Abstract
A one-step, large-scale preparation of alkyne-containing hyper-branched polyglycerols (HPG) is reported. The HPGs undergo click reactions to organic azides allowing a range of applications.
Polyglycerol dendrimers and hyperbranched polyglycerols (HPGs) have a number of attractive properties that have led to their use in a broad range of applications from drug delivery devices to catalysis to diversity platforms for synthesis.1 Arguably, the most important property is the biocompatibility afforded by the water-soluble, highly branched polyether–polyol structure. Indeed, HPGs resist protein adsorption and show toxicity profiles in cell studies that are at least as good as polyethylene glycol (PEG). Further, no toxicity in mice was observed even at high doses for a prolonged period.2 Beyond their biocompatibility, HPGs can be prepared on kilogram scale3 in one step from commercial reagents.
Because of these favourable features, much effort has focused on specific functionalization of HPGs.4 For example, 8 step routes to azide and alkyne-cored polyglycerol dendrons and their subsequent click reaction were recently reported.5 Herein we report the straight forward and scalable preparation of alkyne-cored HPG. Both high (>50 000 Mn) and low (<10 000 Mn) molecular weight polymers could be accessed with higher Mn material synthesized using an emulsion polymerization method.6 Details on the preparation, characterization, and chemistry of the high Mn HPG can be found in the ESI.‡ The utility of the low Mn HPGs was shown by clicking them to fluorescein and biotin, as well as their use in stabilizing gold nanoparticles (NP) and in a pH-sensitive nanocarrier to catch and release small molecules.
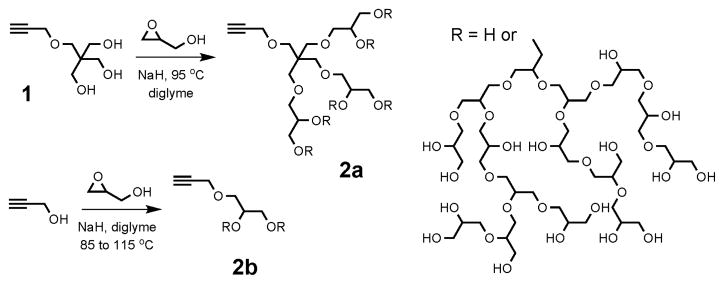
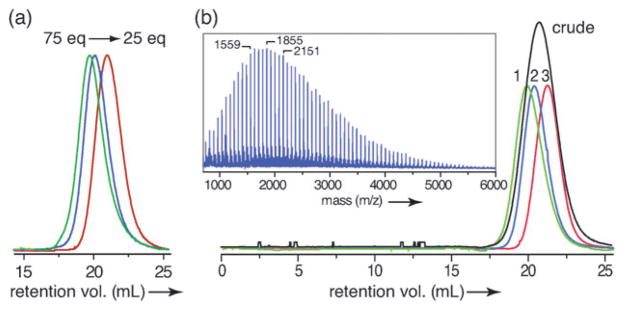
Two routes to alkyne-functionalized HPG are outlined in Fig. 1. The first uses triol initiator 1, prepared in three steps from pentaerythritol. Thus, a diglyme solution of 1 was treated with 10 mol% sodium hydride and glycidol was slowly added to produce HPG 2a. Typical Mn and PDI values (Mw/Mn) for purified polymers are shown in Table 1 with representative SEC traces presented in Fig. 2a. An alternative synthesis uses propargyl alcohol as an alkyne source allowing the HPG to be prepared in a single step. The precipitation process used to purify the polymers reduced the PDI and increased molecular weights by removing lower molecular weight polymer. This process was further refined and used to fractionate polymer 2b giving material with a lower PDI and 77% mass recovery overall (Table 2 and Fig. 2b). The presence of the propargyl group was confirmed using MALDI (inset to Fig. 2b) and NMR, (see ESI‡).
Fig. 1.
Hyperbranched polymerization to form PGs 2a and 2b. Structure of PG is representative.
Table 1.
Molecular weights of HPGs 2a prepared from 1 and various equivalents of glycidol followed by acetone precipitation. SEC determined in DMF with polystyrene calibration
| Equivalents | Mn (theor) | Mn (SEC) | Mn/Mw |
|---|---|---|---|
| 25 | 2027 | 7100 | 1.36 |
| 50 | 3878 | 11 600 | 1.39 |
| 75 | 5729 | 13 100 | 1.49 |
| 100 | 7580 | 14 500 | 1.54 |
Fig. 2.
(a) SEC traces of HPG 2a prepared using 25, 50, and 75 equivalents of glycidol. (b) SEC traces of 2b: crude and fractionated (fractions 1–3). Inset shows MALDI of 2b. Adjacent peaks separated by m/z 74.03, the MW of glycidol. Within 0.1%, (b) indicated peaks correspond to initiator + 20, 24, and 28 glycidol units + Na.
Table 2.
Molecular weights of HPG 2b prepared from propargyl alcohol. SEC determined in DMF with polystyrene calibration
| Polymer | Mn (theor) | Mn (SEC) | Mn (NMR) | Mn/Mw | Mass (g) |
|---|---|---|---|---|---|
| Crude | 2054 | 8200 | 1.5 | 90 | |
| 1st fraction | 14 000 | 7000 | 1.35 | 20.1 | |
| 2nd fraction | 11 600 | 6000 | 1.26 | 29 | |
| 3rd fraction | 7500 | 3000 | 1.25 | 34.5 |
Inverse gated 13C NMRconfirmed the branched structure of HPG 2b and indicated a degree of branching of 58%.3a As has been observed previously for HPGs,3a Mn values determined by SEC are overestimated under these conditions.
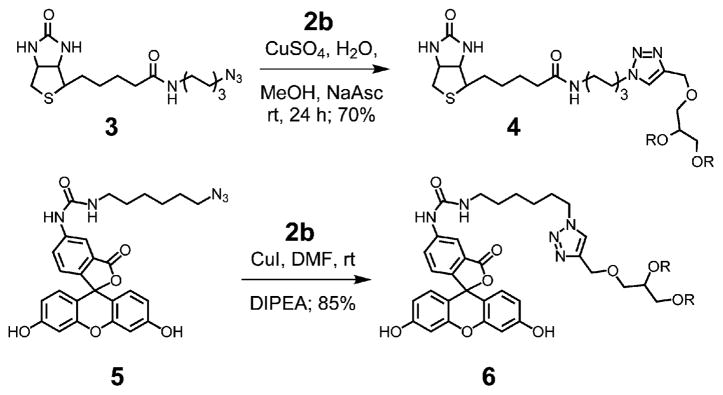
By virtue of the single alkyne group at the focal point, these HPGs can be covalently linked to a broad range of compounds and materials using standard click chemistry.7 To illustrate this potential in a chemical biology context, 2b was reacted with biotin azide 3 to give 4 and also clicked to fluorescein azide 5 giving fluorescent PG 6 (Scheme 1).8 SEC traces of 5 and 6 showed no sign of cross-linking as a result of the orthogonal nature of the click reaction.
Scheme 1.
Click chemistry of HPGs.
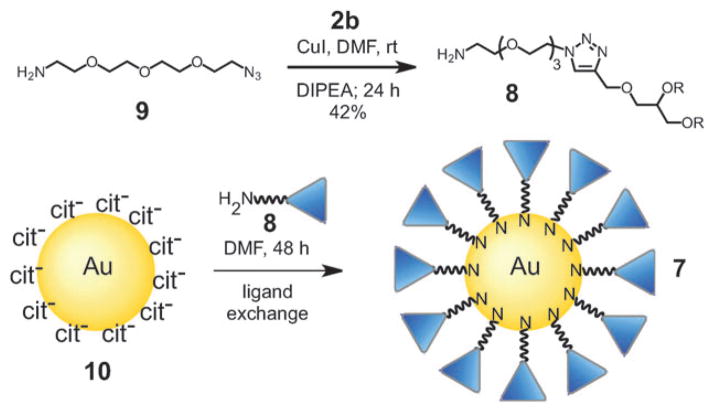
Because of their biocompatibility and resistance to protein adsorption,9 it is expected that HPG 2b will also be useful as a NP based surface coating. This is shown in Scheme 2 for the synthesis of HPG-capped gold NP 2b. This scheme also shows the utility of the click reaction to introduce other functional groups at the focal point of the HPG polymer. Thus, rather than linking 2b to the NP by a click reaction, amine functionalized HPG 8 was synthesized using azide 9. Thiols and amines are well-known to undergo the exchange reaction shown in Scheme 2.10 Thus 8 was used to coat 13.5 ± 1.1 nm diameter, citrate-capped gold NPs 10, prepared using the Frens method.11
Scheme 2.
Synthesis of HPG coated gold nanoparticles.
Addition of HPG 8 to an aqueous suspension of NP 10 led to aggregation, which was confirmed by dynamic light scattering (DLS). However, when 10 was suspended in DMF and treated with 8, a stable solution formed. This allowed the ligand exchange to take place and stable HPG-capped gold NPs were isolated without aggregation and resuspended in DI water.
Functionalization of the NPs was confirmed by an increase of hydrodynamic diameter of 2.7 nm and a change in surface effective charge (zeta potential) from −30.2 ± 0.6 mV to −0.01 ± 4 mV for HPG-capped NPs. Previous reports of HPG-capped gold NPs used random and highly amine functionalized HPG which produced positively charged nanoparticles.12 HPG-capped NPs exhibited enhanced stability to salt-induced aggregation compared to citrate-capped NPs as judged by DLS and UV-Vis spectroscopy (see ESI). The enhanced stability of 7 likely involves a steric protection of the NP surface that is independent of solution ionic strength, thus mimicking the classic PEG NP coating.13
As with NPs, drug delivery agents such as unimolecular micelles rely on a biocompatible surface coating for extended circulation in the body and for minimizing toxicity.14 Recently we reported a method for the synthesis of degradable dendrimers and hydrogels utilizing 1,3,5-triazaadamantane (TAA) monomers.15 These hydrophobic dendrimers are acid-labile but lack water solubility. Because they are built through a series of click reactions, they present an excellent synthetic scaffold for the synthesis of acid-sensitive unimolecular micelles using alkyne-functionalized HPGs.
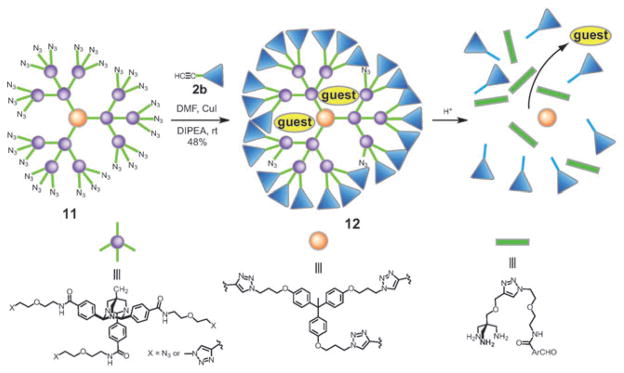
A second generation TAA dendrimer 11 (MW = 11 402; 27 azide groups) was used as the core and was reacted with one equivalent of HPG 2b (fraction 3, Mn =3000, PDI = 1.25) in DMF with CuI and DIPEA (Scheme 3). The reaction was monitored by SEC and was stopped after 24 h. The polymer obtained, 12, was fully water soluble despite the IR spectrum indicating the presence of unreacted azide groups. DLS measurements of concentrated solutions of 12 (2.5 mg/mL) indicated that the polymer is monomeric in phosphate buffer with an average hydrodynamic diameter of 8.4 nm. In addition, a cell viability assay showed that the polymer is well-tolerated by cells at concentrations up to 0.75 mg/mL with a toxicity profile similar to HPG alone.2
Scheme 3.
Surface functionalization of a TAA dendrimer using 2b to produce water-soluble, acid-labile unimolecular micelle.
Titration of 1-anilino-8-sulfonic acid (1,8-ANS) was used to verify that 12 could encapsulate non-polar guest molecules. The 1,8-ANS probe is well-established to show enhanced fluorescence in non-polar environments. A plot of fluorescence against [12] exhibits a 1 : 1 binding isotherm with an apparent Ka=2.0 × 104M−1. To further verify the guest is encapsulated in a single polymer and not in polymer aggregates, the fluorescence enhancement at lower concentrations was measured. Under these conditions, a linear dependence of fluorescence was observed down to 20 nM with no critical micelle concentration, indicating that 12 does not aggregate under these conditions.
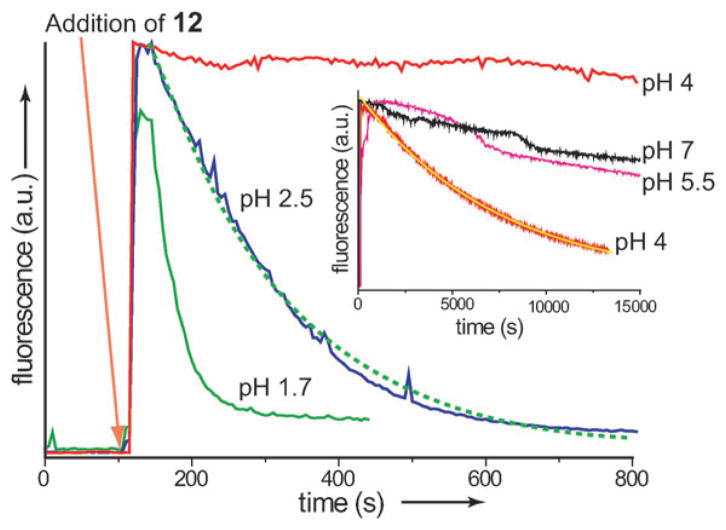
The enhanced fluorescence observed for encapsulated 1,8-ANS provides a convenient method of monitoring dendrimer degradation (Fig. 3). Thus, the fluorescence of 1,8-ANS at various pH values was monitored during the addition of 2.4 equivalents of 12. Time-dependent fluorescence plots show a spike with addition of 12 as a result of the uptake of the dye into the hydrophobic interior. This is followed by a first order decay of the fluorescence as the dendrimer degrades and the dye is released back into solution. The decay is pH dependent with steady state fluorescence reached in 5 min at pH 1.7 and over 4 h at pH 4. When the same experiment was conducted in the absence of 1,8-ANS a marginal increase in fluorescence was observed which quickly reached a steady state indicating that the fluorescence is due to dye encapsulation and not the fluorescence of the polymer itself. The degradation of the TAA core was also monitored by 1H NMR through the appearance of the aldehyde peak at 10.5 ppm and an overall sharpening of the peaks in the aromatic region. A comparison of the dendrimer degradation and the fluorescence decay of ANS showed similar rates (see ESI‡). The results indicate that the two processes are occurring on a similar time scale and that the fluorescence decay is due to dendrimer degradation and not fluorophore degradation.
Fig. 3.
Fluorescence degradation of ANS in the presence of 12. Inset, fluorescence response at higher pH values. Green dashed and yellow solid lines represent a fit to a first order decay curve. Degradation at pH 4 shown in both plots.
Herein we have outlined a simple procedure for the synthesis of alkyne functionalized HPG. These polymers facilitated the preparation of potential biological imaging agents such as monofunctionalized fluorescein and biotin HPG, the stabilization of gold nanoparticles, and the synthesis of unimolecular acid-labile micelles. Because of the diverse applications involving HPG, there is a need for robust chemistry for their synthesis. With this in mind, the preparation of alkyne-cored HPG directly from commercial starting materials is particularly appealing. We are continuing to investigate the use of these polymers as a method for the functionalization and stabilization of nanoparticles, as well as stabilizing agents for water-soluble fluorescent dyes.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (GM087448 and HL097314) and the American Chemical Society Petroleum Research Fund (48907-ND4).
Footnotes
This article is part of a ChemComm ‘Supramolecular Chemistry’ web-based themed issue marking the International Year of Chemistry 2011.
Electronic supplementary information (ESI) available: Synthetic procedures and characterization of organic compounds, high and low molecular weight HPG and nanoparticles. See DOI: 10.1039/c0cc04096g
Contributor Information
Hyunjoon Kong, Email: hjkong06@illinois.edu.
Steven C. Zimmerman, Email: sczimmer@illinois.edu.
Notes and references
- 1.Calderón M, Quadir MA, Sharma SK, Haag R. Adv Mater. 2010;22:190. doi: 10.1002/adma.200902144. [DOI] [PubMed] [Google Scholar]
- 2.(a) Kainthan RK, Janzen J, Levin E, Devine DV, Brooks DE. Biomacromolecules. 2006;7:703. doi: 10.1021/bm0504882. [DOI] [PubMed] [Google Scholar]; b) Kainthan RK, Hester SR, Levin E, Devine DV, Brooks DE. Biomaterials. 2007;28:4581. doi: 10.1016/j.biomaterials.2007.07.011. [DOI] [PubMed] [Google Scholar]; c) Kainthan RK, Brooks DE. Biomaterials. 2007;28:4779. doi: 10.1016/j.biomaterials.2007.07.046. [DOI] [PubMed] [Google Scholar]
- 3.(a) Sunder A, Hanselmann R, Frey H, Mülhaupt R. Macromolecules. 1999;32:4240. [Google Scholar]; b) Frey H, Haag R. Rev Mol Biotechnol. 2002;90:257. doi: 10.1016/s1389-0352(01)00063-0. [DOI] [PubMed] [Google Scholar]
- 4.(a) Yeh PYJ, Kainthan RK, Zou Y, Chiao M, Kizhakkedathu JN. Langmuir. 2008;24:4907. doi: 10.1021/la702867t. [DOI] [PubMed] [Google Scholar]; b) Wyszogrodzka M, Haag R. Biomacromolecules. 2009;10:1043. doi: 10.1021/bm801093t. [DOI] [PubMed] [Google Scholar]
- 5.(a) Wyszogrodzka M, Haag R. Chem Eur J. 2008;14:9202. doi: 10.1002/chem.200800892. [DOI] [PubMed] [Google Scholar]; b) Elmer SL, Man S, Zimmerman SC. Eur J Org Chem. 2008:3845. doi: 10.1002/ejoc.200800401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kainthan RK, Muliawan EB, Hatzikiriakos SG, Brooks DE. Macromolecules. 2006;39:7708. [Google Scholar]
- 7.(a) Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. Angew Chem, Int Ed. 2002;41:2596. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]; b) Font D, Bastero A, Sayalero S, Jimeno C, Pericàs MA. Org Lett. 2007;9:1943. doi: 10.1021/ol070526p. [DOI] [PubMed] [Google Scholar]
- 8.Selected examples of click reactions involving biotin or fluorescein: Wang Q, Chan TR, Hilgraf R, Fokin VV, Sharpless KB, Finn MG. J Am Chem Soc. 2003;125:3192. doi: 10.1021/ja021381e.Sun EY, Josephson L, Weissleder R. Mol Imaging. 2006;5:122.
- 9.Siegers C, Biesalski M, Haag R. Chem–Eur J. 2004;10:2831. doi: 10.1002/chem.200306073. [DOI] [PubMed] [Google Scholar]
- 10.(a) Freeman RG, Grabar KC, Allison KJ, Bright RM, Davis JA, Guthrie AP, Hommer MB, Jackson MA, Smith PC, Walter DG, Natan MJ. Science. 1995;267:1629. doi: 10.1126/science.267.5204.1629. [DOI] [PubMed] [Google Scholar]; b) Nath N, Chilkoti A. Anal Chem. 2002;74:504. doi: 10.1021/ac015657x. [DOI] [PubMed] [Google Scholar]
- 11.Frens G. Nature Phys Sci. 1973;241:20. [Google Scholar]
- 12.Shen Y, Kuang M, Shen Z, Nieberle J, Duan HW, Frey H. Angew Chem, Int Ed. 2008;47:2227. doi: 10.1002/anie.200704572. [DOI] [PubMed] [Google Scholar]
- 13.(a) Doty RC, Tshikhudo TR, Brust M, Fernig DG. Chem Mater. 2005;17:4630. [Google Scholar]; b) Kanaras AG, Kamounah FS, Schaumburg K, Kiely CJ, Brust M. Chem Commun. 2002:2294. doi: 10.1039/b207838b. [DOI] [PubMed] [Google Scholar]
- 14.(a) Torchilin VP, Trubetskoy VS. Adv Drug Delivery Rev. 1995;16:141. [Google Scholar]; b) Woodle MC. Adv Drug Delivery Rev. 1998;32:139. doi: 10.1016/s0169-409x(97)00136-1. [DOI] [PubMed] [Google Scholar]
- 15.(a) Balija AM, Kohman RE, Zimmerman SC. Angew Chem, Int Ed. 2008;47:8072. doi: 10.1002/anie.200802222. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Kohman RE, Zimmerman SC. Chem Commun. 2009:794. doi: 10.1039/b818183g. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.