Abstract
Rationale: Diesel exhaust enhances allergic inflammation, and pollutants are associated with heightened susceptibility to viral respiratory infections. The effects of combined diesel and virus exposure in humans are unknown.
Objectives: Test whether acute exposure to diesel modifies inflammatory responses to influenza virus in normal humans and those with allergies.
Methods: We conducted a double-blind, randomized, placebo-controlled study of nasal responses to live attenuated influenza virus in normal volunteers and those with allergic rhinitis exposed to diesel (100 μg/m3) or clean air for 2 hours, followed by standard dose of virus and serial nasal lavages. Endpoints were inflammatory mediators (ELISA) and virus quantity (quantitative reverse-transcriptase polymerase chain reaction). To test for exposure effect, we used multiple regression with exposure group (diesel vs. air) as the main explanatory variable and allergic status as an additional factor.
Measurements and Main Results: Baseline levels of mediators did not differ among groups. For most postvirus nasal cytokine responses, there was no significant diesel effect, and no significant interaction with allergy. However, diesel was associated with significantly increased IFN-γ responses (P = 0.02), with no interaction with allergy in the regression model. Eotaxin-1 (P = 0.01), eosinophil cationic protein (P < 0.01), and influenza RNA sequences in nasal cells (P = 0.03) were significantly increased with diesel exposure, linked to allergy.
Conclusions: Short-term exposure to diesel exhaust leads to increased eosinophil activation and increased virus quantity after virus inoculation in those with allergic rhinitis. This is consistent with previous literature suggesting a diesel “adjuvant” effect promoting allergic inflammation, and our data further suggest this change may be associated with reduced virus clearance.
Clinical trial registered with www.clinicaltrials.gov (NCT00617110).
Keywords: diesel, influenza, eosinophil cationic protein, eotaxin-1, interferon-γ
At a Glance Commentary
Scientific Knowledge on the Subject
Diesel exhaust enhances allergic inflammation, and pollutants are associated with heightened susceptibility to viral respiratory infections. The effects of combined diesel and virus exposure in humans are unknown.
What This Study Adds to the Field
This study suggests that prior exposure to diesel exhaust exacerbates influenza virus–induced nasal inflammation, in particular by promoting eosinophil activation in allergic rhinitis.
Viral respiratory infections account for a large share of human morbidity and mortality worldwide, particularly during pandemic outbreaks of influenza virus (1). It is well established from epidemiologic data that exposure to air pollutants is associated with increased risk for severe influenza and other respiratory viral infections (2). Although controlled human experiments to define the mechanisms for this are difficult to design, we recently reported reduced virus clearance and suppression of specific innate immune factors in nasal secretions of smokers compared with nonsmokers, after standard inoculation with live attenuated influenza vaccine (LAIV) (3). Influenza virus is also epidemiologically important in asthma (4, 5), and has obvious general public health importance in its annual epidemic and pandemic forms. Epidemiologic studies have demonstrated that exposure to air pollution is associated with increased asthma-related hospital admissions, medication use, and asthma exacerbation (6–8). Because respiratory viral infections are the leading cause of asthma exacerbations, and concurrent exposure to pollutants is likely to occur frequently, it is important to understand how air pollutants further aggravate asthmatic and allergic inflammation and how this impacts viral infection.
Previous studies have demonstrated that exposure to high levels of oxidant air pollutants increases the severity of virus-induced exacerbation of asthma (9). In experimental murine models, diesel exhaust exposure has been well documented to exacerbate allergic inflammation and Th2 immunity (10–14), and we have recently demonstrated that prior exposure to diesel exhaust particles increases influenza-induced exacerbation of allergic inflammation (11). However, few studies have been done to confirm these effects in humans.
We hypothesized that in individuals with allergic rhinitis, exposure to diesel exhaust increases allergic inflammation after subsequent viral infection, which in turn modifies early innate immune responses and virus clearance. To test this, we investigated the effects of short-term, controlled diesel exhaust exposure on nasal inflammatory responses to LAIV in human volunteers. Some of the results of this study have been previously reported in the form of an abstract.
Methods
Study Design and Subjects
This was a randomized, double-blind, prospective study comparing the effects of diesel exhaust (100 μg/m3, 2 h at rest) versus clean air on subsequent nasal inflammatory responses to LAIV. At a screening visit, a medical history was obtained and subjects underwent spirometry, HIV testing, pregnancy test for females, and a panel of allergy skin tests. Subjects returned 2–4 weeks later on “Day 0” to undergo baseline nasal lavage, followed by controlled chamber exposure to diesel exhaust or clean air (randomized) for 2 hours, and a standard intranasal dose of LAIV (FluMist, MedImmune, Inc., Gaithersburg, MD) was given 2 to 3 hours after the end of the chamber exposure. Detailed description of the diesel exposure has been previously described (15) and is in the online supplement. Subjects then returned to the research facility on Days 1, 2, 3, 4, and 9 for repeat nasal lavages. Nasal lavage was performed according to a method previously described (5). Subjects were masked as to exposure, as were all technicians performing assays on nasal lavage fluids (NLFs). Serum was obtained at the screening visit and at 21 days after LAIV inoculation.
Both healthy, nonallergic young adult volunteers, and those with allergic rhinitis, age 18–40 years, were recruited. Allergic rhinitis was defined as a history of seasonal or perennial rhinitis plus at least one positive immediate hypersensitivity reaction to allergen among a standard panel (including Dermatophagoides farinae, Dermatophagoides pteronyssius, grass mix, weed mix, tree mix, dog, cat, Alternaria, and cockroach) administered at screening. Subjects were excluded for acute symptoms within 3 weeks before study participation. Other exclusion criteria were smoking, any cardiorespiratory disease including asthma, immune deficiency, receipt of an influenza vaccine or documented influenza illness in the past 12 months, egg allergy, and pregnancy. Subjects with no asthma history but with abnormal spirometry at screening (FEV1 <75% predicted) were also excluded. Subjects were prohibited from use of inhaled medications of any kind or medications expected to influence nasal inflammation (e.g., nonsteroidal antiinflammatory drugs). A detailed description of the sample estimate calculation can be found in the online supplement.
The study was approved by the University of North Carolina Biomedical Institutional Review Board and the US Environmental Protection Agency.
LAIV and Virus Detection
LAIV (FluMist) was purchased from MedImmune, Inc., and used according to the manufacturer's recommendations. Vaccine composition is described in the online supplement. Virus quantification in NLF cells was done using quantitative reverse-transcriptase polymerase chain reaction for influenza type B hemagglutinin RNA as described previously (5). Serologic response to LAIV was measured by hemagglutination-inhibition assay in sera obtained at screen visit and 21 days after-LAIV (see online supplement for more details).
Measurement of Mediator Levels in NLF
Inflammatory cytokines (IL-1β, IL-6, IL-10, IL-12p70, granulocyte-macrophage colony–stimulating factor, and IFN-γ) and chemokines (IL-8, interferon-inducible protein-10, monocyte chemotactic protein [MCP]-1, thymus and activation-regulated chemokine, eotaxin-1, eotaxin-3, macrophage-derived chemokine, and MCP-4) were measured in NLFs using a multiplex ELISA platform (MesoScale Discovery, Gaithersburg, MD) according to the manufacturer's instructions. Eosinophilic cationic protein (ECP) was measured in NLFs using a specific commercial ELISA kit (Medical and Biological Laboratories Co., Nagoya, Japan), also according to the manufacturer's instructions.
Statistical Analysis
Raw NLF data are presented in descriptive fashion for each mediator endpoint, and levels of most mediators tended to rise and then fall back to baseline levels over the 9 days after LAIV inoculation (see online supplement). To evaluate the effect of exposure and allergic status on the mediators, we reduced the longitudinal observations for each subject to a single point representing response to LAIV, namely the area under curve (AUC), which was calculated for fold change over baseline. To formally test for the exposure effect, we used a sequence of nested multiple regression models with exposure group (diesel or air) as the main explanatory variable and allergic status (normal or allergic rhinitis) as an additional factor. Because body mass index is known to affect both influenza outcomes and vaccine responses in humans (16–18), it was included in all the models. The full model is a two-way analysis of covariance model with interaction of exposure group and allergic status. Subsequent models tested were an additive two-way analysis of covariance model and one-way analysis of covariance model. Additional details of the statistical models can be found in the online supplement.
Results
Subject Characteristics
Demographic characteristics of the subjects completing the protocol are shown in Table 1. Subjects in the normal and allergic rhinitis groups were similar in age and body mass index. Similarly, the normal and allergic rhinitis subgroups exposed to air and diesel also did not differ in age or body mass index. In the normal group, there were more females than males but the distribution was similar between the diesel- and air-exposed subgroups. One subject in the allergic rhinitis and air group developed prominent allergic-type symptoms (sneezing and conjunctivitis) believed to be unrelated to the study exposures. This subject was excluded from the final analysis, making the final n = 7 for this group.
TABLE 1.
DEMOGRAPHIC CHARACTERISTICS OF NORMAL VOLUNTEERS (NV) AND THOSE WITH ALLERGIC RHINITIS (AR) EXPOSED TO AIR OR DIESEL EXHAUST (DE)
| Group/Expos. | NV/Air | NV/DE | AR/Air | AR/DE |
| N | 8 | 8 | 7 | 9 |
| Age, yr | 25.3 (5.8) | 24.3 (5.9) | 24.4 (4.2) | 24.9 (4.6) |
| Body mass index | 23.3 (3.2) | 25.1 (5.5) | 27.3 (6.6) | 23.6 (4.1) |
| Sex, M/F | 3/5 | 2/6 | 4/4 | 4/5 |
Data are shown as mean (SD).
Chamber Exposure Data
Mean measured average particle exposure for subjects exposed to diesel was 93.2 μg/m3 (range, 76.7–108.6 μg/m3), and for air-exposed subjects did not exceed 4.5 μg/m3 in any case. Measured chamber concentration range for NO2 was 0.00–0.01 ppm (air) and 0.86–1.32 ppm (diesel); NO concentration was 0.00–0.03 ppm (air) and 3.26–4.67 ppm (diesel); SO2 concentration was 0.00–0.01 ppm (air) and 0.04–0.06 ppm (diesel); and CO concentration was 0.01–0.08 (air) and 3.08–6.00 ppm (diesel). Subjects did not report significant symptoms with any exposure.
NLF Cytokines
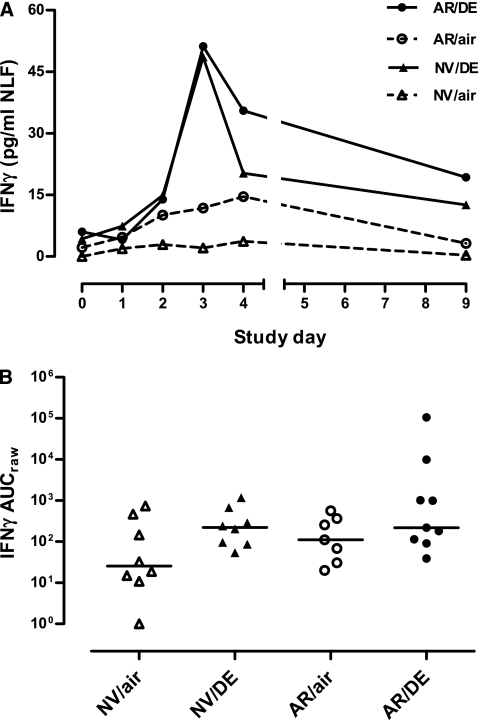
At Day 0, pre-LAIV baseline levels of NLF cytokines did not differ significantly between diesel- and air-exposure groups. For most cytokines, there was an increase in median levels in NLF from Day 0 over the subsequent Days 1–4 after LAIV, with a decline back toward baseline by Day 9 (see Figures E1–E6). Statistical analysis of the diesel effect on post-LAIV cytokine responses based on AUC data for ratio to baseline (AUCratio) for IL-1β, IL-6, IL-10, IL-12p70, and granulocyte-macrophage colony–stimulating factor responses to LAIV suggested no statistically significant effect of diesel (vs. air), and no significant interaction with allergy status. However, for IFN-γ there was a significant diesel effect, not related to allergic status (P = 0.02) (Figure 1). This was true whether AUC included Days 1–9 or only Days 1–4.
Figure 1.
(A) Time course of IFN-γ in nasal lavage fluid after Day 0 inoculation with live attenuated influenza vaccine (LAIV) in subjects exposed just before LAIV inoculation to clean air or to 100 μg/m3 diesel exhaust particles for 2 hours at rest. Data are shown as medians at each time point. (B) Individual area under curve (AUC) data for IFN-γ in nasal lavage fluid. Horizontal lines represent medians for each exposure group. AR/air = those with allergic rhinitis exposed to air; AR/DE = those with allergic rhinitis exposed to diesel exhaust; NV/air = normal volunteers exposed to air; NV/DE = normal volunteers exposed to diesel exhaust.
NLF Chemokines and ECP
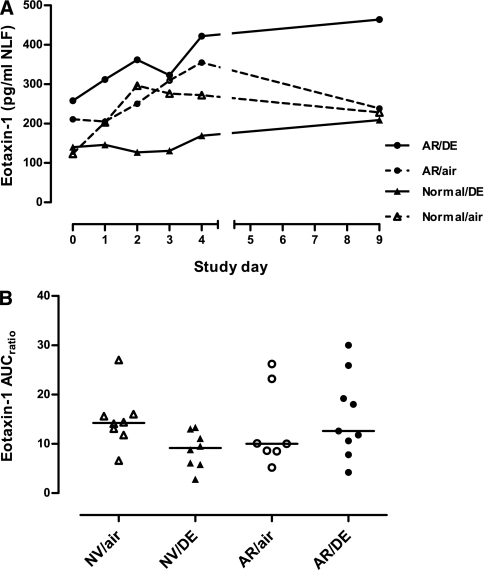
At Day 0, pre-LAIV baseline levels of NLF chemokines did not differ significantly between diesel- and air-exposure groups, except for thymus and activation-regulated chemokine levels, which were higher in the diesel-exposed normal subgroup than the air-exposed normal subgroup (P = 0.01). Similar to the cytokine responses to LAIV, most of the measured chemokines increased during Days 1–4 after LAIV inoculation, then declined back toward baseline by Day 9 (see Figures E7–E12). For the CXC chemokines interferon-inducible protein-10 and IL-8, there was no significant diesel effect in the regression model, although if AUC excluded Day 9, a significant increase with diesel was noted for IL-8 in those with allergic rhinitis. Among the CC chemokines, eotaxin-1 (CCL11) showed a statistically significant diesel-associated increase in the regression model, an effect interacting with allergic status (P = 0.01) (Figure 2). This was true whether AUC included Days 1–9 or only Days 1–4.
Figure 2.
(A) Time course of eotaxin-1 in nasal lavage fluid after Day 0 inoculation with live attenuated influenza vaccine (LAIV) in subjects exposed just before LAIV inoculation to clean air or to 100 μg/m3 diesel exhaust particles for 2 hours at rest. Data are shown as medians at each time point. (B) Individual area under curve (AUC) data for eotaxin-1 in nasal lavage fluid. Horizontal lines represent medians for each exposure group. AR/air = those with allergic rhinitis exposed to air; AR/DE = those with allergic rhinitis exposed to diesel exhaust; NV/air = normal volunteers exposed to air; NV/DE = normal volunteers exposed to diesel exhaust.
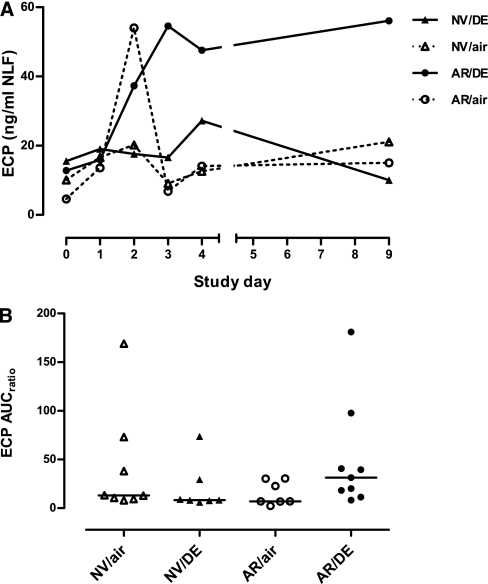
At Day 0, pre-LAIV baseline levels of NLF ECP did not differ significantly between diesel-and air-exposure groups. In subjects with allergic rhinitis exposed to diesel, ECP levels were elevated compared with air-exposed control subjects after LAIV, and persistently elevated at Day 9 (Figure 3A). ECP response expressed as AUC was significantly increased with diesel exposure in the regression model, an effect linked with allergy status (P < 0.01) (Figure 3B). This was true whether AUC included Days 1–9 or only Days 1–4.
Figure 3.
(A) Time course of eosinophil cationic protein (ECP) in nasal lavage fluid after Day 0 inoculation with live attenuated influenza vaccine (LAIV) in subjects exposed just before LAIV inoculation to clean air or to 100 μg/m3 diesel exhaust particles for 2 hours at rest. Data are shown as medians at each time point. (B) Individual area under curve (AUC) data for ECP in nasal lavage fluid. Horizontal lines represent medians for each exposure group. AR/air = those with allergic rhinitis exposed to air; AR/DE = those with allergic rhinitis exposed to diesel exhaust; NV/air = normal volunteers exposed to air; NV/DE = normal volunteers exposed to diesel exhaust.
Virus Quantity and Antiviral Antibody Responses
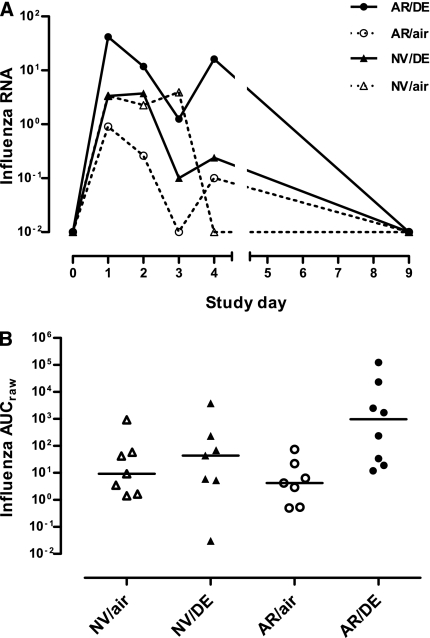
Although within-group data for influenza B hemagglutinin RNA sequences in NLF cells were quite variable, median virus quantity on Days 1–4 after-LAIV inoculation tended to be 1–2 log10 higher in diesel- than in air-exposed groups (Figure 4A). Because there was no virus in NLFs at Day 0 (pre-LAIV) baselines, AUC analysis for influenza B hemagglutinin RNA sequences in NLF cells used only “raw” virus quantity (AUCraw) to assess diesel effects. Levels of viral RNA sequences in NLF cells were significantly increased with diesel exposure, an effect modified by allergy status (P = 0.03) (Figure 4B).
Figure 4.
(A) Time course of influenza virus RNA in nasal lavage fluid after Day 0 inoculation with live attenuated influenza vaccine (LAIV) in subjects exposed just before LAIV inoculation to clean air or to 100 μg/m3 diesel exhaust particles for 2 hours at rest. Data are shown as medians at each time point. (B) Individual area under curve (AUC) data for influenza virus RNA in nasal lavage fluid. Horizontal lines represent medians for each exposure group. AR/air = those with allergic rhinitis exposed to air; AR/DE = those with allergic rhinitis exposed to diesel exhaust; NV/air = normal volunteers exposed to air; NV/DE = normal volunteers exposed to diesel exhaust.
Pre-LAIV hemagglutination-inhibition titers in serum did not differ at baseline among the subject groups. Paired pre-LAIV and 21-day post-LAIV sera were available for testing in most subjects, and all four groups had significantly increased reciprocal titers after LAIV. Mean (SD) fold change increase in reciprocal titers after LAIV were similar among groups: normal and air = 2.5 (1), n = 4; normal and diesel = 2.9 (2.5), n = 7; allergic rhinitis and air = 3 (2.8), n = 5; and allergic rhinitis and diesel = 1.7 (0.5), n = 7.
Discussion
In a randomized, controlled sequential-exposure study in young adults with or without allergic rhinitis, it was found that short-term diesel exhaust exposure in a chamber setting had significant effects on subsequent post-LAIV nasal responses, specifically an increase in IFN-γ levels and in virus quantity. Subjects with allergic rhinitis had particularly elevated quantities of virus, and additionally had statistically significant and prolonged increases in post-LAIV eotaxin-1 and ECP responses after diesel compared with air control subjects. Thus, individuals who are allergic may be especially susceptible to diesel-induced eosinophil recruitment and activation, either as a direct result of diesel exhaust particle interactions with nasal cell types relevant to allergic inflammation, or secondary to diesel-induced reduction in virus clearance. Although it has been previously reported that young adults who smoke also have reduced clearance of LAIV and altered nasal inflammation (5), we believe the current study is the first to measure the impact of an inhaled pollutant on viral respiratory infection in a controlled-exposure setting in human volunteers.
Our results are consistent with data from experimental models suggesting that diesel exhaust exacerbates allergic inflammation. In ovalbumin (OVA)-sensitized BALB/c mice, exposure to diesel exhaust particles at 2,000 μg/m3 for 4 hours per day for 4 days resulted in increased eosinophils, neutrophils, lymphocytes, and IL-6 in bronchoalveolar lavage fluids after OVA challenge, whereas nonsensitized control animals responded to diesel only with increased neutrophils (14). Data from a similar model suggest that diesel exhaust particles may function as an adjuvant in the induction of Th2 immune responses by OVA (13). Two recent studies (10, 12) have reported that repeated exposure to low-dose diesel exhaust after allergen challenge induced increased expression of macrophage-derived chemokine and the Th2 cytokines IL-4, IL-5, and IL-13 in murine lung tissue. Oxidant stress seems to be involved in diesel-associated increases in allergic inflammation and airway hyperresponsiveness as evidenced by experiments in Nrf2 knockout mice (19) and exposure of dendritic cells (DC) to diesel exhaust particle extracts (20, 21). Ambient and diesel particulates instilled into the oropharynx have been shown to activate pulmonary DC and promote eosinophilia and Th2-type responses in mice (22). In a study comparing responses to several chemically distinct preparations of diesel exhaust particles, potentiation of murine allergic immune responses seemed to correlate with diesel exhaust content of polycyclic aromatic hydrocarbons (23). Additionally, nasal challenge studies in humans have shown that diesel exhaust exposure predisposes to heightened allergic responses after allergen stimulation, particularly in GSTM1-null individuals (24, 25). However, conclusions regarding the clinical or mechanistic significance of our data must be made with caution given the fundamental differences between LAIV and wild-type viruses (see caveats below).
Several recent reports have described experiments using human tissue to investigate mechanisms for diesel effects. In experimental in vitro studies using human bronchial epithelial cells in coculture with myeloid DC, it was reported that diesel exhaust particles induced oxidative stress, which up-regulated epithelial production of thymic stromal lymphopoietin, driving myeloid DC to Th2 polarization (26). Incubation of nonatopic human peripheral blood mononuclear cells with diesel exhaust particle–derived polyaromatic hydrocarbons resulted in increased IL-13 and decreased IFN-γ production and increased chemotaxis of Th2 lymphocytes (27). In vitro stimulation of human DC with diesel exhaust particles induced production of several cytokines including IFN-γ, and direction of a Th2-like cytokine production pattern by CD4+ lymphocytes in coculture (28). Thus, limited research using human tissue seems consistent with data from murine studies.
The effects of diesel exhaust exposure on susceptibility to infection have also been investigated in BALB/c mice. Exposure at varying levels and durations increased inflammatory mediators, including IFN-γ, but suppressed expression of surfactant proteins A and D, which function as important host defense factors against both bacterial and viral pathogens in the lung (29). In murine experiments analogous to the design for the current study, we found that OVA-sensitized C57BL/6 mice instilled intratracheally with diesel exhaust particles, then infected with influenza A, had increased lung levels of eosinophils and Th2 cytokines compared with mice exposed to either diesel or virus alone (11). Furthermore, the Th2-like cytokine responses to influenza noted in neonatal mice (compared with adults) are associated with delayed migration of T cells to the lung, and increased susceptibility to influenza virus infection (30). No previous studies have tested the effects of sequential exposures to diesel exhaust and virus in human volunteers.
Epidemiologic evidence exists for an impact of diesel exhaust or “near roadway” effects on asthma in humans, particularly children (31). Behndig and coworkers (32) recently reported a study of normal volunteers and subjects with mild to moderate asthma who were exposed to a similar controlled diesel exhaust exposure as in our study (100 μg/m3, idling truck source, 2 h with intermittent exercise), followed by lung function testing and bronchoscopy 18 hours later. Although the normal volunteers had mild increases in neutrophils in the airways, those with asthma had no change in inflammation or in bronchial hyperreactivity, and the authors concluded that “the increased sensitivity of individuals with asthma to traffic-related air pollution is not necessarily associated with classical acute inflammation or aggravation of standard cellular indicators of allergic asthmatic inflammation.” It is possible that the lower airways are relatively protected from real-world diesel exhaust effects compared with the nasal airway, or that the diesel fuel or engine characteristics used by Behndig and coworkers were significantly different from ours. Furthermore, a study at our center in which diesel exhaust particles were applied directly to the nasal mucosa in volunteers with asthma showed no inflammatory effect (33). However, a reasonable hypothesis, consistent with our current results and with our data in mice (11), is that lower-level exposures may have minimal impact alone, but can predispose to exacerbation of allergic or asthmatic inflammation by increasing virus-induced eosinophilic inflammation and reduced early virus clearance. This might occur by effects of diesel exhaust on airway cells driving Th2 immune responses, by diesel-induced reduction of virus clearance through nonimmune mechanisms, or both. Although such a sequence of events is complex and cannot be proved in an observational human study such as this, it could conceivably be common in individuals with allergies in urban settings. A very similar sequence was suggested in a previous report in which severity of virus-induced exacerbation was increased in children with asthma with recent exposure to elevated levels of ambient NO2 (9).
Several caveats are appropriate for this study. First, the relatively small number of subjects studied may limit definitive conclusions. Baseline levels of most factors in NLF were quite variable, but the main method of data reduction for statistical analysis (AUC for ratios to baseline) was chosen to minimize the impact of baseline variability. Second, the temperature-sensitive LAIV is replication-limited and does not fully mimic a natural influenza infection, although it clearly is immunogenic and simulates many important features of natural mucosal host defense (34). For practical reasons and based on our prior published experience (3), we limited our data collection to the first several days after LAIV inoculation plus a later time point (Day 9), leaving open the possibility that significant effects could have been missed in the Day 5–8 interval. However, it seems unlikely that such changes could have been sufficient to negate the significant results observed in our analysis, which were similar whether AUC data for Days 1–4 only or including Day 9 were used.
Diesel exhaust particles are a complex mixture of organic carbons adsorbed to elemental carbons and small amounts of sulfates, nitrates, and minerals (35). Our study, designed to approximate real-world exposures by using an actual truck engine and levels of particulates reported in common urban situations (36), used a fuel and exposure setup identical to that reported by Sobus and coworkers (37), but different engines and running conditions can alter the chemical composition of diesel exhaust, which in turn may have a significant impact on inflammatory outcomes (23). Our study design did not include control groups for LAIV itself, raising the possibility that any mediator effects observed were related to the diesel or air exposures alone rather than to LAIV. However, the time course and pattern of mediator responses observed in the current study were very similar to those in our previous LAIV study, which did not involve diesel exposure (3), and a previous study at our center showed no effect of diesel exhaust particles alone on nasal inflammation (29). It seems very unlikely that the cytokine elevations observed in the current study were independent of LAIV. A final caveat is that nasal mucosal responses may also not entirely mimic those in the lower airways, although there is evidence from whole-genome studies that nasal epithelial responses resemble bronchial epithelial responses to toxins (38).
In summary, we have observed evidence for reduced nasal virus clearance and increased and prolonged virus-induced eosinophil activation in those with allergic rhinitis randomized to exposure to low-level diesel exhaust compared with air before LAIV inoculation. These results are consistent with data from our previous study conducted in OVA-sensitized mice (11), supporting the relevance of these models, and add to the evidence that diesel exposure at commonly encountered levels may influence the course of infection in humans, particularly in individuals with allergies. Although the clinical importance of this kind of interaction cannot be defined from our data, additional studies regarding the effect of novel preventive or treatment strategies, such as antioxidant up-regulation, seem warranted. Additionally, our data suggest that detailed studies of mucosal responses to respiratory viruses may be safely performed in humans using nasal exposure models, as we have previously shown for LAIV (5) and as recently shown for respiratory syncytial virus by DeVincenzo and coworkers (39). The general experimental approach of sequential controlled exposures to environmental pollutants and attenuated or well-tolerated wild-type virus strains may allow further advances in the understanding of the complex interactions between environmental agents and infectious diseases in humans.
Supplementary Material
Acknowledgments
The authors thank Paula Murphy and Wenli Zhang for expert technical assistance; Margaret Sanders, R.N., Martha Almond, R.R.T., Lynne Newlin-Clapp, Aline Kala, and Sally Ivins for assistance with study coordination; and Jon Berntsen for assistance with chamber exposures and monitoring.
Footnotes
Supported by grants from the National Institute of Environmental Health Sciences (P30 ES010126 and R01 ES013611). Although the research described in this article has also been funded in part by the United States Environmental Protection Agency through cooperative agreement CR83346301 with the Center for Environmental Medicine, Asthma and Lung Biology at the University of North Carolina at Chapel Hill, it has not been subjected to the Agency's required peer and policy review, and therefore does not necessarily reflect the views of the Agency and no official endorsement should be inferred. Mention of trade names or commercial products does not constitute endorsement or recommendation for use.
Author Contributions: T.L.N., study principal investigator, oversight of all facets of study including design, data organization and analysis, and manuscript preparation; K.H., optimization of nasal lavage procedure and manuscript revision; C.R., research coordinator and manuscript revision; H.Z. and H.Z., statistical analysis and manuscript revision; D.D.-S., study design and oversight regarding diesel exhaust exposures and manuscript revision; I.J., study design, oversight of nasal lavage fluid assays, and manuscript revision; and M.K. and M.M., hemagglutination inhibition assay design and interpretation and manuscript revision.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201103-0465OC on October 27, 2011
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Monto AS. Occurrence of respiratory virus: time, place and person. Pediatr Infect Dis J 2004;23:S58–S64 [DOI] [PubMed] [Google Scholar]
- 2.Ciencewicki J, Jaspers I. Air pollution and respiratory viral infection. Inhal Toxicol 2007;19:1135–1146 [DOI] [PubMed] [Google Scholar]
- 3.Noah TL, Zhou H, Monaco J, Horvath K, Herbst M, Jaspers I. Tobacco smoke exposure and altered nasal responses to live attenuated influenza virus. Environ Health Perspect 2011;119:78–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnston SL, Pattemore PK, Sanderson G, Smith S, Lampe F, Josephs L, Symington P, O'Toole S, Myint SH, Tyrrell DA, et al. Community study of role of viral infections in exacerbations of asthma in 9–11 year old children. BMJ 1995;310:1225–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teichtahl H, Buckmaster N, Pertnikovs E. The incidence of respiratory tract infection in adults requiring hospitalization for asthma. Chest 1997;112:591–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peden DB. The epidemiology and genetics of asthma risk associated with air pollution. J Allergy Clin Immunol 2005;115:213–219, quiz 20 [DOI] [PubMed] [Google Scholar]
- 7.Schwartz J, Slater D, Larson TV, Pierson WE, Koenig JQ. Particulate air pollution and hospital emergency room visits for asthma in Seattle. Am Rev Respir Dis 1993;147:826–831 [DOI] [PubMed] [Google Scholar]
- 8.Wong GW, Lai CK. Outdoor air pollution and asthma. Curr Opin Pulm Med 2004;10:62–66 [DOI] [PubMed] [Google Scholar]
- 9.Chauhan AJ, Inskip HM, Linaker CH, Smith S, Schreiber J, Johnston SL, Holgate ST. Personal exposure to nitrogen dioxide (NO2) and the severity of virus-induced asthma in children. Lancet 2003;361:1939–1944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inoue K, Koike E, Yanagisawa R, Takano H. Impact of diesel exhaust particles on th2 response in the lung in asthmatic mice. J Clin Biochem Nutr 2008;43:199–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaspers I, Sheridan PA, Zhang W, Brighton LE, Chason KD, Hua X, Tilley SL. Exacerbation of allergic inflammation in mice exposed to diesel exhaust particles prior to viral infection. Part Fibre Toxicol 2009;6:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsumoto A, Hiramatsu K, Li Y, Azuma A, Kudoh S, Takizawa H, Sugawara I. Repeated exposure to low-dose diesel exhaust after allergen challenge exaggerates asthmatic responses in mice. Clin Immunol 2006;121:227–235 [DOI] [PubMed] [Google Scholar]
- 13.Samuelsen M, Nygaard UC, Lovik M. Allergy adjuvant effect of particles from wood smoke and road traffic. Toxicology 2008;246:124–131 [DOI] [PubMed] [Google Scholar]
- 14.Stevens T, Krantz QT, Linak WP, Hester S, Gilmour MI. Increased transcription of immune and metabolic pathways in naive and allergic mice exposed to diesel exhaust. Toxicol Sci 2008;102:359–370 [DOI] [PubMed] [Google Scholar]
- 15.Sawyer K, Samet JM, Ghio AJ, Pleil JD, Madden MC. Responses measured in the exhaled breath of human volunteers acutely exposed to ozone and diesel exhaust. J Breath Res 2008;2:037019. [DOI] [PubMed] [Google Scholar]
- 16.Hagau N, Slavcovici A, Gonganau DN, Oltean S, Dirzu DS, Brezoszki ES, Maxim M, Ciuce C, Mlesnite M, Gavrus RL, et al. Clinical aspects and cytokine response in severe H1N1 influenza A virus infection. Crit Care 2010;14:R203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karlsson EA, Sheridan PA, Beck MA. Diet-induced obesity impairs the T cell memory response to influenza virus infection. J Immunol 2010;184:3127–3133 [DOI] [PubMed] [Google Scholar]
- 18.Louie JK, Acosta M, Samuel MC, Schechter R, Vugia DJ, Harriman K, Matyas BT. A novel risk factor for a novel virus: obesity and 2009 pandemic influenza A (H1N1). Clin Infect Dis 2011;52:301–312 [DOI] [PubMed] [Google Scholar]
- 19.Li YJ, Takizawa H, Azuma A, Kohyama T, Yamauchi Y, Takahashi S, Yamamoto M, Kawada T, Kudoh S, Sugawara I. Nrf2 is closely related to allergic airway inflammatory responses induced by low-dose diesel exhaust particles in mice. Clin Immunol 2010;137:234–241 [DOI] [PubMed] [Google Scholar]
- 20.Chan RC, Wang M, Li N, Yanagawa Y, Onoe K, Lee JJ, Nel AE. Pro-oxidative diesel exhaust particle chemicals inhibit LPS-induced dendritic cell responses involved in T-helper differentiation. J Allergy Clin Immunol 2006;118:455–465 [DOI] [PubMed] [Google Scholar]
- 21.Williams MA, Rangasamy T, Bauer SM, Killedar S, Karp M, Kensler TW, Yamamoto M, Breysse P, Biswal S, Georas SN. Disruption of the transcription factor Nrf2 promotes pro-oxidative dendritic cells that stimulate Th2-like immunoresponsiveness upon activation by ambient particulate matter. J Immunol 2008;181:4545–4559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bezemer GF, Bauer SM, Oberdorster G, Breysse PN, Pieters RH, Georas SN, Williams MA. Activation of pulmonary dendritic cells and Th2-type inflammatory responses on instillation of engineered, environmental diesel emission source or ambient air pollutant particles in vivo. J Innate Immun 2011;3:150–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stevens T, Cho SH, Linak WP, Gilmour MI. Differential potentiation of allergic lung disease in mice exposed to chemically distinct diesel samples. Toxicol Sci 2009;107:522–534 [DOI] [PubMed] [Google Scholar]
- 24.Gilliland FD, Li YF, Saxon A, Diaz-Sanchez D. Effect of glutathione-S-transferase M1 and P1 genotypes on xenobiotic enhancement of allergic responses: randomised, placebo-controlled crossover study. Lancet 2004;363:119–125 [DOI] [PubMed] [Google Scholar]
- 25.Gilliland FD, Li YF, Gong H, Jr, Diaz-Sanchez D. Glutathione s-transferases M1 and P1 prevent aggravation of allergic responses by secondhand smoke. Am J Respir Crit Care Med 2006;174:1335–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bleck B, Tse DB, Gordon T, Ahsan MR, Reibman J. Diesel exhaust particle-treated human bronchial epithelial cells upregulate Jagged-1 and OX40 ligand in myeloid dendritic cells via thymic stromal lymphopoietin. J Immunol 2010;185:6636–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang Y, Senechal S, de Nadai P, Chenivesse C, Gilet J, Vorng H, Legendre B, Tonnel AB, Wallaert B, Lassalle P, et al. Diesel exhaust exposure favors TH2 cell recruitment in nonatopic subjects by differentially regulating chemokine production. J Allergy Clin Immunol 2006;118:354–360 [DOI] [PubMed] [Google Scholar]
- 28.Porter M, Karp M, Killedar S, Bauer SM, Guo J, Williams D, Breysse P, Georas SN, Williams MA. Diesel-enriched particulate matter functionally activates human dendritic cells. Am J Respir Cell Mol Biol 2007;37:706–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gowdy K, Krantz QT, Daniels M, Linak WP, Jaspers I, Gilmour MI. Modulation of pulmonary inflammatory responses and antimicrobial defenses in mice exposed to diesel exhaust. Toxicol Appl Pharmacol 2008;229:310–319 [DOI] [PubMed] [Google Scholar]
- 30.Lines JL, Hoskins S, Hollifield M, Cauley LS, Garvy BA. The migration of T cells in response to influenza virus is altered in neonatal mice. J Immunol 2010;185:2980–2988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gehring U, Wijga AH, Brauer M, Fischer P, de Jongste JC, Kerkhof M, Oldenwening M, Smit HA, Brunekreef B. Traffic-related air pollution and the development of asthma and allergies during the first 8 years of life. Am J Respir Crit Care Med 2010;181:596–603 [DOI] [PubMed] [Google Scholar]
- 32.Behndig AF, Larsson N, Brown JL, Stenfors N, Helleday R, Duggan ST, Dove RE, Wilson SJ, Sandstrom T, Kelly FJ, et al. Proinflammatory doses of diesel exhaust in healthy subjects fail to elicit equivalent or augmented airway inflammation in subjects with asthma. Thorax 2011;66:12–19 [DOI] [PubMed] [Google Scholar]
- 33.Kongerud J, Madden MC, Hazucha M, Peden D. Nasal responses in asthmatic and nonasthmatic subjects following exposure to diesel exhaust particles. Inhal Toxicol 2006;18:589–594 [DOI] [PubMed] [Google Scholar]
- 34.Boyce TG, Gruber WC, Coleman-Dockery SD, Sannella EC, Reed GW, Wolff M, Wright PF. Mucosal immune response to trivalent live attenuated intranasal influenza vaccine in children. Vaccine 1999;18:82–88 [DOI] [PubMed] [Google Scholar]
- 35.Wichmann HE. Diesel exhaust particles. Inhal Toxicol 2007;19:241–244 [DOI] [PubMed] [Google Scholar]
- 36.Kinney PL, Aggarwal M, Northridge ME, Janssen NA, Shepard P. Airborne concentrations of PM(2.5) and diesel exhaust particles on Harlem sidewalks: a community-based pilot study. Environ Health Perspect 2000;108:213–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sobus JR, Pleil JD, Madden MC, Funk WE, Hubbard HF, Rappaport SM. Identification of surrogate measures of diesel exhaust exposure in a controlled chamber study. Environ Sci Technol 2008;42:8822–8828 [DOI] [PubMed] [Google Scholar]
- 38.Zhang X, Sebastiani P, Liu G, Schembri F, Dumas YM, Langer EM, Alekseyev Y, O'Connor GT, Brooks DR, Lenburg ME, et al. Similarities and differences between smoking-related gene expression in nasal and bronchial epithelium. Physiol Genomics 2010;41:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DeVincenzo JP, Wilkinson T, Vaishnaw A, Cehelsky J, Meyers R, Nochur S, Harrison L, Meeking P, Mann A, Moane E, et al. Viral load drives disease in humans experimentally infected with respiratory syncytial virus. Am J Respir Crit Care Med 2010;182:1305–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.