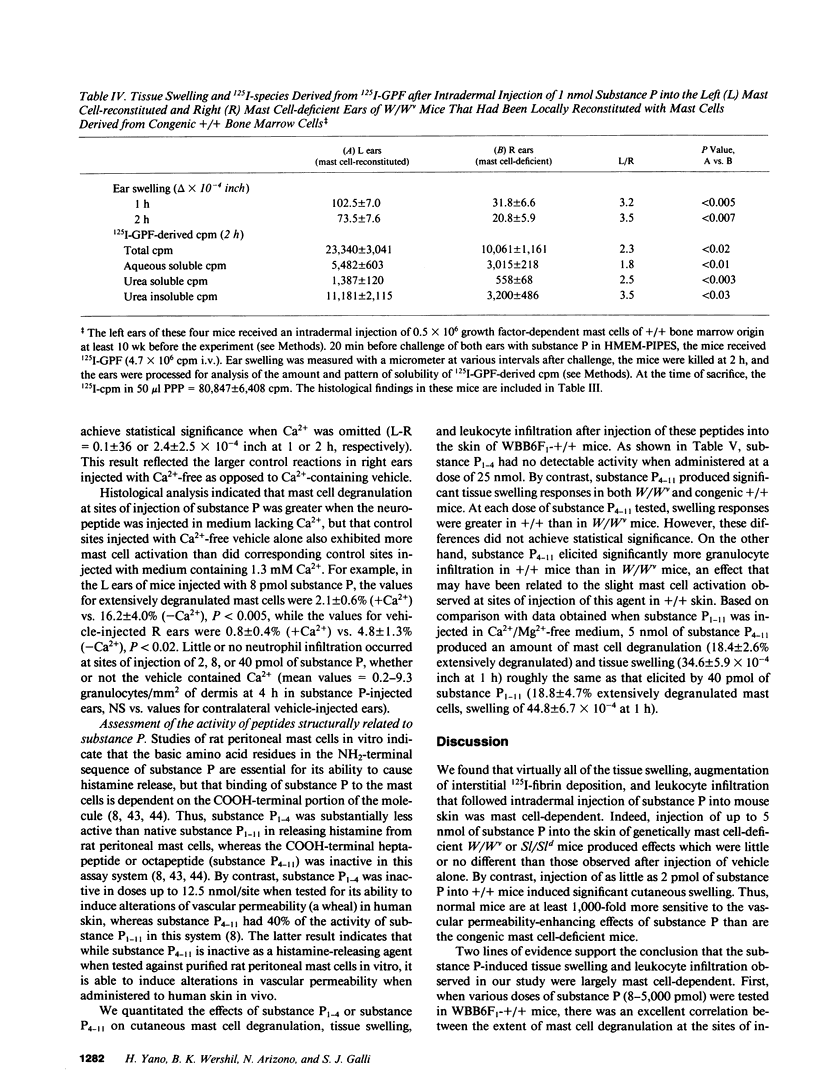
Abstract
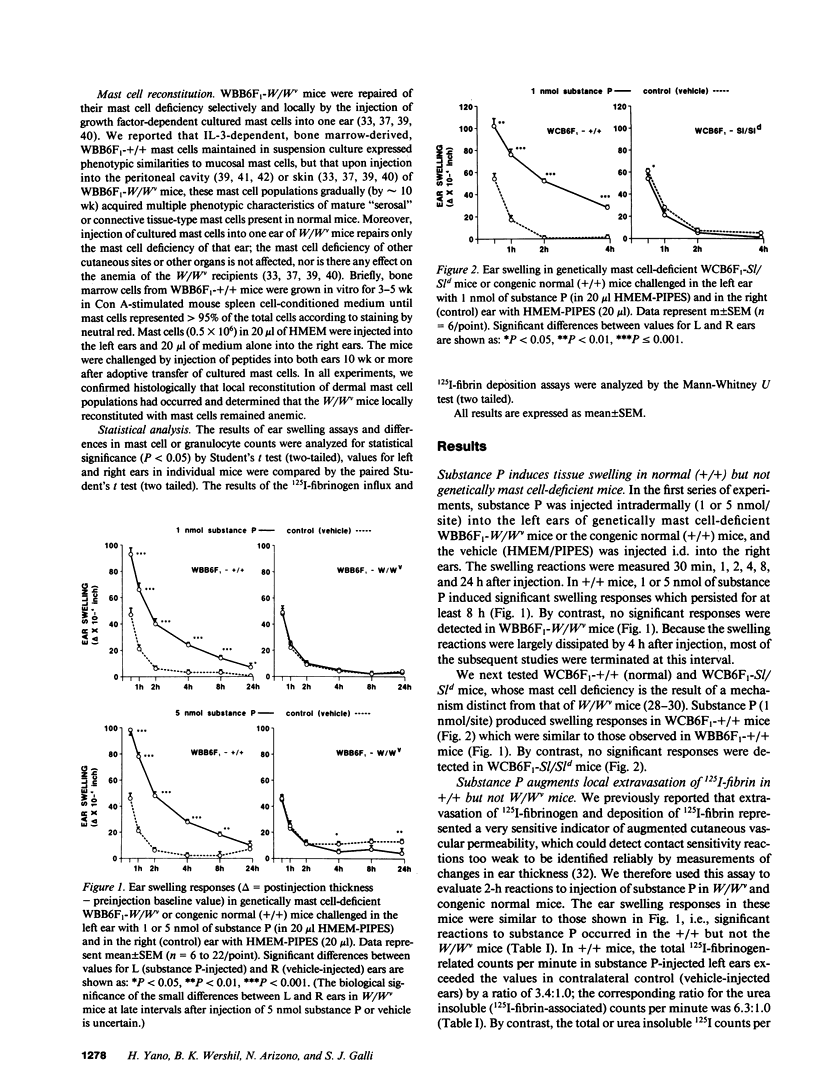
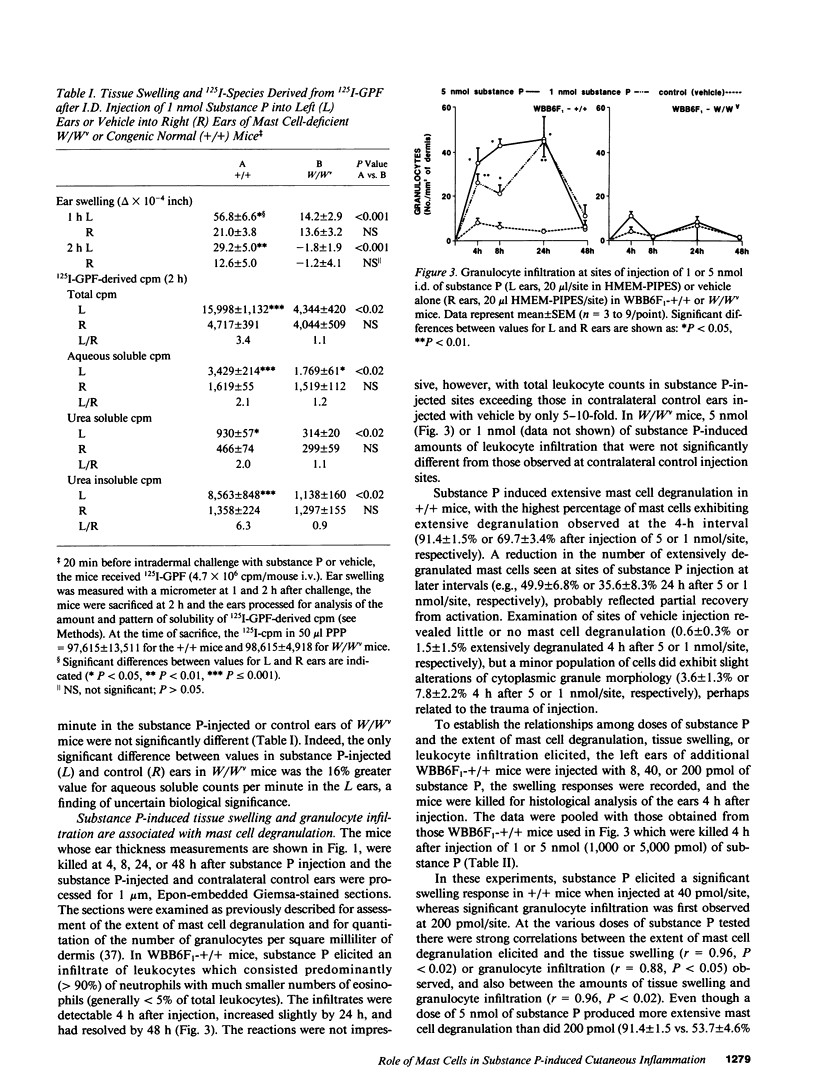
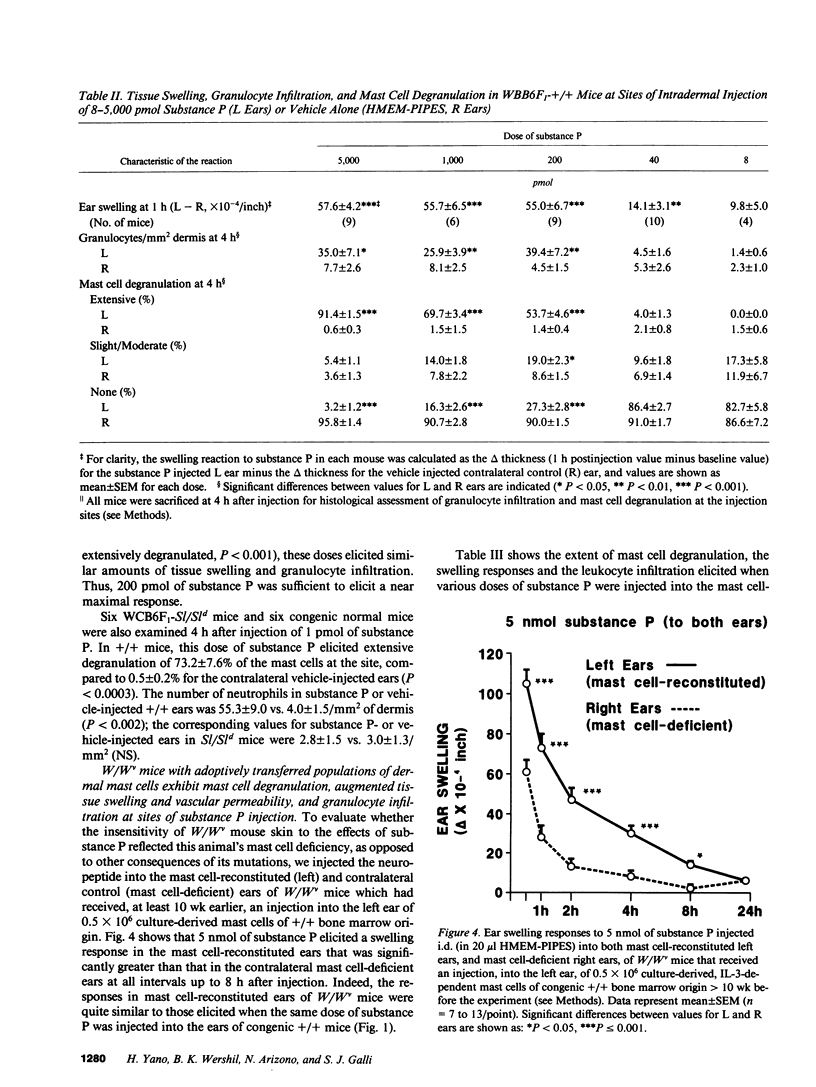
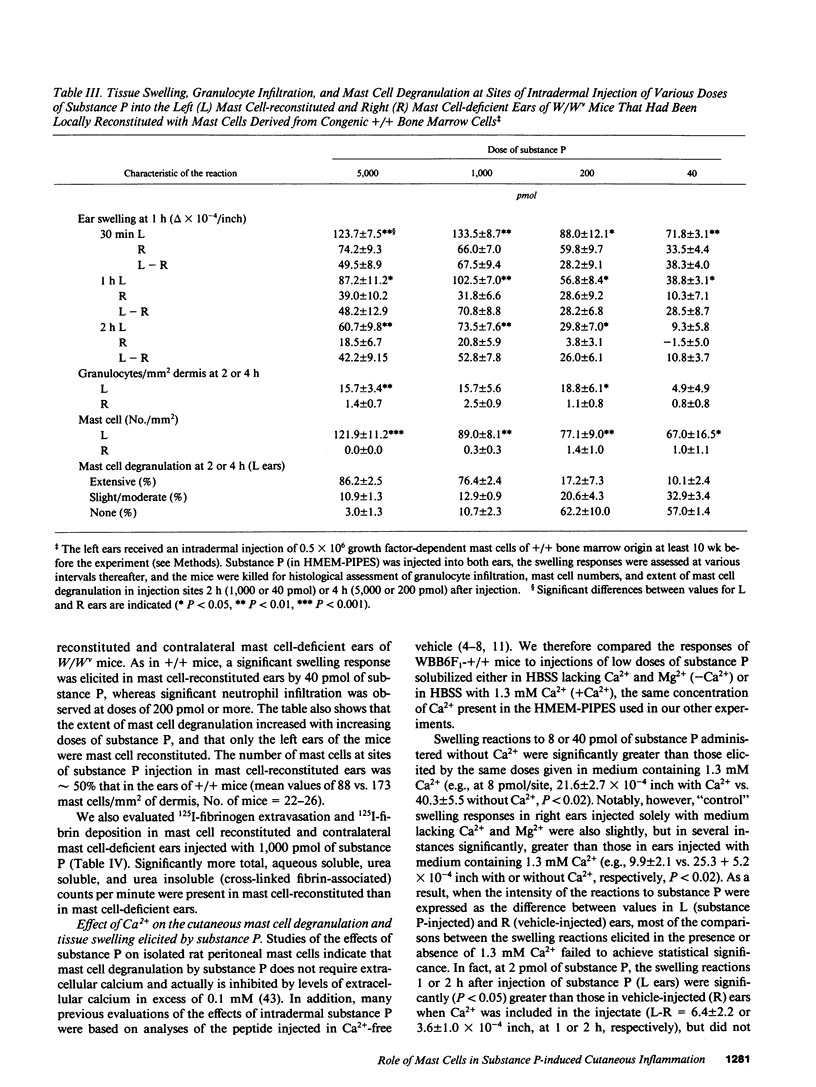
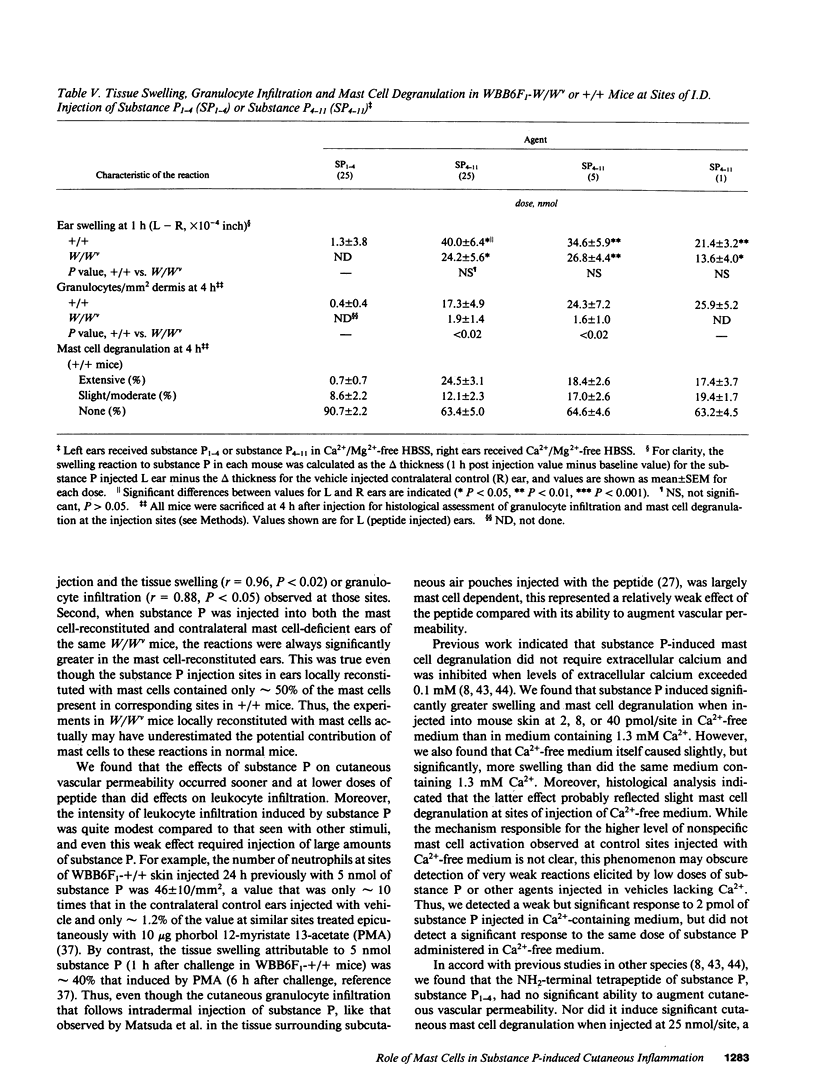
The undecapeptide substance P is thought to mediate both vasodilatation and augmented vascular permeability when released from sensory nerve endings in the skin. Substance P also induces mast cell degranulation in vitro or in vivo. However, the extent to which substance P-induced changes in vascular permeability are mast cell-dependent is unclear. We investigated this issue by injecting substance P and certain related peptides (substance P1-4, substance P4-11) into the skin of genetically mast cell-deficient WBB6F1-W/W or WCB6F1- SI/SId mice the congenic normal (+/+) mice, and W/W mice which had undergone selective local repair of their mast cell deficiency by intradermal injection of IL-3-dependent mast cells generated in vitro from the bone marrow cells of the congenic +/+ mice. Substance P induced significant augmentation of vascular permeability and significant cutaneous swelling when injected into normal mice at doses as low as 2 pmol i.d. Substance P also induced granulocyte infiltration, although the infiltrate were modest and were seen at doses of peptide from 5 to more than 20-fold higher than those required for induction of tissue swelling. The effects of substance P on tissue swelling, vascular permeability, and granulocyte infiltration were virtually entirely mast cell dependent. By contrast, substance P1-4 was inactive in our assays at 25 nmol/site, and substance P4-11 induced modest augmentation of vascular permeability, which was at least in part mast cell independent.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Couture R., Cuello A. C. Studies on the trigeminal antidromic vasodilatation and plasma extravasation in the rat. J Physiol. 1984 Jan;346:273–285. doi: 10.1113/jphysiol.1984.sp015021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak H. F., Harvey V. S., McDonagh J. Quantitation of fibrinogen influx and fibrin deposition and turnover in line 1 and line 10 guinea pig carcinomas. Cancer Res. 1984 Aug;44(8):3348–3354. [PubMed] [Google Scholar]
- Dvorak H. F., Mihm M. C., Jr, Dvorak A. M., Barnes B. A., Manseau E. J., Galli S. J. Rejection of first-set skin allografts in man. the microvasculature is the critical target of the immune response. J Exp Med. 1979 Aug 1;150(2):322–337. doi: 10.1084/jem.150.2.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebertz J. M., Hirshman C. A., Kettelkamp N. S., Uno H., Hanifin J. M. Substance P-induced histamine release in human cutaneous mast cells. J Invest Dermatol. 1987 Jun;88(6):682–685. doi: 10.1111/1523-1747.ep12470339. [DOI] [PubMed] [Google Scholar]
- Erdös E. G., Skidgel R. A. Neutral endopeptidase 24.11 (enkephalinase) and related regulators of peptide hormones. FASEB J. 1989 Feb;3(2):145–151. [PubMed] [Google Scholar]
- Fewtrell C. M., Foreman J. C., Jordan C. C., Oehme P., Renner H., Stewart J. M. The effects of substance P on histamine and 5-hydroxytryptamine release in the rat. J Physiol. 1982 Sep;330:393–411. doi: 10.1113/jphysiol.1982.sp014347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjellner B., Hägermark O. Studies on pruritogenic and histamine-releasing effects of some putative peptide neurotransmitters. Acta Derm Venereol. 1981;61(3):245–250. [PubMed] [Google Scholar]
- Foreman J. C., Jordan C. C., Oehme P., Renner H. Structure-activity relationships for some substance P-related peptides that cause wheal and flare reactions in human skin. J Physiol. 1983 Feb;335:449–465. doi: 10.1113/jphysiol.1983.sp014543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman J. C. Substance P and calcitonin gene-related peptide: effects on mast cells and in human skin. Int Arch Allergy Appl Immunol. 1987;82(3-4):366–371. doi: 10.1159/000234229. [DOI] [PubMed] [Google Scholar]
- Foreman J., Jordan C. Histamine release and vascular changes induced by neuropeptides. Agents Actions. 1983 Apr;13(2-3):105–116. doi: 10.1007/BF01967311. [DOI] [PubMed] [Google Scholar]
- Galli S. J., Hammel I. Unequivocal delayed hypersensitivity in mast cell-deficient and beige mice. Science. 1984 Nov 9;226(4675):710–713. doi: 10.1126/science.6494907. [DOI] [PubMed] [Google Scholar]
- Galli S. J., Kitamura Y. Genetically mast-cell-deficient W/Wv and Sl/Sld mice. Their value for the analysis of the roles of mast cells in biologic responses in vivo. Am J Pathol. 1987 Apr;127(1):191–198. [PMC free article] [PubMed] [Google Scholar]
- Gamse R., Saria A. Antidromic vasodilatation in the rat hindpaw measured by laser Doppler flowmetry: pharmacological modulation. J Auton Nerv Syst. 1987 May;19(2):105–111. doi: 10.1016/0165-1838(87)90003-8. [DOI] [PubMed] [Google Scholar]
- Goetzl E. J., Chernov T., Renold F., Payan D. G. Neuropeptide regulation of the expression of immediate hypersensitivity. J Immunol. 1985 Aug;135(2 Suppl):802s–805s. [PubMed] [Google Scholar]
- Hägermark O., Hökfelt T., Pernow B. Flare and itch induced by substance P in human skin. J Invest Dermatol. 1978 Oct;71(4):233–235. doi: 10.1111/1523-1747.ep12515092. [DOI] [PubMed] [Google Scholar]
- Jancsó N., Jancsó-Gábor A., Szolcsányi J. Direct evidence for neurogenic inflammation and its prevention by denervation and by pretreatment with capsaicin. Br J Pharmacol Chemother. 1967 Sep;31(1):138–151. doi: 10.1111/j.1476-5381.1967.tb01984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A. R., Erdös E. G. Release of histamine from mast cells by vasoactive peptides. Proc Soc Exp Biol Med. 1973 Apr;142(4):1252–1256. doi: 10.3181/00379727-142-37219. [DOI] [PubMed] [Google Scholar]
- Kanakura Y., Thompson H., Nakano T., Yamamura T., Asai H., Kitamura Y., Metcalfe D. D., Galli S. J. Multiple bidirectional alterations of phenotype and changes in proliferative potential during the in vitro and in vivo passage of clonal mast cell populations derived from mouse peritoneal mast cells. Blood. 1988 Sep;72(3):877–885. [PubMed] [Google Scholar]
- Kiernan J. A. A study of chemically induced acute inflammation in the skin of the rat. Q J Exp Physiol Cogn Med Sci. 1977 Apr;62(2):151–161. doi: 10.1113/expphysiol.1977.sp002385. [DOI] [PubMed] [Google Scholar]
- Kiernan J. A. The involvement of mast cells in vasodilatation due to axon reflexes in injured skin. Q J Exp Physiol Cogn Med Sci. 1972 Jul;57(3):311–317. doi: 10.1113/expphysiol.1972.sp002164. [DOI] [PubMed] [Google Scholar]
- Kitamura Y., Go S. Decreased production of mast cells in S1/S1d anemic mice. Blood. 1979 Mar;53(3):492–497. [PubMed] [Google Scholar]
- Kitamura Y., Go S., Hatanaka K. Decrease of mast cells in W/Wv mice and their increase by bone marrow transplantation. Blood. 1978 Aug;52(2):447–452. [PubMed] [Google Scholar]
- Kowalski M. L., Kaliner M. A. Neurogenic inflammation, vascular permeability, and mast cells. J Immunol. 1988 Jun 1;140(11):3905–3911. [PubMed] [Google Scholar]
- LOEFSTROEM B., PERNOW B., WAHREN J. VASODILATING ACTION OF SUBSTANCE P IN THE HUMAN FOREARM. Acta Physiol Scand. 1965 Mar;63:311–324. doi: 10.1111/j.1748-1716.1965.tb04070.x. [DOI] [PubMed] [Google Scholar]
- Lawrence I. D., Warner J. A., Cohan V. L., Hubbard W. C., Kagey-Sobotka A., Lichtenstein L. M. Purification and characterization of human skin mast cells. Evidence for human mast cell heterogeneity. J Immunol. 1987 Nov 1;139(9):3062–3069. [PubMed] [Google Scholar]
- Lembeck F., Holzer P. Substance P as neurogenic mediator of antidromic vasodilation and neurogenic plasma extravasation. Naunyn Schmiedebergs Arch Pharmacol. 1979 Dec;310(2):175–183. doi: 10.1007/BF00500282. [DOI] [PubMed] [Google Scholar]
- Lundberg J. M., Saria A., Rosell S., Folkers K. A substance P antagonist inhibits heat-induced oedema in the rat skin. Acta Physiol Scand. 1984 Jan;120(1):145–146. doi: 10.1111/j.1748-1716.1984.tb07385.x. [DOI] [PubMed] [Google Scholar]
- Martin T. R., Galli S. J., Katona I. M., Drazen J. M. Role of mast cells in anaphylaxis. Evidence for the importance of mast cells in the cardiopulmonary alterations and death induced by anti-IgE in mice. J Clin Invest. 1989 Apr;83(4):1375–1383. doi: 10.1172/JCI114025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H., Kawakita K., Kiso Y., Nakano T., Kitamura Y. Substance P induces granulocyte infiltration through degranulation of mast cells. J Immunol. 1989 Feb 1;142(3):927–931. [PubMed] [Google Scholar]
- Mazurek N., Pecht I., Teichberg V. I., Blumberg S. The role of the N-terminal tetrapeptide in the histamine releasing action of substance P. Neuropharmacology. 1981 Nov;20(11):1025–1027. doi: 10.1016/0028-3908(81)90091-5. [DOI] [PubMed] [Google Scholar]
- Mekori Y. A., Dvorak H. F., Galli S. J. 125I-fibrin deposition in contact sensitivity reactions in the mouse. Sensitivity of the assay for quantitating reactions after active or passive sensitization. J Immunol. 1986 Mar 15;136(6):2018–2025. [PubMed] [Google Scholar]
- Mekori Y. A., Galli S. J. Undiminished immunologic tolerance to contact sensitivity in mast cell-deficient W/Wv and Sl/Sld mice. J Immunol. 1985 Aug;135(2):879–885. [PubMed] [Google Scholar]
- Nakano T., Sonoda T., Hayashi C., Yamatodani A., Kanayama Y., Yamamura T., Asai H., Yonezawa T., Kitamura Y., Galli S. J. Fate of bone marrow-derived cultured mast cells after intracutaneous, intraperitoneal, and intravenous transfer into genetically mast cell-deficient W/Wv mice. Evidence that cultured mast cells can give rise to both connective tissue type and mucosal mast cells. J Exp Med. 1985 Sep 1;162(3):1025–1043. doi: 10.1084/jem.162.3.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newson B., Dahlström A., Enerbäck L., Ahlman H. Suggestive evidence for a direct innervation of mucosal mast cells. Neuroscience. 1983 Oct;10(2):565–570. doi: 10.1016/0306-4522(83)90153-7. [DOI] [PubMed] [Google Scholar]
- Otsu K., Nakano T., Kanakura Y., Asai H., Katz H. R., Austen K. F., Stevens R. L., Galli S. J., Kitamura Y. Phenotypic changes of bone marrow-derived mast cells after intraperitoneal transfer into W/Wv mice that are genetically deficient in mast cells. J Exp Med. 1987 Mar 1;165(3):615–627. doi: 10.1084/jem.165.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanahan F., Denburg J. A., Fox J., Bienenstock J., Befus D. Mast cell heterogeneity: effects of neuroenteric peptides on histamine release. J Immunol. 1985 Aug;135(2):1331–1337. [PubMed] [Google Scholar]
- Skofitsch G., Savitt J. M., Jacobowitz D. M. Suggestive evidence for a functional unit between mast cells and substance P fibers in the rat diaphragm and mesentery. Histochemistry. 1985;82(1):5–8. doi: 10.1007/BF00502084. [DOI] [PubMed] [Google Scholar]
- Stead R. H., Tomioka M., Quinonez G., Simon G. T., Felten S. Y., Bienenstock J. Intestinal mucosal mast cells in normal and nematode-infected rat intestines are in intimate contact with peptidergic nerves. Proc Natl Acad Sci U S A. 1987 May;84(9):2975–2979. doi: 10.1073/pnas.84.9.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stimler-Gerard N. P. Neutral endopeptidase-like enzyme controls the contractile activity of substance P in guinea pig lung. J Clin Invest. 1987 Jun;79(6):1819–1825. doi: 10.1172/JCI113023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wershil B. K., Mekori Y. A., Murakami T., Galli S. J. 125I-fibrin deposition in IgE-dependent immediate hypersensitivity reactions in mouse skin. Demonstration of the role of mast cells using genetically mast cell-deficient mice locally reconstituted with cultured mast cells. J Immunol. 1987 Oct 15;139(8):2605–2614. [PubMed] [Google Scholar]
- Wershil B. K., Murakami T., Galli S. J. Mast cell-dependent amplification of an immunologically nonspecific inflammatory response. Mast cells are required for the full expression of cutaneous acute inflammation induced by phorbol 12-myristate 13-acetate. J Immunol. 1988 Apr 1;140(7):2356–2360. [PubMed] [Google Scholar]
- Wiesner-Menzel L., Schulz B., Vakilzadeh F., Czarnetzki B. M. Electron microscopical evidence for a direct contact between nerve fibres and mast cells. Acta Derm Venereol. 1981;61(6):465–469. doi: 10.2340/0001555561465469. [DOI] [PubMed] [Google Scholar]


