Abstract
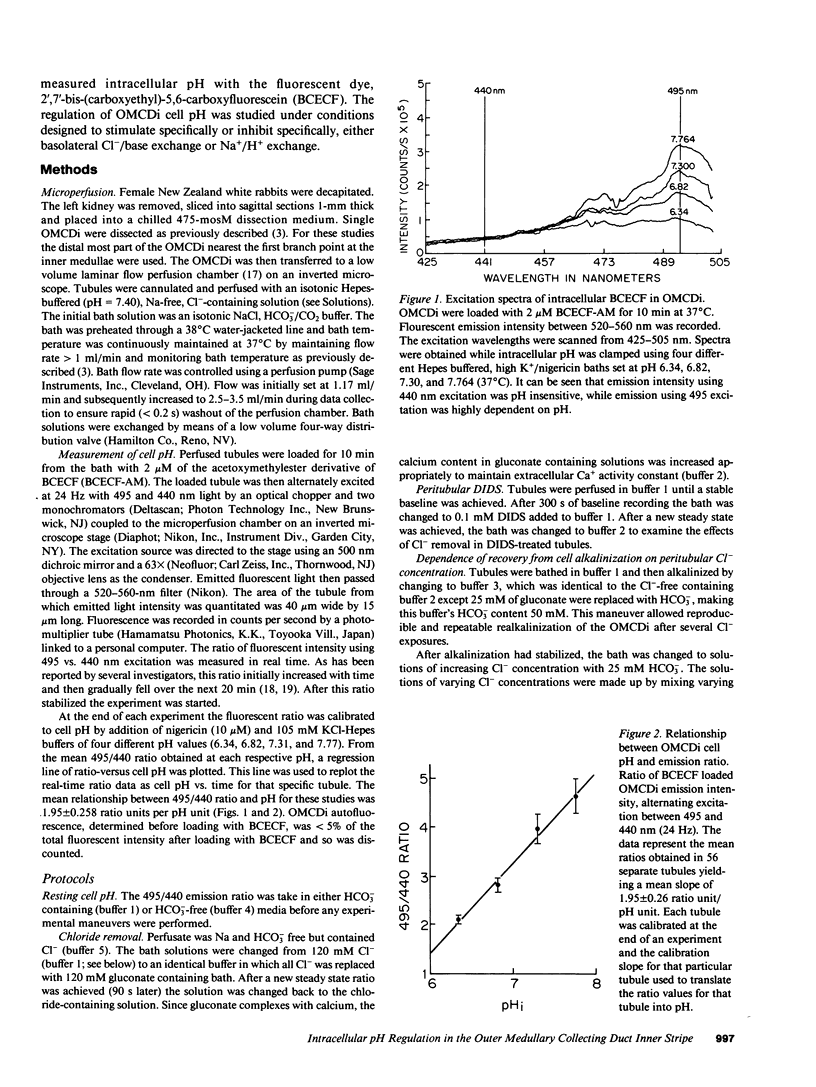
The collecting duct of the inner stripe outer medulla (OMCDi) is a major site of distal nephron acidification. Using the pH sensitive fluorescent dye 2'-7'-bis(carboxyethyl)-5,6,-carboxyfluorescein (BCECF) and quantitative spectrofluorometry to measure intracellular pH in isolated perfused OMCDi, we have characterized basolateral transport processes responsible for regulation of intracellular pH. Experiments suggesting the existence of basolateral Cl-/base exchange were performed. In HCO3- containing buffers, bath Cl- replacement resulted in reversible alkalinization of the OMCDi from 7.22 +/- 0.05 to 7.57 +/- 0.12. Similarly 0.1 mM bath 4',4'-diisothiocyanostilbene-2,2'-disulphonic acid (DIDS) alkalinized the OMCDi from 7.14 +/- 0.09 to 7.34 +/- 0.09 and blocked further alkalinization by bath Cl- removal (delta = + 0.02 pH units). The concentration dependence kinetics of Cl-/base exchange revealed a K1/2 of 10 mM for external Cl- with a Vmax of 0.50 pH U/min. Experiments suggesting the existence of basolateral Na+/H+ exchange were also performed. Replacement of bath Na+ by tetramethylammonium resulted in reversible cell acidification (7.14 +/- 0.09 to 6.85 +/- 0.1). Tubules that were acidified by a brief exposure to NH4Cl displayed recovery of cell pH back to baseline at a rate that was highly dependent on bath Na+ concentration. Half maximal recovery rate was achieved at 7 mM bath Na+ and Vmax was 0.605 pH U/min. The Na+-dependent rate of cell pH recovery after acidification was blocked by 0.2 mM bath amiloride. These results suggest that intracellular pH in the OMCDi is regulated by parallel basolateral Na+/H+ exchange and Cl-/base exchange.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ait-Mohamed A. K., Marsy S., Barlet C., Khadouri C., Doucet A. Characterization of N-ethylmaleimide-sensitive proton pump in the rat kidney. Localization along the nephron. J Biol Chem. 1986 Sep 25;261(27):12526–12533. [PubMed] [Google Scholar]
- Aronson P. S. Kinetic properties of the plasma membrane Na+-H+ exchanger. Annu Rev Physiol. 1985;47:545–560. doi: 10.1146/annurev.ph.47.030185.002553. [DOI] [PubMed] [Google Scholar]
- Boron W. F. Transport of H+ and of ionic weak acids and bases. J Membr Biol. 1983;72(1-2):1–16. doi: 10.1007/BF01870311. [DOI] [PubMed] [Google Scholar]
- Brown D., Hirsch S., Gluck S. An H+-ATPase in opposite plasma membrane domains in kidney epithelial cell subpopulations. Nature. 1988 Feb 18;331(6157):622–624. doi: 10.1038/331622a0. [DOI] [PubMed] [Google Scholar]
- Burnatowska-Hledin M. A., Spielman W. S. Immunodissection of mitochondria-rich cells from rabbit outer medullary collecting tubule. Am J Physiol. 1988 Jun;254(6 Pt 2):F907–F911. doi: 10.1152/ajprenal.1988.254.6.F907. [DOI] [PubMed] [Google Scholar]
- Busa W. B. Mechanisms and consequences of pH-mediated cell regulation. Annu Rev Physiol. 1986;48:389–402. doi: 10.1146/annurev.ph.48.030186.002133. [DOI] [PubMed] [Google Scholar]
- Cabantchik Z. I., Rothstein A. Membrane proteins related to anion permeability of human red blood cells. I. Localization of disulfonic stilbene binding sites in proteins involved in permeation. J Membr Biol. 1974;15(3):207–226. doi: 10.1007/BF01870088. [DOI] [PubMed] [Google Scholar]
- Cassel D., Scharf O., Rotman M., Cragoe E. J., Jr, Katz M. Characterization of Na+-linked and Na+-independent Cl-/HCO3- exchange systems in Chinese hamster lung fibroblasts. J Biol Chem. 1988 May 5;263(13):6122–6127. [PubMed] [Google Scholar]
- Chaillet J. R., Boron W. F. Intracellular calibration of a pH-sensitive dye in isolated, perfused salamander proximal tubules. J Gen Physiol. 1985 Dec;86(6):765–794. doi: 10.1085/jgp.86.6.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaillet J. R., Lopes A. G., Boron W. F. Basolateral Na-H exchange in the rabbit cortical collecting tubule. J Gen Physiol. 1985 Dec;86(6):795–812. doi: 10.1085/jgp.86.6.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L. H., Mueller A., Steinmetz P. R. Inhibition of the bicarbonate exit step in urinary acidification by a disulfonic stilbene. J Clin Invest. 1978 Apr;61(4):981–986. doi: 10.1172/JCI109023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L. H., Steinmetz P. R. Control of active proton transport in turtle urinary bladder by cell pH. J Gen Physiol. 1980 Sep;76(3):381–393. doi: 10.1085/jgp.76.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson A. C., Spring K. R. Volume regulation by Necturus gallbladder: apical Na+-H+ and Cl(-)-HCO-3 exchange. Am J Physiol. 1982 Sep;243(3):C146–C150. doi: 10.1152/ajpcell.1982.243.3.C146. [DOI] [PubMed] [Google Scholar]
- Frelin C., Vigne P., Ladoux A., Lazdunski M. The regulation of the intracellular pH in cells from vertebrates. Eur J Biochem. 1988 May 16;174(1):3–14. doi: 10.1111/j.1432-1033.1988.tb14055.x. [DOI] [PubMed] [Google Scholar]
- GOTTSCHALK C. W., LASSITER W. E., MYLLE M. Localization of urine acidification in the mammalian kidney. Am J Physiol. 1960 Mar;198:581–585. doi: 10.1152/ajplegacy.1960.198.3.581. [DOI] [PubMed] [Google Scholar]
- Gluck S., Caldwell J. Immunoaffinity purification and characterization of vacuolar H+ATPase from bovine kidney. J Biol Chem. 1987 Nov 15;262(32):15780–15789. [PubMed] [Google Scholar]
- Gunn R. B., Fröhlich O. Asymmetry in the mechanism for anion exchange in human red blood cell membranes. Evidence for reciprocating sites that react with one transported anion at a time. J Gen Physiol. 1979 Sep;74(3):351–374. doi: 10.1085/jgp.74.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M., Schuster V. L., Stokes J. B. Absence of transepithelial anion exchange by rabbit OMCD: evidence against reversal of cell polarity. Am J Physiol. 1988 Aug;255(2 Pt 2):F220–F228. doi: 10.1152/ajprenal.1988.255.2.F220. [DOI] [PubMed] [Google Scholar]
- Hebert S. C. Hypertonic cell volume regulation in mouse thick limbs. II. Na+-H+ and Cl(-)-HCO3- exchange in basolateral membranes. Am J Physiol. 1986 Jun;250(6 Pt 1):C920–C931. doi: 10.1152/ajpcell.1986.250.6.C920. [DOI] [PubMed] [Google Scholar]
- Hilden S. A., Johns C. A., Madias N. E. Cl(-)-dependent ATP-driven H+ transport in rabbit renal cortical endosomes. Am J Physiol. 1988 Nov;255(5 Pt 2):F885–F897. doi: 10.1152/ajprenal.1988.255.5.F885. [DOI] [PubMed] [Google Scholar]
- Imbert-Teboul M., Doucet A., Marsy S., Siaume-Perez S. Alterations of enzymatic activities along rat collecting tubule in potassium depletion. Am J Physiol. 1987 Sep;253(3 Pt 2):F408–F417. doi: 10.1152/ajprenal.1987.253.3.F408. [DOI] [PubMed] [Google Scholar]
- Kinsella J. L., Aronson P. S. Amiloride inhibition of the Na+-H+ exchanger in renal microvillus membrane vesicles. Am J Physiol. 1981 Oct;241(4):F374–F379. doi: 10.1152/ajprenal.1981.241.4.F374. [DOI] [PubMed] [Google Scholar]
- Kleinman J. G., Blumenthal S. S., Wiessner J. H., Reetz K. L., Lewand D. L., Mandel N. S., Mandel G. S., Garancis J. C., Cragoe E. J., Jr Regulation of pH in rat papillary tubule cells in primary culture. J Clin Invest. 1987 Dec;80(6):1660–1669. doi: 10.1172/JCI113255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeppen B. M. Conductive properties of the rabbit outer medullary collecting duct: inner stripe. Am J Physiol. 1985 Apr;248(4 Pt 2):F500–F506. doi: 10.1152/ajprenal.1985.248.4.F500. [DOI] [PubMed] [Google Scholar]
- Koeppen B. M. Conductive properties of the rabbit outer medullary collecting duct: outer stripe. Am J Physiol. 1986 Jan;250(1 Pt 2):F70–F76. doi: 10.1152/ajprenal.1986.250.1.F70. [DOI] [PubMed] [Google Scholar]
- Kurtz I. Apical Na+/H+ antiporter and glycolysis-dependent H+-ATPase regulate intracellular pH in the rabbit S3 proximal tubule. J Clin Invest. 1987 Oct;80(4):928–935. doi: 10.1172/JCI113184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz I., Golchini K. Na+-independent Cl(-)-HCO-3- exchange in Madin-Darby canine kidney cells. Role in intracellular pH regulation. J Biol Chem. 1987 Apr 5;262(10):4516–4520. [PubMed] [Google Scholar]
- LeFurgey A., Tisher C. C. Morphology of rabbit collecting duct. Am J Anat. 1979 May;155(1):111–124. doi: 10.1002/aja.1001550108. [DOI] [PubMed] [Google Scholar]
- Lombard W. E., Kokko J. P., Jacobson H. R. Bicarbonate transport in cortical and outer medullary collecting tubules. Am J Physiol. 1983 Mar;244(3):F289–F296. doi: 10.1152/ajprenal.1983.244.3.F289. [DOI] [PubMed] [Google Scholar]
- Lowe A. G., Lambert A. Chloride-bicarbonate exchange and related transport processes. Biochim Biophys Acta. 1982 Dec;694(4):353–374. doi: 10.1016/0304-4157(82)90002-8. [DOI] [PubMed] [Google Scholar]
- Malnic G., De Mello Aires M., Giebisch G. Micropuncture study of renal tubular hydrogen ion transport in the rat. Am J Physiol. 1972 Jan;222(1):147–158. doi: 10.1152/ajplegacy.1972.222.1.147. [DOI] [PubMed] [Google Scholar]
- Nord E. P., Brown S. E., Crandall E. D. Cl-/HCO3- exchange modulates intracellular pH in rat type II alveolar epithelial cells. J Biol Chem. 1988 Apr 25;263(12):5599–5606. [PubMed] [Google Scholar]
- Olsnes S., Tønnessen T. I., Sandvig K. pH-regulated anion antiport in nucleated mammalian cells. J Cell Biol. 1986 Mar;102(3):967–971. doi: 10.1083/jcb.102.3.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinertsen K. V., Tønnessen T. I., Jacobsen J., Sandvig K., Olsnes S. Role of chloride/bicarbonate antiport in the control of cytosolic pH. Cell-line differences in activity and regulation of antiport. J Biol Chem. 1988 Aug 15;263(23):11117–11125. [PubMed] [Google Scholar]
- Ridderstrale Y., Kashgarian M., Koeppen B., Giebisch G., Stetson D., Ardito T., Stanton B. Morphological heterogeneity of the rabbit collecting duct. Kidney Int. 1988 Nov;34(5):655–670. doi: 10.1038/ki.1988.230. [DOI] [PubMed] [Google Scholar]
- Satlin L. M., Schwartz G. J. Postnatal maturation of rabbit renal collecting duct: intercalated cell function. Am J Physiol. 1987 Oct;253(4 Pt 2):F622–F635. doi: 10.1152/ajprenal.1987.253.4.F622. [DOI] [PubMed] [Google Scholar]
- Schuster V. L., Bonsib S. M., Jennings M. L. Two types of collecting duct mitochondria-rich (intercalated) cells: lectin and band 3 cytochemistry. Am J Physiol. 1986 Sep;251(3 Pt 1):C347–C355. doi: 10.1152/ajpcell.1986.251.3.C347. [DOI] [PubMed] [Google Scholar]
- Schuster V. L., Stokes J. B. Chloride transport by the cortical and outer medullary collecting duct. Am J Physiol. 1987 Aug;253(2 Pt 2):F203–F212. doi: 10.1152/ajprenal.1987.253.2.F203. [DOI] [PubMed] [Google Scholar]
- Schwartz G. J., Satlin L. M., Bergmann J. E. Fluorescent characterization of collecting duct cells: a second H+-secreting type. Am J Physiol. 1988 Nov;255(5 Pt 2):F1003–F1014. doi: 10.1152/ajprenal.1988.255.5.F1003. [DOI] [PubMed] [Google Scholar]
- Steinmetz P. R. Cellular organization of urinary acidification. Am J Physiol. 1986 Aug;251(2 Pt 2):F173–F187. doi: 10.1152/ajprenal.1986.251.2.F173. [DOI] [PubMed] [Google Scholar]
- Stokes J. B., Ingram M. J., Williams A. D., Ingram D. Heterogeneity of the rabbit collecting tubule: localization of mineralocorticoid hormone action to the cortical portion. Kidney Int. 1981 Sep;20(3):340–347. doi: 10.1038/ki.1981.144. [DOI] [PubMed] [Google Scholar]
- Stokes J. B., Tisher C. C., Kokko J. P. Structural-functional heterogeneity along the rabbit collecting tubule. Kidney Int. 1978 Dec;14(6):585–593. doi: 10.1038/ki.1978.167. [DOI] [PubMed] [Google Scholar]
- Stone D. K., Seldin D. W., Kokko J. P., Jacobson H. R. Anion dependence of rabbit medullary collecting duct acidification. J Clin Invest. 1983 May;71(5):1505–1508. doi: 10.1172/JCI110905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone D. K., Seldin D. W., Kokko J. P., Jacobson H. R. Mineralocorticoid modulation of rabbit medullary collecting duct acidification. A sodium-independent effect. J Clin Invest. 1983 Jul;72(1):77–83. doi: 10.1172/JCI110986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange K., Spring K. R. Methods for imaging renal tubule cells. Kidney Int. 1986 Aug;30(2):192–200. doi: 10.1038/ki.1986.171. [DOI] [PubMed] [Google Scholar]
- Wall S. M., Kraut J. A., Muallem S. Modulation of Na+-H+ exchange activity by intracellular Na+, H+, and Li+ in IMCD cells. Am J Physiol. 1988 Aug;255(2 Pt 2):F331–F339. doi: 10.1152/ajprenal.1988.255.2.F331. [DOI] [PubMed] [Google Scholar]
- Warnock D. G., Reenstra W. W., Yee V. J. Na+/H+ antiporter of brush border vesicles: studies with acridine orange uptake. Am J Physiol. 1982 Jun;242(6):F733–F739. doi: 10.1152/ajprenal.1982.242.6.F733. [DOI] [PubMed] [Google Scholar]
- Zeidel M. L., Silva P., Seifter J. L. Intracellular pH regulation and proton transport by rabbit renal medullary collecting duct cells. Role of plasma membrane proton adenosine triphosphatase. J Clin Invest. 1986 Jan;77(1):113–120. doi: 10.1172/JCI112264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidel M. L., Silva P., Seifter J. L. Intracellular pH regulation in rabbit renal medullary collecting duct cells. Role of chloride-bicarbonate exchange. J Clin Invest. 1986 May;77(5):1682–1688. doi: 10.1172/JCI112486. [DOI] [PMC free article] [PubMed] [Google Scholar]


