Summary
Shifts of gaze and shifts of attention are closely linked and it is debated whether they result from the same neural mechanisms. Both processes involve the frontal eye fields (FEF), an area which is also a source of top-down feedback to area V4 during covert attention. To test the relative contributions of oculomotor and attention-related FEF signals to such feedback, we recorded simultaneously from both areas in a covert attention task and in a saccade task. In the attention task, only visual and visuomovement FEF neurons showed enhanced responses, whereas movement cells were unchanged. Importantly, visual, but not movement or visuomovement cells, showed enhanced gamma frequency synchronization with activity in V4 during attention. Within FEF, beta synchronization was increased for movement cells during attention but was suppressed in the saccade task. These findings support the idea that the attentional modulation of visual processing is not mediated by movement neurons.
INTRODUCTION
Detailed analysis of a visual scene requires selection of behaviorally relevant objects or locations for further visual processing. Humans and monkeys can orient to interesting objects or parts of the visual field either by making saccades, which bring the object of interest on the fovea (overt orienting) or by shifting attention without shifting gaze (covert orienting). Whether these two processes are independent or nearly identical and whether they rely on the same brain circuitry has been a matter of debate. Motor theories of attention such as the “oculomotor readiness hypothesis” (Klein, 1980) and the “premotor theory of attention” (Rizzolatti et al., 1994) suggest that oculomotor mechanisms play a critical role in the employment of visual attention at least when this is directed to spatial locations. The “premotor theory of attention” of Rizzolatti and colleagues in particular proposes that covert visual spatial attention arises from signals related to the preparation for a saccadic eye movement and thus that neuronal activity during attention can be considered a by-product of activity in the motor system (Rizzolatti, 1983; Rizzolatti et al., 1987).
Psychophysical experiments have provided evidence that covert spatial attention and eye movements are coupled (Deubel and Schneider, 1996; Hoffman and Subramaniam, 1995; Kowler et al., 1995; Sheliga et al., 1994; Shepherd et al., 1986) and neuroimaging studies have demonstrated that the same network of brain areas is activated both for saccades and covert shifts of attention (Beauchamp et al., 2001; Corbetta et al., 1998; Kastner and Ungerleider, 2000; Nobre et al., 2000). Moreover, electrical stimulation of oculomotor centers such as the FEF and the superior colliculus (SC) influences the allocation of spatial attention (Cavanaugh and Wurtz, 2004; Kustov and Robinson, 1996; Moore and Armstrong, 2003; Moore and Fallah, 2001; Muller et al., 2005) while inactivation of the same areas leads to deficits in visual selection in overt (McPeek and Keller, 2004) as well as in covert attention tasks (Wardak et al., 2006).
However, other evidence suggests that overt and covert orienting are functionally distinct processes and are mediated by different neurons. Firstly, shifts of attention can occur without concomitant shifts of gaze (Hoffman and Subramaniam, 1995; Kowler et al., 1995). Secondly, attentional deployment and oculomotor processes can be dissociated even in behavioral paradigms where saccades are performed (Hunt and Kingstone, 2003; Klein, 1980; Posner, 1980). Moreover, the activity of visually responsive neurons in the FEF and SC is related to the selection of a target stimulus and does not depend on saccade production (McPeek and Keller, 2002; Sato and Schall, 2003; Schall and Hanes, 1993; Thompson et al., 1997) indicating that the allocation of attention and saccade preparation are distinct processes. In line with these ideas, a recent study showed that voluntary control of FEF neuronal responses leading to increased activity results in selective visual attention and not oculomotor preparation (Schafer and Moore, 2011)
Despite the accumulating evidence suggesting that saccade preparation and attention are not necessarily interdependent it is still unclear how the diverse neuronal types contribute to each of these processes. Neurons with visual, visuomotor and motor properties have been described in the FEF (Bruce and Goldberg, 1985), but how these different functional classes contribute to attentional selection is not yet fully understood. One study (Thompson et al., 2005) recorded the responses of FEF neurons with visual and saccade-related activity in an exogenous (pop-out) search task and found that only the responses of visual neurons were modulated by attention whereas the responses of movement neurons were suppressed. However, it has been argued that oculomotor mechanisms should be engaged in endogenous rather than in exogeneous (pop-out) attention tasks (Awh et al., 2006; Klein, 1980; Rizzolatti et al., 1994). If so, then movement cells should be active when attention is voluntarily directed to a spatial location covertly, which has not yet been tested.
In addition to modulating firing rates, attention also modulates synchronous activity within and across cortical areas. We have previously shown that attention increases neuronal synchronization within the FEF as well as between FEF and V4 in the gamma frequency range (Gregoriou et al., 2009a), suggesting that top-down feedback enhances visual processing at least partly through synchronization of activity. However, it is not known whether the top-down attentional control of visual cortex results from oculomotor or separate attentional signals in FEF. If movement cells synchronized their activity with V4 during attention, it would strongly support premotor theories.
To address these unresolved issues, we recorded the firing rates and synchrony of FEF and V4 neurons. Our goal was to test the contribution of different classes of FEF neurons to covert attention and saccades. The results suggest that covert and overt selection are not mediated by the same neural elements and can be further dissociated by synchronous interactions.
RESULTS
We recorded single unit activity from FEF and area V4 of two macaque monkeys engaged in two tasks with different eye movement requirements: a covert attention task and a memory guided saccade task (Figure 1). In the attention task, the monkeys were rewarded for detecting a color change of a target stimulus presented among distracters. The location of the target was randomized in different trials so that attention could be directed inside or outside the RF of the recorded neurons. The monkeys were rewarded for releasing a bar as soon as the target stimulus changed color, ignoring color changes of the distracters. Both monkeys performed very well with the first monkey reaching a performance level of 87% correct and the second monkey performing at 82% correct. False alarms to distracter color change were rare (monkey 1: 3.5% and monkey 2: 1% of trials where a distracter changed color). The animals failed to detect the target change and respond to it within 600 ms in 12% of the trials (monkey 1: 8%, monkey 2: 15%). In the memory guided saccade task, a single stimulus was flashed briefly in one of 6 randomly selected positions, and the monkeys were required to memorize the location of the recently presented target and withhold an eye movement until the central fixation spot was turned off. This served as a go signal for the execution of a saccade to the memorized location of the flashed target. The two monkeys performed at 87% and 90% correct, respectively.
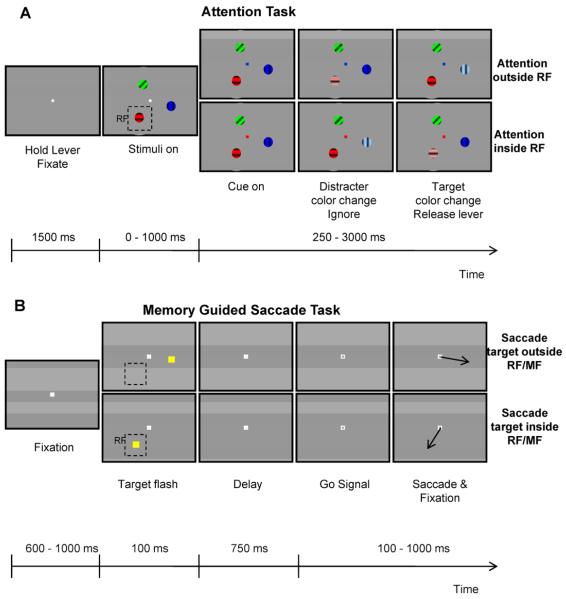
Figure 1. Behavioral Tasks.
(A) Attention task. At the beginning of the trial a central fixation spot appeared. A variable time period after fixation, three sinusoidal drifting gratings of different color appeared. The fixation spot was then replaced by a color cue the color of which indicated the target stimulus (blue grating in upper row, red grating in lower row). Following this any of the three stimuli could change color. The monkey was rewarded if it released a bar to the color change of the target stimulus. (B) Memory guided saccade task. The trial began with fixation of a central white spot. Following fixation, a yellow rectangle was flashed for 100 ms in one of 6 possible positions arranged on a circle and spaced 60° apart. The monkey had to maintain fixation during the delay period and then the fixation spot was turned off to indicate that a saccade should be executed to the memorized location of the flashed stimulus. The monkey was rewarded for making a saccade to the memorized location. Dashed rectangles indicate the position of a hypothetical receptive field (RF).
We recorded from 387 neurons in the FEF from the two monkeys (123 in monkey 1 and 264 in monkey 2) in both tasks. The cells were isolated off-line from the multiunit activity reported in a separate study (Gregoriou et al., 2009a). The neuronal responses in the memory guided saccade task were used in order to classify neurons according to their visual and/or saccade related activity (Bruce and Goldberg, 1985). Using the criteria described in the Methods we found 241 neurons with visual responses and no saccade related activity (visual neurons), 97 neurons with visual as well as saccade-related responses (visuomovement neurons) and 49 neurons with saccade-related activity and no visual responses (movement neurons). Out of the 97 neurons with visual and saccade-related activity, 58 neurons displayed saccade-related responses when saccades were executed toward the visual RF whereas for 39 neurons with significant motor responses there was no significant saccade-related activity toward the visual RF position. In this report, we restrict the analysis of visuomovement neurons to those 58 cells that displayed saccade-related activity when saccades were executed inside the visual RF.
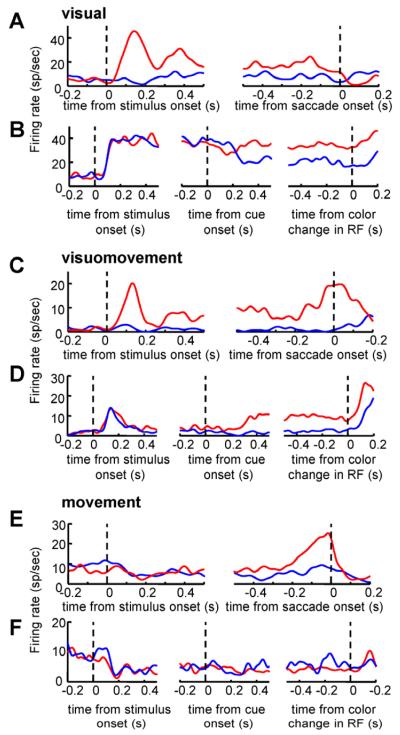
Figure 2 shows typical examples of FEF neurons. Figure 2A and B shows an example of a visual neuron. In the memory guided saccade task (Figure 2A) this neuron responded transiently to the appearance of the peripheral stimulus when this was presented inside the neuron’s RF, maintained an elevated activity during the delay period and showed no enhancement around the beginning of the saccade. When the stimulus was presented outside the neuron’s RF, in the opposite hemifield, no significant increase in activity was present. In the attention task this neuron showed spatially selective responses following the onset of the cue (Figure 2B). Activity was enhanced when attention was directed inside the neuron’s RF and remained elevated for the duration of the trial until the color change. The neuron shown in Figure 2C and D is an example of a visuomovement neuron. During the memory guided saccade task this neuron responded to the onset of the stimulus when this was inside the RF, maintained an elevated level of activity in the delay period and showed an increase in activity around the saccade onset (~−150 ms - 100 ms relative to saccade onset, Figure 2C). In the attention task this neuron too displayed an enhanced response after the cue onset and up until the color change in the RF (Figure 2D). Finally, the movement neuron depicted in Figure 2E and F showed an enhancement in activity only before the onset of the saccade in the memory guided saccade task (Figure 2E) and no spatial selectivity during the attention task (Figure 2F). Interestingly, for this particular neuron there was a suppression of activity relative to the baseline in the attention task after the cue onset and for the duration of the trial. Figure 3 shows the population average response for each class of neurons (visual, visuomovement and movement) in the memory guided saccade task.
Figure 2. Examples of FEF neurons.
(A) Activity of a visual neuron in the memory guided saccade task aligned on stimulus onset (left) and saccade onset (right). (B) Activity of the same visual neuron shown in (A) in the attention task aligned on the onset of the stimuli (left), the onset of the cue (middle) and the color change in the RF (right). (C,D) Activity of a visuomovement neuron in the memory guided saccade task (C), and in the attention task (D). (E,F) Activity of a movement neuron in the memory guided saccade task (E), and in the attention task (F). In all plots, the red line corresponds to the response in the condition in which the target stimulus appeared inside the RF/MF of the recorded neuron with the blue line corresponding to the response of the neuron when the target stimulus appeared outside the RF/MF.
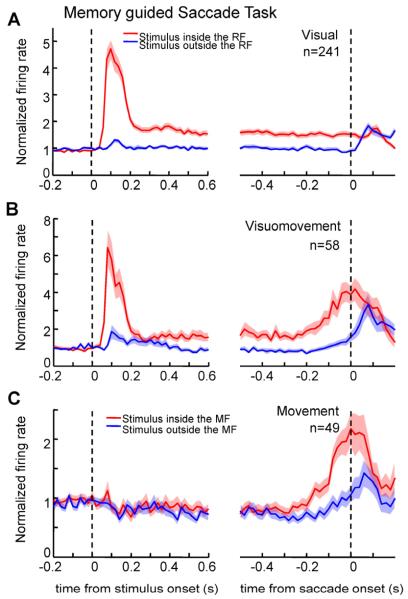
Figure 3. Population average activity for the different FEF neuronal classes in the memory guided saccade task.
Normalized population average activity aligned on stimulus flash (left) and on saccade onset (right) for visual (A), visuomovement (B) and movement neurons (C). Shading over the lines indicates mean ± SEM at each time point. Conventions as in Figure 2.
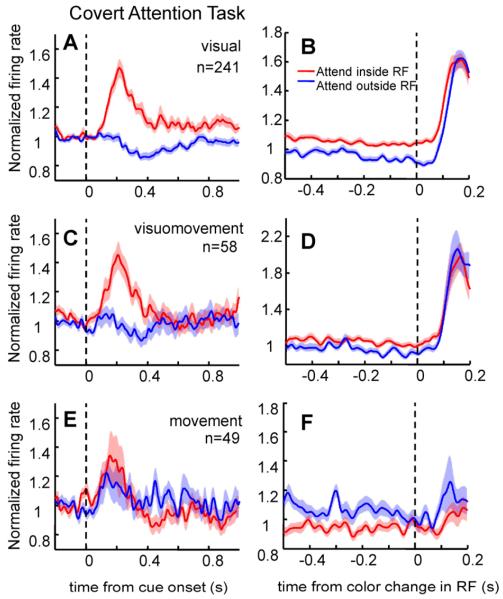
In the covert attention task, 53% of visual neurons and 47% of visuomovement neurons showed a significant enhancement in their firing rates (6% and 8% respectively showed a significant decrease) following the onset of the cue when attention was directed inside the neuron’s RF (average response in a window 100-400 ms after cue onset; Wilcoxon rank-sum test, p<0.05). The number of visual and visuomovement neurons showing significant modulation with attention was above the one predicted by chance (p<0.001 in both cases, see supplementary material). Figure 4, A and C, shows the average normalized response of the population of FEF visual and visuomovement neurons, respectively, following the onset of the cue. At the population level, activity was enhanced with attention by 29% and 20% for visual and visuomovement neurons, respectively following the cue onset (Wilcoxon sign-rank test, p<0.001). This attention-induced increase in response was maintained for the duration of the trial as shown in the population average of firing rate responses before the color change in the RF (Figure 4B and D). The enhancement was significant for visual neurons (average response in a 400 ms window preceding the color change, Wilcoxon sign-rank test, p<0.001) but did not reach significance for visuomovement neurons (Wilcoxon sign-rank test, p=0.08).
Figure 4. Population average activity for the different FEF neuronal classes in the covert attention task.
Normalized population average activity of visual (A), visuomovement (C) and movement neurons (E) aligned on the onset of the cue. Normalized population average activity of visual (B), visuomovement (D) and movement neurons (F) aligned on the color change in the RF/MF. Conventions as in Figure 3. See also Figures S1, S2.
Movement neurons displayed a strikingly different pattern of activity in the attention task. Figure 4E shows the population average of firing rate responses following the cue onset. No significant modulation with attention was found at the population level following the onset of the cue (Wilcoxon sign-rank test, p=0.14) with only 6 movement neurons (12%) showing a significant increase in activity. The number of movement neurons with significant enhancement in firing rate was not significantly higher than that predicted by chance (p>0.05, see supplementary material). The absence of attentional effects following the cue suggests that movement neurons are not directly involved in directing attention to the target stimulus. Moreover, movement neurons showed a decrease in activity with attention later in the trial (Figure 4F; average response 400ms before color change in RF, Wilcoxon sign-rank test, p<0.05). In fact, 35% of movement neurons showed a significant decrease in activity when attention was directed inside the movement field during sustained attention.
We performed a non-parametric one-way ANOVA (Kruskal-Wallis) to compare the attentional modulation in the firing rate of the three different groups (visual, visuomovement and movement cells). The results showed a significant main effect of cell class on attentional enhancement following the cue onset as well as later in the trial (p<0.01). Significant differences were found between visual and movement neurons as well as between visuomovement and movement neurons (Tukey-Kramer, p<0.05 for both comparisons) but not between visual and visuomovement neurons (p>0.45).
Taking all the results from the movement neurons together, these cells increased their activity during saccade preparation in the memory guided saccade task but showed no change or decreased their activity when attention was directed into their movement field but with saccades inhibited. This strongly supports the idea that saccade execution and covert attention to a location in the visual field can be decoupled at the neuronal level in FEF (Thompson et al., 2005). For a distribution of attentional effects on firing rates see supplementary material (Figure S1). Interestingly, about 34% of the movement neurons in our sample showed a statistically significant suppression in activity in the attention task relative to the prestimulus period (Wilcoxon sign-rank test, p<0.05) similar to that shown for the neuron in Figure 2F. The decrease in activity following the presentation of the stimuli was not spatially selective. This suppression in activity relative to the baseline is in agreement with results from a previous study (Thompson et al., 2005). About 42% of the neurons in our sample showed no statistically significant difference from baseline following the presentation of the stimuli. In sum, the type of firing rate changes by movement neurons in the attention task argues against a role of movement neurons in either shifts or maintenance of attention to spatial locations.
The enhancement of firing rate with attention for visual and visuomovement neurons following the cue onset was accompanied by a transient suppression of the response when attention was directed away from the RF (Figure 4A and C, blue line). Interestingly, the suppression in the “attend out” condition did not occur concurrently with the “attend in” enhancement but followed it. A similar effect has been described after cued shifts of feature-selective attention in a human EEG study (Andersen and Muller, 2010) and it has been suggested that it reflects competitive interactions between neuronal populations encoding the attended and unattended stimulus. It is indeed possible that the enhanced response for the attended location caused the suppression of the unattended location through competitive interactions between groups of FEF neurons that encode different spatial locations. The suppression effect we measured was statistically significant only for visual neurons (average response −150-0 ms and 250-400 ms relative to cue onset, Wilcoxon sign-rank test, visual: p<0.001, visuomovement: p=0.09, movement: p=0.39).
The differential modulation of responses with attention for the three classes of FEF neurons raised the possibility that the effect of attention on firing rates depended not so much on the cell class, but on the relative size of visual and saccade-related responses for a given cell. Indeed, FEF cells display a continuum of visual and motor responses (Bruce and Goldberg, 1985; Thompson et al., 2005). We therefore quantified this continuum using a visuomovement index (VMI), and we examined the correlation between the VMI and the attentional effect in firing rate. The VMI could take values between −1 and 1 with positive values indicating stronger visual responses and negative values corresponding to stronger saccade-related responses. The attentional effect was calculated as an attentional index (AI) and could also take values between −1 and 1, with positive values indicating an increase in activity when attention was directed inside the RF/MF and negative values indicating a stronger response when attention was directed outside the RF/MF.
We calculated the correlation between the AI for the time period 100-400 ms after the cue onset and the VMI for all recorded neurons. The correlation between the two variables was statistically significant (r=0.30, p<0.001; Figure S2A). A similar significant correlation was found between the VMI and the AI calculated in a window 400 ms before the color change in the RF (Figure S2B; r=0.21, p<0.001). These results indicate that the stronger the visual response of the cell relative to the saccade-related response the larger the increase in firing rate is when attention is directed inside the RF. Thus, cells with predominantly visual responses are more involved in the selection of the target and in the maintenance of attention to a spatial location.
In addition to attentional effects on firing rates, we and others have shown that neuronal synchronization is enhanced with attention both within areas which have been implicated in visual attention as well as across distant areas of the attentional network in both humans and monkeys (Bichot et al., 2005; Buschman and Miller, 2007; Fries et al., 2001; Gregoriou et al., 2009a; Lakatos et al., 2008; Saalmann et al., 2007; Siegel et al., 2008). Recently, we showed that oscillatory coupling between FEF and V4 in the gamma frequency range is enhanced with attention and that this coupling is initiated by the FEF (Gregoriou et al., 2009a). We therefore asked whether the coupling between the two areas is cell type dependent or whether all FEF neurons regardless of their functional properties are equally likely to be phase coupled to V4 activity.
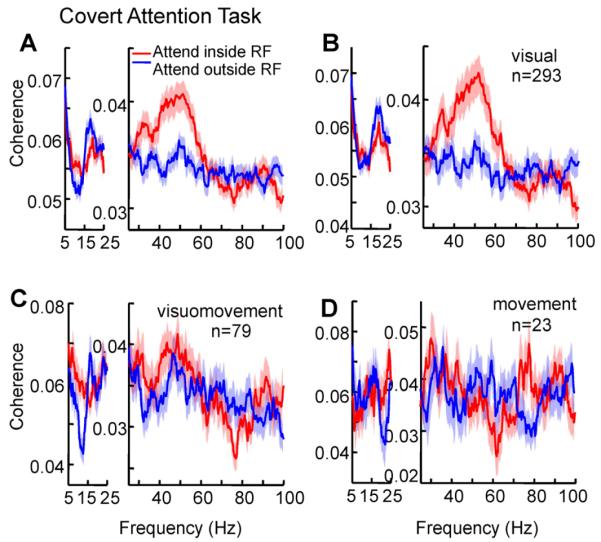
To measure synchrony between FEF and V4, we used multi-taper spectral methods to compute coherence between spikes from well isolated single units in the FEF and local field potentials (LFPs) in V4. First taking all types of FEF cells together, we found that spike-field coherence in the gamma frequency range was significantly enhanced between FEF and V4 when attention was directed inside the joint RF (Figure 5A; coherence averaged between 35 and 60 Hz; paired t-test p<0.001). At the population level gamma band coherence increased by 13%. This result confirms and extends findings from our recent study based on multi-unit activity that demonstrated enhanced neural synchrony between FEF and V4 with attention (Gregoriou et al., 2009a).
Figure 5. Effect of attention on synchronization between FEF and V4 in the attention task.
Spike-field coherence between: (A) spikes of FEF neurons and V4 LFPs (B) spikes of FEF visual neurons and V4 LFPs (C) spikes of FEF visuomovement neurons and V4 LFPs, and (D) spikes of FEF movement neurons and V4 LFPs. Spike and LFP signals from 300 ms after cue onset up to the earliest color change (target or distracter) were used for the coherence calculation. Conventions as in Figure 4. Tapers providing smoothing of ±10 Hz were used for spectral estimation of frequencies above 25 Hz (right part of each graph), whereas for frequencies below 25 Hz, tapers providing smoothing of ±4Hz were used (left part of each graph). See also Figure S3.
After subdividing the coherence spectra in FEF by cell class, the results showed that visual, visuomovement and movement neurons display distinct FEF-V4 coherence profiles. Coherence between the spikes of purely visual FEF neurons and LFPs in V4 showed a 16% enhancement with attention in the gamma range and this increase was statistically significant (Figure 5B; 35-60Hz, paired t-test, p<0.001). In agreement with our previous results we found that the distribution of the average (between 35 and 60 Hz) relative phase between FEF spikes and V4 LFPs had a median close to half a gamma cycle (attend-in condition; median = 176°, Rayleigh test, p<0.001). This phase shift corresponds to a time delay of ~10ms between spikes in the FEF and the phase of maximum depolarization in the V4 LFP, and we have previously suggested that a 10 ms time delay is needed to account for conduction and synaptic delays between the two areas (Gregoriou et al., 2009a). Spike-field coherence between FEF neurons with saccade related activity (visuomovement and movement neurons) and V4 LFPs did not display any significant gamma band modulation with attention (Figure 5 C and D; paired t-test, visuomovement cells: p=0.22, 7% increase; movement cells: p=0.87, 1% decrease with attention). For a distribution of attentional effects in gamma coherence see supplementary material Figure S3. The attentional enhancement of gamma coherence was significantly different across the three FEF cell classes (Kruskal-Wallis, p<0.001). Coherence between visual FEF cells and V4 LFPs was significantly enhanced relative to that between visuomovement or movement FEF cells and V4 (Tukey-Kramer, p<0.001 for both pair comparisons), whereas attentional effects on FEF-V4 coherence were not significantly different for visuomovement and movement FEF cells (Tukey-Kramer, p=0.69). We also confirmed that the absence of gamma coherence modulation with attention between FEF movement neurons and V4 cannot be attributed to low firing rate (see Supplemental Information). The dependence of the gamma band attentional effect on the visual response was further confirmed by estimating the correlation between an attentional index (AICOH) and the visuomovent index (VMI). The correlation between the two variables was statistically significant (r=0.14, p<0.01) indicating that the stronger the visual response relative to the motor response, the stronger the coupling with V4 during attention. It should be noted, that in contrast to the results in the covert attention task, no prominent synchrony was found in the memory guided saccade task between any type of FEF neuron and V4 LFPs, and there was no spatial effect on coherence, suggesting that the processes involved in the two tasks are markedly different.
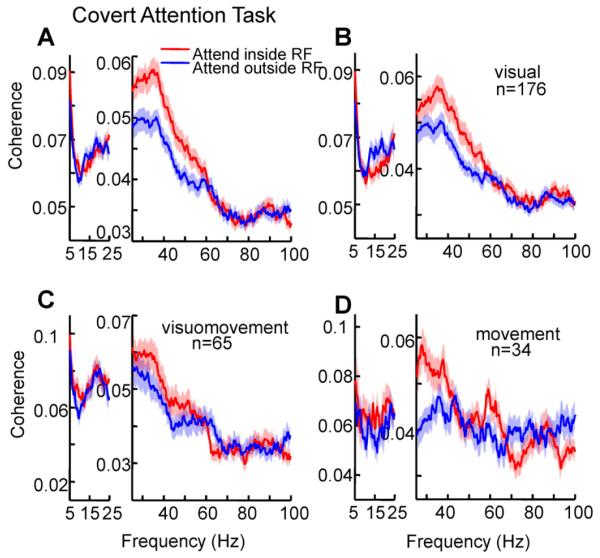
We next examined the effects of attention on spike-field coherence within FEF. First taking all cells together, we found that single unit spike-field coherence in the gamma frequency range was significantly enhanced with attention (Figure 6A; coherence averaged between 35 and 60 Hz; paired t-test p<0.001), consistent with our previous multi-unit results (Gregoriou et al., 2009a). At the population level gamma band coherence increased by 12%. However, this enhancement of gamma synchrony with attention in FEF was specific to just the visual cells. Pure visual neurons showed a significant, 13%, enhancement with attention in the gamma range (Figure 6B; 35-60Hz, paired t-test, p<0.01), whereas visuomovement and movement neurons did not display significant modulation of synchrony in the gamma band with attention (Figure 6 C and D; paired t-test, visuomovement cells: p=0.14, 9% increase; movement cells: p=0.21, 9% increase with attention). Moreover, when the attentional effect on gamma synchrony was compared across the three neuronal classes a significant main effect of cell type was found (Kruskal-Wallis, p<0.01) with visual to visuomovement and movement FEF neurons comparisons revealing a significant difference (Tukey-Kramer, p < 0.05 for both comparisons) and no difference between visuomovement and movement neurons (p = 0.61).
Figure 6. Effect of attention on synchronization within the FEF in the attention task.
Spike-field coherence between: (A) spikes of FEF neurons and FEF LFPs (B) spikes of FEF visual neurons and FEF LFPs (C) spikes of FEF visuomovement neurons and FEF LFPs, and (D) spikes of FEF movement neurons and FEF LFPs. Spike and LFP signals from 300 ms after cue onset up to the earliest color change (target or distracter) were used for the coherence calculation. Conventions as in Figure 5. See also Figure S4.
Interestingly, however, movement cells did show a significant, 28%, increase in coherence with attention inside their movements fields at lower frequencies, spanning beta and lower gamma frequencies (15-35 Hz, paired t-test, p<0.001). For a distribution of attentional effects on frequencies from 35-60 Hz and 15-35 Hz see supplemental information Figure S4. Although the increase in synchrony between 15 and 35 Hz could be attention-related, we also considered whether it might be caused by the inhibition of saccades into the movement field in the attention task, given that the task required that the animal attended to the stimulus in the field but suppressed any saccade to it.
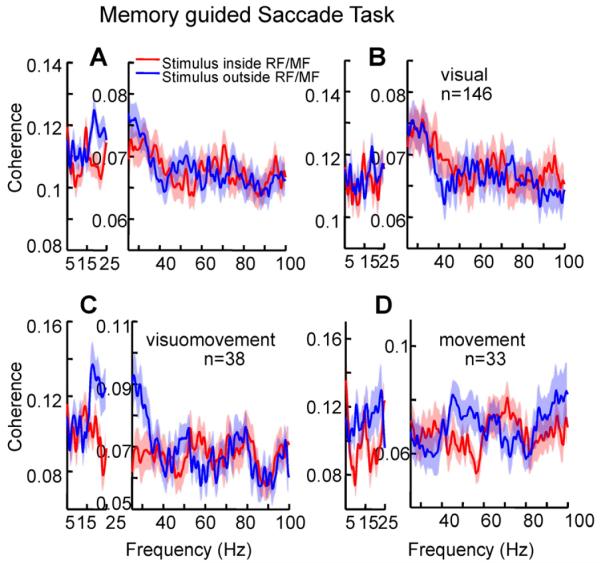
To distinguish whether the increase in synchrony between 15 and 35 Hz was due to attention to the movement field or inhibition of saccades into the movement field in the attention task, we examined coherence within FEF in the delayed saccade task. According to the enhanced attention hypothesis, synchrony should be enhanced in both tasks, because attention was directed into the movement field in both tasks. According to the saccade inhibition hypothesis, it should not be enhanced, or should even be reduced in the delayed saccade task because the animal was planning a saccade to the movement field stimulus in that task. As shown in Figure 7, the results supported the saccade inhibition hypothesis, in that for all FEF cells combined, spike-field beta coherence in the delayed saccade task was significantly decreased by 10% (coherence averaged 17-23 Hz; paired t-test, p<0.01), when the stimulus had appeared inside the visual RF and the saccade was planned to be executed within the movement field of the neuron (Figure 7A). Considering coherence by cell type, beta coherence was significantly decreased by 23% for visuomovement cells and by 19% for purely movement cells (paired t-test; visuomovement cells: p<0.01, movement cells: p<0.05), but there was only a small, 4%, decrease for visual cells, which did not reach significance (paired t-test, p=0.36). However, these spatial effects on beta synchrony were not significantly different across groups (Kruskal-Wallis, p=0.31). A distribution of the spatial effects on beta synchrony for the different classes of neurons in the memory guided saccade task is shown in Figure S5.
Figure 7. Spatial effects on synchronization within the FEF in the memory guided saccade task.
Spike-field coherence between: (A) spikes of FEF neurons and FEF LFPs (B) spikes of FEF visual neurons and FEF LFPs (C) spikes of FEF visuomovement neurons and FEF LFPs, and (D) spikes of FEF movement neurons and FEF LFPs. Spike and LFP signals from 350 ms after the target flash to the go cue were used for the coherence calculation. Conventions as in Figure 5. See also Figure S5.
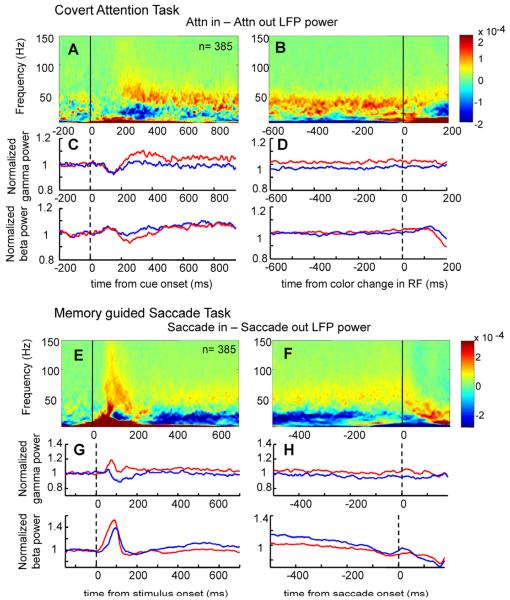
The time course of LFP power paralleled the results from the trial-averaged spike-field coherence of all FEF cell types taken together (Figure 8). In the attention task, gamma power (35-60 Hz) increased with attention after cue onset and was maintained enhanced for the remainder of the trial (8% increase with attention, 300-700ms after cue onset; paired t-test, p<0.001) (Figure 8 A-D). After a small dip in beta power with attention following the onset of the cue, beta power was largely unaffected by the direction of attention, except that there was a small but significant increase later in the trial, in the period just before the color change (−400-0 ms relative to color change; 15-25 Hz, paired t-test, p<0.001, 3% increase, Figure 8 B and D). No significant modulation in alpha frequencies (9-14 Hz) was measured during sustained attention (300-700ms after cue onset; paired t-test, p=0.08; −400-0 ms relative to color change; paired t-test, p=0.09). By contrast, in the memory guided saccade task a desynchronization in beta frequencies was the most prominent feature during the delay period in the FEF (Figure 8 E-H). This reduction in beta power became evident about 300 ms after the stimulus flash but was maintained throughout the delay period. When the saccade was planned towards the RF/MF, beta power in the FEF was decreased by 9% and this difference was statistically significant (−600-(−200)ms relative to saccade onset, beta power averaged 15-25 Hz; paired t-test, p<0.001). Alpha band power was also differentially modulated in the memory guided saccade task compared to the covert attention task. We found a significant 5% decrease in alpha power during the delay period when the saccade was planned towards the RF/MF (paired t-test, p<0.001) Gamma power increased shortly following the stimulus flashed in the RF/MF, and was maintained at a higher rate until the onset of the saccade.
Figure 8. LFP power in the attention and memory guided saccade tasks.
(A) Population average of attentional effects (attention inside RF-attention outside RF) on FEF LFP power time course aligned on cue onset. (B) Population average of attentional effects (attention inside RF-attention outside RF) on FEF LFP power time course aligned on color change in RF. (C) Normalized FEF LFP gamma power averaged between 35-60 Hz (upper graph) and beta power averaged over 15-25 Hz (lower graph) aligned on the cue onset in the attention task. (D) Normalized FEF LFP gamma power averaged between 35-60 Hz (upper graph) and beta power averaged over 15-25 Hz (lower graph) aligned on the color change in RF in the attention task. (E) Population average of spatial effects (saccade inside RF/MF-saccade outside RF/MF) on FEF LFP power time course aligned on stimulus flash. (F) Population average of spatial effects (saccade inside RF/MF-saccade outside RF/MF) on FEF LFP power time course aligned on saccade onset. (G) Normalized FEF LFP gamma power averaged between 35-60 Hz (upper graph) and beta power averaged over 15-25 Hz (lower graph) aligned on stimulus flash. (H) Normalized FEF LFP gamma power averaged between 35-60 Hz (upper graph) and beta power averaged over 15-25 Hz (lower graph) aligned on saccade onset. Conventions as in Figure 3.
DISCUSSION
The present study provides new evidence on the cellular substrate of attention and how different neuronal types contribute to long range interactions between different nodes of the attentional network. As a group, only visual neurons in FEF show significant synchronous oscillations with cells in V4 with attention. This coherent activity between the FEF visual cells and V4 was confined to the gamma frequency range. Cells with movement related activity have synchronous oscillations within FEF, not with V4. This coherent activity within FEF occurs in the beta frequency range and is consistent with the inhibition of saccades. Furthermore, only neurons with visual activity enhanced their firing rate when attention was directed inside their RF as well as during the maintenance of attention within the RF. The vast majority of movement neurons was either suppressed when attention was maintained inside their movement field or was unaffected by the locus of attention. These results together with those from previous studies argue against motor theories of attention that attribute a direct causal role of saccadic activity to attentional processes and provide new insight into the neural mechanisms of attention at the cellular and network level.
Previous studies have established a role of FEF in covert attention in both humans and monkeys. Neuroimaging studies in humans have shown that the FEF is activated in both covert and overt shifts of attention (Astafiev et al., 2003; Beauchamp et al., 2001; Corbetta et al., 1998; Nobre et al., 2000). Moreover, transcranial magnetic stimulation over FEF facilitates visual detection in a covert attention task and reduces reaction times showing that FEF activity is not only correlated with the generation of saccades but it is causally related to covert visual attention (Grosbras and Paus, 2002). Likewise, electrical stimulation of FEF in monkeys elicits both eye movements (Bruce et al., 1985; Tehovnik et al., 2000) and shifts in covert attention (Moore and Fallah, 2001, 2004). Specifically, Moore and colleagues have demonstrated that subthreshold stimulation of the FEF improves detection thresholds and also modulates responses in visual area V4 mimicking the effects of spatial attention (Armstrong et al., 2006; Moore and Armstrong, 2003; Moore and Fallah, 2001). Clearly, FEF plays a role in both saccadic eye movements and covert attention, but the important mechanistic question is whether it is the same neural circuitry in FEF that mediates both.
Neurophysiological studies in FEF have indicated that visual selection and saccade production are different processes and can be dissociated. FEF neurons with visual responses can discriminate a target among distracters in a pop-out task at a latency that is independent of the saccade latency toward the same target (Sato et al., 2001; Sato and Schall, 2003; Thompson et al., 1996) and these selection signals do not depend on the generation of a saccade (Thompson et al., 1997). Moreover, when the saccade is directed to a stimulus outside the RF, FEF neurons are activated by distracters similar to the target (Bichot and Schall, 1999) confirming that visual selection signals are independent of saccade production signals in the FEF. Finally, electrical microstimulation of the FEF in an antisaccade task demonstrated that covert attention is independent of the actual saccade preparation (Juan et al., 2004)
Although the evidence listed above argues against a causal role of saccadic activity in attentional processes, a direct test should include a comparison of the responses of all classes of FEF neurons (Bruce and Goldberg, 1985) in both covert attention and saccade tasks, as well as a comparison of their roles in top-down attentional feedback to visual cortex. Our study is the first to do that. We employed an endogeneous attention task and a manual response, to preclude any preparation for a saccade.
An earlier study also examined the source of attentional signals among FEF neurons (Thompson et al., 2005). Using a pop-out visual search task that required no saccadic response the authors showed that only cells with visual responses in the FEF (visual and visuomovement) modulated their activity with the locus of attention. Saccade-related movement neurons were suppressed in the attention task and this suppression was not spatially selective. Our data on firing rates are in large agreement with Thompson et al, and extend their results in two ways. First, during sustained attention, we found that only purely visual neurons increased their activity with attention to the RF and at this time the activity of movement neurons decreased when attention was directed toward their movement field. The suppression of saccade-related movement neurons with attention may be the result of local processing within the FEF so that saccades are inhibited downstream based on behavioral context. Indeed, SC, which lies closer to the brainstem saccade generator, receives projections mainly from the infragranular layers of the FEF where most movement neurons lie (Fries, 1984; Pouget et al., 2009; Segraves and Goldberg, 1987). Second, while Thompson et al used a task characterized by exogenous shifts of attention (pop-out), we used a task that required endogenous shifts of attention. It has been previously suggested that endogenous, rather than exogenous, shifts of attention are mediated by oculomotor processes related to the preparation for a saccade (Awh et al., 2006; Klein, 1980; Rizzolatti et al., 1994). The two studies together, therefore, demonstrate that neither in exogenous nor in endogenous attention do FEF saccade-related movement neurons contribute to shifts of attention.
The selective coupling of FEF visual neurons with V4 during sustained attention adds further evidence to the distinct contribution of FEF visual neurons to attentional mechanisms. Our finding that enhanced coupling occurs with attention only between FEF visual neurons and V4 suggests that V4 neurons have preferential connections with FEF visual neurons rather than any other FEF cell type. The pattern of anatomical connections between FEF and V4 supports this conclusion. The majority of FEF projections to V4 arise from the supragranular layers (Barone et al., 2000; Pouget et al., 2009), and neurons in the supragranular layers of the FEF subserve visual selection (Thompson et al., 1996). With attention, an increase in gamma synchrony between FEF supragranular-layer visual cells and V4 with the appropriate phase relationships may increase effective communication between the two areas to enhance processing of signals related to the attended location (Fries, 2005; Gregoriou et al., 2009a; Gregoriou et al., 2009b). Moreover, the absence of any effect of attention on synchrony between FEF movement cells and V4 further indicates that attentional mechanisms at the network level are largely independent and distinct from movement processing.
If visual FEF cells subserve visual selection and provide top-down inputs to extrastriate cortex whereas movement FEF neurons mediate saccade execution via projections to oculomotor centers what is the role of visuomovement neurons? Previous studies have indicated that the responses of visuomovement neurons do not mediate saccade preparation and have suggested that they may provide a corollary discharge to update the visual representations every time the eyes move (Ray et al., 2009). Similar presaccadic enhancements have also been recorded in areas that are anatomically distant from the brainstem saccade generator such as area V4, and area 46 (Boch and Golbberg, 1989; Fischer and Boch, 1981; Moore et al., 1998). It is thus possible that such a corollary discharge signal is provided by FEF visuomovement neurons once a saccade is bound to occur. Our task was not designed to test this possibility. Given that no saccades were executed during our attention task the absence of coupling between FEF visuomovement neurons and V4 is not surprising.
A very recent study showed that FEF cells mediating saccade selection are affected by activation of both D1 and D2 dopamine receptors whereas those contributing to visual modulation of V4 are sensitive only to D1 receptor agonists (Noudoost and Moore, 2011). This is in line with the finding that in infragranular layers, source of saccade related signals in the FEF, both D1 and D2 receptors are found, whereas in supragranular layers, source of FEF signals responsible for the enhancement of activity in V4, D2 receptors are less frequent (Lidow et al., 1991; Santana et al., 2009). The results support the idea that the visual cells found to have synchronous activity with cells in V4 in the present study are superficial layers cells in FEF.
Within FEF we found attentional effects on synchrony in different frequency ranges for visual and movement neurons. An increase in gamma spike-field coherence with attention for visual neurons parallels our own previous findings in the FEF using multi unit activity (Gregoriou et al., 2009a) as well as similar effects measured in visual area V4 with attention (Bichot et al., 2005; Fries et al., 2001; Fries et al., 2008). It was also accompanied by an increase in gamma power of the LFP. Gamma frequency synchronization has been suggested to reflect local computations which mediate the enhancement of sensory representations (Buschman and Miller, 2007; Kopell et al., 2000). Such an enhancement of sensory representations would be in agreement with the role of visual neurons in the covert attention task. The enhancement in gamma synchrony for visual neurons was contrasted by an increase in synchrony in lower frequencies including the beta band for FEF movement neurons and a small but significant increase in LFP beta power within the FEF.
A different pattern of beta band modulation was found in the memory guided saccade task. A desynchronization in beta frequencies within the FEF was measured specifically for neurons with saccade-related movement activity and a decrease in LFP beta power was found during the delay period. The increase in beta (and lower gamma) synchrony and beta power with attention and the decrease in the memory guided saccade task suggest that the contribution of FEF neurons with movement activity is different in the two tasks and thus confirm that the two processes are subserved by different mechanisms. Given that the exact frequency range at which beta coherence modulation was found was somewhat different in the two tasks (saccade task: 17-23 Hz, covert attention task: 15-35 Hz) we can’t rule out the possibility that other factors besides saccade inhibition contribute to the increase in coherence in the covert attention task for movement cells. However, the fact that LFP beta power (15-25Hz) was also differentially affected in the two tasks indicates that beta band modulation reflects the distinct motor requirements of the two tasks.
One could argue that preparing a saccade to a visible stimulus (in a covert attention task) could differ fundamentally from preparing a saccade to a remembered location (as in the memory guided saccade task). If this is the case then the differential beta band modulation in the two tasks could reflect processes not related to the current state of the oculomotor network. However, the existing literature on the role of beta oscillations and synchrony in motor processes supports our suggestion. An increase in beta frequency oscillations has been associated with an inactive state of the motor system while a decrease of beta power has been reported to reflect motor preparation and motor execution in skeletomotor tasks (Baker et al., 1997; Gilbertson et al., 2005; Pfurtscheller et al., 1996; Tkach et al., 2007; Zhang et al., 2008). Beta band oscillations may promote a steady motor output, maintain the status quo or contribute to a mechanism that calibrates the sensorimotor system (Androulidakis et al., 2007; Baker, 2007; Engel and Fries, 2010; Gilbertson et al., 2005). Our experiments were not designed to answer this question. However, the current findings indicate that similar principles may govern oculomotor and skeletomotor functions. Moreover, our results establish that beta band synchrony and LFP power can be used as an index of the state of the local network in an oculomotor structure such as the FEF. Interestingly, we also found a selective decrease in alpha power in the memory guided saccade task, a finding that is in accord with human motor studies showing a reduction in alpha power during motor preparation and execution (Neuper et al., 2006). How a decrease in alpha and beta power and synchrony may be used in saccade preparation remains to be explored in subsequent studies.
In conclusion, the data provided here reveal that saccadic and attentional processes can be dissociated at the cellular and population dynamics level. Although we cannot rule out the possibility that the two mechanisms are linked during visually-guided saccades in ways not observed here, the results suggest that distinct neuronal circuits between FEF and V4 mediate motor processes and covert shifts of attention. Whether oculomotor and attentional control is mediated by separate functional cell types in other structures remains to be determined. Initial evidence suggests that distinct cell types in SC subserve target selection (Ignashchenkova et al., 2004; McPeek and Keller, 2002).
EXPERIMENTAL PROCEDURES
Subjects
Two male rhesus monkeys (Macaca mulatta) weighing 8-10 kg were used. A post to fix the head and two recording chambers, one over FEF and one over area V4 were implanted under general anesthesia and aseptic conditions. The positioning of the chambers was based on MRI scans obtained before surgery. All procedures and animal care were in accordance with the NIH guidelines.
Behavioral Tasks
The monkeys faced a computer monitor (resolution 800×600 pixels and refresh rate 100Hz) at a distance of 57cm with their heads fixed. Behavioral parameters and presentation of visual stimuli were controlled by the CORTEX software package. Eye position was monitored by an infrared based eye-tracking system at 60 Hz (ISCAN).
Receptive fields (RFs) were mapped by flashing stimuli while the monkeys were fixating centrally. RFs were further examined in a memory guided saccade task.
In each session we recorded activity first from the memory guided saccade and then from the attention task.
Memory Guided Saccade Task
At the beginning of the trial the monkeys had to fixate (within a 3×3° window) a white spot presented at the center of the screen for 600-1000 ms. Successful fixation was followed by presentation of a yellow stimulus 1.5×1.5° which was flashed for 100 ms in one of six positions arranged on a circle with radius equal to the eccentricity that elicited the maximal response in the RF mapping task. Monkeys were required to maintain fixation of the central spot. After a delay of 750 ms, the fixation spot was turned off and the monkeys had to saccade to the memorized position of the peripheral stimulus and maintain their gaze at the peripheral location within a 3×3° window for 200 ms in order to be rewarded with juice.
Attention Task
Monkeys were required to hold a bar to initiate the trial and subsequently fixate a central spot (0.4×0.4°) on the screen. Successful fixation within a 3×3° window for 1500 ms was followed by the appearance of three isoluminant, sinusoidal, drifting gratings (2° diameter, drifting rate 1cycle/s), one red, one blue and one green, positioned at the same distance from the center of the screen (usually within 4-8°) and distributed radially around the fixation point at 120° intervals. Following a variable period of time (0-1000 ms), the fixation spot was replaced by a small square cue whose color indicated the stimulus to be attended. The monkeys had to shift their attention to the target stimulus (while maintaining fixation of the central cue) and wait for the target to change color. The color change could happen any time between 250 and 3000 ms after the cue onset. In one third of the trials one distracter changed color before the target, in one third both distracters changed color before the target (with a minimum delay of 400 ms) and in one third only the target changed color. The animals were required to ignore any color changes of the distracter stimuli and respond only to the target color change by releasing the bar within 600 ms. Successful completion of the trial was rewarded with a drop of juice. If the monkeys released the bar prematurely, did not respond to the target color change within the specified time, or broke fixation, the trial was aborted. We manipulated task difficulty by making the color changes subtle so that the monkeys needed to attend to the target in order to detect the change and respond correctly. We decreased the magnitude of color change to the point that the monkeys performed between 80 and 85% to ensured that they did not rely on a bottom-up, stimulus driven approach but they rather used the cue to attend to the target.
Recording
We used a Multichannel Acquisition Processor system (Plexon) to record spikes and local field potentials (LFPs) from FEF and V4 simultaneously using up to four tungsten microelectrodes in each area. The recording procedure has been described in detail before (Gregoriou et al., 2009a) and is briefly outlined in the Supplemental Information. Briefly, spike data were obtained after filtering between 250 Hz-8 kHz, amplifying and digitizing the signal at 40 kHz. Spikes were selected offline to include multi-unit activity on each electrode and were sorted offline using the Offline Sorter software (Plexon, Inc) to isolate spike trains from single units. For the LFP, the signals were filtered between 0.7-170 Hz, amplified and digitized at 1 kHz. LFP data were post-processed to correct for the known phase shifts as previously described (Gregoriou et al., 2009a).
Data Analysis
Firing Rates
In each correct trial of the memory guided saccade task we detected the beginning of the saccade as the time after the go signal at which eye velocity exceeded 300°/s and the amplitude of the resulted deviation of the eye position was greater than 1°. A semi-automatic process allowed us to optimize these parameters in order to avoid including noise or fixational saccades in the analysis.
To classify neurons as visual, visuomovement and movement we measured spike counts within specified windows. Visual responses were measured between 50 and 150 ms after the target flash. Baseline activity was measured between 150 ms and 0 ms before the target flash. Movement responses were measured between 100 ms before and 20 ms after the initiation of the saccade. Premovement activity was measured between 350 ms and 200 ms before the initiation of the saccade. A neuron was classified as visual if the visual response was significantly greater than baseline activity (p<0.05, Wilcoxon sign rank test) in at least one target location and the movement response was not significantly greater than the premovement activity at any target location. Accordingly, a neuron was classified as movement related if the movement response was significantly greater than the premovement activity (p<0.05) for saccades to at least one target location. Visuomovement neurons displayed significant visual and movement responses. The center of the visual RF of each signal was defined to be the location that elicited the maximal visual response (averaged across trials) in the memory guided saccade task. Likewise, movement field (MF) location was defined as the location that elicited the maximal movement response. To quantify the relative magnitude of visual and motor responses we computed a visuomovement index for each neuron as VMI = (visual response − movement response)/(visual response + movement response) with visual and movement responses measured between 50 and 150 ms following the target flash and between 100 ms before the onset of the saccade and 20 ms after the onset of the saccade, respectively.
To quantify the attentional effect for each neuron an attention index was computed as AI = (Response in Attend In- Response in Attend Out)/(Response in Attend In + Response in Attend Out). Responses were averaged within a window 100-400 ms after cue onset for effects early in the trial and −400-0 ms relative to the color change inside the RF (or MF) for effects assessed later in the trial. The location on the opposite hemifield to the neuron’s RF, 120° away from the RF location was considered as “attend out” location in the attention task and “saccade out” location in the memory guided saccade task. For all statistical comparisons throughout the paper significance values below the 0.001 level are reported at this cutoff point.
Data were normalized to the mean pre-cue activity (−200-0 ms relative to cue onset) or the mean pre- color change activity (−400-0 ms relative to color change in RF) across both attention conditions. In the memory guided saccade task data were normalized to the mean pre-stimulus activity (−200-0 ms relative to stimulus flash).
Coherence Analysis
We calculated spike-LFP coherency, which is a measure of phase locking between two signals as a function of frequency. Coherency for two signals x and y is calculated as
where Sx(f), and Sy(f) represent the auto-spectra and Sxy(f) the cross-spectrum of the two signals x and y averaged across trials. Coherency is a complex quantity. Its absolute value (coherence) ranges from 0 (when there is no consistent phase relationship between the two signals) to 1 (when the two signals have a constant phase relationship). To achieve optimal spectral concentration we used multi-taper methods for spectral estimation providing a smoothing of ±10Hz in frequencies above 25Hz and ±4Hz for lower frequencies. An optimal family of orthogonal tapers given by the discrete prolate spheroid sequences (Slepian functions) was used as described before (Fries et al., 2008; Gregoriou et al., 2009a; Jarvis and Mitra, 2001). Sample size bias and the effect of firing rate differences was treated as previously described (Gregoriou et al., 2009a) (see Supplemental Information).
To examine the correlation between attentional effects and the visuomovement index we computed an attention index as AICOH = (Coherence in Attend In- Coherence in Attend Out)/(Coherence in Attend In + Coherence in Attend Out). Coherence was averaged within the frequency range we found a significant attentional effect.
LFP power
To compute the time course of the LFP power spectra we used the Hilbert-Huang Transform (HHT) (Huang et al., 1998). This approach employs the Empirical Mode Decomposition (EMD) method and the Hilbert transform. The Hilbert spectrum was calculated for each trial employing matlab functions. The resulting three dimensional time-frequency spectra were smoothed using a 2D Gaussian filter (sigma = [4ms, 2Hz], size = [10ms, 5Hz]). For each signal the LFP power within the frequency range of interest per condition was normalized to the average power within the frequency range of interest across both conditions in a 200 ms window before cue onset for data aligned on cue onset and in a 500ms window before the color change in RF for data aligned on color change in the attention task. In the memory guided saccade task the data were normalized to the average power within either a 200ms window before the stimulus flash or within a 500ms window before the saccade onset.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Grant Pielli, Donovan Stock and Courtney Alfes for help with the animal training and Steve Stefanou and George Spiropoulos for help with preliminary analysis. The authors were supported by a 5R01EY017921 Grant to R.D, by the European Community’s Seventh Framework Programme (Grant PIRG05-GA-2009-246761), the General Secretariat for Research and Technology (Grant 9FR27), and the Special Account of Research Funds, University of Crete (Grant 3004) to G.G.G. S.J.G. was supported initially by MH64445 from the National Institutes of Health (USA) and later by the National Institute of Mental Health, Division of Intramural Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Andersen SK, Muller MM. Behavioral performance follows the time course of neural facilitation and suppression during cued shifts of feature-selective attention. Proc Natl Acad Sci U S A. 2010;107:13878–13882. doi: 10.1073/pnas.1002436107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Androulidakis AG, Doyle LM, Yarrow K, Litvak V, Gilbertson TP, Brown P. Anticipatory changes in beta synchrony in the human corticospinal system and associated improvements in task performance. Eur J Neurosci. 2007;25:3758–3765. doi: 10.1111/j.1460-9568.2007.05620.x. [DOI] [PubMed] [Google Scholar]
- Armstrong KM, Fitzgerald JK, Moore T. Changes in visual receptive fields with microstimulation of frontal cortex. Neuron. 2006;50:791–798. doi: 10.1016/j.neuron.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Astafiev SV, Shulman GL, Stanley CM, Snyder AZ, Van Essen DC, Corbetta M. Functional organization of human intraparietal and frontal cortex for attending, looking, and pointing. J Neurosci. 2003;23:4689–4699. doi: 10.1523/JNEUROSCI.23-11-04689.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awh E, Armstrong KM, Moore T. Visual and oculomotor selection: links, causes and implications for spatial attention. Trends Cogn Sci. 2006;10:124–130. doi: 10.1016/j.tics.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Baker SN. Oscillatory interactions between sensorimotor cortex and the periphery. Curr Opin Neurobiol. 2007;17:649–655. doi: 10.1016/j.conb.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SN, Olivier E, Lemon RN. Coherent oscillations in monkey motor cortex and hand muscle EMG show task-dependent modulation. J Physiol. 1997;501(Pt 1):225–241. doi: 10.1111/j.1469-7793.1997.225bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone P, Batardiere A, Knoblauch K, Kennedy H. Laminar distribution of neurons in extrastriate areas projecting to visual areas V1 and V4 correlates with the hierarchical rank and indicates the operation of a distance rule. J Neurosci. 2000;20:3263–3281. doi: 10.1523/JNEUROSCI.20-09-03263.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp MS, Petit L, Ellmore TM, Ingeholm J, Haxby JV. A parametric fMRI study of overt and covert shifts of visuospatial attention. Neuroimage. 2001;14:310–321. doi: 10.1006/nimg.2001.0788. [DOI] [PubMed] [Google Scholar]
- Bichot NP, Rossi AF, Desimone R. Parallel and serial neural mechanisms for visual search in macaque area V4. Science. 2005;308:529–534. doi: 10.1126/science.1109676. [DOI] [PubMed] [Google Scholar]
- Bichot NP, Schall JD. Effects of similarity and history on neural mechanisms of visual selection. Nat. Neurosci. 1999;2:549–554. doi: 10.1038/9205. [DOI] [PubMed] [Google Scholar]
- Boch RA, Golbberg ME. Participation of prefrontal neurons in the preparation of visually guided eye movements in the rhesus monkey. J. Neurophysiol. 1989;61:1064–1084. doi: 10.1152/jn.1989.61.5.1064. [DOI] [PubMed] [Google Scholar]
- Bruce CJ, Goldberg ME. Primate frontal eye fields. I. Single neurons discharging before saccades. J. Neurophysiol. 1985;53:603–635. doi: 10.1152/jn.1985.53.3.603. [DOI] [PubMed] [Google Scholar]
- Bruce CJ, Goldberg ME, Bushnell MC, Stanton GB. Primate frontal eye fields. II. Physiological and anatomical correlates of electrically evoked eye movements. J. Neurophysiol. 1985;54:714–734. doi: 10.1152/jn.1985.54.3.714. [DOI] [PubMed] [Google Scholar]
- Buschman TJ, Miller EK. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science. 2007;315:1860–1862. doi: 10.1126/science.1138071. [DOI] [PubMed] [Google Scholar]
- Cavanaugh J, Wurtz RH. Subcortical modulation of attention counters change blindness. J Neurosci. 2004;24:11236–11243. doi: 10.1523/JNEUROSCI.3724-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Akbudak E, Conturo TE, Snyder AZ, Ollinger JM, Drury HA, Linenweber MR, Petersen SE, Raichle ME, Van Essen DC, Shulman GL. A common network of functional areas for attention and eye movements. Neuron. 1998;21:761–773. doi: 10.1016/s0896-6273(00)80593-0. [DOI] [PubMed] [Google Scholar]
- Deubel H, Schneider WX. Saccade target selection and object recognition: evidence for a common attentional mechanism. Vision Res. 1996;36:1827–1837. doi: 10.1016/0042-6989(95)00294-4. [DOI] [PubMed] [Google Scholar]
- Engel AK, Fries P. Beta-band oscillations--signalling the status quo? Curr Opin Neurobiol. 2010;20:156–165. doi: 10.1016/j.conb.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Fischer B, Boch R. Enhanced activation of neurons in prelunate cortex before visually guided saccades of trained rhesus monkeys. Exp Brain Res. 1981;44:129–137. doi: 10.1007/BF00237333. [DOI] [PubMed] [Google Scholar]
- Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn Sci. 2005;9:474–480. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Fries P, Reynolds JH, Rorie AE, Desimone R. Modulation of oscillatory neuronal synchronization by selective visual attention. Science. 2001;291:1560–1563. doi: 10.1126/science.1055465. [DOI] [PubMed] [Google Scholar]
- Fries P, Womelsdorf T, Oostenveld R, Desimone R. The effects of visual stimulation and selective visual attention on rhythmic neuronal synchronization in macaque area V4. J Neurosci. 2008;28:4823–4835. doi: 10.1523/JNEUROSCI.4499-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries W. Cortical projections to the superior colliculus in the macaque monkey: a retrograde study using horseradish peroxidase. J Comp Neurol. 1984;230:55–76. doi: 10.1002/cne.902300106. [DOI] [PubMed] [Google Scholar]
- Gilbertson T, Lalo E, Doyle L, Di Lazzaro V, Cioni B, Brown P. Existing motor state is favored at the expense of new movement during 13-35 Hz oscillatory synchrony in the human corticospinal system. J Neurosci. 2005;25:7771–7779. doi: 10.1523/JNEUROSCI.1762-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoriou GG, Gotts SJ, Zhou H, Desimone R. High frequency long range coupling between prefrontal cortex and visual cortex during attention. Science. 2009a;324:1207–1210. doi: 10.1126/science.1171402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoriou GG, Gotts SJ, Zhou H, Desimone R. Long-range neural coupling through synchronization with attention. Prog Brain Res. 2009b;176:35–45. doi: 10.1016/S0079-6123(09)17603-3. [DOI] [PubMed] [Google Scholar]
- Grosbras MH, Paus T. Transcranial magnetic stimulation of the human frontal eye field: effects on visual perception and attention. J Cogn Neurosci. 2002;14:1109–1120. doi: 10.1162/089892902320474553. [DOI] [PubMed] [Google Scholar]
- Hoffman JE, Subramaniam B. The role of visual attention in saccadic eye movements. Percept Psychophys. 1995;57:787–795. doi: 10.3758/bf03206794. [DOI] [PubMed] [Google Scholar]
- Huang NE, Shen Z, Long SR, Wu MLC, Shih HH, Zheng QN, Yen NC, Tung CC, Liu HH. The empirical mode decomposition and the Hilbert spectrum for nonlinear and non-stationary time series analysis. Proceedings of the Royal Society of London Series a-Mathematical Physical and Engineering Sciences. 1998;454:903–995. [Google Scholar]
- Hunt AR, Kingstone A. Covert and overt voluntary attention: linked or independent? Brain Res Cogn Brain Res. 2003;18:102–105. doi: 10.1016/j.cogbrainres.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Ignashchenkova A, Dicke PW, Haarmeier T, Thier P. Neuron-specific contribution of the superior colliculus to overt and covert shifts of attention. Nat Neurosci. 2004;7:56–64. doi: 10.1038/nn1169. [DOI] [PubMed] [Google Scholar]
- Jarvis MR, Mitra PP. Sampling properties of the spectrum and coherency of sequences of action potentials. Neural Comput. 2001;13:717–749. doi: 10.1162/089976601300014312. [DOI] [PubMed] [Google Scholar]
- Juan CH, Shorter-Jacobi SM, Schall JD. Dissociation of spatial attention and saccade preparation. Proc Natl Acad Sci U S A. 2004;101:15541–15544. doi: 10.1073/pnas.0403507101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Annu Rev Neurosci. 2000;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- Klein RM. Does oculomotor readiness mediate cognitive control of visual attention. In: Nickerson R, editor. Attention and Performance VIII. Academic Press; New York: 1980. pp. 259–276. [Google Scholar]
- Kopell N, Ermentrout GB, Whittington MA, Traub RD. Gamma rhythms and beta rhythms have different synchronization properties. Proc Natl Acad Sci U S A. 2000;97:1867–1872. doi: 10.1073/pnas.97.4.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowler E, Anderson E, Dosher B, Blaser E. The role of attention in the programming of saccades. Vision Res. 1995;35:1897–1916. doi: 10.1016/0042-6989(94)00279-u. [DOI] [PubMed] [Google Scholar]
- Kustov AA, Robinson DL. Shared neural control of attentional shifts and eye movements. Nature. 1996;384:74–77. doi: 10.1038/384074a0. [DOI] [PubMed] [Google Scholar]
- Lakatos P, Karmos G, Mehta AD, Ulbert I, Schroeder CE. Entrainment of neuronal oscillations as a mechanism of attentional selection. Science. 2008;320:110–113. doi: 10.1126/science.1154735. [DOI] [PubMed] [Google Scholar]
- Lidow MS, Goldman-Rakic PS, Gallager DW, Rakic P. Distribution of dopaminergic receptors in the primate cerebral cortex: quantitative autoradiographic analysis using [3H]raclopride, [3H]spiperone and [3H]SCH23390. Neuroscience. 1991;40:657–671. doi: 10.1016/0306-4522(91)90003-7. [DOI] [PubMed] [Google Scholar]
- McPeek RM, Keller EL. Saccade target selection in the superior colliculus during a visual search task. J Neurophysiol. 2002;88:2019–2034. doi: 10.1152/jn.2002.88.4.2019. [DOI] [PubMed] [Google Scholar]
- McPeek RM, Keller EL. Deficits in saccade target selection after inactivation of superior colliculus. Nat Neurosci. 2004;7:757–763. doi: 10.1038/nn1269. [DOI] [PubMed] [Google Scholar]
- Moore T, Armstrong KM. Selective gating of visual signals by microstimulation of frontal cortex. Nature. 2003;421:370–373. doi: 10.1038/nature01341. [DOI] [PubMed] [Google Scholar]
- Moore T, Fallah M. Control of eye movements and spatial attention. Proc Natl Acad Sci U S A. 2001;98:1273–1276. doi: 10.1073/pnas.021549498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T, Fallah M. Microstimulation of the frontal eye field and its effects on covert spatial attention. J Neurophysiol. 2004;91:152–162. doi: 10.1152/jn.00741.2002. [DOI] [PubMed] [Google Scholar]
- Moore T, Tolias AS, Schiller PH. Visual representations during saccadic eye movements. Proc Natl Acad Sci U S A. 1998;95:8981–8984. doi: 10.1073/pnas.95.15.8981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller JR, Philiastides MG, Newsome WT. Microstimulation of the superior colliculus focuses attention without moving the eyes. Proc Natl Acad Sci U S A. 2005;102:524–529. doi: 10.1073/pnas.0408311101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuper C, Wortz M, Pfurtscheller G. ERD/ERS patterns reflecting sensorimotor activation and deactivation. Prog Brain Res. 2006;159:211–222. doi: 10.1016/S0079-6123(06)59014-4. [DOI] [PubMed] [Google Scholar]
- Nobre AC, Gitelman DR, Dias EC, Mesulam MM. Covert visual spatial orienting and saccades: overlapping neural systems. Neuroimage. 2000;11:210–216. doi: 10.1006/nimg.2000.0539. [DOI] [PubMed] [Google Scholar]
- Noudoost B, Moore T. Control of visual cortical signals by prefrontal dopamine. Nature. 2011;474:372–375. doi: 10.1038/nature09995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfurtscheller G, Stancak A, Jr., Neuper C. Post-movement beta synchronization. A correlate of an idling motor area? Electroencephalogr Clin Neurophysiol. 1996;98:281–293. doi: 10.1016/0013-4694(95)00258-8. [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Q J Exp Psychol. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Pouget P, Stepniewska I, Crowder EA, Leslie MW, Emeric EE, Nelson MJ, Schall JD. Visual and motor connectivity and the distribution of calcium-binding proteins in macaque frontal eye field: implications for saccade target selection. Front Neuroanat. 2009;3:2. doi: 10.3389/neuro.05.002.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S, Pouget P, Schall JD. Functional distinction between visuomovement and movement neurons in macaque frontal eye field during saccade countermanding. J Neurophysiol. 2009;102:3091–3100. doi: 10.1152/jn.00270.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G. Mechanisms of selective attention in mammals. In: Ewert JP, Capranica RR, Ingle DJ, editors. Advances in Vertebrate Neuroethology. Plenum Press; London: 1983. pp. 261–297. [Google Scholar]
- Rizzolatti G, Riggio L, Dascola I, Umilta C. Reorienting attention across the horizontal and vertical meridians: evidence in favor of a premotor theory of attention. Neuropsychologia. 1987;25:31–40. doi: 10.1016/0028-3932(87)90041-8. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Riggio L, Sheliga BM. Space and selective attention. In: Umilta C, editor. Attention and Performance XV. MIT Press; Cambridge, MA: 1994. pp. 231–265. [Google Scholar]
- Saalmann YB, Pigarev IN, Vidyasagar TR. Neural mechanisms of visual attention: how top-down feedback highlights relevant locations. Science. 2007;316:1612–1615. doi: 10.1126/science.1139140. [DOI] [PubMed] [Google Scholar]
- Santana N, Mengod G, Artigas F. Quantitative analysis of the expression of dopamine D1 and D2 receptors in pyramidal and GABAergic neurons of the rat prefrontal cortex. Cereb Cortex. 2009;19:849–860. doi: 10.1093/cercor/bhn134. [DOI] [PubMed] [Google Scholar]
- Sato T, Murthy A, Thompson KG, Schall JD. Search efficiency but not response interference affects visual selection in frontal eye field. Neuron. 2001;30:583–591. doi: 10.1016/s0896-6273(01)00304-x. [DOI] [PubMed] [Google Scholar]
- Sato TR, Schall JD. Effects of stimulus-response compatibility on neural selection in frontal eye field. Neuron. 2003;38:637–648. doi: 10.1016/s0896-6273(03)00237-x. [DOI] [PubMed] [Google Scholar]
- Schafer RJ, Moore T. Selective attention from voluntary control of neurons in prefrontal cortex. Science. 2011;332:1568–1571. doi: 10.1126/science.1199892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schall JD, Hanes DP. Neural basis of saccade target selection in frontal eye field during visual search. Nature. 1993;366:467–469. doi: 10.1038/366467a0. [DOI] [PubMed] [Google Scholar]
- Segraves MA, Goldberg M. Functional properties of corticotectal neurons in the monkey’s frontal eye field. J. Neurophysiol. 1987;58:1387–1419. doi: 10.1152/jn.1987.58.6.1387. [DOI] [PubMed] [Google Scholar]
- Sheliga BM, Riggio L, Rizzolatti G. Orienting of attention and eye movements. Exp Brain Res. 1994;98:507–522. doi: 10.1007/BF00233988. [DOI] [PubMed] [Google Scholar]
- Shepherd M, Findlay JM, Hockey RJ. The relationship between eye movements and spatial attention. Q J Exp Psychol A. 1986;38:475–491. doi: 10.1080/14640748608401609. [DOI] [PubMed] [Google Scholar]
- Siegel M, Donner TH, Oostenveld R, Fries P, Engel AK. Neuronal synchronization along the dorsal visual pathway reflects the focus of spatial attention. Neuron. 2008;60:709–719. doi: 10.1016/j.neuron.2008.09.010. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ, Sommer MA, Chou IH, Slocum WM, Schiller PH. Eye fields in the frontal lobes of primates. Brain Res Brain Res Rev. 2000;32:413–448. doi: 10.1016/s0165-0173(99)00092-2. [DOI] [PubMed] [Google Scholar]
- Thompson KG, Bichot NP, Schall JD. Dissociation of visual discrimination from saccade programming in macaque frontal eye field. J. Neurophysiol. 1997;77:1046–1050. doi: 10.1152/jn.1997.77.2.1046. [DOI] [PubMed] [Google Scholar]
- Thompson KG, Biscoe KL, Sato TR. Neuronal basis of covert spatial attention in the frontal eye field. J Neurosci. 2005;25:9479–9487. doi: 10.1523/JNEUROSCI.0741-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson KG, Hanes DP, Bichot NP, Schall JD. Perceptual and motor processing stages identified in the activity of macaque frontal eye field neurons during visual search. J Neurophysiol. 1996;76:4040–4055. doi: 10.1152/jn.1996.76.6.4040. [DOI] [PubMed] [Google Scholar]
- Tkach D, Reimer J, Hatsopoulos NG. Congruent activity during action and action observation in motor cortex. J Neurosci. 2007;27:13241–13250. doi: 10.1523/JNEUROSCI.2895-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardak C, Ibos G, Duhamel JR, Olivier E. Contribution of the monkey frontal eye field to covert visual attention. J Neurosci. 2006;26:4228–4235. doi: 10.1523/JNEUROSCI.3336-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chen Y, Bressler SL, Ding M. Response preparation and inhibition: the role of the cortical sensorimotor beta rhythm. Neuroscience. 2008;156:238–246. doi: 10.1016/j.neuroscience.2008.06.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.