Abstract
Rationale: The immunologic events surrounding primary Mycobacterium tuberculosis infection and development of tuberculosis remain controversial. Young children who develop tuberculosis do so quickly after first exposure, thus permitting study of immune response to primary infection and disease. We hypothesized that M. tuberculosis–specific CD8+ T cells are generated in response to high bacillary loads occurring during tuberculosis.
Objectives: To determine if M. tuberculosis–specific T cells are generated among healthy children exposed to M. tuberculosis and children with tuberculosis.
Methods: Enzyme-linked immunosorbent spot assays were used to measure IFN-γ production in response to M. tuberculosis–specific proteins ESAT-6/CFP-10 by peripheral blood mononuclear cells and CD8+ T cells isolated from Ugandan children hospitalized with tuberculosis (n = 96) or healthy tuberculosis contacts (n = 62).
Measurements and Main Results: The proportion of positive CD8+ T-cell assays and magnitude of CD8+ T-cell responses were significantly greater among young (<5 yr) tuberculosis cases compared with young contacts (P = 0.02, Fisher exact test, P = 0.01, Wilcoxon rank-sum, respectively). M. tuberculosis–specific T-cell responses measured in peripheral blood mononuclear cells were equivalent between groups.
Conclusions: Among young children, M. tuberculosis–specific CD8+ T cells develop in response to high bacillary loads, as occurs during tuberculosis, and are unlikely to be found after M. tuberculosis exposure. T-cell responses measured in peripheral blood mononuclear cells are generated after M. tuberculosis exposure alone, and thus cannot distinguish exposure from disease. In young children, IFN-γ–producing M. tuberculosis–specific CD8+ T cells provide an immunologic signature of primary M. tuberculosis infection resulting in disease.
Keywords: Mycobacterium tuberculosis, infant, child, CD8-positive T lymphocytes, enzyme-linked immunosorbent spot
At a Glance Commentary
Scientific Knowledge on the Subject
Production of IFN-γ by host T cells is known to be essential to the human immune response to Mycobacterium tuberculosis (Mtb). However, little is understood regarding the immune response to a primary Mtb exposure that results in disease, an outcome that is common in young children.
What This Study Adds to the Field
Mtb-specific CD8+ T cells were present among young children with tuberculosis disease, but not healthy children recently exposed to Mtb. This indicates that an Mtb-specific CD8+ T cell response is generated in response to high bacillary load and may be able to distinguish children with TB disease from those with Mtb exposure.
Mycobacterium tuberculosis (Mtb), the etiology of tuberculosis (TB), causes over 9 million cases of disease and 1.7 million deaths annually (1). The only available vaccine to prevent TB, bacillus Calmette-Guérin, offers little protection against the most common disease manifestations (2) and efforts to develop an improved vaccine are hampered by poor understanding of immunologic events that occur after Mtb exposure. Scientific studies of immunologic responses to initial Mtb infection are difficult because most individuals living in TB-endemic settings have experienced multiple Mtb exposures. Young children, however, suffer disproportionately after exposure to Mtb, because they are at substantial risk for developing TB after primary infection (3–5). Therefore, young children with TB offer a valuable window into the human immune response to primary Mtb infection.
The fundamental importance of IFN-γ production in response to Mtb has been shown in animal models, and through identification of severe mycobacterial disease in humans with genetically altered IFN-γ signaling pathways (6). Although CD4+ T cells are an important source of IFN-γ, evidence suggests that CD8+ T cells also contribute to Mtb containment (7–13). Because our prior work has shown that Mtb-specific CD8+ T-cell clones preferentially recognize dendritic cells heavily infected with Mtb (9), we hypothesized that IFN-γ producing Mtb-specific CD8+ T cells are generated in response to high bacillary burden. Therefore, Mtb-specific CD8+ T cells would be present in individuals with TB and, unlike Mtb-specific T-cell responses elicited from unfractionated peripheral blood mononuclear cell (PBMC) populations, absent in healthy individuals with recent Mtb exposure.
To test our hypotheses, we studied children because their unique vulnerability to develop TB after initial exposure offers an opportunity to capture the immunologic response to a recently established Mtb infection. In Uganda, we compared Mtb-specific T-cell responses elicited from unfractionated PBMC with those elicited from CD8+ T cells, between young and older healthy children exposed to Mtb. In addition, we compared Mtb-specific T-cell responses elicited from PBMC with those elicited from CD8+ T cells, between healthy children exposed to Mtb and age-matched acutely ill children with probable and confirmed pulmonary TB. We provide evidence that young children with a heavy burden of disease harbor Mtb-specific CD8+ T cells, thus offering insight into the human immune response to primary Mtb infection. Some of these results have been previously reported in poster and platform presentations (14, 15).
Methods
Participants and Procedures
Healthy children exposed to Mtb (HE) and children acutely ill with confirmed or probable pulmonary TB (CP-TB) were enrolled from separate recruitment sites in Kampala, Uganda (Figure 1). For the HE group, we evaluated child household contacts (age <15 yr) of adults with culture-confirmed pulmonary TB (16). For the CP-TB group, we enrolled acutely ill, HIV-uninfected, hospitalized children (age <10 yr) meeting World Health Organization criteria for confirmed or probable intrathoracic TB (see Table E1 in the online supplement) (17). Subject recruitment and study coordination were sponsored by the Tuberculosis Research Unit at Case Western Reserve University, Cleveland, OH.
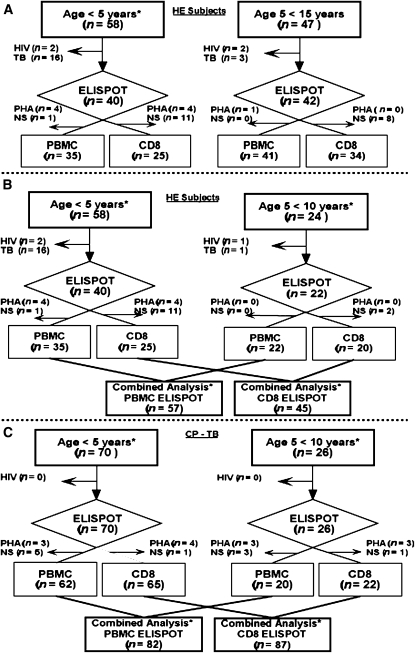
Figure 1.
Flow diagram depicting enrollment into the study. Flow diagram of enrollment of (A) healthy exposed (HE) children stratified for ages 0–5 and 5–15 years, (B) HE children stratified for ages 0–5 and 5–10 years, and (C) children with confirmed or probable tuberculosis (CP-TB) stratified for ages 0–5 and 5–10 years. Children excluded because of HIV infection or TB (HE group only), and children excluded because the positive control response was low (phytohemagglutinin [PHA] response ≤20 spot-forming units) or peripheral blood mononuclear cell (PBMC) sample was insufficient (NS) for analysis, are shown. *Age groups of HE children used for comparison with the CP-TB group.
For all participants, demographic and clinical information were collected and a physical examination and anterior chest radiograph were performed. Blood was drawn at enrollment for enzyme-linked immunosorbent spot (ELISPOT) analysis and HIV testing, before or within 72 hours of tuberculin skin test (TST) placement. Nutritional status was determined by comparing individuals’ body mass index (BMI) with World Health Organization child growth standards to generate a Z-score. Hospitalized children with suspected TB had a mycobacterial smear and culture of one induced sputum sample performed.
For the HE cohort, children were followed for 6–24 months and those with symptoms concerning for TB evaluated. Children with a positive TST, and children less than 5 years, were offered 9 months of isoniazid prophylaxis after TB was excluded. Children with a history of prior or current TB, children who developed TB within 6 months of enrollment, and children who were HIV-infected or immunosuppressed were excluded.
Children with suspected TB received a standard four-drug regimen per Ugandan national treatment guidelines. Children received a final designation of confirmed TB, probable TB, or not TB based on results of their diagnostic work-up and response to treatment at 2 months. Children classified as not TB were excluded.
IFN-γ ELISPOT Assay
Overnight IFN-γ ELISPOT assays were performed as described previously (18) using a single synthetic peptide pool consisting of 15-mers overlapping by 11 aa, representing Mtb-specific proteins CFP-10 and ESAT-6. For the CD8 ELISPOT assay, CD8+ T cells were negatively selected from PBMCs using a combination of CD4 and CD56 magnetic beads (Miltenyi Biotec, Auburn, CA). For the PBMC ELISPOT, unfractionated PBMCs were used as the source of responding T cells.
Study Design and Statistical Analysis
We performed a cross-sectional study examining Mtb-specific T-cell responses elicited from PBMC (PBMC ELISPOT]) and isolated CD8+ T cells (CD8 ELISPOT) at baseline from two clinical study groups: children with CP-TB and HE children. All analysis was performed using SAS version 9.1 (SAS Institute Inc., Cary, NC). All categorical comparisons between study groups were performed using Student t test for continuous variables, and chi-square test (or Fisher exact test where indicated) for categorical variables. The magnitude of spot-forming units (SFU) above background between groups was compared using a nonparametric analysis (Wilcoxon rank-sum test). A positive ELISPOT assay was defined by an antigen-specific response at least two SD above the background control plus 5 SFU. If these criteria were met, the background was subtracted out to determine the antigen-specific response.
To study the factors associated with CP-TB, logistic regression models were constructed to examine the independent influence of the CD8+ T-cell response (CD8 ELISPOT, categorical), the PBMC response (PBMC ELISPOT, categorical), age group (<5 and >5), nutritional status (BMI Z score, continuous), and TST (categorical) on the odds of having TB compared with the reference group (HE). Backward logistic regression was performed with significance level of 0.05. The online supplement provides additional details.
Ethics Approval
This study received Institutional Review Board approval at all sponsoring institutions. Written, informed consent was obtained from participants’ parents or guardians before enrollment.
Role of the Funding Source
The funders of the study had no role in study design; in the collection, analysis, and interpretation of data; in writing of the report; or in the decision to submit the paper for publication.
Results
Patient Enrollment and Clinical Characteristics
To study the effect of age on PBMC and CD8+ T-cell responses within the HE group, we evaluated 105 child household contacts age less than 15 years (Figure 1A). Exclusions included 19 children who developed TB within 6 months of enrollment and four children infected with HIV. Thus, we performed ELISPOT assays on 82 HE children age less than 15 years; 76 PBMC ELISPOT assays and 59 CD8 ELISPOT assays were included in the final analysis. To compare PBMC and CD8+ T-cell responses between the HE and CP-TB group (age <10 yr), we included ELISPOT assay data from 62 eligible HE children who were age less than 10 years (Figure 1B).
Regarding the CP-TB group, 101 children uninfected with HIV with suspected pulmonary TB (see Table E1) were assessed for eligibility. Of these, 96 children were enrolled into the CP-TB group with 28 (29%) classified as confirmed TB (C-TB) and 68 (71%) as probable TB (Prob-TB) at 2 months (see Table E1; 5 of 101 children with suspected TB were classified as not TB). There were 82 interpretable PBMC ELISPOT assays and 87 interpretable CD8 ELISPOT assays included in the final analysis (Figure 1C). There was no significant difference between the number of interpretable ELISPOTs between children with C-TB and Prob-TB.
Characteristics of children age less than 10 years enrolled in the HE and CP-TB groups, after exclusions for HIV infection (both groups) and TB (HE children only), are compared in Table 1. Children with CP-TB were more malnourished (P = 0.006) than the HE group and were slightly younger (P = 0.01). The frequency of a positive TST was equivalent between groups, as was the presence of bacillus Calmette-Guérin scar (data not shown). Baseline clinical characteristics (age, sex, BMI Z-score, and TST status) of children included in the final analysis did not differ from those of children excluded because of failed ELISPOT assays (data not shown).
TABLE 1.
BASELINE CHARACTERISTICS OF HEALTHY EXPOSED CHILDREN COMPARED WITH CHILDREN WITH CONFIRMED AND PROBABLE TB, AGE <10 YEARS
| Category | Healthy Exposed (n = 62) | CP-TB (n = 96) | P Value |
| Female, n (%) | 29 (47) | 51 (53) | 0.4* |
| Mean age, yr | 4.7 | 3.6 | 0.01† |
| Age IQR | 2.5 to 6 | 1 to 6 | |
| Mean BMI Z score | 0.09 | −0.8 | 0.006† |
| BMI Z score IQR | −0.6 to 0.6 | −2.1 to 0.6 | |
| TST+, n (%) | 28 (45) | 40 (42) | 0.6* |
Definition of abbreviations: BMI = body mass index; CP = confirmed and probable; IQR = inter-quartile range; TB = tuberculosis; TST = tuberculin skin test.
Chi-square test.
Student t test Satterthwaite/unequal variances.
Mtb-specific PBMC and CD8+ T-cell Responses in Healthy Children Exposed to Mtb
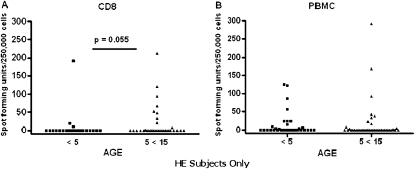
We sought to determine if Mtb-specific PBMC and CD8+ T-cell responses differed between healthy individuals who had experienced their primary exposure to Mtb, and healthy individuals who had experienced multiple, lifetime exposures to Mtb and would be at increased risk for persistent infection. Therefore, we analyzed the magnitude of PBMC and CD8+ T-cell responses in the HE cohort by age group, because younger (<5 yr) children were less likely to have been exposed to Mtb previously compared with their older (5–15 yr), school-aged counterparts (4). We observed robust PBMC (PBMC ELISPOT) responses in both age groups, whereas CD8+ T-cell (CD8 ELISPOT) responses were decreased in children age less than 5 years compared with older children (P = 0.055) (Figure 2).
Figure 2.
Comparison of Mycobacterium tuberculosis–specific T-cell responses in healthy exposed (HE) children stratified by age. (A) CD8 enzyme-linked immunosorbent spot (ELISPOT) assay results for HE children age less than 5 years (n = 25) compared with those for HE children age 5–15 years (n = 34) are shown. (B) Peripheral blood mononuclear cell (PBMC) ELISPOT assay results for HE children less than 5 years (n = 35) compared with those for HE children age 5–15 years (n = 41) are shown. Spot-forming units per 250,000 cells above background. Wilcoxon rank-sum test (one-sided) was performed comparing HE children less than 5 years with those age 5–15 years for the CD8 ELISPOT assay (P = 0.055) and for the PBMC ELISPOT assay (P = 0.2).
Comparison of Mtb-specific PBMC and CD8+ T-cell Responses Between Healthy Children Exposed to Mtb and Children with TB
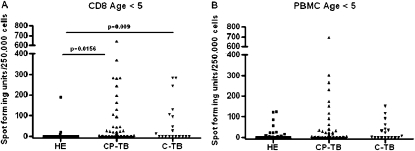
To determine if Mtb-specific CD8+ T-cell responses are associated with a high bacillary load, we compared Mtb-specific PBMC and CD8+ T-cell responses between HE children and children with TB age less than 10 years. We determined if the magnitude of Mtb-specific PBMC and CD8+ T-cell responses differed among HE, C-TB, and CP-TB cohorts (Figure 3). For children age less than 5 years, the magnitude of the CD8+ T-cell response (CD8 ELISPOT) was greater in children with TB (CP-TB, P = 0.0156; C-TB, P = 0.009) than HE children, whereas PBMC (PBMC ELISPOT) responses were equivalent between groups (Figures 3A and 3B). For children age 5–10 years, the magnitude of CD8+ T-cell (CD8 ELISPOT) and PBMC (PBMC ELISPOT) responses were equivalent between the HE and CP-TB cohorts; however, because of the small number in the C-TB cohort age 5–10 yr statistical comparison was not performed (see Figure E1). These data demonstrate that among children age less than 5 years, Mtb-specific CD8+ T cells productive of IFN-γ are generated in response to TB but not Mtb exposure. This is in contrast to the Mtb-specific T-cell response elicited from PBMC that seems to be generated equally by young children with TB and those exposed to Mtb.
Figure 3.
Magnitude of Mycobacterium tuberculosis (Mtb)–specific T-cell responses in young children with tuberculosis (TB) or health exposed (HE) children. The magnitude of M. tuberculosis–specific T-cell responses is shown. Results of CD8 enzyme-linked immunosorbent spot (ELISPOT) (A) and peripheral blood mononuclear cell (PBMC) ELISPOT (B) assays among children age less than 5 years are shown. The sample size of the subgroups is (A) HE (n = 25), CP-TB (n = 65), C-TB (n = 19); (B) HE (n = 35), CP-TB (n = 62), C-TB (n = 18). Spot-forming units per 250,000 cells above background. Two-sided Wilcoxon rank-sum test was performed comparing the HE with CP-TB, and the HE with C-TB groups. C-TB = confirmed tuberculosis; CP-TB = confirmed and probable tuberculosis.
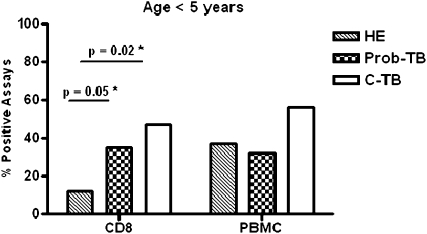
We next examined the proportion of positive CD8 and PBMC ELISPOT assays between young children with TB and HE children (Figure 4). The proportion of positive CD8 ELISPOT assays was significantly greater in children with C-TB (47%; n = 9/19; confidence interval [CI], 0.24–0.71; P = 0.02) and children with Prob-TB (35%; n = 16/46; CI, 0.21–0.50; P = 0.05) compared with HE children (12%; n = 3/25; CI, 0.03–0.31). The proportion of positive CD8 ELISPOTs was also significantly greater in young children with CP-TB compared with young HE children (data not shown). However, when we compared children age less than 5 years between cohorts, the proportion of positive PBMC ELISPOT assays was equivalent between HE children (37%; n = 13/35; CI, 0.21–0.55) and children with C-TB (56%; n = 10/18; CI, 0.31–0.78; P = 0.2) (Figure 4), and between HE children and children with Prob-TB (32%; n = 14/44; CI, 0.18–0.47; P = 0.6). The proportion of positive PBMC ELISPOT assays was also similar between children with CP-TB and the HE children age less than 5 years (data not shown). Therefore, our analysis suggests that in young children less than 5 years, who have likely experienced their primary exposure to Mtb, a detectable CD8+ T-cell response to Mtb distinguishes TB from a history of Mtb exposure. The small number of children enrolled in the 5- to 10-year cohort precluded formal statistical analysis.
Figure 4.
Proportion of positive Mycobacterium tuberculosis–specific T-cell responses in young children with tuberculosis (TB) versus healthy exposed (HE) children. The proportion of positive CD8 and peripheral blood mononuclear cell (PBMC) enzyme-linked immunosorbent spot (ELISPOT) assay results for HE children and children in the probable TB (Prob-TB) and confirmed TB (C-TB) groups age less than 5 years are shown. *The differences in proportions were tested using Fisher exact test.
Statistical Associations with PBMC and CD8+ T-cell Responses to Mtb Antigens
Logistic regression analysis was performed to identify variables associated with CP-TB and included CD8 and PBMC ELISPOT assay results as a binary variable (positive or negative); age group (age <5 yr as reference); nutritional status (BMI Z score); and TST results (positive or negative) (see Table E2). Children with a positive CD8 ELISPOT assay result had a 3.7 times greater odds of having CP-TB compared with that of children who are healthy and exposed (P = 0.005) adjusted for age, nutritional status, and TST result (Hosmer-Lemeshow goodness-of-fit test, P = 0.2). By comparison, children with a positive PBMC ELISPOT assay did not have increased odds of having CP-TB compared with HE children (Hosmer-Lemeshow goodness-of-fit test, P = 0.15). In a model including both CD8 and PBMC ELISPOT assay results as covariates, a positive CD8 ELISPOT assay was significantly associated with CP-TB, adjusted for PBMC ELISPOT assay result, age, nutritional status, and TST result (odds ratio, 5.3; 95% CI, 1.7–15.8; P = 0.003; Hosmer-Lemeshow goodness-of-fit test, P = 0.13). To increase model fit, backward logistic regression was used. Here, the odds of having TB (CP-TB cohort) in someone with a positive CD8 ELISPOT assay was 3.9 times (CI, 1.5–9.8) that of someone in the HE group adjusted for age group only (P = 0.003; data not shown). The PBMC ELISPOT assay result did not add to the overall model fit and was eliminated in the backward iterative selection process along with nutritional status and TST result (Hosmer-Lemeshow goodness-of-fit test, P = 0.9; data not shown).
Discussion
The evaluation of Mtb-specific immune responses in young children offers a unique window of opportunity to characterize the immunologic signatures of Mtb exposure, primary infection, and disease. Young children who are recently infected often progress to TB disease, providing a more accurate assignment of the timing of their primary infection (3, 5). In addition, young children by virtue of their age are less likely to have had repeated exposures and reinfection that confounds similar studies of adults performed in TB-endemic settings (4).
Previous work has shown that CD8+ T cells preferentially recognize dendritic cells harboring intracellular bacteria, especially cells heavily infected with Mtb (9). Although the in vivo relevance of such observations remains difficult to assess, the magnitude of the CD8+ T-cell response has been shown to correlate with mycobacterial burden in TB animal models (19, 20). Moreover, these findings mirror work shown in HIV where there is a robust association between CD8+ T-cell responses and viral set point (21), viral load dynamics (22), and viral control (23). Therefore, we postulated that an Mtb-specific CD8+ T-cell response indicates true intracellular infection and that the magnitude of this response reflects bacillary burden. This is biologically plausible because CD8+ T cells sample the intracellular environment by recognizing antigens presented by major histocompatibility complex class I; thus presence of such a response is both necessary and sufficient to discern an intracellular Mtb infection.
Our data show that Mtb-specific CD8+ T-cell responses immunologically distinguish young children with TB from HE children. We postulate that HE children who demonstrate detectable T-cell responses in PBMC, but absent CD8+ T-cell responses, may not have persistent intracellular infection. Rather, we suggest this PBMC response is dominated by CD4+ T cells and reflects prior exposure or priming to Mtb antigens. Children with TB, however, who are expected to have a high intracellular bacillary burden, demonstrate robust CD8+ and PBMC responses. Therefore, detection of an Mtb-specific CD8+ T-cell response is not reflective of protective immunity, but rather increased mycobacterial load.
Although controversial, the possibility that Mtb exposure does not result in persistent infection is supported by animal models. Transient TST conversions have been observed in healthy guinea pigs with no evidence of underlying Mtb infection that had been exposed to patients with TB (24). More recent work has shown that roughly 30% of guinea pigs exposed to subjects with drug-resistant TB have transient TST conversions but no pathologic or microbiologic evidence of Mtb infection (25). In addition, high rates of TST reversions have been noted in employees with documented Mtb-exposure who undergo repeat TST testing (26). We propose that after Mtb exposure three distinct outcomes are possible: (1) an individual remains uninfected, (2) Mtb infection takes place but is resolved through a sterilizing immune response, or (3) a persistent Mtb infection is established. Therefore, HE children who demonstrated a positive TST or detectable Mtb-specific PBMC response but a negative CD8+ T-cell response may have experienced previous antigen exposure to Mtb, but no longer harbor viable bacilli (the second outcome). Although an alternative interpretation of our data is that CD8+ T cells are insensitive in detecting the low bacillary load associated with clinically asymptomatic Mtb infection, we propose that Mtb-specific CD8+ T-cell responses do not develop unless a persistent intracellular infection has occurred (the third outcome) as is expected during TB disease or after multiple exposures to Mtb. We speculate that HE children who demonstrated CD8+ T-cell responses (see Figure E1), most of whom were school aged, had experienced multiple lifetime exposures to Mtb resulting in persistent infection.
It is unclear why young children are highly susceptible to develop TB. Evidence suggesting that a reduced Th1 cell response predisposes young children to fail to contain Mtb is controversial (27–30), and there are little data regarding the role of CD8+ T cells in the immune response to Mtb in young children. In adults, CD8+ T cells are observed in high frequency in individuals with latent TB infection and TB disease (12, 31–33) and CD8+ T cells are present at extrapulmonary sites of infection (13). Although bacillus Calmette-Guérin vaccination induces IFN-γ–producing CD8+ T cells in infants (34) and clonal expansion of Mtb-reactive CD8+ T cells has been demonstrated in children with TB (35), this is the first study comparing antigen-specific CD8+ T-cell responses between HE children and children with TB. Our finding that young children with TB disease have Mtb-specific IFN-γ–producing T cells suggests that failure to generate a Th1-type response is not responsible for the increased susceptibility of young children to TB.
In young children, infection with Mtb is associated with severe manifestations of TB, highlighting the importance of accurate and timely diagnosis. However, current diagnostic methods have limited use, because only 5–16% of children with suspected TB have positive acid fast bacillis smears and positive cultures are reported in only 20–50% of suspected cases (36–44). Although commercial IFN-γ release assays are more specific than the TST in adults (45) and children (46), IFN-γ release assays have limited use in TB-endemic settings because they do not reliably differentiate TB from latent TB (47–49). In this regard, we also found that measurement of ESAT-6/CFP-10–specific PBMC responses did not distinguish healthy children exposed to Mtb from children with TB. In contrast, we found that detection of an ESAT-6/CFP-10–specific CD8+ T-cell response was specifically associated with TB rather than Mtb exposure. Our results suggest that measurement of CD8+ T-cell responses could be developed as a more accurate diagnostic tool for childhood TB.
Our study has several limitations. First, the cross-sectional design of our study does not permit any insight into the kinetics of T-cell responses in children with Mtb exposure or after treatment for TB. Second, because our only measure of T-cell responses to Mtb was IFN-γ production, we may have failed to detect Mtb-specific T-cell responses characterized by the production of alternative cytokines or cytolytic markers. Third, the small number of children greater than 5 years in our CP-TB cohort limited statistical analysis of the results stratified by age within this cohort. Fourth, although unlikely because all children were recruited from the same geographic area, it is possible that our different recruitment strategies for children in the HE and suspected TB groups introduced biases because of differences in socioeconomic factors or duration of Mtb exposure. Lastly, this current study was not designed to evaluate the diagnostic accuracy of Mtb-specific PBMC or CD8+ T-cell responses for childhood TB. Therefore the potential use of measurement of CD8+ T-cell responses to diagnose childhood TB requires further study.
This is the first study to demonstrate that measurement of a CD8+ T-cell response to ESAT-6 and CFP-10 by ELISPOT assay distinguishes acutely ill, young children with TB from healthy, exposed household contacts in a TB-endemic region. We believe our results are supportive of the hypothesis that Mtb-specific CD8+ T cells develop in response to an established intracellular infection, and that the magnitude of the CD8+ T-cell response is reflective of bacillary burden. This is distinctive from Mtb-specific responses found in PBMC that are generated in response to current or prior antigen exposure and therefore cannot discern exposure from true infection. Finally, these findings may have practical applications for development of a CD8+ T-cell immunodiagnostic for TB in young children, as well as immune-based assays with the capacity to identify latently infected individuals at high risk for reactivation disease and assays that measure response to TB treatment.
Supplementary Material
Acknowledgments
The authors acknowledge the invaluable contribution made by the study medical officers, health visitors, and laboratory and data personnel: Dr. Lorna Nshuti, Dr. Roy Mugerwa, Dr. Christina Hirsch, Allan Chiunda, Brenda Okware, Mark Breda, Dennis Dobbs, Hussein Kisingo, Albert Muganda, Richard Bamuhimbisa, Yusuf Mulumba, Deborah Nsamba, Barbara Kyeyune, Faith Kintu, Mary Nsereko, Gladys Mpalanyi, Janet Mukose, Grace Tumusiime, Pierre Peters, Alphonse Okwera, Keith Chervenak, Karen Morgan, Moses Joloba, Alfred Etwom, Micheal Angel Mugerwa, and Lisa Kucharski. They also acknowledge and thank Dr. Francis Adatu Engwau, Head of the Uganda National Tuberculosis and Leprosy Program, for his support of this project. They acknowledge the medical officers, nurses, and counselors at the National Tuberculosis Treatment Centre, Mulago Hospital, the Ugandan National Tuberculosis and Leprosy Program, and the Uganda Tuberculosis Investigation Bacteriological Unit, Wandegeya, for their contributions to this study. This study would not be possible without the generous participation of the Ugandan patients and families. Finally, they acknowledge Dr. Shannon McWeeney for advice on statistical methods.
Footnotes
Supported in part by the Tuberculosis Research Unit (TBRU), established with Federal funds from US NIAID, under contract HHSN266200700022C/N01-AI-70022. This work was also supported by the following agencies and institutes: NIH grants AI079847, HHSN266200400081C/NO1-A1–400081, AI48090, and AI054474; The National Center For Research Resources KL2RR024141; VA Merit Review Grant and the Portland VA Medical Center; ALA Career Investigator Award; and the Rainbow Babies and Children's Hospital Fellowship Research Award. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author Contributions: C.L., data analysis, article drafting and revisions, and final manuscript approval; M.N., data analysis, article drafting and revisions, and final manuscript approval; S.K., conception and design, acquisition of data, article drafting and revisions, and final manuscript approval; S.Z., acquisition of data, article drafting and revisions, and final manuscript approval; T.M., data analysis, article drafting and revisions, and final manuscript approval; H.M.-K., conception and design, article drafting and revisions, and final manuscript approval; S.B., acquisition of data, article drafting and revisions, and final manuscript approval; M.N., acquisition of data, article drafting and revisions, and final manuscript approval; J.B., acquisition of data, article drafting and revisions, and final manuscript approval; D.M., data analysis, article drafting and revisions, and final manuscript approval; L.B., acquisition of data, article drafting and revisions, and final manuscript approval; G.S., acquisition of data, article drafting and revisions, and final manuscript approval; C.S., data analysis, article drafting and revisions, and final manuscript approval; D.F.J., data analysis, article drafting and revisions, and final manuscript approval; L.M., data analysis, article drafting and revisions, and final manuscript approval; P.M.-M., data analysis, article drafting and revisions, and final manuscript approval; W.H.B., conception and design, article drafting and revisions, and final manuscript approval; D.M.L., conception and design, article drafting and revisions, and final manuscript approval; and D.A.L., conception and design, article drafting and revisions, and final manuscript approval.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201107-1355OC on October 27, 2011
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.World Health Organization Global tuberculosis control: surveillance, planning, financing: WHO report 2008. WHO/HTM/TB/2008393 Geneva, Switzerland: World Health Organization; 2008 [Google Scholar]
- 2.Colditz GA, Berkey CS, Mosteller F, Brewer TF, Wilson ME, Burdick E, Fineberg HV. The efficacy of bacillus Calmette-Guerin vaccination of newborns and infants in the prevention of tuberculosis: meta-analyses of the published literature. Pediatrics 1995;96:29–35 [PubMed] [Google Scholar]
- 3.Marais BJ, Gie RP, Schaaf HS, Hesseling AC, Obihara CC, Starke JJ, Enarson DA, Donald PR, Beyers N. The natural history of childhood intra-thoracic tuberculosis: a critical review of literature from the pre-chemotherapy era. Int J Tuberc Lung Dis 2004;8:392–402 [PubMed] [Google Scholar]
- 4.Marais BJ, Gie RP, Schaaf HS, Hesseling AC, Obihara CC, Nelson LJ, Enarson DA, Donald PR, Beyers N. The clinical epidemiology of childhood pulmonary tuberculosis: a critical review of literature from the pre-chemotherapy era. Int J Tuberc Lung Dis 2004;8:278–285 [PubMed] [Google Scholar]
- 5.Marais BJ, Gie RP, Schaaf HS, Hesseling AC, Enarson DA, Beyers N. The spectrum of disease in children treated for tuberculosis in a highly endemic area. Int J Tuberc Lung Dis 2006;10:732–738 [PubMed] [Google Scholar]
- 6.Newport MJ, Huxley CM, Huston S, Hawrylowicz CM, Oostra BA, Williamson R, Levin M. A mutation in the interferon-gamma-receptor gene and susceptibility to mycobacterial infection. N Engl J Med 1996;335:1941–1949 [DOI] [PubMed] [Google Scholar]
- 7.Flynn JLGM, Triebold KJ, Koller B, Bloom BR. Major histocompatibility complex class I-restricted T cells are required for resistance to Mycobacterium tuberculosis infection. Proc Natl Acad Sci USA 1992;89:12013–12017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan JS, Canaday DH, Boom WH, Balaji KN, Schwander SK, Rich EA. Human alveolar T lymphocyte responses to Mycobacterium tuberculosis antigens: role for CD4+ and CD8+ cytotoxic T cells and relative resistance of alveolar macrophages to lysis. J Immunol 1997;159:290–297 [PubMed] [Google Scholar]
- 9.Lewinsohn DA, Heinzel AS, Gardner JM, Zhu L, Alderson MR, Lewinsohn DM. Mycobacterium tuberculosis-specific CD8+ T cells preferentially recognize heavily infected cells. Am J Respir Crit Care Med 2003;168:1346–1352 [DOI] [PubMed] [Google Scholar]
- 10.Lewinsohn DM, Alderson MR, Briden AL, Riddell SR, Reed SG, Grabstein KH. Characterization of human CD8+ T cells reactive with Mycobacterium tuberculosis-infected antigen-presenting cells. J Exp Med 1998;187:1633–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cho S, Mehra V, Thoma-Uszynski S, Stenger S, Serbina N, Mazzaccaro RJ, Flynn JL, Barnes PF, Southwood S, Celis E, et al. Antimicrobial activity of MHC class I-restricted CD8+ T cells in human tuberculosis. Proc Natl Acad Sci USA 2000;97:12210–12215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lalvani A, Brookes R, Wilkinson RJ, Malin AS, Pathan AA, Andersen P, Dockrell H, Pasvol G, Hill AV. Human cytolytic and interferon gamma-secreting CD8+ T lymphocytes specific for Mycobacterium tuberculosis. Proc Natl Acad Sci USA 1998;95:270–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caccamo N, Meraviglia S, La Mendola C, Guggino G, Dieli F, Salerno A. Phenotypical and functional analysis of memory and effector human CD8+ T cells specific for mycobacterial antigens. J Immunol 2006;177:1780–1785 [DOI] [PubMed] [Google Scholar]
- 14.Lancioni C. CD8+ T cell responses accurately identify young children with tuberculosis. Presented at the annual meeting of the Pediatric Academic Societies; April 30, 2011, Denver, CO [Google Scholar]
- 15.Lewinsohn DA. Improved diagnostic accuracy for active tuberculosis in Ugandan children using a CD8 ELISPOT assay. Presented in abstract form at the American Thoracic Society annual meeting; May 17–19, 2009, San Diego, CA [Google Scholar]
- 16.Guwatudde D, Nakakeeto M, Jones-Lopez EC, Maganda A, Chiunda A, Mugerwa RD, Ellner JJ, Bukenya G, Whalen CC. Tuberculosis in household contacts of infectious cases in Kampala, Uganda. Am J Epidemiol 2003;158:887–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization Provisional guidelines for the diagnosis and classification of the EPI target diseases for primary health care, surveillance, and special studies. EPI/GEN/83/4 Geneva, Switzerland: World Health Organization; 1983 [Google Scholar]
- 18.Heinzel AS, Grotzke JE, Lines RA, Lewinsohn DA, McNabb AL, Streblow DN, Braud VM, Grieser HJ, Belisle JT, Lewinsohn DM. HLA-e-dependent presentation of Mtb-derived antigen to human CD8+ T cells. J Exp Med 2002;196:1473–1481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Billeskov R, Vingsbo-Lundberg C, Andersen P, Dietrich J. Induction of CD8+ T cells against a novel epitope in tb10.4: correlation with mycobacterial virulence and the presence of a functional region of difference-1. J Immunol 2007;179:3973–3981 [DOI] [PubMed] [Google Scholar]
- 20.Lewinsohn DM, Tydeman IS, Frieder M, Grotzke JE, Lines RA, Ahmed S, Prongay KD, Primack SL, Colgin LM, Lewis AD, et al. High resolution radiographic and fine immunologic definition of TB disease progression in the rhesus macaque. Microbes Infect 2006;8:2587–2598 [DOI] [PubMed] [Google Scholar]
- 21.Burgers WA, Riou C, Mlotshwa M, Maenetje P, de Assis Rosa D, Brenchley J, Mlisana K, Douek DC, Koup R, Roederer M, et al. Association of HIV-specific and total CD8+ T memory phenotypes in subtype c HIV-1 infection with viral set point. J Immunol 2009;182:4751–4761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turnbull EL, Wong M, Wang S, Wei X, Jones NA, Conrod KE, Aldam D, Turner J, Pellegrino P, Keele BF, et al. Kinetics of expansion of epitope-specific T cell responses during primary HIV-1 infection. J Immunol 2009;182:7131–7145 [DOI] [PubMed] [Google Scholar]
- 23.Saez-Cirion A. The characteristic CD8+ T cell response in HIV controllers: an objective to achieve? Pathol Biol (Paris) 2008;56:251–253 [DOI] [PubMed] [Google Scholar]
- 24.Riley RL, Wells WF, Mills CC, Nyka W, McLean RL. Air hygiene in tuberculosis: quantitative studies of infectivity and control in a pilot ward. Am Rev Tuberc 1957;75:420–431 [DOI] [PubMed] [Google Scholar]
- 25.Dharmadhikari AS, Basaraba RJ, Van Der Walt ML, Weyer K, Mphahlele M, Venter K, Jensen PA, First MW, Parsons S, McMurray DN, et al. Natural infection of guinea pigs exposed to patients with highly drug-resistant tuberculosis. Tuberculosis (Edinb) 2011;91:329–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Friedman LN, Nash ER, Bryant J, Henry S, Shi J, D'Amato J, Khaled GH, Russi MB, O'Connor PG, Edberg SC, et al. High rate of negative results of tuberculin and quantiferon tests among individuals with a history of positive skin test results. Infect Control Hosp Epidemiol 2006;27:436–441 [DOI] [PubMed] [Google Scholar]
- 27.Kori M, Barak V, Leibovitz E, Altman Y, Eliraz A, Handzel ZT. Specific in vitro proliferative immune responses and lymphokine production in Ethiopian children with and without tuberculosis. Infection 2000;28:42–45 [DOI] [PubMed] [Google Scholar]
- 28.Soares AP, Scriba TJ, Joseph S, Harbacheuski R, Murray RA, Gelderbloem SJ, Hawkridge A, Hussey GD, Maecker H, Kaplan G, et al. Bacillus Calmette-Guerin vaccination of human newborns induces T cells with complex cytokine and phenotypic profiles. J Immunol 2008;180:3569–3577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watkins ML, Semple PL, Abel B, Hanekom WA, Kaplan G, Ress SR. Exposure of cord blood to mycobacterium bovis BCG induces an innate response but not a T-cell cytokine response. Clin Vaccine Immunol 2008;15:1666–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lewinsohn DA, Zalwango S, Stein CM, Mayanja-Kizza H, Okwera A, Boom WH, Mugerwa RD, Whalen CC. Whole blood interferon-gamma responses to Mycobacterium tuberculosis antigens in young household contacts of persons with tuberculosis in Uganda. PLoS ONE 2008;3:e3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pathan AA, Wilkinson KA, Wilkinson RJ, Latif M, McShane H, Pasvol G, Hill AV, Lalvani A. High frequencies of circulating IFN-gamma-secreting CD8 cytotoxic T cells specific for a novel MHC class I-restricted Mycobacterium tuberculosis epitope in M. tuberculosis-infected subjects without disease. Eur J Immunol 2000;30:2713–2721 [DOI] [PubMed] [Google Scholar]
- 32.Klein MR, Smith SM, Hammond AS, Ogg GS, King AS, Vekemans J, Jaye A, Lukey PT, McAdam KP. HLA-b*35-restricted CD8+ T cell epitopes in the Antigen 85 complex of Mycobacterium tuberculosis. J Infect Dis 2001;183:928–934 [DOI] [PubMed] [Google Scholar]
- 33.Lewinsohn DA, Winata E, Swarbrick GM, Tanner KE, Cook MS, Null MD, Cansler ME, Sette A, Sidney J, Lewinsohn DM. Immunodominant tuberculosis CD8 antigens preferentially restricted by HLA-b. PLoS Pathog 2007;3:1240–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hanekom WA. The immune response to BCG vaccination of newborns. Ann N Y Acad Sci 2005;1062:69–78 [DOI] [PubMed] [Google Scholar]
- 35.Jacobsen M, Detjen AK, Mueller H, Gutschmidt A, Leitner S, Wahn U, Magdorf K, Kaufmann SH. Clonal expansion of CD8+ effector T cells in childhood tuberculosis. J Immunol 2007;179:1331–1339 [DOI] [PubMed] [Google Scholar]
- 36.Iriso R, Mudido PM, Karamagi C, Whalen C. The diagnosis of childhood tuberculosis in an HIV-endemic setting and the use of induced sputum. Int J Tuberc Lung Dis 2005;9:716–726 [PubMed] [Google Scholar]
- 37.Marais BJ, Gie RP, Hesseling AC, Schaaf HS, Lombard C, Enarson DA, Beyers N. A refined symptom-based approach to diagnose pulmonary tuberculosis in children. Pediatrics 2006;118:e1350–e1359 [DOI] [PubMed] [Google Scholar]
- 38.Marais BJ, Gie RP, Schaaf HS, Beyers N, Donald PR, Starke JR. Childhood pulmonary tuberculosis: old wisdom and new challenges. Am J Respir Crit Care Med 2006;173:1078–1090 [DOI] [PubMed] [Google Scholar]
- 39.Marais BJ, Hesseling AC, Gie RP, Schaaf HS, Enarson DA, Beyers N. The bacteriologic yield in children with intrathoracic tuberculosis. Clin Infect Dis 2006;42:e69–e71 [DOI] [PubMed] [Google Scholar]
- 40.Merino JM, Alvarez T, Marrero M, Anso S, Elvira A, Iglesias G, Gonzalez JB. Microbiology of pediatric primary pulmonary tuberculosis. Chest 2001;119:1434–1438 [DOI] [PubMed] [Google Scholar]
- 41.Nelson LJ, Wells CD. Tuberculosis in children: considerations for children from developing countries. Semin Pediatr Infect Dis 2004;15:150–154 [DOI] [PubMed] [Google Scholar]
- 42.Owens S, Abdel-Rahman IE, Balyejusa S, Musoke P, Cooke RP, Parry CM, Coulter JB. Nasopharyngeal aspiration for diagnosis of pulmonary tuberculosis. Arch Dis Child 2007;92:693–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salazar GE, Schmitz TL, Cama R, Sheen P, Franchi LM, Centeno G, Valera C, Leyva M, Montenegro-James S, Oberhelman R, et al. Pulmonary tuberculosis in children in a developing country. Pediatrics 2001;108:448–453 [DOI] [PubMed] [Google Scholar]
- 44.Zar HJ, Hanslo D, Apolles P, Swingler G, Hussey G. Induced sputum versus gastric lavage for microbiological confirmation of pulmonary tuberculosis in infants and young children: a prospective study. Lancet 2005;365:130–134 [DOI] [PubMed] [Google Scholar]
- 45.Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med 2008;149:177–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Detjen AK, Keil T, Roll S, Hauer B, Mauch H, Wahn U, Magdorf K. Interferon-gamma release assays improve the diagnosis of tuberculosis and nontuberculous mycobacterial disease in children in a country with a low incidence of tuberculosis. Clin Infect Dis 2007;45:322–328 [DOI] [PubMed] [Google Scholar]
- 47.Eum SY, Lee YJ, Kwak HK, Min JH, Hwang SH, Via LE, Barry CE, III, Cho SN. Evaluation of the diagnostic utility of a whole-blood interferon-gamma assay for determining the risk of exposure to Mycobacterium tuberculosis in bacille Calmette-Guerin (BCG)-vaccinated individuals. Diagn Microbiol Infect Dis 2008;61:181–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kampmann B, Whittaker E, Williams A, Walters S, Gordon A, Martinez-Alier N, Williams B, Crook AM, Hutton AM, Anderson ST. Interferon-gamma release assays do not identify more children with active tuberculosis than the tuberculin skin test. Eur Respir J 2009;33:1374–1382 [DOI] [PubMed] [Google Scholar]
- 49.Liebeschuetz S, Bamber S, Ewer K, Deeks J, Pathan AA, Lalvani A. Diagnosis of tuberculosis in South African children with a T-cell-based assay: a prospective cohort study. Lancet 2004;364:2196–2203 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.