Abstract
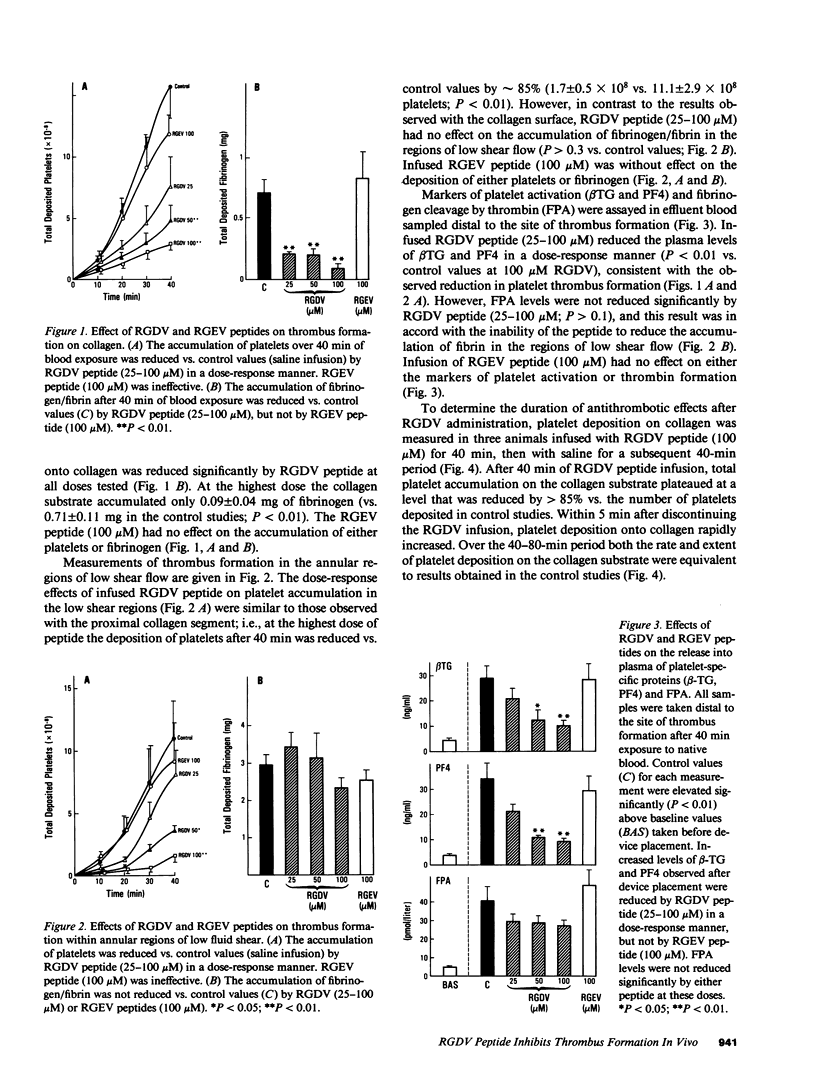
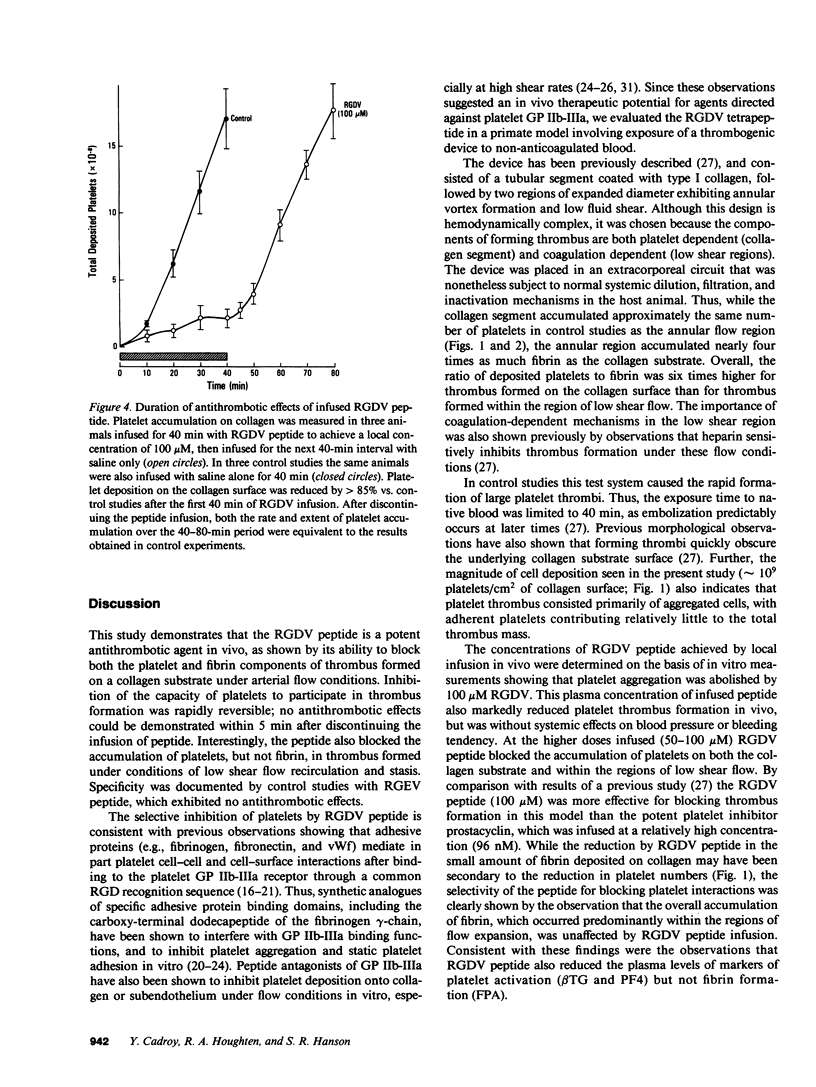
Since platelet hemostatic functions are mediated in part through the binding of adhesive proteins containing an RGD (Arg-Gly-Asp) recognition sequence, and since platelet reactions may be inhibited in vitro by RGD-containing peptides, we assessed in vivo the antithrombotic activity of RGDV (Arg-Gly-Asp-Val) tetrapeptide using a baboon thrombosis model. Thrombus formation was induced by a device consisting of a tubular segment coated with type I collagen, followed by two regions of expanded diameter exhibiting disturbed flow and stasis. The thrombogenic device was incorporated into femoral arteriovenous shunts under conditions of intermediate wall shear rate (100 s-1). Thrombus formation was measured by scintillation camera imaging of 111In-platelets and by counting of 125I-fibrinogen/fibrin. Thrombus that formed on the collagen substrate was rich in platelets, while thrombus formed in the disturbed flow regions was rich in fibrin and red cells. RGDV peptide was infused proximal to the thrombogenic device to maintain local plasma concentrations of 25, 50, and 100 microM. Infused RGDV decreased the accumulation of both platelets and fibrin on the collagen substrate in a dose-response manner. At the highest dose platelet and fibrin deposition after 40 min was reduced by greater than 80% (P less than 0.01). In the region of disturbed flow, RGDV (100 microM) reduced platelet deposition by 85% (P less than 0.01) but did not reduce the accumulation of fibrin (P less than 0.3). Similarly, the peptide inhibited the release of granular proteins from platelets associated with thrombus (platelet factor 4, beta-thromboglobulin; P less than 0.01), but did not prevent the appearance of fibrinopeptide A in circulating blood (P greater than 0.1). No systemic alterations in blood pressure, bleeding time, or platelet aggregation ex vivo were produced by locally infused RGDV. The antithrombotic effects of RGDV peptide disappeared within 5 min after discontinuing the infusion. In control studies infused RGEV (Arg-Gly-Glu-Val, 100 microM) showed no antithrombotic activity. Thus, RGDV selectively blocks platelet-dependent thrombus formation in vivo.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balduini C. L., Grignani G., Sinigaglia F., Bisio A., Pacchiarini L., Rota Scalabrini D., Balduini C., Mauri C., Ascari E. Severe platelet dysfunction in a patient with autoantibodies against membrane glycoproteins IIb-IIIa. Haemostasis. 1987;17(1-2):98–104. doi: 10.1159/000215564. [DOI] [PubMed] [Google Scholar]
- Cadroy Y., Horbett T. A., Hanson S. R. Discrimination between platelet-mediated and coagulation-mediated mechanisms in a model of complex thrombus formation in vivo. J Lab Clin Med. 1989 Apr;113(4):436–448. [PubMed] [Google Scholar]
- Coller B. S., Folts J. D., Scudder L. E., Smith S. R. Antithrombotic effect of a monoclonal antibody to the platelet glycoprotein IIb/IIIa receptor in an experimental animal model. Blood. 1986 Sep;68(3):783–786. [PubMed] [Google Scholar]
- Coller B. S., Scudder L. E., Berger H. J., Iuliucci J. D. Inhibition of human platelet function in vivo with a monoclonal antibody. With observations on the newly dead as experimental subjects. Ann Intern Med. 1988 Oct 15;109(8):635–638. doi: 10.7326/0003-4819-109-8-635. [DOI] [PubMed] [Google Scholar]
- Coller B. S., Scudder L. E. Inhibition of dog platelet function by in vivo infusion of F(ab')2 fragments of a monoclonal antibody to the platelet glycoprotein IIb/IIIa receptor. Blood. 1985 Dec;66(6):1456–1459. [PubMed] [Google Scholar]
- De Marco L., Girolami A., Zimmerman T. S., Ruggeri Z. M. von Willebrand factor interaction with the glycoprotein IIb/IIa complex. Its role in platelet function as demonstrated in patients with congenital afibrinogenemia. J Clin Invest. 1986 Apr;77(4):1272–1277. doi: 10.1172/JCI112430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fressinaud E., Baruch D., Girma J. P., Sakariassen K. S., Baumgartner H. R., Meyer D. von Willebrand factor-mediated platelet adhesion to collagen involves platelet membrane glycoprotein IIb-IIIa as well as glycoprotein Ib. J Lab Clin Med. 1988 Jul;112(1):58–67. [PubMed] [Google Scholar]
- Gartner T. K., Bennett J. S. The tetrapeptide analogue of the cell attachment site of fibronectin inhibits platelet aggregation and fibrinogen binding to activated platelets. J Biol Chem. 1985 Oct 5;260(22):11891–11894. [PubMed] [Google Scholar]
- Gold H. K., Coller B. S., Yasuda T., Saito T., Fallon J. T., Guerrero J. L., Leinbach R. C., Ziskind A. A., Collen D. Rapid and sustained coronary artery recanalization with combined bolus injection of recombinant tissue-type plasminogen activator and monoclonal antiplatelet GPIIb/IIIa antibody in a canine preparation. Circulation. 1988 Mar;77(3):670–677. doi: 10.1161/01.cir.77.3.670. [DOI] [PubMed] [Google Scholar]
- Hanson S. R., Pareti F. I., Ruggeri Z. M., Marzec U. M., Kunicki T. J., Montgomery R. R., Zimmerman T. S., Harker L. A. Effects of monoclonal antibodies against the platelet glycoprotein IIb/IIIa complex on thrombosis and hemostasis in the baboon. J Clin Invest. 1988 Jan;81(1):149–158. doi: 10.1172/JCI113286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harfenist E. J., Packham M. A., Mustard J. F. Effects of the cell adhesion peptide, Arg-Gly-Asp-Ser, on responses of washed platelets from humans, rabbits, and rats. Blood. 1988 Jan;71(1):132–136. [PubMed] [Google Scholar]
- Haverstick D. M., Cowan J. F., Yamada K. M., Santoro S. A. Inhibition of platelet adhesion to fibronectin, fibrinogen, and von Willebrand factor substrates by a synthetic tetrapeptide derived from the cell-binding domain of fibronectin. Blood. 1985 Oct;66(4):946–952. [PubMed] [Google Scholar]
- Houdijk W. P., Sixma J. J. Fibronectin in artery subendothelium is important for platelet adhesion. Blood. 1985 Mar;65(3):598–604. [PubMed] [Google Scholar]
- Houghten R. A. General method for the rapid solid-phase synthesis of large numbers of peptides: specificity of antigen-antibody interaction at the level of individual amino acids. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5131–5135. doi: 10.1073/pnas.82.15.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloczewiak M., Timmons S., Lukas T. J., Hawiger J. Platelet receptor recognition site on human fibrinogen. Synthesis and structure-function relationship of peptides corresponding to the carboxy-terminal segment of the gamma chain. Biochemistry. 1984 Apr 10;23(8):1767–1774. doi: 10.1021/bi00303a028. [DOI] [PubMed] [Google Scholar]
- Malpass T. W., Hanson S. R., Savage B., Hessel E. A., 2nd, Harker L. A. Prevention of acquired transient defect in platelet plug formation by infused prostacyclin. Blood. 1981 Apr;57(4):736–740. [PubMed] [Google Scholar]
- Marguerie G. A., Edgington T. S., Plow E. F. Interaction of fibrinogen with its platelet receptor as part of a multistep reaction in ADP-induced platelet aggregation. J Biol Chem. 1980 Jan 10;255(1):154–161. [PubMed] [Google Scholar]
- Nievelstein P. F., Sixma J. J. Glycoprotein IIb-IIIa and RGD(S) are not important for fibronectin-dependent platelet adhesion under flow conditions. Blood. 1988 Jul;72(1):82–88. [PubMed] [Google Scholar]
- Peerschke E. I., Zucker M. B., Grant R. A., Egan J. J., Johnson M. M. Correlation between fibrinogen binding to human platelets and platelet aggregability. Blood. 1980 May;55(5):841–847. [PubMed] [Google Scholar]
- Plow E. F., McEver R. P., Coller B. S., Woods V. L., Jr, Marguerie G. A., Ginsberg M. H. Related binding mechanisms for fibrinogen, fibronectin, von Willebrand factor, and thrombospondin on thrombin-stimulated human platelets. Blood. 1985 Sep;66(3):724–727. [PubMed] [Google Scholar]
- Plow E. F., Pierschbacher M. D., Ruoslahti E., Marguerie G. A., Ginsberg M. H. The effect of Arg-Gly-Asp-containing peptides on fibrinogen and von Willebrand factor binding to platelets. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8057–8061. doi: 10.1073/pnas.82.23.8057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pytela R., Pierschbacher M. D., Ginsberg M. H., Plow E. F., Ruoslahti E. Platelet membrane glycoprotein IIb/IIIa: member of a family of Arg-Gly-Asp--specific adhesion receptors. Science. 1986 Mar 28;231(4745):1559–1562. doi: 10.1126/science.2420006. [DOI] [PubMed] [Google Scholar]
- Ruggeri Z. M., Houghten R. A., Russell S. R., Zimmerman T. S. Inhibition of platelet function with synthetic peptides designed to be high-affinity antagonists of fibrinogen binding to platelets. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5708–5712. doi: 10.1073/pnas.83.15.5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakariassen K. S., Fressinaud E., Girma J. P., Meyer D., Baumgartner H. R. Role of platelet membrane glycoproteins and von Willebrand factor in adhesion of platelets to subendothelium and collagen. Ann N Y Acad Sci. 1987;516:52–65. doi: 10.1111/j.1749-6632.1987.tb33029.x. [DOI] [PubMed] [Google Scholar]
- Todd M. E., McDevitt E., Goldsmith E. I. Blood-clotting mechanisms of nonhuman primates. Choice of the baboon model to simulate man. J Med Primatol. 1972;1(3):132–141. doi: 10.1159/000460376. [DOI] [PubMed] [Google Scholar]
- Weiss H. J., Hawiger J., Ruggeri Z. M., Turitto V. T., Thiagarajan P., Hoffmann T. Fibrinogen-independent platelet adhesion and thrombus formation on subendothelium mediated by glycoprotein IIb-IIIa complex at high shear rate. J Clin Invest. 1989 Jan;83(1):288–297. doi: 10.1172/JCI113871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda T., Gold H. K., Fallon J. T., Leinbach R. C., Guerrero J. L., Scudder L. E., Kanke M., Shealy D., Ross M. J., Collen D. Monoclonal antibody against the platelet glycoprotein (GP) IIb/IIIa receptor prevents coronary artery reocclusion after reperfusion with recombinant tissue-type plasminogen activator in dogs. J Clin Invest. 1988 Apr;81(4):1284–1291. doi: 10.1172/JCI113446. [DOI] [PMC free article] [PubMed] [Google Scholar]


