy worsening appears to be somewhat greater than that reported for healthy older adults. Specifically, Brodaty et al. (2010) reported an average yearly increase on the AES of .92 points (1.7% total score), while the 12-month change (time 2 to time 3) in the present study was 1 point on the AS (2.4% total score).
A low level of education was associated with higher levels of all symptoms. Previous studies have demonstrated a similar relationship between education and several non-motor aspects of Parkinson's disease, including depression (Klepac & Trkulja, 2009), psychosis and sleep disturbance (Cohen et al., 2007), as well as between education and health-related quality of life (Carod-Artal, Vargas & Martinez-Martin, 2007; Cubo, et al., 2002). In this study, the slope of motor changes was attenuated by female sex and longer disease duration. These findings are in line with reports that the clinical progression of PD is more rapid during the earlier stages of the disease and with the potential protective effect of estrogen (Schrag, et al., 2007; Bourque, Dluzen & Di Paolo, 2009).
There was some evidence that the quadratic slope for depression related to changes in depressive treatments during the period of observation. Specifically, the initiation of antidepressants at six months was associated with higher BDI scores, and accounting for antidepressant regime eliminated the quadratic effect of depressive symptoms. With the current data, it cannot be ruled out that depressive symptoms would also have worsened linearly in the absence of antidepressants. However, the fact that depressive symptoms exhibited different relationships with variables indexing age and antidepressant use than did apathy argues against the possibility that apathy and depression reflect the same neuropsychiatric state. The use of antidepressants was unrelated to apathy or motor symptoms. The lack of relationship between antidepressant use and apathy seems to support reports that symptoms of apathy may not respond to depression treatments in the same way as do depressive symptoms. Rather, certain antidepressants (i.e., selective serotonin reuptake inhibitors) may even worsen apathy (Barnhart, Makela & Latocha, 2004; Wongpakaran et al., 2007). However, we did not find an association between antidepressant initiation and apathy worsening during our 1.5 year study period.
While the changes in motor functioning and apathy observed over our relatively short study period were small, their linear increases over this period were statistically significant. The detection of significant changes over such a small time period highlights the potential magnitude of physical and psychological alterations endured by patients who live with PD for 10-20 years, on average. In this study, we found that 43% of patients reported experiencing clinically-significant apathy at baseline. After only 18 months, an additional 10% of patients had transitioned to experiencing clinically-significant apathy. In contrast, only 18% of patients reported experiencing clinically-significant depression at baseline, and an additional 3% had transitioned after 18 months, perhaps as a result of increased use of antidepressants in the sample. These statistics emphasize the need for empirically-supported treatments for apathy in PD, for which there are none at present.
A limitation of the present study is its inability to estimate the effects of covariates on the rates of change in the mood variables. Future studies should endeavor to obtain larger samples in order to more fully explore the effects of patient characteristics and clinical covariates on the trajectories of mood symptoms in PD. A prospective design in which participants are followed and drop-out is accounted for would be a stronger methodology. In addition, the present study did not account for cognitive dysfunction, which is relatively common in PD and has been associated with mood disturbances and greater motor impairment. In the future, longitudinal studies designed to assess mood, motor and cognitive functioning over time would improve our understanding of the progression of a greater constellation of symptoms. A major strength of this study was its use of sophisticated statistical modeling based on three occasions of measurement, in which levels of the three variables of interest are controlled for in the estimation of all effects.
In conclusion, the present study provides evidence for a tighter coupling of apathy and motor symptoms than of depression and motor symptoms in medically-managed PD. Future research is necessary to determine the relationship between the progression and underlying neuropathophysiologies of these symptoms in PD.
Acknowledgments
This work was supported by the University of Florida (UF) Foundation; UF National Parkinson Center of Excellence; and National Institute on Aging (UF-L.B.Z., T32-AG020499). A preliminary version of this work was presented at the 39th annual meeting of the International Neuropsychological Society in Boston, MA. The authors acknowledge the assistance of Ms. Stephanie Gocklin in preparing the figures. Authors also acknowledge the UF INFORM database.
Appendix
In the present study, if Yit is assumed to represent the three repeated assessment measures of a variable (e.g., apathy), where i represents each observed individual in the study and t represents the time-ordered measurements, a simple longitudinal model equation for describing an individual's development over the repeated measurements (i.e., level 1 or within-person model) is:
| (1) |
where αyi is the initial status measured at baseline (i.e., intercept) of an individual's change trajectory, and βyi is the slope of the change trajectory. The parameter λt represents the measured time points, and εit represents the model residual for each individual. Because αyi and βyi are random variables, these model parameters can be represented by a group mean intercept (μαy) and mean slope (μβy) plus the components of individual intercept (ζαyi) and slope (ζβyi) variation, as shown in the following model equations for which sample-based estimates are obtained:
| (2) |
| (3) |
When time-varying covariates are considered both as a separate model and incorporated into the initial latent change model, the model is commonly referred to as a multivariate latent growth curve model. The relationships between the three repeatedly- measured variables (motor severity, apathy, and depression) are related to one another via the factors of each measured variable and are based on the covariance structure of the level 2 models.
Footnotes
Author Note: None of the authors have conflicts of interest directly related to the content of this article.
Footnotes: The greatest amount of missing data was found in UPDRS scores at time 1. Mann-Whitney tests revealed that the 47 individuals for whom baseline motor scores were not available did not differ from the rest of the sample in age, disease duration, education, or severity of mood symptoms (all p>.10). Similarly, the 24 individuals for whom BDI scores at the third occasion were not available did not differ from the rest of the sample on any of these variables (all p>.10).
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/neu
References
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual Review of Neuroscience. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Andersson S, Berstad J, Finset A, Grimsmo J. Amantadine treatment of severe cognitive disorders in traumatic brain injury patients. Journal of the Norwegian Medical Association. 1992;112:2070–2072. [Google Scholar]
- Bar-Gad I, Bergman H. Stepping out of the box: information processing in the neural networks of the basal ganglia. Current Opinion in Neurobiology. 2001;11:689–695. doi: 10.1016/s0959-4388(01)00270-7. [DOI] [PubMed] [Google Scholar]
- Barnhart WJ, Makela EH, Latocha MJ. SSRI-induced apathy syndrome: a clinical review. Journal of Psychiatric Practice. 2004;10:196–199. doi: 10.1097/00131746-200405000-00010. [DOI] [PubMed] [Google Scholar]
- Becker T, Becker G, Seufert J, Hogmann E, Lange KW, Naumann M, … Reiners K. Parkinson's disease and depression: evidence for an alteration of the basal limbic system detected by transcranial sonography. Journal of Neurology, Neurosurgery, and Psychiatry. 1997;63:590–595. doi: 10.1136/jnnp.63.5.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque M, Dluzen DE, Di Paolo T. Neuroprotective actions of sex steroids in Parkinson's disease. Frontiers in Neuroendocrinology. 2009;30:142–157. doi: 10.1016/j.yfrne.2009.04.014. [DOI] [PubMed] [Google Scholar]
- Brodaty H, Altendorf A, Withall A, Sachdev P. Do people become more apathetic as they grow older? A longitudinal study in healthy individuals. International Psychogeriatrics. 2010;22:426–436. doi: 10.1017/S1041610209991335. [DOI] [PubMed] [Google Scholar]
- Brown R, Jahanshahi M. Depression in Parkinson's disease: a psychosocial viewpoint. In: Weinter J, Lang AE, editors. Behavioral Neurology of Movement Disorders. Advances in Neurology. Vol. 65. New York: Raven; 1995. pp. 61–84. [PubMed] [Google Scholar]
- Brown RG, MacCarthy B, Gotham AM, Der GJ, Marsden CD. Depression and disability in Parkinson's disease: a follow-up of 132 cases. Psychological Medicine. 1988;18:49–55. doi: 10.1017/s0033291700001872. [DOI] [PubMed] [Google Scholar]
- Cardoso EF, Fregni F, Martins Maia F, Boggio PS, Luis Myczkowski M, Coracini K, Amaro E., Jr rTMS treatment for depression in Parkinson's disease increases BOLD responses in the left prefrontal cortex. International Journal of Neuropsychopharmacology. 2008;11(2):173–183. doi: 10.1017/S1461145707007961. [DOI] [PubMed] [Google Scholar]
- Carod-Artal FJ, Vargas AP, Martinez-Martin Determinants of quality of life in Brazilian patients with Parkinson's disease. Movement Disorders. 2007;22:1408–1415. doi: 10.1002/mds.21408. [DOI] [PubMed] [Google Scholar]
- Cohen OS, Vakil E, Tanne D, Nitsan Z, Schwartz R, Hassin-Baer S. Educational level as a modulator of cognitive performance and neuropsychiatric features in Parkinson's disease. Cognitive and Behavioral Neurology. 2007;20:68–72. doi: 10.1097/WNN.0b013e3180335f8e. [DOI] [PubMed] [Google Scholar]
- Cubo E, Rojo A, Ramos S, Quintana S, Gonzalez M, Kompoliti K, Aguilar M. The importance of education and psychological factors in Parkinson's disease quality of life. European Journal of Neurology. 2002;9:589–593. doi: 10.1046/j.1468-1331.2002.00484.x. [DOI] [PubMed] [Google Scholar]
- Czernecki V, Pillon B, Houeto JL, Pochon JB, Levy R, Dubois B. Motivation, reward, and Parkinson's disease: Influence of dopatherapy. Neuropsychologia. 2002;40:2257–2267. doi: 10.1016/s0028-3932(02)00108-2. [DOI] [PubMed] [Google Scholar]
- Fahn S, Elton RL, Committee . Unified Parkinson's Disease Rating Scale. In: Fahn S, Marsden CD, Golstein M, Clane DB, editors. Recent developments in Parkinson's disease. Florham Park: Macmillan Health Care Information; 1987. pp. 153–163. [Google Scholar]
- Fibiger HC. The neurobiological substrates of depression in Parkinson's disease. Canadian Journal of Neurological Sciences. 1984;11:105–107. doi: 10.1017/s0317167100046230. [DOI] [PubMed] [Google Scholar]
- Gibb WR. Functional neuropathology in Parkinson's disease. European Journal of Neurology. 1997;38:21–25. doi: 10.1159/000113472. [DOI] [PubMed] [Google Scholar]
- Hama S, Yamashita H, Shigenobu M, Watanabe A, Kurisu K, Yamawaki S, Kitaoka T. Post-stroke affect or apathetic depression and lesion location: left frontal lobe and bilateral basal ganglia. European Archives of Psychiatry and Clinical Neurosciences. 2007;257:149–152. doi: 10.1007/s00406-006-0698-7. [DOI] [PubMed] [Google Scholar]
- Hermann N, Rothenburg LS, Black SE, Ryan M, Liu BA, Busto UE, Lanctot KL. Methyphenidate for the treatment of apathy in Alzheimer disease: prediction of response using dextroamphetamine challenge. Journal of Clinical Psychopharmacology. 2008;28(3):296–301. doi: 10.1097/JCP.0b013e318172b479. [DOI] [PubMed] [Google Scholar]
- Hertzog C, Lindenberger U, Ghisletta P, von Oertzen T. On the power of multivariate latent growth curve models to detect correlated change. Psychological Methods. 2006;11:244–252. doi: 10.1037/1082-989X.11.3.244. [DOI] [PubMed] [Google Scholar]
- Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. Journal of Neurology, Neurosurgery and Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch-Darrow L, Fernandez HH, Marsiske M, Okun MS, Bowers D. Dissociating apathy and depression in Parkinson disease. Neurology. 2006;67:33–38. doi: 10.1212/01.wnl.0000230572.07791.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klepac N, Trkulja V. Education effect on depression and quality of life in nondemented Parkinonson's disease patients. Journal of Neuropsychiatry and Clinical Neurosciences. 2009;21:314–322. doi: 10.1176/jnp.2009.21.3.314. [DOI] [PubMed] [Google Scholar]
- Lavretsky H, Ballmaier M, Pham D, Toga A, Kumar A. Neuroanatonical characteristics of geriatric apathy and depression: a magnetic resonance imaging study. American Journal of Geriatric psychiatry. 2007;15:386–394. doi: 10.1097/JGP.0b013e3180325a16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leentjens AFG. Depression in Parkinson's disease: conceptual issues and clinical challenges. Journal of Geriatric Psychiatry and Neurology. 2004;17:120–126. doi: 10.1177/0891988704267456. [DOI] [PubMed] [Google Scholar]
- Leentjens AF, Dujardin K, Marsh L, Martinez-Martin P, Richard IH, Starkstein SE, Goetz CG. Apathy and anhedonia rating scales in Parkinson's disease: critique and recommendations. Movement Disorders. 2008;23:2004–2014. doi: 10.1002/mds.22229. [DOI] [PubMed] [Google Scholar]
- Mayeux R. The “serotonergic hypothesis” for depression in Parkinson's disease. Advances in Neurology. 1990;53:163–166. [PubMed] [Google Scholar]
- Mayeux R, Stern Y, Cote L, Williams BW. Altered serotonin metabolism in depressed patients with Parkinson's disease. Neurology. 1984;34:642–646. doi: 10.1212/wnl.34.5.642. [DOI] [PubMed] [Google Scholar]
- Marin RS. Apathy: a neuropsychiatric syndrome. Journal of Neuropsychiatry and Clinical Neurosciences. 1991;3:243–254. doi: 10.1176/jnp.3.3.243. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Research Reviews. 2000;31:236–250. doi: 10.1016/s0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- Morisette M, Di Paolo T. Sex and estrous cycle variations of rat striatal dopamine uptake sites. Neuroendocrinology. 1993;58:16–22. doi: 10.1159/000126507. [DOI] [PubMed] [Google Scholar]
- Obeso JA, Rodríguez-Oroz MC, Benitez-Temino B, Blesa FJ, Guridi J, Marin C, Rodriguez M. Functional organization of the basal ganglia: therapeutic implications for Parkinson's disease. Movement Disorders. 2008;23:S548–S559. doi: 10.1002/mds.22062. [DOI] [PubMed] [Google Scholar]
- Oguru M, Tachibana H, Toda K, Okuda B, Oka N. Apathy and depression in parkinson disease. Journal of Geriatric Psychiatry and Neurology. 2010;23:35–41. doi: 10.1177/0891988709351834. [DOI] [PubMed] [Google Scholar]
- Pavon JM, Whitson HE, Okun MS. Parkinson's disease in women: a call for improved clinical studies and for comparative effectiveness research. Maturitas. 2010;65:352–358. doi: 10.1016/j.maturitas.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen KF, Alves G, Bronnick K, Aarsland D, Tysnes OB, Larsen JP. Apathy in drug-naïve patients with incident Parkinson's disease: the Norwegian ParkWest study. Journal of Neurology. 2010;257:217–223. doi: 10.1007/s00415-009-5297-x. [DOI] [PubMed] [Google Scholar]
- Poewe W. The natural history of Parkinson's disease. Journal of Neurology. 2006;253:VII/2–VII/6. doi: 10.1007/s00415-006-7002-7. [DOI] [PubMed] [Google Scholar]
- Powell JH, al-Adawai S, Morgan J, Greenwood RJ. Motivational deficits after brain injury: effect of bromocriptine in 11 patients. Journal of Neurology, Neurosurgery and Psychiatry. 1996;60:416–421. doi: 10.1136/jnnp.60.4.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravina B, Elm J, Camicioli R, Como PG, Marsh L, Jankovic J, Weintraub D. The course of depressive symptoms in early Parkinson's disease. Movement Disorders. 2009;24:1306–1311. doi: 10.1002/mds.22572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remy P, Doder M, Lees A, Turjanski N, Brooks D. Depression in Parkinson's disease: loss of dopamine and noradrenaline innervations in the limbic system. Brain. 2005;128:1314–1322. doi: 10.1093/brain/awh445. [DOI] [PubMed] [Google Scholar]
- Schrag A, Dodel R, Spottke A, Borchschein B, Siebert U, Quinn NP. Rate of clinical progression in Parkinson's disease. A prospective study. Movement Disorders. 2007;22:938–945. doi: 10.1002/mds.21429. [DOI] [PubMed] [Google Scholar]
- Schrag A, Jahanshahi M, Quinn NP. What contributes to depression in Parkinson's disease? Psychological Medicine. 2001;31:65–73. doi: 10.1017/s0033291799003141. [DOI] [PubMed] [Google Scholar]
- Simuni T Sethi K. Nonmotor manifestations of Parkinson's disease. Annals of Neurology. 2008;64:S65–S80. doi: 10.1002/ana.21472. [DOI] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Applied longitudinal data analysis. Oxford, UK: Oxford University Press; 2003. [Google Scholar]
- Starkstein SE, Mayberg HS, Leiguarda R, Preziosi TJ, Robinson RG. A prospective longitudinal study of depression, cognitive decline, and physical impairments in patients with Parkinson's disease. Journal of Neurology, Neurosurgery, and Psychiatry. 1992;55:377–382. doi: 10.1136/jnnp.55.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkstein SE, Mayberg HS, Preziosi TJ, Andrezejewski P, Leiguarda R, Robinson RG. Reliability, validity, and clinical correlates of apathy in Parkinson's disease. Journal of Neuropsychiatry and Clinical Neuroscience. 1992;4:134–139. doi: 10.1176/jnp.4.2.134. [DOI] [PubMed] [Google Scholar]
- Starkstein SE, Merello M, Brockman S, Bruce D, Petracca G, Power BD. Apathy predicts more severe parkinsonism in Alzheimer's disease. American Journal of Geriatric Psychiatry. 2009;17:291–298. doi: 10.1097/jgp.0b013e31818a0e35. [DOI] [PubMed] [Google Scholar]
- Visser M, Leentjens AFG, Marinus J, Stiggelbout AM, van Hilten JJ. Reliability and validity of the Beck Depression Inventory in patients with Parkinson's disease. Movement Disorders. 2006;21:668–672. doi: 10.1002/mds.20792. [DOI] [PubMed] [Google Scholar]
- Walter U, Skoloudik D, Berg D. Transcranial sonography findings related to non-motor features of Parkinson's disease. Journal of Neurological Science. 2010;289:123–127. doi: 10.1016/j.jns.2009.08.027. [DOI] [PubMed] [Google Scholar]
- Watanabe MD, Martin EM, DeLeon OA, Gaviria M, Pavel DG, Trepashko DW. Successful methylphenidate treatment of apathy after subcortical infarcts. Journal of Neuropsychiatry and Clinical Neurosciences. 1995;7:502–504. doi: 10.1176/jnp.7.4.502. [DOI] [PubMed] [Google Scholar]
- Wongpakaran N, van Reedkum R, Wongpakaran T, Clarke D. Selective serotonin reuptake inhibitor use associates with apathy among depressed elderly: a case-control study. Annals of General Psychiatry. 2007;6:7. doi: 10.1186/1744-859X-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


