Abstract
Skin fibroblasts grown from three individuals with osteogenesis imperfecta (OI) each synthesized a population of normal type I collagen molecules and additional molecules that had one or two alpha 1(I) chains that contained a cysteine residue within the triple-helical domain, a region from which cysteine normally is excluded. The patients had very different phenotypes. One patient with OI type I had a population of alpha 1(I) chains in which glycine at position 94 of the triple helix was substituted by cysteine; a patient with OI type III had a population of alpha 1(I) chains in which glycine at position 526 of the triple helix was substituted by cysteine; and the third patient, with OI type II, had a cysteine for glycine substitution at position 718 of the alpha 1(I) chain. From all three patients, molecules that contained two mutant chains formed interchain, intramolecular disulfide bonds, and although less stable to thermal denaturation than normal molecules, they were more stable than molecules that contained only a single mutant chain. These findings indicate that substitutions for glycine within the triple-helical domain of the alpha 1(I) chain are not invariably lethal and that their phenotypic effect largely depends on the nature of the substituting residue and its location in the chain.
Full text
PDF

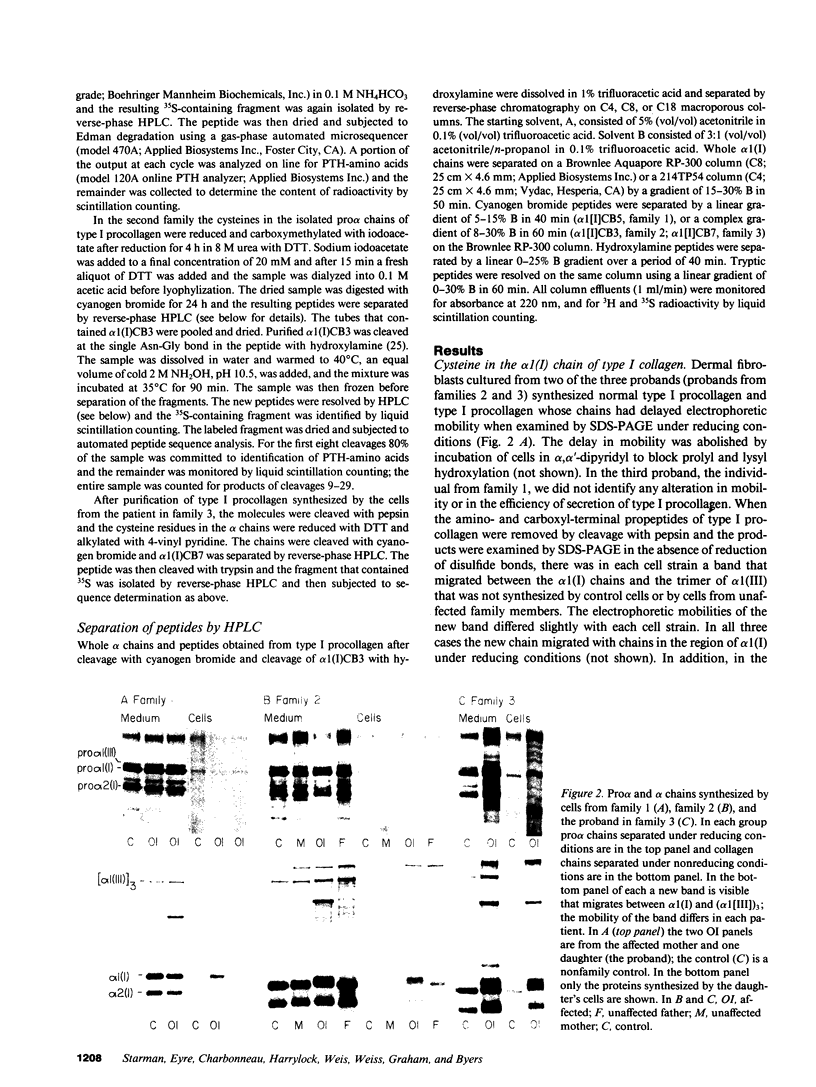






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. C., Dixon J. E. A procedure for in situ alkylation of cystine residues on glass fiber prior to protein microsequence analysis. Anal Biochem. 1987 Mar;161(2):524–528. doi: 10.1016/0003-2697(87)90484-2. [DOI] [PubMed] [Google Scholar]
- Barsh G. S., David K. E., Byers P. H. Type I osteogenesis imperfecta: a nonfunctional allele for pro alpha 1 (I) chains of type I procollagen. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3838–3842. doi: 10.1073/pnas.79.12.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsh G. S., Roush C. L., Bonadio J., Byers P. H., Gelinas R. E. Intron-mediated recombination may cause a deletion in an alpha 1 type I collagen chain in a lethal form of osteogenesis imperfecta. Proc Natl Acad Sci U S A. 1985 May;82(9):2870–2874. doi: 10.1073/pnas.82.9.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman J. F., Chan D., Walker I. D., Rogers J. G., Cole W. G. Lethal perinatal osteogenesis imperfecta due to the substitution of arginine for glycine at residue 391 of the alpha 1(I) chain of type I collagen. J Biol Chem. 1987 May 25;262(15):7021–7027. [PubMed] [Google Scholar]
- Bateman J. F., Lamande S. R., Dahl H. H., Chan D., Cole W. G. Substitution of arginine for glycine 664 in the collagen alpha 1(I) chain in lethal perinatal osteogenesis imperfecta. Demonstration of the peptide defect by in vitro expression of the mutant cDNA. J Biol Chem. 1988 Aug 25;263(24):11627–11630. [PubMed] [Google Scholar]
- Bonadio J., Byers P. H. Subtle structural alterations in the chains of type I procollagen produce osteogenesis imperfecta type II. Nature. 1985 Jul 25;316(6026):363–366. doi: 10.1038/316363a0. [DOI] [PubMed] [Google Scholar]
- Bonadio J., Holbrook K. A., Gelinas R. E., Jacob J., Byers P. H. Altered triple helical structure of type I procollagen in lethal perinatal osteogenesis imperfecta. J Biol Chem. 1985 Feb 10;260(3):1734–1742. [PubMed] [Google Scholar]
- Bornstein P. Structure of alpha-1-CB8, a large cyanogen bromide produced fragment from the alpha-1 chain of rat collagen. The nature of a hydroxylamine-sensitive bond and composition of tryptic peptides. Biochemistry. 1970 Jun 9;9(12):2408–2421. doi: 10.1021/bi00814a004. [DOI] [PubMed] [Google Scholar]
- Bruckner P., Prockop D. J. Proteolytic enzymes as probes for the triple-helical conformation of procollagen. Anal Biochem. 1981 Jan 15;110(2):360–368. doi: 10.1016/0003-2697(81)90204-9. [DOI] [PubMed] [Google Scholar]
- Byers P. H., Shapiro J. R., Rowe D. W., David K. E., Holbrook K. A. Abnormal alpha 2-chain in type I collagen from a patient with a form of osteogenesis imperfecta. J Clin Invest. 1983 Mar;71(3):689–697. doi: 10.1172/JCI110815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers P. H., Starman B. J., Cohn D. H., Horwitz A. L. A novel mutation causes a perinatal lethal form of osteogenesis imperfecta. An insertion in one alpha 1(I) collagen allele (COL1A1). J Biol Chem. 1988 Jun 5;263(16):7855–7861. [PubMed] [Google Scholar]
- Chu M. L., Gargiulo V., Williams C. J., Ramirez F. Multiexon deletion in an osteogenesis imperfecta variant with increased type III collagen mRNA. J Biol Chem. 1985 Jan 25;260(2):691–694. [PubMed] [Google Scholar]
- Cohn D. H., Apone S., Eyre D. R., Starman B. J., Andreassen P., Charbonneau H., Nicholls A. C., Pope F. M., Byers P. H. Substitution of cysteine for glycine within the carboxyl-terminal telopeptide of the alpha 1 chain of type I collagen produces mild osteogenesis imperfecta. J Biol Chem. 1988 Oct 15;263(29):14605–14607. [PubMed] [Google Scholar]
- Cohn D. H., Byers P. H., Steinmann B., Gelinas R. E. Lethal osteogenesis imperfecta resulting from a single nucleotide change in one human pro alpha 1(I) collagen allele. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6045–6047. doi: 10.1073/pnas.83.16.6045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinou C. D., Nielsen K. B., Prockop D. J. A lethal variant of osteogenesis imperfecta has a single base mutation that substitutes cysteine for glycine 904 of the alpha 1(I) chain of type I procollagen. The asymptomatic mother has an unidentified mutation producing an overmodified and unstable type I procollagen. J Clin Invest. 1989 Feb;83(2):574–584. doi: 10.1172/JCI113920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyre D. R., Shapiro F. D., Aldridge J. F. A heterozygous collagen defect in a variant of the Ehlers-Danlos syndrome type VII. Evidence for a deleted amino-telopeptide domain in the pro-alpha 2(I) chain. J Biol Chem. 1985 Sep 15;260(20):11322–11329. [PubMed] [Google Scholar]
- Eyre D. R., Wu J. J. Collagen of fibrocartilage: a distinctive molecular phenotype in bovine meniscus. FEBS Lett. 1983 Jul 25;158(2):265–270. doi: 10.1016/0014-5793(83)80592-4. [DOI] [PubMed] [Google Scholar]
- Genovese C., Rowe D. Analysis of cytoplasmic and nuclear messenger RNA in fibroblasts from patients with type I osteogenesis imperfecta. Methods Enzymol. 1987;145:223–235. doi: 10.1016/0076-6879(87)45012-x. [DOI] [PubMed] [Google Scholar]
- Kuivaniemi H., Sabol C., Tromp G., Sippola-Thiele M., Prockop D. J. A 19-base pair deletion in the pro-alpha 2(I) gene of type I procollagen that causes in-frame RNA splicing from exon 10 to exon 12 in a proband with atypical osteogenesis imperfecta and in his asymptomatic mother. J Biol Chem. 1988 Aug 15;263(23):11407–11413. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lichtenstein J. R., Byers P. H., Smith B. D., Martin G. R. Identification of the collagenous proteins synthesized by cultured cells from human skin. Biochemistry. 1975 Apr 22;14(8):1589–1594. doi: 10.1021/bi00679a007. [DOI] [PubMed] [Google Scholar]
- Nicholls A. C., Osse G., Schloon H. G., Lenard H. G., Deak S., Myers J. C., Prockop D. J., Weigel W. R., Fryer P., Pope F. M. The clinical features of homozygous alpha 2(I) collagen deficient osteogenesis imperfecta. J Med Genet. 1984 Aug;21(4):257–262. doi: 10.1136/jmg.21.4.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihlajaniemi T., Dickson L. A., Pope F. M., Korhonen V. R., Nicholls A., Prockop D. J., Myers J. C. Osteogenesis imperfecta: cloning of a pro-alpha 2(I) collagen gene with a frameshift mutation. J Biol Chem. 1984 Nov 10;259(21):12941–12944. [PubMed] [Google Scholar]
- Sillence D. O., Barlow K. K., Cole W. G., Dietrich S., Garber A. P., Rimoin D. L. Osteogenesis imperfecta type III. Delineation of the phenotype with reference to genetic heterogeneity. Am J Med Genet. 1986 Mar;23(3):821–832. doi: 10.1002/ajmg.1320230309. [DOI] [PubMed] [Google Scholar]
- Sillence D. O., Senn A., Danks D. M. Genetic heterogeneity in osteogenesis imperfecta. J Med Genet. 1979 Apr;16(2):101–116. doi: 10.1136/jmg.16.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes B., Ogilvie D., Wordsworth P., Anderson, Jones N. Osteogenesis imperfecta is linked to both type I collagen structural genes. Lancet. 1986 Jul 12;2(8498):69–72. doi: 10.1016/s0140-6736(86)91609-0. [DOI] [PubMed] [Google Scholar]
- Tromp G., Prockop D. J. Single base mutation in the pro alpha 2(I) collagen gene that causes efficient splicing of RNA from exon 27 to exon 29 and synthesis of a shortened but in-frame pro alpha 2(I) chain. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5254–5258. doi: 10.1073/pnas.85.14.5254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel B. E., Doelz R., Kadler K. E., Hojima Y., Engel J., Prockop D. J. A substitution of cysteine for glycine 748 of the alpha 1 chain produces a kink at this site in the procollagen I molecule and an altered N-proteinase cleavage site over 225 nm away. J Biol Chem. 1988 Dec 15;263(35):19249–19255. [PubMed] [Google Scholar]
- Vogel B. E., Minor R. R., Freund M., Prockop D. J. A point mutation in a type I procollagen gene converts glycine 748 of the alpha 1 chain to cysteine and destabilizes the triple helix in a lethal variant of osteogenesis imperfecta. J Biol Chem. 1987 Oct 25;262(30):14737–14744. [PubMed] [Google Scholar]
- Wallis G., Beighton P., Boyd C., Mathew C. G. Mutations linked to the pro alpha 2(I) collagen gene are responsible for several cases of osteogenesis imperfecta type I. J Med Genet. 1986 Oct;23(5):411–416. doi: 10.1136/jmg.23.5.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenstrup R. J., Cohn D. H., Cohen T., Byers P. H. Arginine for glycine substitution in the triple-helical domain of the products of one alpha 2(I) collagen allele (COL1A2) produces the osteogenesis imperfecta type IV phenotype. J Biol Chem. 1988 Jun 5;263(16):7734–7740. [PubMed] [Google Scholar]
- Wenstrup R. J., Tsipouras P., Byers P. H. Osteogenesis imperfecta type IV. Biochemical confirmation of genetic linkage to the pro alpha 2(I) gene of type I collagen. J Clin Invest. 1986 Dec;78(6):1449–1455. doi: 10.1172/JCI112735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willing M. C., Cohn D. H., Starman B., Holbrook K. A., Greenberg C. R., Byers P. H. Heterozygosity for a large deletion in the alpha 2(I) collagen gene has a dramatic effect on type I collagen secretion and produces perinatal lethal osteogenesis imperfecta. J Biol Chem. 1988 Jun 15;263(17):8398–8404. [PubMed] [Google Scholar]
- de Vries W. N., de Wet W. J. The molecular defect in an autosomal dominant form of osteogenesis imperfecta. Synthesis of type I procollagen containing cysteine in the triple-helical domain of pro-alpha 1(I) chains. J Biol Chem. 1986 Jul 5;261(19):9056–9064. [PubMed] [Google Scholar]