Abstract
The cell wall peptidoglycan of Gram-positive bacteria functions as a surface organelle for the transport and assembly of proteins that interact with the environment, in particular, the tissues of an infected host. Signal peptide-bearing precursor proteins are secreted across the plasma membrane of Gram-positive bacteria. Some precursors carry C-terminal sorting signals with unique sequence motifs that are cleaved by sortase enzymes and linked to the cell wall peptidoglycan of vegetative forms or spores. The sorting signals of pilin precursors are cleaved by pilus-specific sortases, which generate covalent bonds between proteins leading to the assembly of fimbrial structures. Other precursors harbour surface (S)-layer homology domains (SLH), which fold into a three-pronged spindle structure and bind secondary cell wall polysaccharides, thereby associating with the surface of specific Gram-positive microbes. Type VII secretion is a non-canonical secretion pathway for WXG100 family proteins in mycobacteria. Gram-positive bacteria also secrete WXG100 proteins and carry unique genes that either contribute to discrete steps in secretion or represent distinctive substrates for protein transport reactions.
Keywords: type VII secretion, WXG protein, sortase, sorting signal, surface-layer homology domain, surface layer
1. Introduction
Hans Christian Gram used light microscopy to detect microbes that were stained with crystal violet/iodine [1]. Microbes that cannot retain this dye following treatment with ethanol were counterstained with safranin (or fuchsin), thereby distinguishing Gram-positive from Gram-negative bacteria. The differential staining property is based on the peptidoglycan layer, which is considerably thicker in Gram-positive microbes [2]. Another difference is that Gram-positive bacteria elaborate a single membrane, whereas Gram-negative microbes harbour a plasma membrane and an additional outer membrane with lipopolysaccharides [3,4]. The secretion of signal peptide-bearing precursor proteins in Gram-positive microbes leads by default to their release into extracellular medium [5]. In contrast, signal peptide-mediated translocation in Gram-negative bacteria leads to protein localization within the periplasm, a compartment between their inner and outer membranes that encompasses a thin peptidoglycan layer [6].
In the past few years, molecular biology approaches have been applied to many Gram-positive bacteria and the ensuing research evolved into some of the most exciting fields of microbiology and microbial pathogenesis, including Actinomyces spp., Bacillus anthracis, Bacillus cereus, Bacillus subtilis, Clostridium perfringens, Clostridium difficile, Corynebacterium diphtheriae, Enterococcus faecalis, Enterococcus faecium, Listeria monocytogenes, Streptococcus agalactiae, Streptococcus gordonii, Streptococcus pyogenes and Streptomyces spp. This review summarizes briefly what is known about the secretion or assembly of proteins in the envelope of Gram-positive microbes. Our goal was to provide a comparative synopsis of parallel fields that may benefit from each other and to define key research frontiers. Because our discussion had to be brief, we apologize for not being able to provide a more exhaustive analysis and for not discussing many important advances in each field.
2. Envelope structures in Gram-positive bacteria
Peptidoglycan is synthesized from nucleotide precursors [7], the modified amino sugar N-acetylmuramic acid (MurNAc) [8] as well as d- or l-amino acids (l-Ala-d-iGlu-m-Dpm-d-Ala-d-Ala, here abbreviated AGDA2) [9] in the bacterial cytoplasm to generate Park's nucleotide (UDP-MurNAc-AGDA2) [10]. Park's nucleotide is linked to the lipid carrier undecaprenyl-pyrophosphate to generate lipid I [C55-PP-MurNAc-AGDA2] [11]. Modification of lipid I with UDP-GlcNAc produces lipid II [C55-PP-MurNAc(AGDA2)-GlcNAc] [12], which is polymerized by transglycosylases and transpeptidases (penicillin-binding proteins) to generate peptidoglycan strands [MurNAc(AGDA2)-GlcNAc-MurNAc(AGDA2)-GlcNAc] that are crosslinked with other strands [13,14].
The peptidoglycan layer protects Gram-positive bacteria from osmotic lysis and serves as a barrier against environmental hydrolases or membrane toxic compounds [2]. Peptidoglycan also functions as a scaffold for the immobilization of capsular polysaccharides [15], cell wall teichoic acids (WTAs) [16] and proteins that are either bound to specific envelope structures [17,18], covalently linked to peptidoglycan [19], assembled into pili [20] or distributed into S-layer structures [21,22]. Proteins in any one of the aforementioned locations fulfil unique physiological roles that aid bacteria in their interaction with the environment, most notably the tissues and immune cells of an infected host [23] (figure 1).
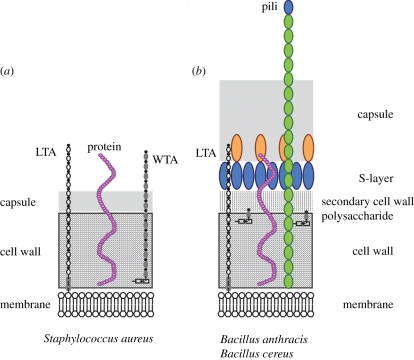
Figure 1.
Envelope structures of Gram-positive bacteria. (a) Staphylococcus aureus elaborates a plasma membrane and thick peptidoglycan layer that encompasses polyribitol-phosphate wall teichoic acids (WTA), proteins and capsular polysaccharides. Lipoteichoic acids (LTA) are poly-glycerolphosphates tethered to a diglucosyl-diacylglycerate membrane anchor. (b) Bacillus cereus as well as Bacillus anthracis elaborate a plasma membrane and thick peptidoglycan layer. Secondary cell wall polysaccharides (SCWPs) are linked to peptidoglycan and serve as a ligand for the SLH domains of S-layer proteins. In addition to cell wall anchored proteins, the peptidoglycan of bacilli also functions as an anchoring point for capsule and pili.
3. Protein translocation across the plasma membrane
The genetic requirements for the secretion of signal peptide-bearing precursors have not been examined in Gram-positive bacteria. What has been learned from Escherichia coli suppressor analyses on the involvement of sec genes is thought to apply also to bacilli, staphylococci or any other Gram-positive microbe [24,25]. Further, in vitro translocation experiments with inverted membrane vesicles of E. coli analysed the biochemical attributes of the Sec pathway [26]. In brief, these lines of investigation identified SecYEG as the translocon channel for precursor movement across the plasma membrane [27], which also involves the cytoplasmic ATPase SecA for pushing precursor proteins into the channel [28] and the SecDF/YajC complex for releasing them from the translocon [29]. Signal peptidase acts on the translocated precursor to release the mature polypeptide into the periplasmic space [30]. The signal recognition particle (SRP) of E. coli is a ribonucleoprotein complex comprising Ffh and 4.5S RNA, which interacts with the nascent precursors of membrane proteins to regulate translation and deliver the ribosome to the SRP receptor (FtsY) and eventually the SecYEG translocon for co-translational secretion of membrane proteins [31,32]. Several chaperones, including SecB [33], heat shock proteins (DnaK/DnaJ/GrpE) [34] as well as trigger factor, a peptidyl-prolyl isomerase [35], contribute to secretion by maintaining specific substrate proteins in a secretion competent state [4,36].
Most Gram-positive bacteria harbour secA, secD, secE, secF, secG, secY, ffh, ftsY and yajC genes and are thought to catalyse protein secretion by pathways similar to those described for E. coli [37]. A notable difference is that Gram-positives lack the secB gene and require prsA, a lipoprotein peptidyl-prolyl isomerase for secretion of some polypeptides into the extracellular media [37,38]. Some Gram-positive bacteria express accessory secretion genes designated secA2 or secY2. In S. gordonii, the secA2 and secY2 gene products are essential for the secretion of the large glycoprotein GspB, which, owing to its heavy glycosylation, cannot be transported via the SecA pathway [39,40]. In addition to its 90 residue N-terminal signal peptide, the first 20 amino acids of mature GspB are required for SecA2/SecY2-mediated translocation by a process that involves also the accessory secretion proteins Asp1–5 [41,42]. Several Gram-positive microbes also use accessory secretion genes, secA2 and secY2 (either alone or together), for the selective transport of specific substrates [43]. Unlike E. coli [44], the SRP pathway of Streptococcus mutans is dispensable for the growth of this bacterium [45,46]. Nevertheless, ffh, ftsY or scRNA mutants display defects in acid tolerance and ATPase activity [45,46]. Some Gram-positive microbes harbour two genes encoding YidC homologues (YidC1 and YidC2) where one of the YidC isoforms appears to specialize in a cotranslational secretion pathway that operates independently of SRP [47].
4. Protein traffic across the cell wall and its associated structures
The peptidoglycan layer of Gram-positive bacteria is much thicker than that of Gram-negative microbes; in some cases, it can be 50–100 nm in diameter [48]. Cell wall envelopes can be isolated by first physically breaking cells with glass or aluminium beads and then purifying murein sacculi, which are impenetrable to proteins [49]. One wonders whether some Gram-positive bacteria have evolved channels for the release of precursors that have been translocated across the plasma membrane into the extracellular milieu. A simple argument in favour of protein transport channels across peptidoglycan is the finding that boiling staphylococci in hot SDS does not release membrane or lipoproteins from the murein sacculus [50]. However, puncturing murein with specific hydrolases does provide for the detergent extraction of such lipoproteins. The peptidoglycan layer functions also as a surface organelle that enables assembly of capsules [51], secondary cell wall polysaccharides (SCWPs) and S-layers [22,52,53], WTAs [16] and of many different sortase-anchored proteins [23,54]. Whether peptidoglycan associated polymers of proteins, polysaccharides or teichoic acids provide a barrier to protein translocation has not been studied.
Using electron microscopy and immunogold labelling techniques in S. pyogenes, Rosch & Caparon [55,56] reported the accumulation of immune-reactive signals at membrane sites (microdomains) that include SecA and HtrA (DepP). Both of these proteins are involved in precursor protein secretion in E. coli and fulfil similar functions in streptococci [57]. The authors proposed the existence of a macromolecular structure designated the ExPortal, which could be responsible for the secretion of precursor proteins in Gram-positive microbes with thick peptidoglycan layers [56]. In agreement with this hypothesis, similar accumulations of immune-reactive membrane signals have been observed in E. faecalis [58]. The molecular compositon, structural features or genes responsible for the formation of the proposed ExPortal have not yet been revealed. The ExPortal hypothesis has been challenged. Others reported that SecA of S. pyogenes is not localized to microdomains but distributed throughout the streptococcal membranes [59].
5. Protein secretion into the cross wall of Gram-positive cocci
Bioinformatic analyses identified two types of signal peptides in genes for precursor proteins of Staphylococcus aureus, Streptococcus pneumoniae and S. pyogenes [60–62]. Lindahl and co-workers [59] discovered that M protein, a cell wall anchored surface protein of S. pyogenes that is secreted via a signal peptide harbouring the YSIRK/GS sequence motif, is deposited near the cell division site, whereas protein F, whose precursor harbours a conventional signal peptide, is deposited near the cell poles. The unique distribution of surface proteins is imposed by the two types of signal peptides, as its switching redirects mutant M protein and protein F precursors to the other location [59]. A similar phenomenon has been reported for S. aureus where precursors with YSIRK/GS signal peptides are secreted into the cross wall, the peptidoglycan synthesis compartment that is formed at midcell following FtsZ-mediated cytokinesis [63]. Surface proteins that are targeted into the cross wall because of their YSIRK/GS signal peptides are eventually distributed over the entire bacterial surface, whereas those that are immobilized following secretion via canonical signal peptides reside only at the cell poles [63,64].
Recent work identified genes for membrane proteins with abortive-infectivity domains, designated spdABC (surface protein display), as being required for the trafficking of YSIRK/GS proteins into the cross wall compartment [65]. Mutants that lack any one of the three spdABC genes display increased thickness of the cross wall compartment and delayed cell separation during staphylococcal cell division [65]. LytN is a murein hydrolase that is secreted into the cross wall of S. aureus via its YSIRK/GS signal peptide [66]. Staphylococcal lytN mutants display cell separation and cross wall structural defects, suggesting that LytN plays a key role in completing cross wall formation and cell separation [66]. Two other secreted murein hydrolases, Sle1 and Atl, also contribute to cross wall separation [67,68]. These enzymes act on the outside the cell wall envelope in the immediate vicinity of the cell division site and split the cross wall from the outside [69,70]. Although these experiments point to various secreted products that are essential in cell wall synthesis and cross wall physiology, the fundamental question of how precursors with YSIRK/GS signal peptides are secreted into the cross wall compartment has not yet been addressed.
6. Sortase A-anchoring of proteins to the cell wall envelope
All Gram-positive bacteria express sortase A, a membrane anchored transpeptidase that cleaves the C-terminal LPXTG sorting signals of precursor proteins destined for cell wall anchoring [71,72]. Sortase A scans secreted polypeptides for its recognition sequence with a unique fold of parallel and anti-parallel β-sheets [73] (figure 2). A scissile peptide bond between the threonyl and the glycyl of LPXTG motif sorting signals is accommodated in direct proximity of the active site cysteine of sortase A [74]. Following nucleophilic attack from the active site, sortase forms a thioester linked intermediate with the carboxyl group of threonine at the C-terminal end of surface proteins [72]. The sortase acyl intermediate is resolved by the nucleophilic attack of the free amino group within lipid II, thereby releasing surface protein linked to the crossbridge and restoring the enzyme active site as reaction products [75,76]. The crossbridge of lipid II precursors varies in structure between bacterial species [77]. In bacilli and listeria, the crossbridge amino group is derived from the side chain of m-diaminopimelic acid (m-Dpm). In contrast, the crossbridge of staphylococci comprises five glycyl residues that are tethered to the ɛ-amino group of lysine within lipid II [C55-(PO3)2-MurNac(l-Ala-d-iGln-Lys(NH2-Gly5)-d-Ala-d-Ala)-GlcNAc] [78,79]. Sortase A enzymes of different bacterial species have evolved to recognize their corresponding crossbridge structures of lipid II [80,81]. The sortase A reaction products, surface protein linked to lipid II, are then incorporated into the cell wall envelope via the transglycosylation and transpeptidation reactions of cell wall synthesis [82–84].
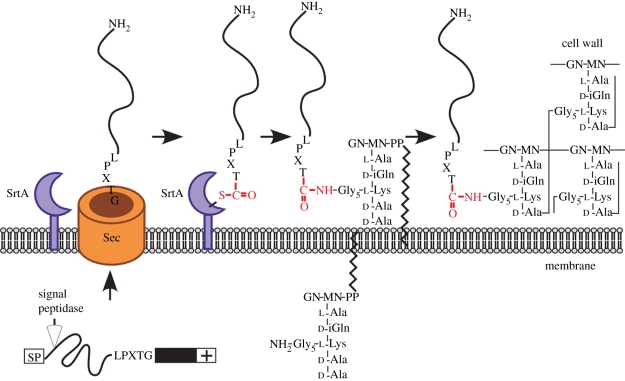
Figure 2.
The role of sortase A in anchoring proteins to the cell wall envelope of Gram-positive bacteria. Surface proteins are synthesized as precursors with an N-terminal signal peptide and a C-terminal LPXTG motif sorting signal, including a hydrophobic domain (black box) and positively charged tail (+). Following initiation into the secretory pathway, the signal peptide is cleaved, while sortase A scans translocated precursors for LPXTG motif sequences. Following cleavage of the precursor by sortase A between the threonine (T) and the glycine (G) of the LPXTG motif, an acyl-enzyme intermediate is formed between the active site cysteine (C) of sortase A and the carboxyl-group of threonine at the C-terminal end of the surface protein. The amino group of cell wall crossbridges within lipid II, pentaglycine (Gly5) in S. aureus, performs a nucleophilic attack at the thioester bond between sortase A and its cleaved substrate, thereby forming an amide (isopeptide) bond between the C-terminal threonine and lipid II. Transglycosylation and transpeptidation reactions promote the incorporation of surface proteins into the cell wall envelope of Gram-positive bacteria (see text for details). MN and GN denote N-acetylmuramic acid and N-acetylglucosamine, respectively.
Staphylococcus aureus sortase A mutants (srtA) are unable to anchor any one of 19 surface proteins with LPXTG sorting signals to the cell wall envelope [85]. These sortase A mutants display a large defect in virulence as the variants cannot cause lethal sepsis or form abscesses in mouse models for staphylococcal disease [86–88]. Sortase-anchored products play an important role in the pathogenesis of disease caused by many different Gram-positive pathogens [54]. There are, however, also some exceptions. For example, B. anthracis sortase A mutants do not display significant virulence defects [89], whereas proteins in the S-layer, for example, the S-layer-associated protein BslA, make significant contributions to the pathogenesis of anthrax [90,91].
The distribution of sortase A in the envelope of S. pyogenes was examined with fluorescence microscopy at various stages of cell growth [92]. During cell division, most sortase A molecules can be found in the cross wall compartment but also at polar sites of surface protein anchoring [92]. This accumulation decays once cell division is complete. Surprisingly, by applying electron microscopy and immunogold labelling techniques, sortase A could be observed at a single site within the plasma membrane of E. faecalis [58]. As this location coincided with the location of pilin-specific sortase, sortase substrates and SecA, sortase A has been proposed to associate with the aforementioned ExPortal structure [58]. The discrepancy of the data on sortase localization for S. pyogenes and E. faecalis can currently not be reconciled.
7. Sortase B-anchoring of haem scavenging factors
The gene for sortase B (srtB) is located within the iron-regulated surface determinant (isd) gene cluster of bacilli, clostridia, listeria and staphylococci [93]. In S. aureus, the cluster (isdA–isdB- isdCDEF-srtB-isdG) encodes for two sortase A anchored products, IsdA and IsdB, as well as three proteins in the plasma membrane (IsdD and the ABC transporter IsdEF) that transport haem across the plasma membrane [93] (figure 3). Haem-binding NEAr-iron Transporter (NEAT) domains are a common feature of IsdA, IsdB, IsdC and IsdD [93]. IsdG is a cytosolic protein with a unique structure that cleaves the tetrapyrrole ring of haem to release iron for bacterial growth [94,95]. Sortase B cleaves the NPQTN sorting signal of a single substrate protein, eIsdC [85]. SrtB assumes a similar fold and catalytic reaction mechanism as SrtA [96]; however, the enzyme appears to accept the crossbridge amino groups of assembled cell wall as a nucleophile to anchor IsdC in the immediate vicinity of the membrane [97]. SrtB activity and the differential distribution of IsdC compared with the SrtA-anchored products (IsdA and IsdB) enable passage of haem-iron across the bacterial cell wall envelope [98]. IsdB as well as IsdH, another SrtA-anchored protein whose gene is located elsewhere on the bacterial chromosome, remove haem from the host proteins haemoglobin and haptoglobin and transfer the polypeptide to IsdC [93,99–101]. IsdC in turn transfers haem to the membrane protein IsdD for subsequent import of the nutrient into the bacterial cytoplasm [102–104].
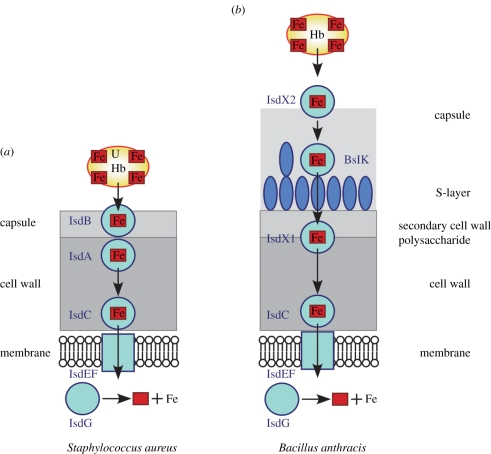
Figure 3.
Haem-iron scavenging in Gram-positive bacteria. (a) The IsdB haemophore of S. aureus removes haem (red box Fe) from haemoglobin (Hb) and transfers the liberated compound to IsdA; both proteins are linked to the cell wall by sortase A. IsdA, in turn, transfers haem to IsdC, a sortase B anchored product that is located in the vicinity of the plasma membrane. Transfer of haem from IsdC to the membrane transporter IsdEF enables staphylococcal import of haem and cleavage by the mono-oxygenase IsdG, which liberates iron (Fe) from haem for staphylococcal growth. (b) Bacillus anthracis secretes IsdX1 and IsdX2, which function as haemophores and haem transfer units. IsdX2 enables transfer of haem to BaslK, an S-layer protein, and then to IsdC. Bacillus anthracis sortase B promotes the anchoring of IsdC to the cell wall envelope. Following haem import across the plasma membrane, the tetrapyrrole is cleaved by IsdG and iron (Fe) is liberated.
Several other Gram-positive microbes express sortase B homologues and have evolved isd gene clusters to accommodate their unique envelope structures [98]. For example, B. anthracis sortase B anchors IsdC to the bacterial peptidoglycan by cleaving its NPKTG sorting signal [105]. Bacilli secrete two haemophores, IsdX1 and IsdX2, into the envelope as well as the extracellular milieu [106]. BasK is a NEAT domain protein in the S-layer of B. anthracis [107]. Haem scavenging of bacilli requires the haemophore activities of IsdX1 and IsdX2 [106], followed by haem transfer to BasK and then to IsdC [107]. Following haem import across the plasma membrane by the ABC transporter IsdEF, the tetrapyrrole ring structure of haem is cleaved by IsdG [108,109].
8. Sortase C-anchoring of proteins to the envelope of spores
The genes for sortase C and its homologues are found only in spore-forming microbes, including B. anthracis and Streptomyces coelicolor [110]. In B. anthracis, the sortase C gene (srtC) is part of the basI-srtC-sctR-sctS operon, which is expressed in a manner requiring the two component regulator SctRS during the onset of sporulation [111] (figure 4). Expression of srtC is initiated precisely 2 h prior to the establishment of the asymmetric septum that initiates B. anthracis spore formation in the carcasses of animals that have succumbed to anthrax disease [111]. SrtC products are first detected in the plasma membrane of sporulating bacilli and, following asymmetric cell division, are subsequently inherited by the mother cell and its endspore [111]. The basI gene product is anchored by SrtC to the peptidoglycan crossbridges of the mother cell envelope [112]. A second substrate gene, basH, is expressed via σF RNA polymerase only in the forespore compartment [111]. BasH precursor is secreted across the plasma membrane into a peptidoglycan compartment bounded by both endospore and mother cell membranes [111]. SrtC cleaves the LPNTA motif of BasI and BasH between the threonyl and the alanyl residues of their LPNTA sorting signals [111,112]. Its acyl-enzyme intermediate accepts the m-Dpm of crossbridges as a nucleophile for the anchoring of mature proteins to either the mother cell (BasI) or the endospore (BasH) peptidoglycan [112]. Sortase C is required for the formation of infectious B. anthracis spores [111].
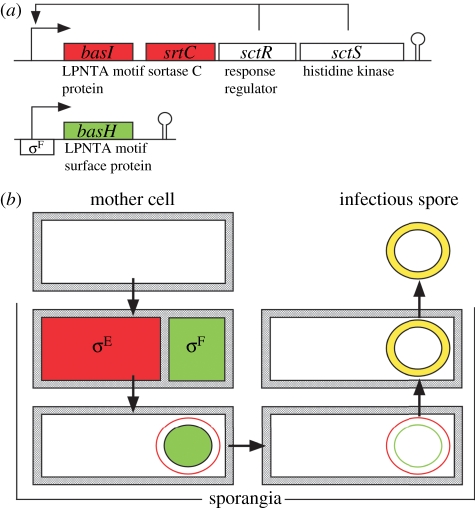
Figure 4.
Role of sortase C in anchoring B. anthracis proteins to the envelope of sporangia or forespores. (a) During sporulation, B. anthracis activates the expression of basI and sortase C (srtC) in the mother cell compartment via the two component regulatory system sctRS. The basH gene is expressed via σF-RNA polymerase in the developing forespore. (b) Sortase C cleaves the LPNTA motif sorting signals of BasI and BasH, anchoring the two polypeptides to the cell wall peptidoglycan of mother cells or the developing forespore, respectively. Sortase C ensures the formation of infectious spores in carcass tissues, i.e. under conditions where oxygen has become limiting.
Streptomyces coelicolor, a high GC content Gram-positive, filamentous soil bacterium, forms mycelia that submerge in liquid or semiliquid environments [113]. The mycelia differentiate to generate aerial hyphae that septate and then develop spore chains for their eventual dissemination in air [114]. Hyphal surfaces are highly hydrophobic, a property that promotes outgrowth into the air and dispersion of spores. Chaplins, a family of surface proteins, are required for aerial hyphae formation, and their anchoring to the cell wall envelope is catalysed by a homologue of sortase C [113,114]. Presumably, chaplins function to lower the aqueous surface tension for the emergence of aerial hyphae [113,114]. Of note, B. anthracis srtC anchored products fulfil a similar role, as sporulating bacilli must satisfy their requirements for oxygen to complete a developmental programme in carcasses where the circulation of oxygenated blood has stopped [111]. Although this has not been shown directly, it is presumed that SrtC anchored products enable bacilli to access air–tissue interfaces.
9. Pilus assembly in Gram-positive bacteria
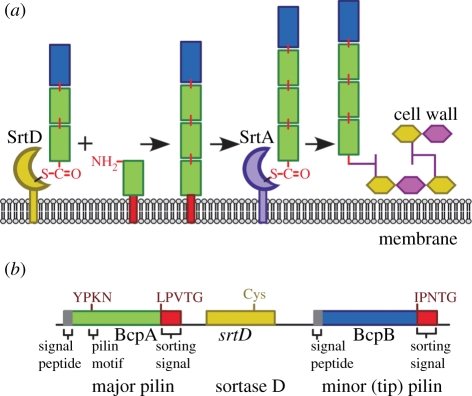
Several different Gram-positive bacteria elaborate pili via a sortase-assembly mechanism, including Actinomyces spp. [115], B. cereus [116], C. diphtheriae [20], Enterococcus spp. [117], Lactobacillus rhamnosus [118], S. agalactiae [119], Streptococcus gallolyticus [120], S. pneumoniae [121] and S. pyogenes [122] (reviewed in Kang et al. [123] and Hendrickx et al. [124]). Gram-positive pili are composed of pilin proteins, whose precursors harbour N-terminal signal peptides and C-terminal sorting signals [20]. The shaft of these 0.1–2 µm pilus filaments is composed of subunits of the major pilin protein [20]. The tip of each filament is decorated with a minor pilin, which promotes attachment of bacteria to host tissues [20,125]. Some, but not all, pili of Gram-positive bacteria harbour yet another pilin subunit, which resides at the base of filaments, providing a link with the cell wall envelope [126]. In microbes whose pili are elaborated from one major and one minor pilin, the filament is tethered to the cell wall envelope through a link with the final major pilin subunit at the base of the structure [127]. For example, pilus assembly in B. cereus involves two pilins, the major (BcpA) and minor (BcpB) subunits, as well as two sortase enzymes [116] (figure 5). The pilin-specific sortase (SrtD) recognizes as a nucleophile the ɛ-amino group of a conserved lysine residue (K) within the YPKN pilin motif, which is present only in BcpA but not in BcpB [128]. SrtD cleaves the C-terminal sorting signals of both BcpA and BcpB to generate acyl-enzyme intermediates [128]. These enzyme intermediates can only be resolved through the nucleophilic attack of the YPKN pilin motif, thereby generating amide (isopeptide) bonds between the C-terminal threonine of the LPXTG sorting signal and the lysine side chain of the YPKN motif in another pilin subunit (intermolecular amide bonds) [128,129]. The second enzyme essential for pilus assembly is sortase A, which recognizes the lipid II amino group as a nucleophile for its acyl-enzyme intermediates [127]. A key feature of B. cereus pilus assembly is the BcpA sorting signal, which has evolved as a substrate for both SrtD and SrtA [128]. Sortase A cleavage of the BcpA sorting signal within a growing pilus filament leads to cell wall anchoring of the pilus, effectively ending the polymerization of pilins. In other words, the substrate properties of BcpA for two sortases are determinants of pilus polymerization and length [130]. The sorting signal of BcpB cannot be cleaved by sortase A [129]. The SrtD acyl intermediate with BcpB requires the BcpA nucleophile for resolution, a substrate property that ensures BcpB deposition at the tip of pili [129].
Figure 5.
Pilus-specific sortase catalyses the polymerization of pili in B. cereus and other Gram-positive bacteria. (a) Pilus-specific sortase (SrtD) cleaves the LPVTG and IPNTG sorting signals of the major (BcpA) and minor (BcpB) pilins, respectively. The acyl-enzyme intermediate is resolved by the nucleophilic attack of the ɛ-amino group of lysine within the YPKN pilin motif. The sorting signal of BcpA, but not of BcpB, can be cleaved by sortase A (SrtA), which promotes cell wall anchoring and effectively terminates pilus assembly. (b) Pilus genes are organized into clusters encompassing the major and minor pilin genes with signal peptides, sorting signals and the YPKN pilin motif (major pilin), as well as the pilus-specific sortase. The structural gene for sortase A (srtA) is located elsewhere on the chromosome.
Corynebacterium diphtheriae assemble pili from three subunits [131]. The sorting signals of the major pilin (SpaA) and the tip adhesin (SpaC) are cleaved by pilin-specific sortase [20]. Here, too, pilus assembly occurs through the nucleophilic attack of the ɛ-amino group of lysine within the YPKN pilin motif of the major pilin (SpaA). SpaB is cleaved by the house-keeping sortase of corynebacteria and anchored to the cell wall envelope [126,132]. A unique feature of SpaB is its substrate property of presenting the ɛ-amino group of a lysine residue as a nucleophile to pilin-specific sortases [126]. This mechanism ensures that transfer of polymerized pili to SpaB leads to the cell wall anchoring of the polymerized structure [126].
X-ray crystallography of recombinant (non-polymerized) pilin subunits provided key insights into the assembly of pili and surface proteins of Gram-positive bacteria [133]. Gbs52, the minor pilin from S. agalactiae, folds into two adjacent immunoglobulin (Ig)-like domains with seven anti-parallel β-strands [134]. One of the two Ig-like domains harbours an intramolecular isopeptide bond [134]. Similar intramolecular bonds were identified in the major pilin subunit of S. pyogenes (Spy0128) [133]. These bonds are formed autocatalyically from the side chains of lysine (Lys) and asparagine (Asp) residues via a mechanism requiring close proximity of the carboxylate of glutamic acid (Glu) or aspartic acid (Asp) [123,133,135]. Intramolecular isopeptide bonds are a stabilizing feature of Cna-B type domains, i.e. Ig-like domains that were first described in the Cna surface protein of S. aureus [136]. Cna-B type domains as well as other folds with intramolecular isopeptide bonds are found in many surface proteins of Gram-positive bacteria [133]. They appear to function as stabilizers of secreted proteins, which, in many Gram-positive bacteria, cannot acquire disulphide bonds, as occurs via the DsbAB pathway for proteins secreted into the periplasm of Gram-negative bacteria [137]. X-ray crystallography also provided insights into the catalysis of pilus assembly by revealing the structure of pilin-specific sortases [138]. The overall structure of pilin-specific sortases is similar to that of sortase A and sortase B [74,138,139]. A distinguishing feature of the pilin-specific sortases of S. pneumoniae and S. agalactiae is a flexible lid that covers the active site of the enzyme [138,140]. The contributions of the lid towards sortase recognition of the side chain of lysine within the YPKN motif of major pilins are not yet appreciated. X-ray crystallography of Spy0129, the pilus-specific sortase of M1 strain S. pyogenes SF370 (SrtC1) revealed a structure more similar to that of sortase B and without a lid over the active site [141,142]. Current work on pilus formation in Gram-positive bacteria focuses on in vitro assembly assays and a complete structural appreciation of how sortases recognize pilin subunits and assemble the pilus fibre [143–145].
10. S-layer assembly in bacilli and clostridia
Surface layers (S-layers) are para-crystalline sheets of proteins that assemble on the surface of bacteria [21,146]. Bacteria elaborate S-layers by abundantly secreting one or two proteins that self assemble into a two-dimensional lattice on the microbial surface [147]. Other proteins associate with the main S-layer proteins and fulfil variable functions in that they act either as a scaffold or enzyme in the bacterial envelope [148], promote nutrient diffusion or transport [107], or contribute to virulence by enabling microbial adhesion to infected host tissues [91]. S-layer proteins and S-layer-associated proteins of many bacteria share three tandem approximately 55 amino acid repeats of the surface-layer homology (SLH) domain [149–151]. Secreted proteins harbouring three tandem SLH domains are tethered to the bacterial envelope by non-covalent interactions between the SLH domains and a secondary cell wall carbohydrate [22]. SLH domains are remarkable for being both necessary and sufficient for the incorporation of chimeric proteins into S-layers [152].
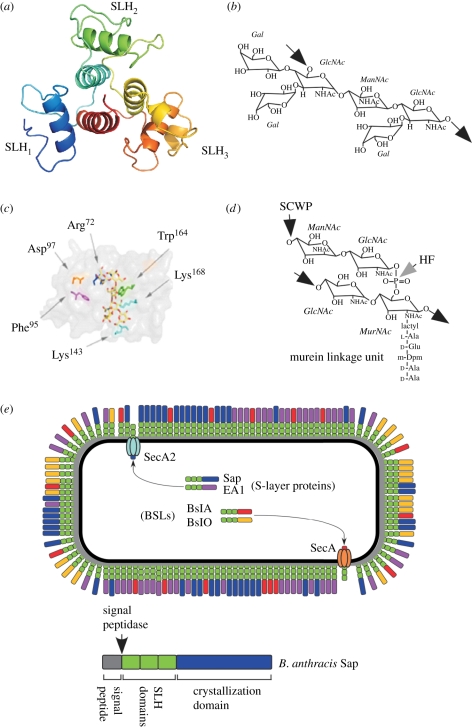
Members of the B. cereus sensu lato group are rod-shaped, spore-forming bacteria [153]. The envelope of their vegetative forms is composed of a plasma membrane and peptidoglycan layer with attached SCWP [154] and capsules composed of polysaccharides, hyaluronic acid or poly-d-γ-glutamic acid [51,155–157]. The genome of B. anthracis, a member of the B. cereus sensu lato group, encompasses 24 open reading frames whose predicted translation products each contain a secretion signal and three tandem SLH domains [90,158] (figure 6). The B. anthracis csaA-csaB-sap-eag gene cluster encodes the two S-layer proteins, surface array protein (Sap) and extractable antigen 1 (EA1) [148,159,160], as well as CsaB, a pyruvyl transferase responsible for decorating SCWP with ketal-pyruvate [22,53]. Bacillus anthracis SCWP is a polymer with the repeating structure [→6)-α-GlcNAc-(1→4)-β-ManNAc-(1→4)-β-GlcNAc-(1→]n, where α-GlcNAc is substituted with α-Gal and β-Gal at O3 and O4, respectively, and the β-GlcNAc is substituted with α-Gal at O3 [52] (figure 6b). The SCWP is linked to the peptidoglycan layer via murein linkage units [53], i.e. a GlcNAc-ManNAc moiety that is phosphodiester linked to the C6 hydroxyl of MurNAc [16] (figure 6d). Bacillus anthracis forms S-layers from both Sap and EA1 by tethering the SLH domains of these polypeptides to pyruvylated SCWP [53,159,161] (figure 6a–c). C-terminal to the SLH domains, Sap and EA1 harbour crystallization domains that engage in recriprocal subunit–subunit interactions to constitute the S-layer [159,162,163] (figure 6e). A current model for S-layer assembly is that secreted subunits are recruited to the edge of an extant S-layer network via enthalpy-driven interactions between crystallization domains and are then tethered to the SCWP via the SLH domains [22]. This model matches growth of the S-layer(s) with increases in the avidity of these networks for the cell wall. This model can also explain how S-layers assemble on top of the peptidoglycan layer and thread capsule between individual S-layer subunits [51,160].
Figure 6.
S-layer assembly occurs through the association of secreted proteins with SLH domains and the SCWP. (a) Three pronged spindle structure of the SLH domains of S-layer and S-layer-associated proteins in B. anthracis. (b) Structure of the SCWP of B. anthracis. (c) Conserved residues of the SLH domains of bacilli and the location within the inter-prong grooves of SLH domains. The SCWP was modelled into the inter-prong grooves of the SLH domain structure. (d) Structure of the murein linkage unit that tethers the SCWP to the peptidoglycan of bacilli. (e) Model for the secretion and assembly of S-layer proteins in clostridia and bacilli, which appears to involve one or two major S-layer proteins as well as S-layer-associated proteins. Precursors can travel via the canonical secretory pathway involving SecA or a SecA2 pathway that appears dedicated for the secretion of S-layer proteins. The distribution of S-layer and S-layer-associated proteins is thought to be organized, albeit that the mechanisms for such organization have not yet been revealed.
The overall structure of the three SLH domains resembles a three-pronged spindle, where each prong is derived from a single SLH domain [164] (figure 6a). The base of the spindle is assembled from all three domains, each of which contributes a single helix that associates into a three-helical bundle [164]. As each of the three SLH domains assumes a nearly identical fold, one can consider the entire structure as a pseudo-trimer. A group of five residues, designated the ITRAE motif for its consensus sequence, is partially conserved among the SLH domains of bacterial S-layer proteins [151]. The motif occupies the last four residues of loop B and the first residue of the central helix bundle. Within the SLH domains of B. anthracis Sap, these motifs have the sequences LTRAE, IDRVS and VTKAE, and contain the cationic residues Arg72, Arg131 and Lys193, respectively [164]. The corresponding positively charged residues of the ITRAE motif are necessary for the incorporation of SLH domain proteins into the S-layer [164].
A B. anthracis csaB mutant, which cannot pyruvylate the SCWP, is unable to assemble secreted Sap or EA1 into S-layers and forms elongated chains of vegetative forms that fail to separate [22]. This phenotype suggests a key function of S-layer and S-layer-associated proteins during the cell cycle of bacilli. The csaB phenotype suggests further that proteins within the S-layer may not be randomly distributed but rather assume discrete positions to fulfil their function. For example, the S-layer-associated protein BslO is deposited near the cell wall septa of bacilli [165]. Mutants lacking bslO display an elongated chain phenotype that can be complemented in trans with purified BslO. The glucosaminidase domain of BslO is thought to cleave septal peptidoglycan to promote the separation of vegetative forms [165]. The mechanism whereby BslO is localized to the septal portion of the bacillus S-layer has not yet been elucidated. Clostridium difficile, an anaerobic microbe that also forms spores, encodes two secA genes, one of which (secA2) is required for assembly of its S-layer proteins and the cell wall protein CwpV [166]. It is not yet clear how SecA2 selects S-layer protein precursors for secretion and why this mechanism contributes to S-layer assembly. Nevertheless, other S-layer producing microbes, for example, B. anthracis, also harbour secA2 genes [158]. Thus, it is conceivable that S-layer assembly in many microbes involves a dedicated secretion pathway for the abundant transport of S-layer proteins with SLH domains.
Not all bacterial S-layers are assembled from proteins with SLH domains. The SbsC protein of Geobacillus stearothermophilus is an example for a class of protein that forms S-layers without SLH domains [167]. SbsC binds to the SCWP of G. stearothermophilus via its N-terminal domain, which consists of three triple-helical bundles connected by two contiguous helices [167]. The N-terminal domain of SbsC has high similarity with S-layer proteins from G. stearothermophilus, Geobacillus kaustophilus and Geobacillus tepidamans, suggesting that its mechanisms of assembly are conserved in other microbes [167].
11. Type VII secretion systems in Gram-positive bacteria
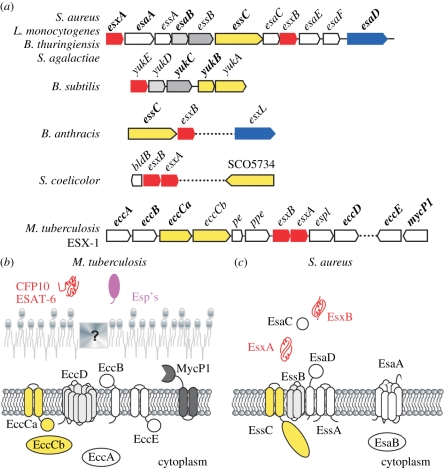
ESAT-6 (EsxA) and its homologue CFP-10 (EsxB) are small α-helical polypeptides and founding members of the WXG100 motif family [168,169]. ESAT-6 (Early Secreted Antigen 6 kDa) and CFP-10 (Culture Filtrate Protein 10 kDa) are secreted by Mycobacterium tuberculosis. Mark Pallen first suggested that genes clustering with esxA and esxB in the genome of M. tuberculosis may represent a novel secretion system (figure 7) [168]. This conjecture was proven to be correct when mutations in this gene cluster caused secretion defects for ESAT-6 and CFP-10 [173–175]. Nevertheless, the molecular mechanisms and the biochemical identity of the proposed ESAT-6 secretion machinery responsible for WXG100 protein secretion have not been revealed. Available models are derived from genetic variations and observations of mutant phenotypes when specific genes in the ESAT-6 and CFP-10 clusters are disrupted (figure 7b) [170]. It was recently suggested that ESAT-6 secretion should be referred to as a type VII secretion system [170,176]. The numerical classification is derived from Gram-negative bacteria, where polypeptides are transported across double membrane envelopes using mechanisms that are either independent of or expand the canonical Sec pathway [177]. Mycobacterial cell walls also encompass a double membrane envelope, including the plasma membrane and the mycolic acid layer with long aliphatic lipids [178–180]. Thus, the term type VII secretion appeared to fit with the previously discovered type I–VI pathways [170]. Nevertheless, as already noted by Pallen, genes encoding for putative WXG100 proteins are also found in the genomes of Gram-positive bacteria lacking double membrane envelopes (figure 7a) [168]. For example, two small WXG100 proteins, EsxA and EsxB, are secreted by S. aureus in a manner depending on genes that are clustered with esxA and esxB [171,172,181]. This gene cluster has been named for its function: ESAT-6 secretion system (Ess) [171] and a model for protein secretion were again derived from the genetic analysis of mutations in this cluster (figure 7c) [171,172]. It is difficult to draw parallels and commonalities between the WXG100 secretion systems for mycobacteria and staphylococci. Although WXG100 proteins carry a typical amino acid WXG signature, they share very little overall identity. Their striking similarity lies in the α-helical hairpin structure adopted by these proteins [169,182,183]. Further, only genes specifying for a predicted ATPase with FtsK-SpoIIIE domain are shared between staphylococcal Ess and mycobacterial T7SS. In mycobacteria, one of the ATPases appears to select substrates for secretion, whereas in S. aureus it is simply required for the secretion of EsxA and EsxB. Unlike CFP-10, where a C-terminal sequence was shown to be necessary for the secretion of CFP-10:ESAT-6 complexes [184], such an element has not be observed in staphylococcal EsxA and EsxB (M. Anderson 2011, personal communication). Other mycobacterial components, among them the EspACD proteins, also seem necessary for the secretion of ESAT-6 and CFP-10 [185]. Even more intriguing is the disparity in genetic composition of putative Ess pathways among mycobacteria and Gram-positive bacteria. Mycobacteria, but perhaps not other Gram-positive bacteria, harbour gene clusters that appear to encode five distinct type VII secretion systems (ESX1-5) [186]. Of these, the ESX-4 system may be the simplest system whose components also display the highest degree of conservation with the type VII systems of other Gram-positive bacteria [186]. Experimental proof for WXG100 protein secretion has thus far been garnered in two other Gram-positive organisms, B. anthracis and S. coelicolor. B. anthracis encodes six proteins with a WXG100 domain, only one of them, Ba-EsxB, is as short as ESAT-6 or CFP-10 (90 amino acids). This protein does not require the FtsK-SpoIIIE ATPase in the Ess cluster for its secretion [187]. However, Ba-EsxB is essential for the secretion of Ba-EsxW, a protein with an N-terminal WXG100 domain and a large C-terminal domain of unknown function encoded outside the B. anthracis Ess cluster. Intriguingly, only B. anthracis appears to encode WXG100 proteins with large C-terminal extensions. Several pathogenic bacilli (B. cereus and B. thuringiensis) encode an Ess-like gene cluster, whereas the non-pathogenic B. subtilis encodes a minimal Ess gene cluster with only one WXG100 gene (yukE), a split FtsK-SpoIIIE ATPase gene and essB esaB like genes (yukC yukD) (figure 7a). The S. coelicolor WXG100 proteins EsxA and EsxB have also been shown to be secreted [188]. Streptomyces esxA and esxB genes are located on a region of the chromosome that includes the regulator BlbD and the FtsK-SpoIIIE ATPase. Other genes within this cluster encode for proteins with domains of unknown function. Streptomyces coelicolor EsxA and EsxB are involved in the morphogenetic development that supports aerial hyphae and spore formation [188].
Figure 7.
Genetic organization and models for WXG100 protein secretion in M. tuberculosis and S. aureus. (a) Gene clusters showing WXG100 products (in red) along with predicted FtsK-SpoIIIE ATPases (in yellow) are shown. The top diagram uses the nomenclature established experimentally for S. aureus and depicts ess genes required for ESAT-6-like secretion, esa genes playing an accessory role for ESAT-6 like secretion and esx genes encoding WXG100 proteins (ESAT-6 like). Gene names shown shaded represent putative plasma membrane proteins. Light and dark grey colours show genes conserved among Gram-positive bacteria while white colour is used when the function of the gene is unknown. The genetic organization for M. tuberculosis is shown only for ESX-1 (one of the five WXG100 secretion systems) and the nomenclature is as described by Bitter et al. [170]. Several Esp encoding genes encoded outside the ESX-1 cluster are not shown. (b,c) Models showing the cellular localization and predicted topology of conserved (Ecc), associated (Esp) and the MycP1 proteins of the ESX-1 gene cluster as well as conserved and accessory (Ess, Esa) proteins of the secretion system in S. aureus. The depiction of EccD and EssB as translocons is speculated based on prediction of transmembrane spanning sequences for these proteins. In S. aureus, the possible WXG100 translocon could be constituted of EssB, EsaD and EssA [171,172].
Loss of ESAT-6 and CFP-10 secretion affects the ability of M. tuberculosis to replicate in macrophages and to suppress innate and adaptive immune responses [175,189–192]. Loss of Ess-dependent secretion in S. aureus affects the developmental programme of abscess formation and staphylococcal persistence in host tissues [181]. A function for WXG100 proteins in B. anthracis could not be deduced. In spite of their dissimilarities, Ess or type VII secretion systems in mycobacteria and Gram-positive bacteria must share some functional properties when supporting the secretion of WXG100 proteins with similar structure. Nevertheless, Ess/type VII pathways certainly fulfil different functions, considering their wide distribution among Gram-positive bacteria and lack of a convergent phenotypic trait for mutants in this pathway in different bacteria. Much remains to be discovered regarding the molecular mechanisms that support substrate recognition, secretion or the pathophysiological attributes of the secreted products. Considering that conserved genes (FtsK/SpoIIIE type ATPase and WXG-100 proteins) are shared among bacterial pathogens that generate distinctive disease features, the effectors of type VII and type VII-like secretion systems may be proteins that do not belong to the WXG-100 family, as has been reported for M. tuberculosis and S. aureus [172,193].
Acknowledgements
The authors wish to thank members of their laboratories for contributions to the field of protein secretion in Gram-positive bacteria. Work described herein was supported by awards from the National Institutes of Allergy and Infectious Diseases, Infectious Diseases Branch AI38897, AI52474, AI52767 and AI69227. The authors acknowledge membership within and support from the Region V ‘Great Lakes’ Regional Center of Excellence in Biodefense and Emerging Infectious Diseases Consortium (NIH Award 1-U54-AI-057153).
References
- 1.Gram H. C. J. 1884. Über die isolierte Färbung der Schizomyceten in Schnitt- und Trockenpräparaten. Fortschritte der Medizin. 2, 185–189 10.1016/S0168-6445(03)00047-0 (doi:10.1016/S0168-6445(03)00047-0) [DOI] [Google Scholar]
- 2.Shockman G. D., Barrett J. F. 1983. Structure, function, and assembly of cell walls of Gram-positive bacteria. Annu. Rev. Microbiol. 37, 501–527 10.1146/annurev.mi.37.100183.002441 (doi:10.1146/annurev.mi.37.100183.002441) [DOI] [PubMed] [Google Scholar]
- 3.Osborn M. J., Gander J. E., Parisi E., Carson J. 1972. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J. Biol. Chem. 247, 3962–3972 10.2174/1568026013394831 (doi:10.2174/1568026013394831) [DOI] [PubMed] [Google Scholar]
- 4.Duong F., Eichler J., Price A., Leonard M. R., Wickner W. 1997. Biogenesis of the Gram-negative bacterial envelope. Cell 91, 567–573 10.1016/S0092-8674(00)80444-4 (doi:10.1016/S0092-8674(00)80444-4) [DOI] [PubMed] [Google Scholar]
- 5.Schneewind O., Model P., Fischetti V. A. 1992. Sorting of protein A to the staphylococcal cell wall. Cell 70, 267–281 10.1016/0092-8674(92)90101-H (doi:10.1016/0092-8674(92)90101-H) [DOI] [PubMed] [Google Scholar]
- 6.Model P., Russel M. 1990. Prokaryotic secretion. Cell 61, 739–741 10.1016/0092-8674(90)90180-M (doi:10.1016/0092-8674(90)90180-M) [DOI] [PubMed] [Google Scholar]
- 7.Park J. T., Johnson M. J. 1949. Accumulation of labile phosphate in Staphylococcus aureus grown in the presence of penicillin. J. Biol. Chem. 179, 585–592 10.1128/JB.183.20.5803-5812.2001 (doi:10.1128/JB.183.20.5803-5812.2001) [DOI] [PubMed] [Google Scholar]
- 8.Strange R. E., Kent L. H. 1959. The isolation, characterization and chemical synthesis of muramic acid. Biochem. J. 71, 333–339 10.1038/nature01050 (doi:10.1038/nature01050) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park J. T. 1952. Uridine-5′-pyrophosphate derivatives. III. Amino acid-containing derivatives. J. Biol. Chem. 194, 897–904 10.1126/science.1083137 (doi:10.1126/science.1083137) [DOI] [PubMed] [Google Scholar]
- 10.Park J. T., Strominger J. L. 1957. Mode of action of penicillin. Science 125, 99–101 10.1126/science.125.3238.99 (doi:10.1126/science.125.3238.99) [DOI] [PubMed] [Google Scholar]
- 11.Higashi Y., Strominger J. L., Sweeley C. C. 1967. Structure of a lipid intermediate in cell wall peptidoglycan synthesis: a derivative of C55 isoprenoid alcohol. Proc. Natl Acad. Sci. USA 57, 1878–1884 10.1073/pnas.57.6.1878 (doi:10.1073/pnas.57.6.1878) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson J. S., Matsuhashi M., Haskin M. A., Strominger J. L. 1965. Lipid-phosphoacetylmuramyl-pentapeptide and lipid-phosphodisaccharide-pentapeptide: presumed membrane transport intermediates in cell wall synthesis. Proc. Natl Acad. Sci. USA 53, 881–889 10.1073/pnas.53.4.881 (doi:10.1073/pnas.53.4.881) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tipper D. J., Strominger J. L. 1965. Mechanism of action of penicillins: a proposal based on their structural similarity to acyl-d-alanyl-alanine. Proc. Natl Acad. Sci. USA 54, 1133–1141 10.1073/pnas.54.4.1133 (doi:10.1073/pnas.54.4.1133) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strominger J. L. 1968. Penicillin-sensitive enzymatic reactions in bacterial cell wall synthesis. Harvey Lect. 64, 179–213 10.1016/j.jsb.2006.12.004 (doi:10.1016/j.jsb.2006.12.004) [DOI] [PubMed] [Google Scholar]
- 15.Ghuysen J.-M. 1968. Use of bacteriolytic enzymes in determination of wall structure and their role in cell metabolism. Bacteriol. Rev. 32, 425–464 10.1128/JB.00684-06 (doi:10.1128/JB.00684-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coley J., Archibald A. R., Baddiley J. 1976. A linkage unit joining peptidoglycan to teichoic acid in Staphylococcus aureus H. FEBS Lett. 61, 240–242 10.1016/0014-5793(76)81047-2 (doi:10.1016/0014-5793(76)81047-2) [DOI] [PubMed] [Google Scholar]
- 17.Baba T., Schneewind O. 1996. Target cell specificity of a bacteriocin molecule: a C-terminal signal directs lysostaphin to the cell wall of Staphylococcus aureus. EMBO J. 15, 4789–4797 10.1128/JB.182.6.1754-1756.2000 (doi:10.1128/JB.182.6.1754-1756.2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jonquieres R., Bierne H., Fiedler F., Gounon P., Cossart P. 1999. Interaction between the protein InlB of Listeria monocytogenes and lipoteichoic acid: a novel mechanism of protein association at the surface of Gram-positive bacteria. Mol. Microbiol. 34, 902–914 10.1046/j.1365-2958.1999.01652.x (doi:10.1046/j.1365-2958.1999.01652.x) [DOI] [PubMed] [Google Scholar]
- 19.Schneewind O., Fowler A., Faull K. F. 1995. Structure of the cell wall anchor of surface proteins in Staphylococcus aureus. Science 268, 103–106 10.1126/science.7701329 (doi:10.1126/science.7701329) [DOI] [PubMed] [Google Scholar]
- 20.Ton-That H., Schneewind O. 2003. Assembly of pili on the surface of Corynebacterium diphtheriae. Mol. Microbiol. 50, 1429–1438 10.1046/j.1365-2958.2003.03782.x (doi:10.1046/j.1365-2958.2003.03782.x) [DOI] [PubMed] [Google Scholar]
- 21.Houwink A. L. 1953. A macromolecular monolayer in the cell wall of Spirillium spec. Biochim. Biophys. Acta 10, 360–366 10.1016/0006-3002(53)90266-2 (doi:10.1016/0006-3002(53)90266-2) [DOI] [PubMed] [Google Scholar]
- 22.Mesnage S., Fontaine T., Mignot T., Delepierre M., Mock M., Fouet A. 2000. Bacterial SLH domain proteins are non-covalently anchored to the cell surface via a conserved mechanism involving wall polysaccharide pyruvylation. EMBO J. 19, 4473–4484 10.1093/emboj/19.17.4473 (doi:10.1093/emboj/19.17.4473) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Navarre W. W., Schneewind O. 1999. Surface proteins of Gram-positive bacteria and the mechanisms of their targeting to the cell wall envelope. Microbiol. Mol. Biol. Rev. 63, 174–229 10.1093/jac/dkl426 (doi:10.1093/jac/dkl426) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Emr S. D., Hanley-Way S., Silhavy T. J. 1981. Suppressor mutations that restore export of a protein with a defective signal sequence. Cell 23, 79–88 10.1016/0092-8674(81)90272-5 (doi:10.1016/0092-8674(81)90272-5) [DOI] [PubMed] [Google Scholar]
- 25.Oliver D. B., Beckwith J. 1981. E. coli mutant pleiotropically defective in the export of secreted proteins. Cell 25, 765–772 10.1016/0092-8674(81)90184-7 (doi:10.1016/0092-8674(81)90184-7) [DOI] [PubMed] [Google Scholar]
- 26.Hartl F. U., Lecker S., Schiebel E., Hendrick J. P., Wickner W. 1990. The binding cascade of SecB to SecA to SecY/E mediates preprotein targeting to the E. coli plasma membrane. Cell 63, 269–279 10.1016/0092-8674(90)90160-G (doi:10.1016/0092-8674(90)90160-G) [DOI] [PubMed] [Google Scholar]
- 27.Gorlich D., Rapoport T. A. 1993. Protein translocation into proteoliposomes reconstituted from purified components of the endoplasmic reticulum membrane. Cell 75, 615–630 10.1016/0092-8674(93)90483-7 (doi:10.1016/0092-8674(93)90483-7) [DOI] [PubMed] [Google Scholar]
- 28.Economou A., Pogliano J. A., Beckwith J., Oliver D. B., Wickner W. 1995. SecA membrane cycling at SecYEG is driven by distinct ATP binding and hydrolysis events and is regulated by SecD and SecF. Cell 83, 1171–1181 10.1016/0092-8674(95)90143-4 (doi:10.1016/0092-8674(95)90143-4) [DOI] [PubMed] [Google Scholar]
- 29.Duong F., Wickner W. 1997. The SecDFYajC domain of preprotein translocase controls preprotein movement by regulating SecA membrane cycling. EMBO J. 16, 4871–4879 10.1093/emboj/16.16.4871 (doi:10.1093/emboj/16.16.4871) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dalbey R. E., Wickner W. 1985. Leader peptidase catalyzes the release of exported proteins from the outer surface of the Escherichia coli plasma membrane. J. Biol. Chem. 260, 15 925–15 931 10.1038/35016007 (doi:10.1038/35016007) [DOI] [PubMed] [Google Scholar]
- 31.Miller J. D., Bernstein H. D., Walter P. 1994. Interaction of E. coli Ffh/4.5S ribonucleoprotein and FtsY mimics that of mammalian signal recognition particle and its receptor. Nature 367, 657–659 10.1038/367657a0 (doi:10.1038/367657a0) [DOI] [PubMed] [Google Scholar]
- 32.Halic M., Blau M., Becker T., Mielke T., Pool M., Wild K., Sinning I., Beckmann R. 2006. Following the signal sequence from ribosomal tunnel exit to signal recognition particle. Nature 444, 507–511 10.1038/nature05326 (doi:10.1038/nature05326) [DOI] [PubMed] [Google Scholar]
- 33.Randall L. L. 1992. Peptide binding by chaperone SecB: implications for recognition of non-native structure. Science 257, 241–245 10.1126/science.1631545 (doi:10.1126/science.1631545) [DOI] [PubMed] [Google Scholar]
- 34.Wild J., Rossmeissl P., Walter W. A., Gross C. A. 1996. Involvement of the DnaK-DnaJ-GrpE chaperone team in protein secretion in Escherichia coli. J. Bacteriol. 178, 3608–3613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stoller G., Rucknagel K. P., Nierhaus K. H., Schmid F. X., Fischer G., Rahfeld J. U. 1995. A ribosome-associated peptidyl-prolyl cis/trans isomerase identified as the trigger factor. EMBO J. 14, 4939–4948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beck K., Wu L.-F., Brunner J., Muller M. 2000. Discrimination of SRP- and SecA/SecB-dependent substrates involves selective recognition of nascent chains by SRP and trigger factor. EMBO J. 19, 134–143 10.1093/emboj/19.1.134 (doi:10.1093/emboj/19.1.134) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sibbald M. J., et al. 2006. Mapping the pathways to staphylococcal pathogenesis by comparative secretomics. Microbiol. Mol. Biol. Rev. 70, 755–788 10.1128/MMBR.00008-06 (doi:10.1128/MMBR.00008-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kontinen V. P., Saris P., Sarvas M. 1991. A gene (prsA) of Bacillus subtilis involved in a novel, late stage of protein export. Mol. Microbiol. 5, 1273–1283 10.1111/j.1365-2958.1991.tb01901.x (doi:10.1111/j.1365-2958.1991.tb01901.x) [DOI] [PubMed] [Google Scholar]
- 39.Bensing B. A., Sullam P. M. 2002. An accessory sec locus of Streptococcus gordonii is required for export of the surface protein GspB and for normal levels of binding to human platelets. Mol. Microbiol. 44, 1081–1094 10.1046/j.1365-2958.2002.02949.x (doi:10.1046/j.1365-2958.2002.02949.x) [DOI] [PubMed] [Google Scholar]
- 40.Siboo I. R., Chaffin D. O., Rubens C. E., Sullam P. M. 2008. Characterization of the accessory Sec system of Staphylococcus aureus. J. Bacteriol. 190, 6188–6196 10.1128/JB.00300-08 (doi:10.1128/JB.00300-08) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bensing B. A., Sullam P. M. 2010. Transport of preproteins by the accessory Sec system requires a specific domain adjacent to the signal peptide. J. Bacteriol. 192, 4223–4232 10.1128/JB.00373-10 (doi:10.1128/JB.00373-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seepersaud R., Bensing B. A., Yen Y. T., Sullam P. M. 2010. Asp3 mediates multiple protein-protein interactions within the accessory Sec system of Streptococcus gordonii. Mol. Microbiol. 78, 490–505 10.1111/j.1365-2958.2010.07346.x (doi:10.1111/j.1365-2958.2010.07346.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rigel N. W., Braunstein M. 2008. A new twist on an old pathway—accessory secretory systems. Mol. Microbiol. 69, 291–302 10.1111/j.1365-2958.2008.06294.x (doi:10.1111/j.1365-2958.2008.06294.x) [Erratum in Mol. Microbiol. 2008 70, 271. (doi:10.1111/j.1365-2958.2008.06433.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Phillips G. J., Silhavy T. J. 1992. The E. coli ffh gene is necessary for viability and efficient protein export. Nature 359, 744–746 10.1038/359744a0 (doi:10.1038/359744a0) [DOI] [PubMed] [Google Scholar]
- 45.Crowley P. J., Svensäter G., Snoep J. L., Bleiweis A. S., Brady L. J. 2004. An ffh mutant of Streptococcus mutans is viable and able to physiologically adapt to low pH in continuous culture. FEMS Microbiol. Lett. 234, 315–324 10.1111/j.1574-6968.2004.tb09550.x (doi:10.1111/j.1574-6968.2004.tb09550.x) [DOI] [PubMed] [Google Scholar]
- 46.Hasona A., Crowley P. J., Levesque C. M., Mair R. W., Cvitkovitch D. G., Bleiweis A. S., Brady L. J. 2005. Streptococcal viability and diminished stress tolerance in mutants lacking the signal recognition particle or YidC2. Proc. Natl Acad. Sci. USA 102, 17 466–17 471 10.1073/pnas.0508778102 (doi:10.1073/pnas.0508778102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Funes S., et al. 2009. Independent gene duplications of the YidC/Oxa/Alb3 family enabled specialized contranslational function. Proc. Natl Acad. Sci. USA 106, 6656–6661 10.1073/pnas.0809951106 (doi:10.1073/pnas.0809951106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Giesbrecht P., Kersten T., Maidhof H., Wecke J. 1998. Staphylococcal cell wall: morphogenesis and fatal variations in the presence of penicillin. Microbiol. Mol. Biol. Rev. 62, 1371–1414 10.1016/S0006-3495(01)76033-X (doi:10.1016/S0006-3495(01)76033-X) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salton M. R. J. 1952. Cell wall of Micrococcus lysodeikticus as the substrate of lysozyme. Nature 170, 746–747 10.1038/170746a0 (doi:10.1038/170746a0) [DOI] [PubMed] [Google Scholar]
- 50.Navarre W. W., Daefler S., Schneewind O. 1996. Cell wall sorting of lipoproteins in Staphylococcus aureus. J. Bacteriol. 178, 441–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Richter G. S., Anderson V. J., Garufi G., Lu L., Joachimiak A., He C., Schneewind O., Missiakas D. 2009. Capsule anchoring in Bacillus anthracis occurs by a transpeptidation mechanism that is inhibited by capsidin. Mol. Microbiol. 71, 404–420 10.1111/j.1365-2958.2008.06533.x (doi:10.1111/j.1365-2958.2008.06533.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Choudhury B., Leoff C., Saile E., Wilkins P., Quinn C. P., Kannenberg E. L., Carlson R. W. 2006. The structure of the major cell wall polysaccharide of Bacillus anthracis is species specific. J. Biol. Chem. 281, 27 932–27 941 10.1074/jbc.M605768200 (doi:10.1074/jbc.M605768200) [DOI] [PubMed] [Google Scholar]
- 53.Kern J., Ryan C., Faull K., Schneewind O. 2010. Bacillus anthracis surface-layer proteins assemble by binding to the secondary cell wall polysaccharide in a manner that requires csaB and tagO. J. Mol. Biol. 401, 757–775 10.1016/j.jmb.2010.06.059 (doi:10.1016/j.jmb.2010.06.059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marraffini L. A., Dedent A. C., Schneewind O. 2006. Sortases and the art of anchoring proteins to the envelopes of Gram-positive bacteria. Microbiol. Mol. Biol. Rev. 70, 192–221 10.1128/MMBR.70.1.192-221.2006 (doi:10.1128/MMBR.70.1.192-221.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rosch J., Caparon M. 2004. A microdomain for protein secretion in Gram-positive bacteria. Science 304, 1513–1515 10.1126/science.1097404 (doi:10.1126/science.1097404) [DOI] [PubMed] [Google Scholar]
- 56.Rosch J. W., Caparon M. G. 2005. The ExPortal: an organelle dedicated to the biogenesis of secreted proteins in Streptococcus pyogenes. Mol. Microbiol. 58, 959–968 10.1111/j.1365-2958.2005.04887.x (doi:10.1111/j.1365-2958.2005.04887.x) [DOI] [PubMed] [Google Scholar]
- 57.Lyon W. R., Caparon M. G. 2004. Role for serine protease HtrA (DegP) of Streptococcus pyogenes in the biogenesis of virulence factors SpeB and the hemolysin streptolysin S. Infect Immun. 72, 1618–1625 10.1128/IAI.72.3.1618-1625.2004 (doi:10.1128/IAI.72.3.1618-1625.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kline K. A., et al. 2009. Mechanism for sortase localization and the role of sortase localization in efficient pilus assembly in Enterococcus faecalis. J. Bacteriol. 191, 3237–3247 10.1128/JB.01837-08 (doi:10.1128/JB.01837-08) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carlsson F., Stalhammar-Carlemalm M., Flardh K., Sandin C., Carlemalm E., Lindahl G. 2006. Signal sequence directs localized secretion of bacterial surface proteins. Nature 442, 943–946 10.1038/nature05021 (doi:10.1038/nature05021) [DOI] [PubMed] [Google Scholar]
- 60.Rosenstein R., Gotz F. 2000. Staphylococcal lipases: biochemical and molecular characterization. Biochimie 82, 1005–1014 10.1016/S0300-9084(00)01180-9 (doi:10.1016/S0300-9084(00)01180-9) [DOI] [PubMed] [Google Scholar]
- 61.Tettelin H., et al. 2001. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science 293, 498–506 10.1126/science.1061217 (doi:10.1126/science.1061217) [DOI] [PubMed] [Google Scholar]
- 62.Bae T., Schneewind O. 2003. The YSIRK-G/S motif of staphylococcal protein A and its role in efficiency of signal peptide processing. J. Bacteriol. 185, 2910–2919 10.1128/JB.185.9.2910-2919.2003 (doi:10.1128/JB.185.9.2910-2919.2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dedent A. C., Missiakas D. M., Schneewind O. 2008. Signal peptides direct surface proteins to two distinct envelope locations of Staphylococcus aureus. EMBO J. 27, 2656–2668 10.1038/emboj.2008.185 (doi:10.1038/emboj.2008.185) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dedent A. C., Mcadow M., Schneewind O. 2007. Distribution of protein A on the surface of Staphylococcus aureus. J. Bacteriol. 189, 4473–4484 10.1128/JB.00227-07 (doi:10.1128/JB.00227-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Frankel M. B., Wojcik B. M., Dedent A. C., Missiakas D. M., Schneewind O. 2010. ABI-domain containing proteins contribute to surface protein display and cell division in Staphylococcus aureus. Mol. Microbiol. 78, 238–252 10.1111/j.1365-2958.2010.07334.x (doi:10.1111/j.1365-2958.2010.07334.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Frankel M. B., Hendrickx A. P., Missiakas D. M., Schneewind O. 2011. LytN, a murein hydrolase in the cross-wall compartment of Staphylococcus aureus, is involved in proper bacterial growth and envelope assembly. J. Biol. Chem. 286, 32 593–32 605 10.1074/jbc.M111.258863 (doi:10.1074/jbc.M111.258863) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oshida T., Sugai M., Komatsuzawa H., Hong Y. M., Suginaka H., Tomasz A. 1995. A Staphylococcus aureus autolysin that has an N-acetylmuramoyl-l-alanine amidase domain and an endo-β-N-acetylglucosaminidase domain: cloning, sequence analysis, and characterization. Proc. Natl Acad. Sci. USA 92, 285–289 10.1073/pnas.92.1.285 (doi:10.1073/pnas.92.1.285) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kajimura J., et al. 2005. Identification and molecular characterization of an N-acetylmuramyl-l-alanine amidase Sle1 involved in cell separation of Staphylococcus aureus. Mol. Microbiol. 58, 1087–1101 10.1111/j.1365-2958.2005.04881.x (doi:10.1111/j.1365-2958.2005.04881.x) [DOI] [PubMed] [Google Scholar]
- 69.Yamada S., Sugai M., Komatsuzawa H., Nakashima S., Oshida T., Matsumoto A., Suginaka H. 1996. An autolysin ring associated with cell separation of Staphylococcus aureus. J. Bacteriol. 178, 1565–1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Komatsuzawa H., Sugai M., Nakashima S., Yamada S., Matsumoto A., Oshida T., Suginaka H. 1997. Subcellular localization of the major autolysin, ATL and its processed proteins in Staphylococcus aureus. Microbiol. Immunol. 41, 469–479 [DOI] [PubMed] [Google Scholar]
- 71.Mazmanian S. K., Liu G., Ton-That H., Schneewind O. 1999. Staphylococcus aureus sortase, an enzyme that anchors surface proteins to the cell wall. Science 285, 760–763 10.1126/science.285.5428.760 (doi:10.1126/science.285.5428.760) [DOI] [PubMed] [Google Scholar]
- 72.Ton-That H., Liu G., Mazmanian S. K., Faull K. F., Schneewind O. 1999. Purification and characterization of sortase, the transpeptidase that cleaves surface proteins of Staphylococcus aureus at the LPXTG motif. Proc. Natl Acad. Sci. USA 96, 12 424–12 429 10.1073/pnas.96.22.12424 (doi:10.1073/pnas.96.22.12424) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ilangovan U., Ton-That H., Iwahara J., Schneewind O., Clubb R. T. 2001. Structure of sortase, the transpeptidase that anchors proteins to the cell wall of Staphylococcus aureus. Proc. Natl Acad. Sci. USA 98, 6056–6061 10.1073/pnas.101064198 (doi:10.1073/pnas.101064198) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zong Y., Bice T. W., Ton-That H., Schneewind O., Narayana S. V. 2004. Crystal structures of Staphylococcus aureus sortase A and its substrate complex. J. Biol. Chem. 279, 31 383–31 389 10.1074/jbc.M401374200 (doi:10.1074/jbc.M401374200) [DOI] [PubMed] [Google Scholar]
- 75.Ruzin A., Severin A., Ritacco F., Tabei K., Singh G., Bradford P. A., Siegel M. M., Projan S. J., Shlaes D. M. 2002. Further evidence that a cell wall precursor [C(55)-MurNAc-(peptide)-GlcNAc] serves as an acceptor in a sorting reaction. J. Bacteriol. 184, 2141–2147 10.1128/JB.184.8.2141-2147.2002 (doi:10.1128/JB.184.8.2141-2147.2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Perry A. M., Ton-That H., Mazmanian S. K., Schneewind O. 2002. Anchoring of surface proteins to the cell wall of Staphylococcus aureus. III. Lipid II is an in vivo peptidoglycan substrate for sortase-catalyzed surface protein anchoring. J. Biol. Chem. 277, 16 241–16 248 10.1074/jbc.M109194200 (doi:10.1074/jbc.M109194200) [DOI] [PubMed] [Google Scholar]
- 77.Schleifer K. H., Kandler O. 1972. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol. Rev. 36, 407–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Matsuhashi M., Dietrich C. P., Strominger J. L. 1965. Incorporation of glycine into the cell wall glycopeptide in Staphylococcus aureus: role of sRNA and lipid intermediates. Proc. Natl Acad. Sci. USA 54, 587–594 10.1073/pnas.54.2.587 (doi:10.1073/pnas.54.2.587) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Berger-Bächi B. 1994. Expression of resistance to methicillin. Trends Microbiol. 2, 389–393 10.1016/0966-842X(94)90617-3 (doi:10.1016/0966-842X(94)90617-3) [DOI] [PubMed] [Google Scholar]
- 80.Dhar G., Faull K. F., Schneewind O. 2000. Anchor structure of cell wall surface proteins in Listeria monocytogenes. Biochemistry 39, 3725–3733 10.1021/bi992347o (doi:10.1021/bi992347o) [DOI] [PubMed] [Google Scholar]
- 81.Bierne H., et al. 2002. Inactivation of the srtA gene in Listeria monocytogenes inhibits anchoring of surface proteins and affects virulence. Mol. Microbiol. 43, 869–881 10.1046/j.1365-2958.2002.02798.x (doi:10.1046/j.1365-2958.2002.02798.x) [DOI] [PubMed] [Google Scholar]
- 82.Ton-That H., Faull K. F., Schneewind O. 1997. Anchor structure of staphylococcal surface proteins. I. A branched peptide that links the carboxyl terminus of proteins to the cell wall. J. Biol. Chem. 272, 22 285–22 292 [DOI] [PubMed] [Google Scholar]
- 83.Ton-That H., Labischinski H., Berger-Bachi B., Schneewind O. 1998. Anchor structure of staphylococcal surface proteins. III. Role of the FemA, FemB, and FemX factors in anchoring surface proteins to the bacterial cell wall. J. Biol. Chem. 273, 29 143–29 149 [DOI] [PubMed] [Google Scholar]
- 84.Ton-That H., Schneewind O. 1999. Anchor structure of staphylococcal surface proteins. IV. Inhibitors of the cell wall sorting reaction. J. Biol. Chem. 274, 24 316–24 320 [DOI] [PubMed] [Google Scholar]
- 85.Mazmanian S. K., Ton-That H., Su K., Schneewind O. 2002. An iron-regulated sortase enzyme anchors a class of surface protein during Staphylococcus aureus pathogenesis. Proc. Natl Acad. Sci. USA 99, 2293–2298 10.1073/pnas.032523999 (doi:10.1073/pnas.032523999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mazmanian S. K., Liu G., Jensen E. R., Lenoy E., Schneewind O. 2000. Staphylococcus aureus mutants defective in the display of surface proteins and in the pathogenesis of animal infections. Proc. Natl Acad. Sci. USA 97, 5510–5515 10.1073/pnas.080520697 (doi:10.1073/pnas.080520697) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cheng A. G., Kim H. K., Burts M. L., Krausz T., Schneewind O., Missiakas D. M. 2009. Genetic requirements for Staphylococcus aureus abscess formation and persistence in host tissues. FASEB J. 23, 3393–3404 10.1096/fj.09-135467 (doi:10.1096/fj.09-135467) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mcadow M., Kim H. K., Dedenta A. C., Hendrickx A. P. A., Schneewind O., Missiakas D. M. 2011. Preventing Staphylococcus aureus sepsis through the inhibition of its agglutination in blood. PLoS Pathog. 7, e1002307. 10.1371/journal.ppat.1002307 (doi:10.1371/journal.ppat.1002307) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gaspar A. H., Marraffini L. A., Glass E. M., Debord K. L., Ton-That H., Schneewind O. 2005. Bacillus anthracis sortase A (SrtA) anchors LPXTG motif-containing surface proteins to the cell wall envelope. J. Bacteriol. 187, 4646–4655 10.1128/JB.187.13.4646-4655.2005 (doi:10.1128/JB.187.13.4646-4655.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kern J. W., Schneewind O. 2008. BslA, a pXO1-encoded adhesin of Bacillus anthracis. Mol. Microbiol. 68, 504–515 10.1111/j.1365-2958.2008.06169.x (doi:10.1111/j.1365-2958.2008.06169.x) [DOI] [PubMed] [Google Scholar]
- 91.Kern J. W., Schneewind O. 2009. BslA, the S-layer adhesin of Bacillus anthracis, is a virulence factor for anthrax pathogenesis. Mol. Microbiol. 75, 324–332 10.1111/j.1365-2958.2009.06958.x (doi:10.1111/j.1365-2958.2009.06958.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Raz A., Fischetti V. A. 2008. Sortase A localizes to distinct foci on the Streptococcus pyogenes membrane. Proc. Natl Acad. Sci. USA 105, 18 549–18 554 10.1073/pnas.0808301105 (doi:10.1073/pnas.0808301105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mazmanian S. K., Skaar E. P., Gaspar A. H., Humayun M., Gornicki P., Jelenska J., Joachmiak A., Missiakas D. M., Schneewind O. 2003. Passage of heme-iron across the envelope of Staphylococcus aureus. Science 299, 906–909 10.1126/science.1081147 (doi:10.1126/science.1081147) [DOI] [PubMed] [Google Scholar]
- 94.Skaar E. P., Gaspar A. H., Schneewind O. 2004. IsdG and IsdI, heme degrading enzymes in the cytoplasm of Staphylococcus aureus. J. Biol. Chem. 279, 436–443 10.1074/jbc.M307952200 (doi:10.1074/jbc.M307952200) [DOI] [PubMed] [Google Scholar]
- 95.Wu R., Skaar E. P., Zhang R., Joachimiak G., Gornicki P., Schneewind O., Joachimiak A. 2005. Staphylococcus aureus IsdG and IsdI, heme degrading enzymes with structural similarity to monooxygenases. J. Biol. Chem. 280, 2840–2846 10.1074/jbc.M409526200 (doi:10.1074/jbc.M409526200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zong Y., Mazmanian S. K., Schneewind O., Narayana S. V. 2004. The structure of sortase B, a cysteine transpeptidase that tethers surface protein to the Staphylococcus aureus cell wall. Structure 12, 105–112 10.1016/j.str.2003.11.021 (doi:10.1016/j.str.2003.11.021) [DOI] [PubMed] [Google Scholar]
- 97.Marraffini L. A., Schneewind O. 2005. Anchor structure of staphylococcal surface proteins. V. Anchor structure of the sortase B substrate IsdC. J. Biol. Chem. 280, 16 263–16 271 [DOI] [PubMed] [Google Scholar]
- 98.Skaar E. P., Schneewind O. 2004. Iron-regulated surface determinants (Isd) of Staphylococcus aureus: stealing iron from heme. Microbes Infect. 6, 390–397 10.1016/j.micinf.2003.12.008 (doi:10.1016/j.micinf.2003.12.008) [DOI] [PubMed] [Google Scholar]
- 99.Dryla A., Gelbmann D., Von Gabain A., Nagy E. 2003. Identification of a novel iron regulated staphylococcal surface protein with haptoglobin–haemoglobin binding activity. Mol. Microbiol. 49, 37–53 10.1046/j.1365-2958.2003.03542.x (doi:10.1046/j.1365-2958.2003.03542.x) [DOI] [PubMed] [Google Scholar]
- 100.Torres V. J., Pishchany G., Humayun M., Schneewind O., Skaar E. P. 2006. Staphylococcus aureus IsdB is a hemoglobin receptor required for heme-iron utilization. J. Bacteriol. 188, 8421–8429 10.1128/JB.01335-06 (doi:10.1128/JB.01335-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Villareal V. A., Pilpa R. M., Robson S. A., Fadeev E. A., Clubb R. T. 2008. The IsdC protein from Staphylococcus aureus uses a flexible binding pocket to capture heme. J. Biol. Chem. 283, 31 591–31 600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Muryoi N., Tiedemann M. T., Pluym M., Cheung J., Heinrichs D. E., Stillman M. J. 2008. Demonstration of the iron-regulated surface determinant (Isd) heme transfer pathway in Staphylococcus aureus. J. Biol. Chem. 283, 28 125–28 136 10.1074/jbc.M802171200 (doi:10.1074/jbc.M802171200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu M., Tanaka W. N., Zhu H., Dooley D. M., Lei B. 2008. Direct hemin transfer from IsdA to IsdC in the iron-regulated surface determinant (Isd) heme acquisition system of Staphylococcus aureus. J. Biol. Chem. 283, 6668–6676 10.1074/jbc.M708372200 (doi:10.1074/jbc.M708372200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sharp K. H., Schneider S., Cockayne A., Paoli M. 2007. Crystal structure of the heme-IsdC complex, the central conduit of the Isd iron/heme uptake system in Staphylococcus aureus. J. Biol. Chem. 282, 10 625–10 631 10.1074/jbc.M700234200 (doi:10.1074/jbc.M700234200) [DOI] [PubMed] [Google Scholar]
- 105.Maresso A. W., Chapa T. J., Schneewind O. 2006. Surface protein IsdC and sortase B are required for heme-iron scavenging of Bacillus anthracis. J. Bacteriol. 188, 8145–8152 10.1128/JB.01011-06 (doi:10.1128/JB.01011-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Maresso A. W., Garufi G., Schneewind O. 2008. Bacillus anthracis secretes proteins that mediate heme acquisition from hemoglobin. PLoS Pathogens. 4, e1000132. 10.1371/journal.ppat.1000132 (doi:10.1371/journal.ppat.1000132) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tarlovsky Y., Fabian M., Solomaha E., Honsa E., Olson J. S., Maresso A. W. 2010. A Bacillus anthracis S-layer homology protein that binds heme and mediates heme delivery to IsdC. J. Bacteriol. 192, 3503–3511 10.1128/JB.00054-10 (doi:10.1128/JB.00054-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Skaar E. P., Gaspar A. H., Schneewind O. 2006. Bacillus anthracis IsdG, a heme degrading monooxygenase. J. Bacteriol. 188, 1071–1080 10.1128/JB.188.3.1071-1080.2006 (doi:10.1128/JB.188.3.1071-1080.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Reniere M. L., Ukpabi G. N., Harry S. R., Stec D. F., Krull R., Wright D. W., Bachmann B. O., Murphy M. E., Skaar E. P. 2010. The IsdG-family of haem oxygenases degrades haem to a novel chromophore. Mol. Microbiol. 75, 1529–1538 10.1111/j.1365-2958.2010.07076.x (doi:10.1111/j.1365-2958.2010.07076.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dramsi S., Trieu-Cuot P., Bierne H. 2005. Sorting sortases: a nomenclature proposal for the various sortases of Gram-positive bacteria. Res. Microbiol. 156, 289–297 10.1016/j.resmic.2004.10.011 (doi:10.1016/j.resmic.2004.10.011) [DOI] [PubMed] [Google Scholar]
- 111.Marraffini L. A., Schneewind O. 2006. Targeting proteins to the cell wall of sporulating Bacillus anthracis. Mol. Microbiol. 62, 1402–1417 10.1111/j.1365-2958.2006.05469.x (doi:10.1111/j.1365-2958.2006.05469.x) [DOI] [PubMed] [Google Scholar]
- 112.Marraffini L. A., Schneewind O. 2007. Sortase C-mediated anchoring of BasI to the cell wall envelope of Bacillus anthracis. J. Bacteriol. 189, 6425–6436 10.1128/JB.00702-07 (doi:10.1128/JB.00702-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Elliot M. A., Karoonuthaisiri N., Huang J., Bibb M. J., Cohen S. N., Kao C. M., Buttner M. J. 2003. The chaplins: a family of hydrophobic cell-surface proteins involved in aerial mycelium formation in Streptomyces coelicolor. Genes Dev. 17, 1727–1740 10.1101/gad.264403 (doi:10.1101/gad.264403) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Claessen D., Rink R., De Jong W., Siebring J., De Vreugd P., Boersma F. G., Dijkhuizen L., Wosten H. A. 2003. A novel class of secreted hydrophobic proteins is involved in aerial hyphae formation in Streptomyces coelicolor by forming amyloid-like fibrils. Genes Dev. 17, 1714–1726 10.1101/gad.264303 (doi:10.1101/gad.264303) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mishra A., Das A., Cisar J. O., Ton-That H. 2007. Sortase-catalyzed assembly of distinct heteromeric fimbriae in Actinomyces naeslundii. J. Bacteriol. 189, 3156–3165 10.1128/JB.01952-06 (doi:10.1128/JB.01952-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Budzik J. M., Marraffini L. A., Schneewind O. 2007. Assembly of pili on the surface of Bacillus cereus vegetative cells. Mol. Microbiol. 66, 495–510 10.1111/j.1365-2958.2007.05939.x (doi:10.1111/j.1365-2958.2007.05939.x) [DOI] [PubMed] [Google Scholar]
- 117.Nallapareddy S. R., Singh K. V., Sillanpää J., Garsin D. A., Höök M., Erlandsen S. L., Murray B. E. 2006. Endocarditis of biofilm-associated pili of Enterococcus faecalis. J. Clin. Invest. 116, 2799–2807 10.1172/JCI29021 (doi:10.1172/JCI29021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Von Ossowski I., et al. 2010. Mucosal adhesion properties of the probiotic Lactobacillus rhamnosus GG SpaCBA and SpaFED pilin subunits. Appl. Environ. Microbiol. 76, 2049–2057 10.1128/AEM.01958-09 (doi:10.1128/AEM.01958-09) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lauer P., et al. 2005. Genome analysis reveals pili in Group B streptococcus. Science 309, 105. 10.1126/science.1111563 (doi:10.1126/science.1111563) [DOI] [PubMed] [Google Scholar]
- 120.Sillanpaa J., Xu Y., Nallapareddy S. R., Murray B. E., Hook M. 2004. A family of putative MSCRAMMs from Enterococcus faecalis. Microbiology 150, 2069–2078 10.1099/mic.0.27074-0 (doi:10.1099/mic.0.27074-0) [DOI] [PubMed] [Google Scholar]
- 121.Barocchi M. A., et al. 2006. A pneumococcal pilus influences virulence and host inflammatory responses. Proc. Natl Acad. Sci. USA 103, 2857–2862 10.1073/pnas.0511017103 (doi:10.1073/pnas.0511017103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mora M., et al. 2005. Group A Streptococcus produce pilus-like structures containing protective antigens and Lancefield T antigens. Proc. Natl Acad. Sci. USA 102, 15 641–15 646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kang H. J., Middleditch M., Proft T., Baker E. N. 2009. Isopeptide bonds in bacterial pili and their characterization by X-ray crystallography and mass spectrometry. Biopolymers 91, 1126–1134 10.1002/bip.21170 (doi:10.1002/bip.21170) [DOI] [PubMed] [Google Scholar]
- 124.Hendrickx A. P., Budzik J. M., Oh S. Y., Schneewind O. 2011. Architects at the bacterial surface — sortases and the assembly of pili with isopeptide bonds. Nat. Rev. Microbiol. 9, 166–176 10.1038/nrmicro2520 (doi:10.1038/nrmicro2520) [DOI] [PubMed] [Google Scholar]
- 125.Mandlik A., Swierczynski A., Das A., Ton-That H. 2007. Corynebacterium diphtheriae employs specific minor pilins to target human pharyngeal epithelial cells. Mol. Microbiol. 64, 111–124 10.1111/j.1365-2958.2007.05630.x (doi:10.1111/j.1365-2958.2007.05630.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mandlik A., Das A., Ton-That H. 2008. The molecular switch that activates the cell wall anchoring step of pilus assembly in Gram-positive bacteria. Proc. Natl Acad. Sci. USA 105, 14 147–14 152 10.1073/pnas.0806350105 (doi:10.1073/pnas.0806350105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Budzik J. M., Oh S. Y., Schneewind O. 2008. Cell wall anchor structure of BcpA pili in Bacillus anthracis. J. Biol. Chem. 283, 36 676–36 686 10.1074/jbc.M806796200 (doi:10.1074/jbc.M806796200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Budzik J. M., Marraffini L. A., Souda P., Whitelegge J. P., Faull K. F., Schneewind O. 2008. Amide bonds assemble pili on the surface of bacilli. Proc. Natl Acad. Sci. USA 105, 10 215–10 220 10.1073/pnas.0803565105 (doi:10.1073/pnas.0803565105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Budzik J. M., Oh S. Y., Schneewind O. 2009. Sortase D forms the covalent bond that links BcpB to the tip of Bacillus cereus pili. J. Biol. Chem. 284, 12 989–12 997 10.1074/jbc.M900927200 (doi:10.1074/jbc.M900927200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Oh S.-Y., Budzik J. M., Schneewind O. 2008. Sortases make pili from three ingredients. Proc. Natl Acad. Sci. USA 105, 14 147–14 152 10.1073/pnas.0807334105 (doi:10.1073/pnas.0807334105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mandlik A., Swierczynski A., Das A., Ton-That H. 2008. Pili in Gram-positive bacteria: assembly, involvement in colonization and biofilm development. Trends Microbiol. 16, 33–40 10.1016/j.tim.2007.10.010 (doi:10.1016/j.tim.2007.10.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ton-That H., Marraffini L., Schneewind O. 2004. Sortases and pilin elements involved in pilus assembly of Corynebacterium diphtheriae. Mol. Microbiol. 53, 1147–1156 10.1111/j.1365-2958.2004.04193.x (doi:10.1111/j.1365-2958.2004.04193.x) [DOI] [PubMed] [Google Scholar]
- 133.Kang H. J., Coulibaly F., Clow F., Proft T., Baker E. N. 2007. Stabilizing isopeptide bonds revealed in Gram-positive bacterial pilus structure. Science 318, 1625–1628 10.1126/science.1145806 (doi:10.1126/science.1145806) [DOI] [PubMed] [Google Scholar]
- 134.Krishnan V., Gaspar A. H., Ye N., Mandlik A., Ton-That H., Narayana S. V. 2007. An IgG-like domain in the minor pilin GBS52 of Streptococcus agalactiae mediates lung epithelial cell adhesion. Structure 15, 893–903 10.1016/j.str.2007.06.015 (doi:10.1016/j.str.2007.06.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kang H. J., Baker E. N. 2009. Intramolecular isopeptide bonds give thermodynamic and proteolytic stability to the major pilin protein of Streptococcus pyogenes. J. Biol. Chem. 284, 20 729–20 737 10.1074/jbc.M109.014514 (doi:10.1074/jbc.M109.014514) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Deivanayagam C. C., Rich R. L., Carson M., Owens R. T., Danthuluri S., Bice T., Höök M., Narayana S. V. 2000. Novel fold and assembly of the repetitive B region of the Staphylococcus aureus collagen-binding surface protein. Structure 8, 67–78 10.1016/S0969-2126(00)00081-2 (doi:10.1016/S0969-2126(00)00081-2) [DOI] [PubMed] [Google Scholar]
- 137.Budzik J. M., Poor C. B., Faull K. F., Whitelegge J. P., He C., Schneewind O. 2009. Intramolecular amide bonds stabilize pili on the surface of bacilli. Proc. Natl Acad. Sci. USA 106, 19 992–19 997 10.1073/pnas.0910887106 (doi:10.1073/pnas.0910887106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Manzano C., Contreras-Martel C., El Mortaji L., Izoré T., Fenel D., Vernet T., Schoehn G., Di Guilmi A. M., Dessen A. 2008. Sortase-mediated pilus fiber biogenesis in Streptococcus pneumoniae. Structure 16, 1838–1848 10.1016/j.str.2008.10.007 (doi:10.1016/j.str.2008.10.007) [DOI] [PubMed] [Google Scholar]
- 139.Zhang R., Wu R., Joachimiak G., Mazmanian S. K., Missiakas D. M., Gornicki P., Schneewind O., Joachimiak A. 2004. Structures of sortase B from Staphylococcus aureus and Bacillus anthracis reveal catalytic amino acid triad in the active site. Structure 12, 1147–1156 10.1016/j.str.2004.06.001 (doi:10.1016/j.str.2004.06.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Khare B., Krishnan V., Rajashankar K. R., I-Hsiu H., Xin M., Ton-That H., Narayana S. V. 2011. Structural differences between the Streptococcus agalactiae housekeeping and pilus-specific sortases: SrtA and SrtC1. PLoS ONE 6, 22 995. 10.1371/journal.pone.0022995 (doi:10.1371/journal.pone.0022995) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhang R.-G., Joachimiak G., Wu R.-Y., Mazmanian S. K., Missiakas D. M., Schneewind O., Joachimiak A. 2004. Structures of sortase B from Staphylococcus aureus and Bacillus anthracis reveal catalytic amino acid triad in the active site. Structure 12, 1147–1156 10.1016/j.str.2004.06.001 (doi:10.1016/j.str.2004.06.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kang H. J., Coulibaly F., Proft T., Baker E. N. 2011. Crystal structure of Spy0129, a Streptococcus pyogenes class B sortase involved in pilus assembly. PLoS ONE 6, e15969. 10.1371/journal.pone.0015969 (doi:10.1371/journal.pone.0015969) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Manzano C., Izoré T., Job V., Di Guilmi A. M., Dessen A. 2009. Sortase activity is controlled by a flexible lid in the pilus biogenesis mechanism of Gram-positive pathogens. Biochemistry 48, 10 549–10 557 10.1021/bi901261y (doi:10.1021/bi901261y) [DOI] [PubMed] [Google Scholar]
- 144.El Mortaji L., Terrasse R., Dessen A., Vernet T., Di Guilmi A. M. 2010. Stability and assembly of pilus subunits of Streptococcus pneumoniae. J. Biol. Chem. 285, 12 405–12 415 10.1074/jbc.M109.082776 (doi:10.1074/jbc.M109.082776) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kang H. J., Baker E. N. 2011. Intramolecular isopeptide bonds: protein crosslinks built for stress? Trends Biochem. Sci. 36, 229–237 10.1016/j.tibs.2010.09.007 (doi:10.1016/j.tibs.2010.09.007) [DOI] [PubMed] [Google Scholar]
- 146.Engelhardt H. 2007. Are S-layers exoskeletons? The basic function of protein surface layers revisited. J. Struct. Biol. 160, 115–124 10.1016/j.jsb.2007.08.003 (doi:10.1016/j.jsb.2007.08.003) [DOI] [PubMed] [Google Scholar]
- 147.Sleytr U. B. 1997. Basc and applied S-layer research: an overview. FEMS Microbiol. Rev. 20, 5–12 10.1016/S0168-6445(97)00039-9 (doi:10.1016/S0168-6445(97)00039-9) [DOI] [Google Scholar]
- 148.Mesnage S., Tosi-Couture E., Fouet A. 1999. Production and cell surface anchoring of functional fusions between the SLH motifs of the Bacillus anthracis S-layer proteins and the Bacillus subtilis levansucrase. Mol. Microbiol. 31, 927–936 10.1046/j.1365-2958.1999.01232.x (doi:10.1046/j.1365-2958.1999.01232.x) [DOI] [PubMed] [Google Scholar]
- 149.Fujino T., Béguin P., Aubert J. P. 1993. Organization of a Clostridium thermocellum gene cluster encoding the cellulosomal scaffolding protein CipA and a protein possibly involved in attachment of the cellulosome to the cell surface. J. Bacteriol. 175, 1891–1899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lupas A., Engelhardt H., Peters J., Santarius U., Volker S., Baumeister W. 1994. Domain structure of the Acetogenium kivui surface layer revealed by electron crystallography and sequence analysis. J. Bacteriol. 176, 1224–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lupas A. 1996. A circular permutation event in the evolution of the SLH domain? Mol. Microbiol. 20, 897–898 10.1111/j.1365-2958.1996.tb02528.x (doi:10.1111/j.1365-2958.1996.tb02528.x) [DOI] [PubMed] [Google Scholar]
- 152.Mesnage S., Weber-Levy M., Haustant M., Mock M., Fouet A. 1999. Cell surface-exposed tetanus toxin fragment C produced by recombinant Bacillus anthracis protects against tetanus toxin. Infect. Immun. 67, 4847–4850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jensen G. B., Hensen B. M., Eilenberg J., Mahillon J. 2003. The hidden lifestyles of Bacillus cereus and relatives. Environ. Microbiol. 5, 631–640 10.1046/j.1462-2920.2003.00461.x (doi:10.1046/j.1462-2920.2003.00461.x) [DOI] [PubMed] [Google Scholar]
- 154.Leoff C., Saile E., Sue D., Wilkins P. P., Quinn C. P., Carlson R. W., Kannenberg E. L. 2008. Cell wall carbohydrate compositions of strains from Bacillus cereus group of species correlate with phylogenetic relatedness. J. Bacteriol. 190, 112–121 10.1128/JB.01292-07 (doi:10.1128/JB.01292-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bruckner V., Kovacs J., Denes G. 1953. Structure of poly-d-glutamic acid isolated from capsulated strains of B. anthracis. Nature 172, 508. 10.1038/172508a0 (doi:10.1038/172508a0) [DOI] [PubMed] [Google Scholar]
- 156.Candela T., Fouet A. 2005. Bacillus anthracis CapD, belonging to the gamma-glutamyltranspeptidase family, is required for the covalent anchoring of capsule to peptidoglycan. Mol. Microbiol. 57, 717–726 10.1111/j.1365-2958.2005.04718.x (doi:10.1111/j.1365-2958.2005.04718.x) [DOI] [PubMed] [Google Scholar]
- 157.Oh S. Y., Budzik J. M., Garufi G., Schneewind O. 2011. Two capsular polysaccharides enable Bacillus cereus G9241 to cause anthrax-like disease. Mol. Microbiol. 79, 455–470 10.1111/j.1365-2958.2011.07582.x (doi:10.1111/j.1365-2958.2011.07582.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Read T. D., et al. 2003. The genome sequence of Bacillus anthracis Ames and comparison to closely related bacteria. Nature 423, 81–86 10.1038/nature01586 (doi:10.1038/nature01586) [DOI] [PubMed] [Google Scholar]
- 159.Mesnage S., Tosi-Couture E., Mock M., Gounon P., Fouet A. 1997. Molecular characterization of the Bacillus anthracis main S-layer component: evidence that it is the major cell-associated antigen. Mol. Microbiol. 23, 1147–1155 10.1046/j.1365-2958.1997.2941659.x (doi:10.1046/j.1365-2958.1997.2941659.x) [DOI] [PubMed] [Google Scholar]
- 160.Mesnage S., Tosi-Couture E., Gounon P., Mock M., Fouet A. 1998. The capsule and S-layer: two independent and yet compatible macromolecular structures in Bacillus anthracis. J. Bacteriol. 180, 52–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Mignot T., Mesnage S., Couture-Tosi E., Mock M., Fouet A. 2002. Developmental switch of S-layer protein synthesis in Bacillus anthracis. Mol. Microbiol. 43, 1615–1627 10.1046/j.1365-2958.2002.02852.x (doi:10.1046/j.1365-2958.2002.02852.x) [DOI] [PubMed] [Google Scholar]
- 162.Couture-Tosi E., Delacroix H., Mignot T., Mesnage S., Chami M., Fouet A., Mosser G. 2002. Structural analysis and evidence for dynamic emergence of Bacillus anthracis S-layer networks. J. Bacteriol. 184, 6448–6456 10.1128/JB.184.23.6448-6456.2002 (doi:10.1128/JB.184.23.6448-6456.2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Candela T., Mignot T., Hagenrelle X., Haustant M., Fouet A. 2005. Genetic analysis of Bacillus anthracis Sap S-layer protein crystallization domain. Microbiology 151, 1485–1490 10.1099/mic.0.27832-0 (doi:10.1099/mic.0.27832-0) [DOI] [PubMed] [Google Scholar]
- 164.Kern J. W., Wilton R., Zhang R., Binkowski A., Joachimiak A., Schneewind O. 2011. Structure of the SLH domains from Bacillus anthracis surface array protein. J. Biol. Chem. 286, 26 042–26 049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Anderson V. J., Kern J. W., Mccool J. W., Schneewind O., Missiakas D. M. 2011. The SLH domain protein BslO is a determinant of Bacillus anthracis chain length. Mol. Microbiol. 81, 192–205 10.1111/j.1365-2958.2011.07688.x (doi:10.1111/j.1365-2958.2011.07688.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Fagan R. P., Fairweather N. F. 2011. Clostridium difficile has two parallel and essential Sec secretion systems. J. Biol. Chem. 286, 27 483–27 493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Pavkov T., Egelseer E. M., Tesarz M., Svergun D. I., Sleytr U. B., Keller W. 2008. The structure and binding behavior of the bacterial cell surface layer protein SbcC. Structure 16, 1226–1237 10.1016/j.str.2008.05.012 (doi:10.1016/j.str.2008.05.012) [DOI] [PubMed] [Google Scholar]
- 168.Pallen M. J. 2002. The ESAT-6/WXG100 superfamily—and a new Gram-positive secretion system? Trends Microbiol. 10, 209–212 10.1016/S0966-842X(02)02345-4 (doi:10.1016/S0966-842X(02)02345-4) [DOI] [PubMed] [Google Scholar]
- 169.Renshaw P. S., et al. 2005. Structure and function of the complex formed by the tuberculosis virulence factors CFP-10 and ESAT-6. EMBO J. 24, 2491–2498 10.1038/sj.emboj.7600732 (doi:10.1038/sj.emboj.7600732) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bitter W., et al. 2009. Systematic genetic nomenclature for type VII secretion systems. PLoS Pathogens. 5, e1000507. 10.1371/journal.ppat.1000507 (doi:10.1371/journal.ppat.1000507) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Burts M. L., Williams W. A., Debord K., Missiakas D. M. 2005. EsxA and EsxB are secreted by an ESAT-6-like system that is required for the pathogenesis of Staphylococcus aureus infections. Proc. Natl Acad. Sci. USA 102, 1169–1174 10.1073/pnas.0405620102 (doi:10.1073/pnas.0405620102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Anderson M., Chen Y. H., Butler E. K., Missiakas D. M. 2011. EsaD, a secretion factor for the Ess pathway in Staphylococcus aureus. J. Bacteriol. 193, 1583–1589 10.1128/JB.01096-10 (doi:10.1128/JB.01096-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hsu T., et al. 2003. The primary mechanism of attenuation of bacillus Calmette-Guerin is a loss of secreted lytic function required for invasion of lung interstitial tissue. Proc. Natl Acad. Sci. USA 100, 12 420–12 425 10.1073/pnas.1635213100 (doi:10.1073/pnas.1635213100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Pym A. S., et al. 2003. Recombinant BCG exporting ESAT-6 confers enhanced protection against tuberculosis. Nat. Med. 9, 533–539 10.1038/nm859 (doi:10.1038/nm859) [DOI] [PubMed] [Google Scholar]
- 175.Stanley S. A., Raghavan S., Hwang W. W., Cox J. S. 2003. Acute infection and macrophage subversion by Mycobacterium tuberculosis require a specialized secretion system. Proc. Natl Acad. Sci. USA 100, 13 001–13 006 10.1073/pnas.2235593100 (doi:10.1073/pnas.2235593100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Abdallah A. M., Gey Van Pittius N. C., Champion P. A., Cox J., Luirink J., Vandenbroucke-Grauls C. M., Appelmelk B. J., Bitter W. 2007. Type VII secretion–mycobacteria show the way. Nat. Rev. Microbiol. 5, 883–891 10.1038/nrmicro1773 (doi:10.1038/nrmicro1773) [DOI] [PubMed] [Google Scholar]
- 177.Lee V. T., Schneewind O. 2001. Protein secretion and the pathogenesis of bacterial infections. Genes Dev. 15, 1725–1752 10.1101/gad.896801 (doi:10.1101/gad.896801) [DOI] [PubMed] [Google Scholar]
- 178.Brennan P. J., Nikaido H. 1995. The envelope of mycobacteria. Annu. Rev. Biochem. 64, 29–63 10.1146/annurev.bi.64.070195.000333 (doi:10.1146/annurev.bi.64.070195.000333) [DOI] [PubMed] [Google Scholar]
- 179.Zuber B., Chami M., Houssin C., Dubochet J., Griffiths G., Daffé M. 2008. Direct visualization of the outer membrane of mycobacteria and corynebacteria in their native state. J. Bacteriol. 190, 5672–5680 10.1128/JB.01919-07 (doi:10.1128/JB.01919-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hoffmann C., Leis A., Niederweis M., Plitzko J. M., Engelhardt H. 2008. Disclosure of the mycobacterial outer membrane: cryo-electron tomography and vitreous sections reveal the lipid bilayer structure. Proc. Natl Acad. Sci. USA 105, 3963–3967 10.1073/pnas.0709530105 (doi:10.1073/pnas.0709530105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Burts M. L., Dedent A. C., Missiakas D. M. 2008. EsaC substrate for the ESAT-6 secretion pathway and its role in persistent infections of Staphylococcus aureus. Mol. Microbiol. 69, 736–746 10.1111/j.1365-2958.2008.06324.x (doi:10.1111/j.1365-2958.2008.06324.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Callahan B., Nguyen K., Collins A., Valdes K., Caplow M., Crossman D. K., Steyn A. J., Eisele L., Derbyshire K. M. 2010. Conservation of structure and protein-protein interactions mediated by the secreted mycobacterial proteins EsxA, EsxB, and EspA. J. Bacteriol. 192, 326–335 10.1128/JB.01032-09 (doi:10.1128/JB.01032-09) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Sundaramoorthy R., Fyfe P. K., Hunter W. N. 2008. Structure of Staphylococcus aureus EsxA suggests a contribution to virulence by action as a transport chaperone and/or adaptor protein. J. Mol. Biol. 383, 603–614 10.1016/j.jmb.2008.08.047 (doi:10.1016/j.jmb.2008.08.047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Champion P. A., Stanley S. A., Champion M. M., Brown E. J., Cox J. S. 2006. C-terminal signal sequence promotes virulence factor secretion in Mycobacterium tuberculosis. Science 313, 1632–1636 10.1126/science.1131167 (doi:10.1126/science.1131167) [DOI] [PubMed] [Google Scholar]
- 185.Fortune S. M., Jaeger A., Sarracino D. A., Chase M. R., Sassetti C. M., Sherman D. R., Bloom B. R., Rubin E. J. 2005. Mutually dependent secretion of proteins required for mycobacterial virulence. Proc. Natl Acad. Sci. USA 102, 10 676–10 681 10.1073/pnas.0504922102 (doi:10.1073/pnas.0504922102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Simeone R., Bottai D., Brosch R. 2009. ESX/type VII secretion systems and their role in host–pathogen interaction. Curr. Opin. Microbiol. 12, 4–10 10.1016/j.mib.2008.11.003 (doi:10.1016/j.mib.2008.11.003) [DOI] [PubMed] [Google Scholar]
- 187.Garufi G., Butler E., Missiakas D. 2008. ESAT-6-like protein secretion in Bacillus anthracis. J. Bacteriol. 190, 7004–7011 10.1128/JB.00458-08 (doi:10.1128/JB.00458-08) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Akpe San Roman S., Facey P. D., Fernandez-Martinez L., Rodriguez C. A., Vallin C., Del Sol R., Dyson P. 2010. A heterodimer of EsxA and EsxB is involved in sporulation and is secreted by a type VII secretion system in Streptomyces coelicolor. Microbiology 156, 1719–1729 10.1099/mic.0.037069-0 (doi:10.1099/mic.0.037069-0) [DOI] [PubMed] [Google Scholar]
- 189.Delano W. L. 2002. The PyMOL molecular graphics system. Palo Alto, CA: DeLano Scientific [Google Scholar]
- 190.Majlessi L., Brodin P., Brosch R., Rojas M. J., Khun H., Huerre M., Cole S. T., Leclerc C. 2005. Influence of ESAT-6 secretion system 1 (RD1) of Mycobacterium tuberculosis on the interaction between mycobacteria and the host immune system. J. Immunol. 174, 3570–3579 [DOI] [PubMed] [Google Scholar]
- 191.Stanley S. A., Johndrow J. E., Manzanillo P., Cox J. S. 2007. The Type I IFN response to infection with Mycobacterium tuberculosis requires ESX-1-mediated secretion and contributes to pathogenesis. J. Immunol. 178, 3143–3152 [DOI] [PubMed] [Google Scholar]
- 192.Davis J. M., Ramakrishnan L. 2009. The role of the granuloma in expansion and dissemination of early tuberculous infection. Cell 136, 37–49 10.1016/j.cell.2008.11.014 (doi:10.1016/j.cell.2008.11.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Raghavan S., Manzanillo P., Chan K., Dovey C., Cox J. S. 2008. Secreted transcription factor controls Mycobacterium tuberculosis virulence. Nature 454, 717–721 10.1038/nature07219 (doi:10.1038/nature07219) [DOI] [PMC free article] [PubMed] [Google Scholar]