Abstract
Protein tyrosine phosphatases (PTPs) constitute a large and structurally diverse family of signaling enzymes that control the cellular levels of protein tyrosine phosphorylation. Malfunction of PTP activity has significant implications in many human diseases, and the PTP protein family provides an exciting array of validated diabetes/obesity (PTP1B), oncology (SHP2), autoimmunity (Lyp), and infectious disease (mPTPB) targets. However, despite the fact that PTPs have been garnering attention as novel therapeutic targets, they remain largely an untapped resource. The main challenges facing drug developers by the PTPs are inhibitor specificity and bioavailability. Work over the last ten years has demonstrated that it is feasible to develop potent and selective inhibitors for individual members of the PTP family by tethering together small ligands that can simultaneously occupy both the active site and unique nearby peripheral binding sites. Recent results with the bicyclic salicylic acid pharmacophores indicate that the new chemistry platform may provide a potential solution to overcome the bioavailability issue that has plagued the PTP drug discovery field for many years. Structural analysis of PTP-inhibitor complexes reveals molecular determinants important for the development of more potent and selective PTP inhibitors, thus offering hope in the medicinal chemistry of a largely unexploited protein class with a wealth of attractive drug targets.
Keywords: protein tyrosine phosphatases, therapeutic target, salicylic acid, inhibitor specificity and bioavailability, combinatorial chemistry
1. Introduction
Reversible tyrosine phosphorylation, catalyzed by the coordinated action of protein tyrosine kinases (PTKs) and protein tyrosine phosphatases (PTPs), is of critical importance to the regulation of signaling events that control essential cellular processes such as growth, differentiation, metabolism, motility, and apoptosis.1,2 Not surprisingly, disruption of the delicate balance between the action of PTKs and PTPs results in aberrant tyrosine phosphorylation, which has been linked to the etiology of several human diseases, including cancer, diabetes, and autoimmune disorders.1, 3–6 Indeed, cellular pathways regulated by tyrosine phosphorylation offer a rich source of molecular targets for novel therapeutics.7 For example, abnormal expression and activation of many receptor tyrosine kinases, such as the epidermal growth factor (EGF) receptor, and the proto-oncogene nonreceptor tyrosine kinases Src and Abl, are causative factors in cancer.8 The first drugs directed against PTKs have now entered the clinic and represent breakthroughs in cancer therapy. These include Gleevec (STI-571), an inhibitor of the p210 Bcr-Abl oncoprotein for the treatment of chronic myelogenous leukemia, and the EGF receptor kinase inhibitors Iressa (Gefitinib) and Tarceva (Erlotinib) for treatment of non-small cell lung cancer.
Major insights into tyrosine phosphorylation mediated cellular events have been derived from studies of PTKs and it is common to view signaling pathways as cascades of reactions emanating from the PTKs. Understandably, drug discovery efforts to date have emphasized the PTKs. Given the reversible nature of protein tyrosine phosphorylation, characterization of the PTPs is a prerequisite to gaining a complete understanding of the physiological consequences of tyrosine phosphorylation and how such signaling events are abrogated in pathological conditions. This in turn may lead to new, more effective drugs for human diseases. The PTPs constitute a large family of enzymes (>100) that parallel PTKs in their structural diversity and complexity.9 Unlike protein kinases, where tyrosine specific and serine/threonine specific kinases share sequence identity, the PTPs show no sequence similarity with serine/threonine phosphatases, or the broad specificity phosphatases such as acid or alkaline phosphatases. The hallmark that defines the PTP superfamily is the active site sequence (I/V)HCXAGXGR(S/T), the PTP signature motif.
PTPs constitute a diverse family of enzymes that have unique biological functions in vivo.2 In addition, as observed with PTKs, deregulation of PTP activity also contributes to the pathogenesis of human diseases.4,10 By catalyzing the removal of the phosphoryl group from tyrosine residues, the PTPs are generally viewed as negative regulators due to their ability to reverse the actions of PTKs, which usually drive signaling cascades. For example, biochemical and genetic studies indicate that PTP1B down-regulates both insulin and leptin signaling.11 Accordingly, mice lacking functional PTP1B exhibit increased sensitivity toward insulin and are resistant to obesity.12,13 The lymphoid-specific tyrosine phosphatase (Lyp) is a critical negative regulator of T cell receptor signaling,14 possibly through the dephosphorylation and inactivation of the PTKs Lck and ZAP-70.15 Recent genome-wide-association studies identified a missense C1858T single-nucleotide polymorphism in the gene (PTPN22) encoding Lyp, which is associated with an increased risk for the development of several autoimmune diseases, including type I diabetes,16 rheumatoid arthritis,17,18 Graves disease,19 and systemic lupus erythematosus.20 Current data indicate that the autoimmune-predisposing PTPN22-W620 variant is a gain-of-function form of the phosphatase that leads to decreased T cell receptor and B cell receptor signaling, which may in turn regulate the establishment of human T and B cell tolerance.21,22 Given the strong association of the C1858T polymorphism with various autoimmune disorders and the elevated phosphatase activity associated with the resultant PTPN22/W620 variant, Lyp represents an attractive target for a broad spectrum of autoimmune diseases.
Interestingly, mounting evidence shows that some PTPs can potentiate, rather than antagonize, signaling mediated by the PTKs. This mode of synergy enhances mitogenic processes, leading to cell transformation. For example, the Src homology 2 (SH2)-domain containing protein tyrosine phosphatase-2 (SHP2), encoded by the PTPN11 gene, plays a positive role in signal transduction downstream of growth factor and cytokine receptors to regulate proliferation, differentiation, motility, and apoptosis.23 Biochemical and genetic evidence places SHP2 upstream of Ras, an essential component of the signaling pathway that underlies growth factor/cytokine-induced cell proliferation and survival,24 and SHP2 activity is required for full activation of the Ras-extracellular signal-regulated kinase (ERK1/2) cascade.23 The critical role of SHP2 in cell physiology is further emphasized by the identification of mutations within SHP2, which are linked to several human diseases. Thus, germline mutations in SHP2 that cause hyperactivation of its phosphatase activity are associated with 50% Noonan syndrome, an autosomal dominant disorder with increased tendency for hematologic abnormalities, including myeloid disorders and juvenile myelomonocytic leukemia.25 Somatic gain-of-function mutations in SHP2 occur in 35% of individuals with juvenile myelomonocytic leukemia, as well as in acute myeloid leukemia (4%), myelodysplastic syndrome (10%), and acute lymphoid leukemia (7%).26–30 In addition to childhood leukemia, SHP2 mutations also occur in adult acute myeloid leukemia (6%) as well as in solid tumors including lung adenocarcinoma, colon cancer, neuroblastoma, melanoma, and hepatocellular carcinoma.31,32 Collectively, these genetic and biochemical observations identify SHP2 as the first bona fide oncogene in the PTP superfamily.
The importance of the PTPs in cellular physiology is also underscored by the fact that they are often exploited and subverted by pathogenic bacteria to cause infection. For instance, YopH, the PTP from Yersinia, inhibits bacterial phagocytosis by removing phosphates from focal adhesion proteins,33 while the Salmonella tyrosine phosphatase SptP dephosphorylates host AAA+ ATPase to promote its intracellular replicative niche.34 Strikingly, Mycobacterium tuberculosis (Mtb) encodes two PTPs, termed mPTPA and mPTPB,35 both of which are secreted by Mtb into the cytoplasm of the macrophage and are important for persistence of mycobacterial infection.36,37 Given the absence of endogenous tyrosine phosphorylation within Mtb, mPTPA and mPTPB likely modifies macrophage proteins to manipulate host-pathogen interactions. Interestingly, deletion of mPTPB blocks intracellular survival of Mtb in interferon-γ (IFN-γ) activated macrophages and severely reduces the bacterial load in a guinea pig model of TB infection.36 These findings led to the hypothesis that mPTPB may mediate mycobacterial survival in macrophages by targeting host cell processes.38 Our recent study revealed that mPTPB prevents macrophage apoptosis and cytokine production.39 The importance of mPTPB for Mtb survival in macrophages also suggests that specific inhibition of mPTPB activity may augment intrinsic host signaling pathways to eradicate tuberculosis infection. Thus, mPTPB represents an exciting new target for anti- tuberculosis drug development.
2. Challenges in Developing PTP-based Therapeutic
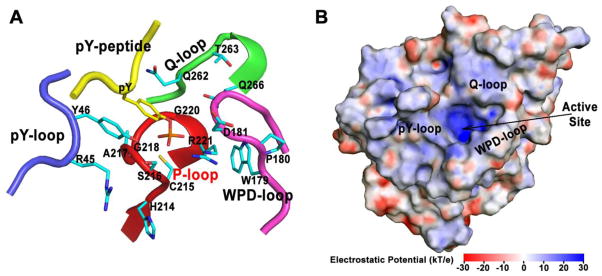
In view of the above discussion, members of the PTP family have been implicated in a wide array of human disorders including diabetes/obesity, oncology, autoimmunity, and infectious diseases. Unfortunately, there is a notable absence of drugs targeting the PTPs. Indeed, the PTPs have proven to be exceptionally challenging targets for the development of new therapeutic agents.40 The major contributing factors to the failure of targeting the PTPs for drug discovery relate to the intrinsic properties of the PTP active site (Figure 1). The catalytic site is highly conserved, so it is not trivial to obtain drugs that can inhibit single PTPs with good selectivity. This is an issue common to most enzyme families that act upon common substrate motifs (such as pTyr for PTPs or ATP for kinases). Moreover, the active site of PTPs is highly positively charged and contains a conserved catalytic cysteine residue, so the brute-force screening of large compound libraries usually leads to initial hits that are either negatively charged or contain oxidizing groups that irreversibly react with the active site cysteine. Strongly polar compounds do not readily cross cell membranes and chemically-reactive compounds with oxidizing activity (e.g. quinones) also have poor safety and selectivity profiles, making them unappealing as drugs. Consequently, despite the fact that PTPs have been garnering attention as potential therapeutic targets, they remain largely an untapped resource.
Figure 1.
Intrinsic properties of the PTP active site. A). The active site of PTP (herein PTP1B as an example) is highly conserved, constituted mainly by four loops, generally named as P-loop, WPD-loop, pY-loop and Q-loop. Several residues essential for pTyr binding and catalysis, showing in cyan stick, are highly conserved. B). The active site of PTP is highly positively charged. The electrostatic potential is calculated using APBS1.3 and is mapped onto the molecular surface of PTP1B.
3. Potent and Selective Inhibitors Can Be Developed for the PTPs
To address the issue of specificity, it has long been recognized that pTyr alone is not sufficient for high-affinity binding and residues flanking the pTyr are important for PTP substrate recognition.41–47 These results indicate that there are subpockets adjacent to the PTP active site that can also be targeted for inhibitor development. Accordingly, a novel paradigm was advanced for the design of potent and specific PTP inhibitors, namely bidentate ligands that bind both the active site and a unique adjacent peripheral site.48 The rationale for the enhanced affinity of bidentate inhibitors is based on the principle of additivity of free energy of binding. The interaction of an inhibitor with two independent sites (e.g., pTyr site and a unique peripheral site) would be expected to confer exquisite specificity, since other PTPs may not possess an identical second site interaction.
Can potent and selective PTP inhibitors be devised by tethering a nonhydrolyzable pTyr mimetic to appropriately functionalized moieties to engage both the active site and unique nearby sub-pockets? To address this question, we initially selected as our starting common molecular motif the nonhydrolyzable pTyr mimetic phosphonodifluoromethyl phenylalanine (F2Pmp).49,50 Although F2Pmp likely interacts in the desired inhibitory fashion with all PTPs, molecular scaffolds attached to the pTyr mimetic may render the inhibitors PTP-selective. Through a focused combinatorial library approach we obtained the most potent and selective PTP1B inhibitor identified to date (Ki = 2.4 nM) that exhibits a 10-fold selectivity against its closest homologue TC-PTP, and over 1,000-fold selectivity against a panel of other PTPs.51 Structural studies reveal that potent and selective PTP1B inhibitory agents are capable of binding both the active site and an adjacent peripheral site.52,53 A number of specific PTP1B inhibitors have since been reported that target both the active site and a nearby peripheral site.11,54 More recently, we have developed a stepwise fluorophore-tagged combinatorial library synthesis and screening strategy that transforms a weak and general PTP inhibitor F2Pmp into a very potent and selective TC-PTP inhibitor.55 Collectively, the results serve as proof-of-concept in PTP inhibitor development and establish the feasibility of acquiring potent, yet highly selective, PTP inhibitory agents.
4. Development of Bicyclic Salicylic Acid Based PTP Inhibitors with Highly Efficacious Cellular Activity
As described above, we and others have shown that it is possible to acquire potent and selective PTP inhibitors by targeting both the active site and an adjacent less-conserved subpocket. Unfortunately, the highly positively charged pTyr-binding pocket impedes the development of inhibitors with favorable bioavailability. Most of the reported PTP inhibitors are not drug-like, and thus are deficient in cell membrane permeability, which has limited further advancement of such compounds as drug candidates.54,56 Thus, one major remaining challenge, which has delayed realization of PTP-based small molecule therapeutics, has been the identification of novel chemical entities with improved physicochemical properties and bioavailability. Indeed, the lack of cellular efficacy of existing PTP inhibitors represents a major obstacle in developing phosphatase-based therapeutics.
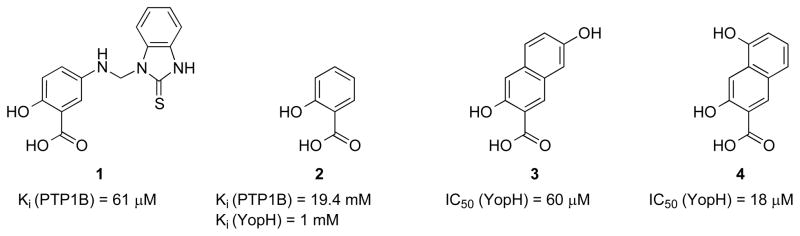
Bioactive natural products are very promising starting points for drug development because they are evolutionarily selected and validated for interfering and interacting with biological targets. Indeed, a disproportionately high percentage of all medicines originated from natural products.57 We discovered from an in silico DOCK screening campaign that the natural product salicylic acid-containing compound 1 (Figure 2) serves as a competitive inhibitor of PTP1B with a Ki of 61 μM.58 Modeling shows that the salicylic acid moiety in 1 approximates the binding mode of pTyr in PTP1B and, consequently, many of the interactions seen between the phosphate moiety and the phosphate binding loop in the PTP1B•pTyr structure are conserved between PTP1B and 1. This suggests that salicylic acid 2 (Figure 2) may serve as a new non-phosphorus-containing pTyr mimic, utilizing carboxylic acid and hydroxyl groups to provide functionality normally afforded by the phosphoryl group in pTyr. As expected, salicylic acid is a competitive inhibitor of PTP1B. However, it only exhibits an inhibition constant of 19.4 mM for PTP1B, similar to the affinity of pTyr and other pTyr surrogates. Thus, additional interactions involving hydrophobic contacts between the 2-thioxo-1-benzimidazolyl fused ring of 1 and surface residues in the immediate vicinity of PTP1B active site are likely responsible for the 320-fold enhancement in the observed binding of 1 versus salicylic acid. We further demonstrated that naphthyl and polyaromatic salicylic acid derivatives (e.g., compounds 3 and 4 in Figure 2) exhibit enhanced affinity for PTPs relative to the corresponding single ring compounds,58,59 indicating that the PTP active site possesses considerable plasticity such that substituted and polyaromatic compounds significantly larger than pTyr can be accommodate to gain additional hydrophobic interactions. Based on these findings, we sought to develop bicyclic salicylic acid-based PTP inhibitors that carry sufficient polar and nonpolar interactions with the active site and yet possess improved pharmacological properties. To this end, we have focused our attention on benzofuran and indole-based salicylic acids as pTyr mimetics. Benzofuran and indole derivatives are of considerable interest because of their widespread occurrence among natural products and synthetic compounds with vital medicinal value.60–62 We hypothesized that a benzofuran or indole-based salicylic acid may bind the active site and interact in the desired inhibitory fashion with the PTPs, and additional diversity elements to which the salicylic core is attached to should render the inhibitors PTP isozyme-selective.
Figure 2.
Salicylic acid derivatives as pTyr mimetics.
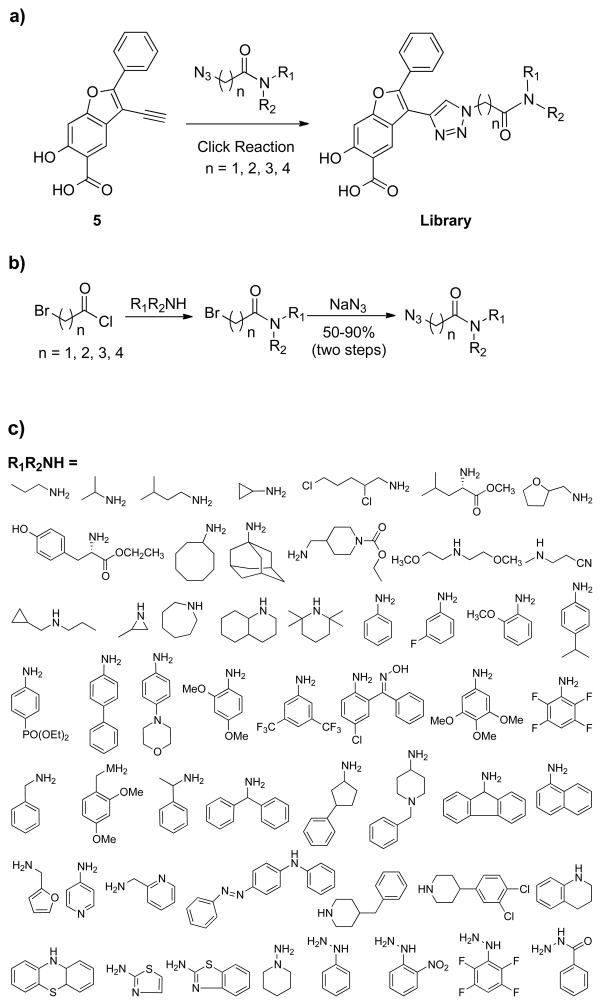
To target both the active site and an adjacent, secondary binding site in PTPs, we have developed a salicylic acid-based combinatorial library approach which entails the following criteria: (1) each member will be of a modular, bidentate structure containing both an active site-directed core (e.g., benzofuran salicylic acid) and a diversity element for interaction with adjacent peripheral sites in PTPs; (2) both the core and the diversity elements will possess favorable pharmacological properties; and (3) each member will be efficiently assembled in situ for facile screening against the PTPs. We initially chose click chemistry for library construction because it offers an expedient way to connect two components together with high yield and purity under extremely mild conditions.63 More importantly, the click reaction can be conducted in aqueous solution in the absence of deleterious reagents, thus allowing direct screening and identification of hits from the library.64–68
Figure 3 depicts a benzofuran salicylic acid based focused library strategy for the acquisition of potent and selective PTP inhibitory agents that are capable of bridging both the active site and an adjacent peripheral site. Thus, the library contains (a) a benzofuran salicylic acid core to engage the active site, and (b) 4 alkyl linkers of 1 to 4 methylene unit to tether the pTyr surrogate to (c) a structurally diverse set of amines and hydrazines, aimed at capturing additional interactions with adjacent pockets surrounding the active site. In the interest of keeping the library to a reasonable size, we selected 53 amines and hydrazines that vary by molecular weight, charge, polarity, hydrophobicity, sterics, etc. and therefore provide a reasonable (albeit limited) structural diversity to increase the number and strength of noncovalent interactions between the PTP and the inhibitor.
Figure 3.
A strategy for the construction of a benzofuran salicylic acid based focused library using click chemistry.
The benzofuran salicylic acid core was prepared from a commercially available compound 4-hydroxysalicylic acid.39 To increase potency and selectivity, the strategically positioned alkyne in the benzofuran salicylic acid core was tethered to 212 different azide-containing diversity elements (53 discrete amines and hydrazines (Figure 3) with 4 alkyl linkers of 1 to 4 methylene length), using click chemistry or the Cu(I)-catalyzed [3+2] azide-alkyne cycloaddition reaction (Figure 3). The azide-containing building blocks were synthesized in a one-pot procedure (Figure 3), in which amines or hydrazines were reacted with the acyl chloride linkers in N, N-dimethylformamide, followed by SN2 reaction with sodium azide to generate the corresponding azides.
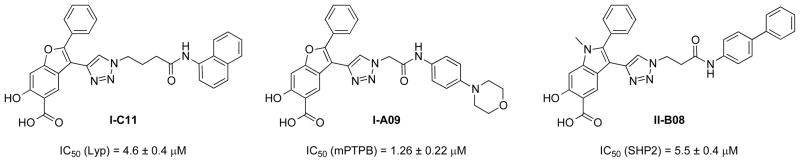
High throughput screening of the benzofuran salicylic acid library let to the identification of a Lyp inhibitor I-C11 (Ki = 2.9±0.5 μM)69 and an mPTPB inhibitor I-A09 (Ki = 1.06±0.06 μM)39 (Figure 4). Similarly, synthesis and screening of an indole-based salicylic acid library furnished a SHP2 inhibitor II-B08 (Ki = 5.2±0.3 μM)70 (Figure 4). Importantly, I-C11, IA-09, and II-B08 possess highly efficacious cellular activity and inhibit their target PTPs in intact cells with similar potencies as those toward the isolated enzymes, whereas previous PTP inhibitors experience 100–10,000 fold loss of potency from biochemical to cellular assays. Thus, treatment of Jurkat T cells by I-C11 increases the T cell receptor mediated Lck phosphorylation on Tyr394 and ERK1/2 activation.69 In addition, inhibition of Lyp by I-C11 reverses that deficit in signaling and proliferation in B cells isolated from PTPN22-W620 subjects at 1–10 μM I-C11 concentrations.22 Similarly, I-A09 possesses highly efficacious cellular activity, capable of reversing the altered host immune responses induced by the bacterial phosphatase in Raw264.7 mouse macrophages in the range of 2–5 μM I-A09 concentrations.39 Moreover, I-A09 blocks mycobacterial growth in host macrophages, which further supports the notion that mPTPB inhibitors could become a valuable weapon in the fight against tuberculosis.39 Finally, the indole-based salicylic acid SHP2 inhibitor II-B08 also possesses highly efficacious cellular activity, and is capable of blocking growth factor stimulated ERK1/2 activation and proliferation in HEK293 cells as well as blocking SHP2 gain-of-function mutant-induced hematopoietic progenitor hyperproliferation in response to GM-CSF in the range of 5–10 μM II-B08 concentrations.70 Again, the observed cellular efficacy for II-B08 is comparable to its biochemical potency. Together, these results provide the necessary proof-of-principle data to support the notion that specific PTP inhibitors with more favorable bioavailability can be developed.
Figure 4.
Structures of I-C11, I-A09, and II-B08.
5. Molecular Basis of PTP Inhibition by Bicyclic Benzofuran and Indole-Based Salicylic Acids
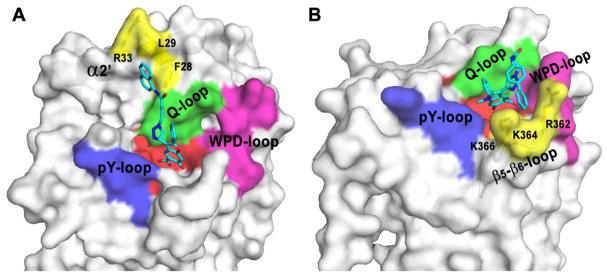
X-ray crystal structural analysis of the Lyp•I-C11 complex indicates that the benzofuran salicylic core occupies the active site whereas the distal naphthalene ring makes hydrophobic interactions with a region unique to Lyp (Figure 5).69 This peripheral site is defined by Phe28, Leu29, and Arg33 in Lyp, which are located in α2′, and border a binding pocket equivalent to the second aryl phosphate-binding site previously identified in PTP1B.48 This second aryl phosphate-binding pocket is important for PTP1B substrate recognition71,72 and has been targeted for PTP1B inhibitor development.11 We have also determined the crystal structure of the catalytic domain of SHP2 in complex with II-B08.70 The structure reveals that the indole salicylic acid core also occupies the SHP2 active site, while the biphenyl group in II-B08 interacts with Arg362, Lys364, and Lys366 in the β5–β6 loop, opposite to the pocket occupied by the naphthyl group in I-C11 (Figure 5). Interestingly, the β5–β6 loop is highly divergent among the PTPs.10 Moreover, it is interesting to note that although both the benzofuran and indole salicylic cores occupy the active site, the distal elements in I-C11 and II-B08 interact with different peripheral sites in the PTPs (Figure 5). These additional non-polar interactions between the distal element in the inhibitor and residues in the peripheral sites contribute to the potency and selectivity of I-C11 and II-B08.69,70 These structural observations highlight the differences in the way individual PTPs interact with the bicyclic salicylic acid based compounds and furnish a solid foundation upon which PTP inhibitors with therapeutic potency and selectivity can be developed based on the bicyclic salicylic acid chemistry platform.
Figure 5.
Crystal structures of Lyp•I-C11 and SHP2•II-B08. Lyp and SHP2 are situated in the same orientation in order to show that although the salicylic cores in I-C11 and II-B08 both occupy the active site, the distal elements in I-C11 and II-B08 interact with different peripheral sites in Lyp and SHP2. A). The structure of Lyp catalytic domain in complex with I-C11. The benzofuran salicylic core occupies the active site whereas the naphthalene ring makes hydrophobic interactions with F28, L29 and R33 locating in the α2′ helix, which includes the unique “Lyp insert” residues. The density for the phenyl group on the bicyclic nucleus is weak in the original structure. Based on its location in I-C11, the phenyl group is not expected to interact with Lyp. The phenyl group depicted in Figure 5A is a model based on its expected position. B). The structure of SHP2 catalytic domain in complex with II-B08. The indole salicylic core also occupies the active site, while the biphenyl group interacts with R362, K364 and K366 locating in the β5–β6-loop.
6. Conclusion
In summary, the PTP family provides an exciting array of validated but previously deemed undruggable targets for diabetes/obesity, oncology, autoimmunity, and infectious diseases. The major challenges presented to the drug developers by the PTPs are specificity and bioavailability. Work over the last ten years has demonstrated that it is feasible to develop potent and selective inhibitors for individual members of the PTP family by tethering together small ligands that can simultaneously occupy both the active site and unique nearby peripheral binding sites. Recent results with the bicyclic salicylic acid pharmacophores indicate that the new chemistry platform may provide a potential solution to overcome the bioavailability issue that has plagued the PTP drug discovery field for many years. The bidentate salicylic acid based PTP inhibitors are sufficiently polar to bind the PTP active site with high affinity, yet remain capable of efficiently crossing cell membranes. X-ray crystallographic analysis of PTP-inhibitor complexes reveals molecular determinants that can be exploited for the acquisition of more potent and selective PTP inhibitors, thus offering hope in the medicinal chemistry of a largely unexploited protein class with a wealth of attractive drug targets.
Acknowledgments
This work was supported by National Institutes of Health Grants CA69202, CA126937, and CA152194.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hunter T. Phil Trans R Soc Lond B Biol Sci. 1998;353:583. doi: 10.1098/rstb.1998.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tonks NK. Nat Rev Mol Cell Biol. 2006;7:833. doi: 10.1038/nrm2039. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Z-Y. Curr Opin Chem Biol. 2001;5:416. doi: 10.1016/s1367-5931(00)00223-4. [DOI] [PubMed] [Google Scholar]
- 4.Arena S, Benvenuti S, Bardelli A. Cell Mol Life Sci. 2005;62:2092. doi: 10.1007/s00018-005-5205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ventura JJ, Nebreda AR. Clin Transl Oncol. 2006;8:153. doi: 10.1007/s12094-006-0005-0. [DOI] [PubMed] [Google Scholar]
- 6.Julien SG, Dubé N, Hardy S, Tremblay ML. Nat Rev Cancer. 2011;11:35. doi: 10.1038/nrc2980. [DOI] [PubMed] [Google Scholar]
- 7.Blume-Jensen P, Hunter T. Nature. 2001;411:355. doi: 10.1038/35077225. [DOI] [PubMed] [Google Scholar]
- 8.Krause DS, Van Etten RA. N Engl J Med. 2005;353:172. doi: 10.1056/NEJMra044389. [DOI] [PubMed] [Google Scholar]
- 9.Alonso A, Sasin J, Bottini N, Friedberg I, Friedberg I, Osterman A, Godzik A, Hunter T, Dixon J, Mustelin T. Cell. 2004;117:699. doi: 10.1016/j.cell.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 10.Andersen JN, Jansen PG, Echwald SM, Mortensen OH, Fukada T, Del Vecchio R, Tonks NK, Moller NP. FASEB J. 2004;18:8. doi: 10.1096/fj.02-1212rev. [DOI] [PubMed] [Google Scholar]
- 11.Zhang S, Zhang Z-Y. Drug Discovery Today. 2007;12:373. doi: 10.1016/j.drudis.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 12.Elchelby M, Payette P, Michaliszyn E, Cromlish W, Collins S, Lee Loy A, Normandin D, Cheng A, Himms-Hagen J, Chan C-C, Ramachandran C, Gresser MJ, Tremblay ML, Kennedy BP. Science. 1999;283:1544. doi: 10.1126/science.283.5407.1544. [DOI] [PubMed] [Google Scholar]
- 13.Klaman LD, Boss O, Peroni OD, Kim JK, Martino JL, Zabolotny JM, Moghal N, Lubkin M, Kim YB, Sharpe AH, Stricker-Krongrad A, Shulman GI, Neel BG, Kahn BB. Mol Cell Biol. 2000;20:5479. doi: 10.1128/mcb.20.15.5479-5489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen S, Dadi H, Shaoul E, Sharfe N, Roifman CM. Blood. 1999;93:2013. [PubMed] [Google Scholar]
- 15.Cloutier JF, Veillette A. J Exp Med. 1999;189:111. doi: 10.1084/jem.189.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bottini N, Musumeci L, Alonso A, Rahmouni S, Nika K, Rostamkhani M, MacMurray J, Meloni GF, Lucarelli P, Pellecchia M, Eisenbarth GS, Comings D, Mustelin T. Nat Genet. 2004;36:337. doi: 10.1038/ng1323. [DOI] [PubMed] [Google Scholar]
- 17.Begovich AB, Carlton VE, Honigberg LA, Schrodi SJ, Chokkalingam AP, Alexander HC, Ardlie KG, Huang Q, Smith AM, Spoerke JM, Conn MT, Chang M, Chang SY, Saiki RK, Catanese JJ, Leong DU, Garcia VE, McAllister LB, Jeffery DA, Lee AT, Batliwalla F, Remmers E, Criswell LA, Seldin MF, Kastner DL, Amos CI, Sninsky JJ, Gregersen PK. Am J Hum Genet. 2004;75:330. doi: 10.1086/422827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carlton VE, Hu X, Chokkalingam AP, Schrodi SJ, Brandon R, Alexander HC, Chang M, Catanese JJ, Leong DU, Ardlie KG, Kastner DL, Seldin MF, Criswell LA, Gregersen PK, Beasley E, Thomson G, Amos CI, Begovich AB. Am J Hum Genet. 2005;77:567. doi: 10.1086/468189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smyth D, Cooper JD, Collins JE, Heward JM, Franklyn JA, Howson JM, Vella A, Nutland S, Rance HE, Maier L, Barratt BJ, Guja C, Ionescu-Tirgoviste C, Savage DA, Dunger DB, Widmer B, Strachan DP, Ring SM, Walker N, Clayton DG, Twells RC, Gough SC, Todd JA. Diabetes. 2004;53:3020. doi: 10.2337/diabetes.53.11.3020. [DOI] [PubMed] [Google Scholar]
- 20.Kyogoku C, Langefeld CD, Ortmann WA, Lee A, Selby S, Carlton VE, Chang M, Ramos P, Baechler EC, Batliwalla FM, Novitzke J, Williams AH, Gillett C, Rodine P, Graham RR, Ardlie KG, Gaffney PM, Moser KL, Petri M, Begovich AB, Gregersen PK, Behrens TW. Am J Hum Genet. 2004;75:504. doi: 10.1086/423790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vang T, Congia M, Macis MD, Musumeci L, Orru V, Zavattari P, Nika K, Tautz L, Tasken K, Cucca F, Mustelin T, Bottini N. Nat Genet. 2005;37:1317. doi: 10.1038/ng1673. [DOI] [PubMed] [Google Scholar]
- 22.Arechiga AF, Habib T, He Y, Zhang X, Zhang Z-Y, Funk A, Buckner JH. J Immunol. 2009;182:3343. doi: 10.4049/jimmunol.0713370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neel BG, Gu H, Pao L. Trends Biochem Sci. 2003;28:284. doi: 10.1016/S0968-0004(03)00091-4. [DOI] [PubMed] [Google Scholar]
- 24.Malumbres M, Barbacid M. Nat Rev Cancer. 2003;3:459. doi: 10.1038/nrc1097. [DOI] [PubMed] [Google Scholar]
- 25.Tartaglia M, Gelb BD. Annu Rev Genomics Hum Genet. 2005;6:45. doi: 10.1146/annurev.genom.6.080604.162305. [DOI] [PubMed] [Google Scholar]
- 26.Tartaglia M, Niemeyer CM, Fragale A, Song X, Buechner J, Jung A, Hahlen K, Hasle H, Licht JD, Gelb BD. Nat Genet. 2003;34:148. doi: 10.1038/ng1156. [DOI] [PubMed] [Google Scholar]
- 27.Tartaglia M, Martinelli S, Cazzaniga G, Cordeddu V, Iavarone I, Spinelli M, Palmi C, Carta C, Pession A, Arico M, Masera G, Basso G, Sorcini M, Gelb BD, Biondi A. Blood. 2004;104:307. doi: 10.1182/blood-2003-11-3876. [DOI] [PubMed] [Google Scholar]
- 28.Loh ML, Vattikuti S, Schubbert S, Reynolds MG, Carlson E, Lieuw KH, Cheng JW, Lee CM, Stokoe D, Bonifas JM, Curtiss NP, Gotlib J, Meshinchi S, Le Beau MM, Emanuel PD, Shannon KM. Blood. 2004;103:2325. doi: 10.1182/blood-2003-09-3287. [DOI] [PubMed] [Google Scholar]
- 29.Loh ML, Reynolds MG, Vattikuti S, Gerbing RB, Alonzo TA, Carlson E, Cheng JW, Lee CM, Lange BJ, Meshinchi S Children’s Cancer Group. Leukemia. 2004;18:1831. doi: 10.1038/sj.leu.2403492. [DOI] [PubMed] [Google Scholar]
- 30.Kratz CP, Niemeyer CM, Castleberry RP, Cetin M, Bergsträsser E, Emanuel PD, Hasle H, Kardos G, Klein C, Kojima S, Stary J, Trebo M, Zecca M, Gelb BD, Tartaglia M, Loh ML. Blood. 2005;106:2183. doi: 10.1182/blood-2005-02-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bentires-Alj M, Paez JG, David FS, Keilhack H, Halmos B, Naoki K, Maris JM, Richardson A, Bardelli A, Sugarbaker DJ, Richards WG, Du J, Girard L, Minna JD, Loh ML, Fisher DE, Velculescu VE, Vogelstein B, Meyerson M, Sellers WR, Neel BG. Cancer Res. 2004;64:8816. doi: 10.1158/0008-5472.CAN-04-1923. [DOI] [PubMed] [Google Scholar]
- 32.Miyamoto D, Miyamoto M, Takahashi A, Yomogita Y, Higashi H, Kondo S, Hatakeyama M. Oncogene. 2008;27:3508. doi: 10.1038/sj.onc.1211019. [DOI] [PubMed] [Google Scholar]
- 33.Black DS, Bliska JB. EMBO J. 1997;16:2730. doi: 10.1093/emboj/16.10.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Humphreys D, Hume PJ, Koronakis V. Cell Host Microbe. 2009;5:225. doi: 10.1016/j.chom.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon SV, Eiglmeier K, Gas S, Barry CE, 3rd, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Krogh A, McLean J, Moule S, Murphy L, Oliver K, Osborne J, Quail MA, Rajandream MA, Rogers J, Rutter S, Seeger K, Skelton J, Squares R, Squares S, Sulston JE, Taylor K, Whitehead S, Barrell BG. Nature. 1998;393:537. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 36.Singh R, Rao V, Shakila H, Gupta R, Khera A, Dhar N, Singh A, Koul A, Singh Y, Naseema M, Narayanan PR, Paramasivan CN, Ramanathan VD, Tyagi AK. Mol Microbiol. 2003;50:751. doi: 10.1046/j.1365-2958.2003.03712.x. [DOI] [PubMed] [Google Scholar]
- 37.Castandet J, Prost JF, Peyron P, Astarie-Dequeker C, Anes E, Cozzone AJ, Griffiths G, Maridonneau-Parini I. Res Microbiol. 2005;156:1005. doi: 10.1016/j.resmic.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 38.Koul A, Herget T, Klebl B, Ullrich A. Nat Rev Microbiol. 2004;2:189. doi: 10.1038/nrmicro840. [DOI] [PubMed] [Google Scholar]
- 39.Zhou B, He Y, Zhang X, Xu J, Luo Y, Wang Y, Franzblau SG, Yang Z, Chan RJ, Liu Y, Zheng J, Zhang Z-Y. Proc Natl Acad Sci USA. 2010;107:4573. doi: 10.1073/pnas.0909133107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barr AJ. Future Med Chem. 2010;2:1563. doi: 10.4155/fmc.10.241. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Z-Y, Maclean D, Thieme-Sefler AM, McNamara D, Dobrusin EM, Sawyer TK, Dixon JE. Proc Natl Acad Sci USA. 1993;90:4446. doi: 10.1073/pnas.90.10.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Z-Y, Maclean D, McNamara DJ, Sawyer TK, Dixon JE. Biochemistry. 1994;33:2285. doi: 10.1021/bi00174a040. [DOI] [PubMed] [Google Scholar]
- 43.Jia Z, Barford D, Flint AJ, Tonks NK. Science. 1995;268:1754. doi: 10.1126/science.7540771. [DOI] [PubMed] [Google Scholar]
- 44.Wu L, Buist A, den Hertog J, Zhang Z-Y. J Biol Chem. 1997;272:6994. doi: 10.1074/jbc.272.11.6994. [DOI] [PubMed] [Google Scholar]
- 45.Sarmiento M, Zhao Y, Gordon SJ, Zhang Z-Y. J Biol Chem. 1998;273:26368. doi: 10.1074/jbc.273.41.26368. [DOI] [PubMed] [Google Scholar]
- 46.Sarmiento M, Puius YA, Vetter SW, Keng Y-F, Wu L, Zhao Y, Lawrence DS, Almo SC, Zhang Z-Y. Biochemistry. 2000;39:8171. doi: 10.1021/bi000319w. [DOI] [PubMed] [Google Scholar]
- 47.Vetter SW, Keng Y-F, Lawrence DS, Zhang Z-Y. J Biol Chem. 2000;275:2265. doi: 10.1074/jbc.275.4.2265. [DOI] [PubMed] [Google Scholar]
- 48.Puius YA, Zhao Y, Sullivan M, Lawrence DS, Almo SC, Zhang Z-Y. Proc Natl Acad Sci USA. 1997;94:13420. doi: 10.1073/pnas.94.25.13420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burke TR, Jr, Kole HK, Roller PP. Biochem Biophys Res Commun. 1994;204:129. doi: 10.1006/bbrc.1994.2435. [DOI] [PubMed] [Google Scholar]
- 50.Chen L, Wu L, Otaka A, Smyth MS, Roller PP, Burke TR, den Hertog J, Zhang Z-Y. Biochem Biophys Res Commun. 1995;216:976. doi: 10.1006/bbrc.1995.2716. [DOI] [PubMed] [Google Scholar]
- 51.Shen K, Keng Y-F, Wu L, Guo X-L, Lawrence DS, Zhang Z-Y. J Biol Chem. 2001;276:47311. doi: 10.1074/jbc.M106568200. [DOI] [PubMed] [Google Scholar]
- 52.Guo X-L, Shen K, Wang F, Lawrence DS, Zhang Z-Y. J Biol Chem. 2002;277:41014. doi: 10.1074/jbc.M207347200. [DOI] [PubMed] [Google Scholar]
- 53.Sun J-P, Fedorov AA, Lee S-Y, Guo X-L, Shen K, Lawrence DS, Almo SC, Zhang Z-Y. J Biol Chem. 2003;278:12406. doi: 10.1074/jbc.M212491200. [DOI] [PubMed] [Google Scholar]
- 54.Combs AP. J Med Chem. 2010;53:2333. doi: 10.1021/jm901090b. [DOI] [PubMed] [Google Scholar]
- 55.Zhang S, Chen L, Luo Y, Gunawan A, Lawrence DS, Zhang Z-Y. J Am Chem Soc. 2009;131:13072. doi: 10.1021/ja903733z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vintonyak VV, Antonchick AP, Rauh D, Waldmann H. Curr Opin Chem Biol. 2009;13:272. doi: 10.1016/j.cbpa.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 57.Newman DJ, Cragg GM. J Nat Prod. 2007;70:461. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- 58.Sarmiento M, Wu L, Keng Y-F, Song L, Luo Z, Huang Z, Wu G-Z, Yuan AK, Zhang Z-Y. J Med Chem. 2000;43:146. doi: 10.1021/jm990329z. [DOI] [PubMed] [Google Scholar]
- 59.Liang F, Huang Z, Lee S-Y, Liang J, Ivanov MI, Alonso A, Bliska JB, Lawrence DS, Mustelin T, Zhang Z-Y. J Biol Chem. 2003;278:41734. doi: 10.1074/jbc.M307152200. [DOI] [PubMed] [Google Scholar]
- 60.Horton DA, Bourne GT, Smythe ML. Chem Rev. 2003;103:893. doi: 10.1021/cr020033s. [DOI] [PubMed] [Google Scholar]
- 61.Gul W, Hamann MT. Life Sci. 2005;78:442. doi: 10.1016/j.lfs.2005.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cho CH, Neuenswander B, Lushington GH, Larock RC. J Comb Chem. 2008;10:941. doi: 10.1021/cc800120y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kolb HC, Finn MG, Sharpless KB. Angew Chem Int Ed. 2001;40:2004. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 64.Lewis WG, Green LG, Grynszpan F, Radić Z, Carlier PR, Taylor P, Finn MG, Sharpless KB. Angew Chem Int Ed. 2002;41:1053. doi: 10.1002/1521-3773(20020315)41:6<1053::aid-anie1053>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 65.Lee LV, Mitchell ML, Huang SJ, Fokin VV, Sharpless KB, Wong CH. J Am Chem Soc. 2003;125:9588. doi: 10.1021/ja0302836. [DOI] [PubMed] [Google Scholar]
- 66.Manetsch R, Krasiński A, Radić Z, Raushel J, Taylor P, Sharpless KB, Kolb HC. J Am Chem Soc. 2004;126:12809. doi: 10.1021/ja046382g. [DOI] [PubMed] [Google Scholar]
- 67.Srinivasan R, Uttamchandani M, Yao SQ. Org Lett. 2006;8:713. doi: 10.1021/ol052895w. [DOI] [PubMed] [Google Scholar]
- 68.Xie J, Seto CT. Bioorg Med Chem. 2007;15:458. doi: 10.1016/j.bmc.2006.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yu X, Sun J-P, He Y, Guo X-L, Liu S, Zhou B, Hudmon A, Zhang Z-Y. Proc Natl Acad Sci USA. 2007;104:19767. doi: 10.1073/pnas.0706233104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang X, He Y, Liu S, Yu Z, Jiang Z-X, Yang Z, Dong Y, Nabinger SC, Wu L, Gunawan AM, Wang L, Chan RJ, Zhang Z-Y. J Med Chem. 2010;53:2482. doi: 10.1021/jm901645u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang Z-Y, Walsh AB, Wu L, McNamara DJ, Dobrusin EM, Miller WT. J Biol Chem. 1996;271:5386. doi: 10.1074/jbc.271.10.5386. [DOI] [PubMed] [Google Scholar]
- 72.Salmeen A, Andersen JN, Myers MP, Tonks NK, Barford D. Mol Cell. 2000;6:1401. doi: 10.1016/s1097-2765(00)00137-4. [DOI] [PubMed] [Google Scholar]