Abstract
Phages are a primary driving force behind the evolution of bacterial pathogens by transferring a variety of virulence genes into their hosts. Similar to other bacterial genomes, the Salmonella enterica serovar Enteritidis LK5 genome contains several regions that are homologous to phages. Although genomic analysis demonstrated the presence of prophages, it was unable to confirm which phage elements within the genome were viable. Genetic markers were used to tag one of the prophages in the genome to allow monitoring of phage induction. Commonly used laboratory strains of Salmonella were resistant to phage infection, and therefore a rapid screen was developed to identify susceptible hosts. This approach showed that a genetically tagged prophage, ELPhiS (Enteritidis lysogenic phage S), was capable of infecting Salmonella serovars that are diverse in host range and virulence and has the potential to laterally transfer genes between these serovars via lysogenic conversion. The rapid screen approach is adaptable to any system with a large collection of isolates and may be used to test the viability of prophages found by sequencing the genomes of various bacterial pathogens.
INTRODUCTION
Bacterial evolution includes the modification of existing functions and the acquisition of new ones. Mutations in preexisting genes, including nucleotide exchanges or frameshifts, are common events and slowly contribute to the development of new phenotypes (45). These events alone, however, do not fully explain the evolving architecture of the bacterial genome (32). Horizontal gene transfer is an important driving force behind the evolution of all bacteria, allowing rapid gains of unique functions (48). The lateral flow of genetic information between two organisms, mediated to a large extent by phage (14), facilitates the emergence of new bacterial species and allows existing strains to adapt to, and thrive in, new environments.
Prophages, or their remnants, are common in bacterial genomes (33). Approximately 60% of the sequenced bacterial genomes carry recognizable prophage elements (9, 23, 46). Prophage genes may constitute as much as 10 to 20% of a bacterial genome (9, 19), and they comprise a large proportion of the “unknown” genes in a genome typically annotated as coding for hypothetical proteins. Furthermore, through the introduction of new genes, prophages are responsible for many of the differences between bacterial strains belonging to the same species (10). While numerous putative prophages have been identified in microbial genome sequences, in most cases it is not known whether a prophage is viable or not unless it can be induced. Furthermore, many viable phages are not inducible using standard techniques.
An essential requirement of any assay for phage viability is the selection of an appropriate host, one in which a successful infection can be observed phenotypically, for example, the formation of plaques on a lawn of host cells. The standard plaque assay has been widely used to identify viable phage. Phages are gathered from environmental samples and then spotted directly onto a bacterial lawn (27, 36). Although this procedure is efficient in isolating lytic phage, it is not always an accurate indicator of lysogenic phage viability because the phages may form lysogens as well as plaques. Ashelford and colleagues (2) showed that substantial populations of bacteriophage are present in soil at levels 350-fold greater than the highest numbers estimated using traditional plaque counts. Systems are needed to rapidly identify appropriate hosts to allow better characterization of these phages.
Salmonella enterica is composed of more than 2,600 genetically related serovars (24).These serovars differ in host range and the types of disease caused, and they are ideal candidates to identify new phage hosts because they differ in the surface receptors used by phages to start the infection process (38). Salmonella serovars are highly similar at the DNA level, typically 95 to 99% identical in conserved regions. However, pairwise comparisons of the Salmonella genomes reveal that the serovars possess large insertions and deletions (indels) relative to each other. The indels are estimated to occupy approximately 500 to 600 kb of the 4.5- to 5-Mb genome, and approximately 60% of these chromosomal insertions are genes acquired from mobile genetic elements, most notably from temperate phage (18).
Most of the phage found integrated into Salmonella chromosomes were considered defective (cryptic). It was hypothesized that these prophages have accrued mutations causing loss of function of genes necessary for lytic growth (7, 25, 44). However, Salmonella enterica serovar Typhimurium strain LT2 harbors four fully functional prophages: Fels-1, Fels-2, Gifsy-1, and Gifsy-2 (1, 20, 21). Furthermore, numerous Salmonella strains have been shown to release phage under standard culturing conditions (42). These prophages carry genes that are beneficial to the host by promoting in vivo survival and pathogenesis (20, 22, 25, 26, 49) and are effective transfer agents of these genes, through either lysogenic conversion or generalized transduction.
Salmonella enterica serovar Enteritidis is the leading cause of food-borne salmonellosis in the United States and is primarily acquired through ingestion of undercooked poultry products (6, 10a). Several phage types (PT) of S. Enteritidis are prevalent worldwide and are distinguished by their sensitivity to a defined set of lytic phage (15). The predominant phage type found in S. Enteritidis outbreaks in Great Britain has been PT4 (47), while PT8 has dominated S. Enteritidis infections in the United States. Although previous attempts to identify viable prophages in S. Enteritidis strains have failed (44), recently it was shown that a phage identified in S. Enteritidis strain NCTC13349 (PT4) and annotated as defective was capable of spontaneous excision (41). However, no efforts were carried out to identify other strains that could act as alternative hosts for the phage.
In this study, we analyzed the sequence of a novel prophage, named ELPhiS (Enteritidis lysogenic phage S), present in the genome of S. enterica serovar Enteritidis PT8 strain LK5. To further characterize ELPhiS, a screening assay was developed to rapidly identify susceptible hosts from a large number of diverse natural Salmonella isolates. The assay results also demonstrated that ELPhiS is capable of transferring genes between Salmonella serovars.
MATERIALS AND METHODS
Selection and construction of bacterial strains.
All bacterial strains and plasmids used are listed in Table S1 in the supplemental material. All strains were grown in Luria-Bertani (LB) medium at 37°C under standard conditions unless specified. LB was supplemented with ampicillin (100 μg/ml), chloramphenicol (30 μg/ml), and kanamycin sulfate (50 μg/ml) as required.
Recipient strains used in lysogenic conversion assays were part of the Salmonella Reference Collection B (SARB) obtained from the Salmonella Genetic Stock Center (http://people.ucalgary.ca/∼kesander/) (4). Several wild-type or mutant strains of serovars S. Enteritidis or S. Typhimurium were also used as recipients. P22 HT105/1 was used for generalized transduction and as a control for phage-mediated transfer.
The phage donor strains, all derivatives of wild-type S. Enteritidis LK5, were constructed by λ-Red site-directed mutagenesis (13). Chloramphenicol or kanamycin resistance cassettes were inserted into defined locations within the ELPhiS prophage genome, one insertion per donor strain. To create the antibiotic resistance gene insertions, segments of DNA containing the antibiotic resistance genes were amplified by PCR. Primers were designed with the 3′ end of the primer bearing 20 bp of homology to the plasmid-priming site and the 5′ end possessing 20 to 50 bp of homology to the gene in the bacterial chromosome to be deleted or disrupted (see Table S2 in the supplemental material). The kanamycin and chloramphenicol genes were amplified from template plasmids pKD4 and pKD3 using the chimeric primers and standard PCR protocols (13, 40).
Helper plasmid pKD46 was introduced into S. Enteritidis LK5 following temporary inactivation of the host restriction system (17). The plasmid-bearing strain was grown at 30°C for 4 h in LB medium containing ampicillin and supplemented with 1 mM l-arabinose to induce gene expression prior to being made electrocompetent (40). PCR products were purified and electroporated into competent cells, and selection for transformants was carried out on the appropriate antibiotics.
Each insertion was backcrossed into wild-type S. Enteritidis LK5 using P22-mediated transduction. The backcross is important to ensure genetic homogeneity (30) because the λ-Red system involves induction of phage genes inside the host cell and the effect of overexpression of these genes on resident prophage has not been determined. Strains were determined to be free of contaminating P22 by streaking on Evans blue-uranine (EBU) plates and cross streaking against P22 as described previously (30). Each insertion or deletion was confirmed by PCR using primers that flank sites of insertion (see Table S3 in the supplemental material).
Genome sequencing and analysis.
The genome of S. Enteritidis LK5 was sequenced by a combination of Sanger sequencing and pyrosequencing (Matthews et al., unpublished data). Subsequent bioinformatic analyses performed using the NUCmer module of MUMmer 3.0 (16, 28), progressiveMauve 2.3.1 (11, 12), Geneious 5.3.6 (Biomatters, Ltd., Auckland, New Zealand), RAST 4.0 (3), and Artemis 12.0 (8) programs revealed the presence and gene organization of ELPhiS in the LK5 genome.
Prophage induction from S. Enteritidis LK5 donor cultures.
Phage donor strains (Table 1), grown overnight in the appropriate antibiotics, were diluted 1:100 in LB medium and grown to early exponential phase. To induce S. Enteritidis LK5 prophages, mitomycin C (Sigma-Genosys, St. Louis, MO) was added to the cultures (final concentration of 1 μg/ml). Mitomycin C-treated cultures were grown for up to 6 h, and cells were lysed by the addition of a few drops of chloroform. Cellular debris was pelleted by centrifugation in a microcentrifuge at maximum speed for 5 min. The aqueous layer was collected and passed through a 0.22-μm-pore-size filter to remove any intact cells or large bacterial debris. Lysates were stored at 4°C.
Table 1.
Positions of Kanr and Camr insertions in the ELPhiS genome and lengths of deleted ELPhiS sequencesa
| Kanr strain | Camr strain | Rightmost position of left end | Leftmost position of right end | Deleted ELPhiS sequence (bp) |
|---|---|---|---|---|
| RE522 | RE641 | 20,423 | 20,892 | 468 |
| RE523 | RE642 | 20,134 | 20,135 | 0 |
| RE548 | 33,955 | 33,963 | 7 | |
| RE574 | 26,349 | 26,350 | 0 | |
| RE579 | RE648 | 25,905 | 26,496 | 590 |
| RE581 | 29,154 | 29,155 | 0 |
Positions (in base pairs) are from the left end of the phage.
Detection of packaged DNA by PCR.
The induction and packaging of S. Enteritidis LK5 prophage was assessed using PCR. A 50-μl aliquot of the phage lysates prepared from S. Enteritidis donor strains tagged with the Kanr gene were treated with 1 μl of RQ1 DNase (Promega, Madison, WI) for 1 h at 37°C. The reaction was stopped by adding stop buffer (supplied with the enzyme) and heating to 65°C for 10 min. As a negative control, a 50-μl aliquot of each lysate was left untreated with DNase, and, as a positive control, a lysate of phage P22 HT was grown on wild-type S. Enteritidis LK5 as described previously (29). Three different sets of primers were used to detect DNA in the lysates (see Table S4 in the supplemental material). Primers specific to the gene thrA were used to detect the presence of chromosomal DNA in the lysates. Primers specific to the P22 eac and int genes were used to detect P22 packaged DNA. Primers specific to the Kanr marker were used to detect the presence of encapsidated prophage DNA. A standard PCR protocol was followed with one modification: the first cycle of PCR included an additional heating step for 10 min at 95°C to break open the phage heads and release packaged DNA.
Rapid screening assay to identify lysogenic conversion in susceptible hosts.
In order to screen a large number of Salmonella strains for their suitability as hosts for the tagged ELPhiS phage, a rapid screening assay was developed. Individual strains (see Table S1 in the supplemental material) were grown to early exponential phase without shaking in wells of a 96-well microtiter plate containing 200 μl of LB. Wells containing only medium and wells containing two wild-type S. Enteritidis strains (TK474 and LK5) (see Table S1) were included as controls. One plate for each phage donor strain was prepared by adding 10 μl of an undiluted lysate freshly collected from an induced S. Enteritidis phage donor strain to each well of a 96-well microtiter plate. After overnight growth, 5 μl of the mixture from each well was spotted onto the appropriate selective medium, with lysogenic conversion to antibiotic resistance indicated by bacterial colony formation.
Determination of phage titer.
EBU top-agar plates were prepared by adding 100 μl of overnight cultures of the host strains identified in the rapid screening assays to the top agar (29). Lysates were serially diluted in 10-fold increments in 0.85% NaCl and spotted on the top agar plates.
Preparation of high-titer phage lysates.
High-titer lysates were prepared as described previously for phage λ (43), with some modification. Lysates were serially diluted 10-fold and spotted onto EBU top agar plates prepared with a host strain previously identified as capable of supporting plaque formation. Following overnight growth, individual plaques were picked and stabbed onto a fresh lawn of the host strain. The same recipient was also grown in 10 ml LB medium overnight at 30°C and then pelleted by centrifugation for 20 min at 4,000 rpm. Pellets were each resuspended in 5 ml of 10 mM MgSO4, and 100 μl of the resuspended cells were added to individual culture tubes. Phages were harvested from the plates by removal of entire lysed areas as agar plugs using sterile glass Pasteur pipettes. The plugs were then added to each of the culture tubes containing the recipient cells. One culture tube was left uninfected as a negative control. After brief vortexing and incubation at room temperature for 5 min, 4 ml of LB liquid medium was added. After 4 to 6 h growth, lysates were collected as described earlier. The titer was determined by spotting serially diluted phage lysates onto a lawn of the appropriate host.
Quantitative lysogenic conversion assays.
The frequency of lysogenic conversion by tagged ELPhiS to Kanr was determined for the host strains identified in the screening assays. Cultures (5 ml) of host bacteria were grown overnight. The concentration of bacteria was determined by serial dilution and plating onto LB agar. High-titer lysates were diluted 1:100 in 0.85% NaCl, and 100 μl of each diluted phage lysate was mixed with 100 μl of an overnight culture of host bacteria, giving an approximate multiplicity of infection (MOI) of 1. The mixtures were incubated for 1 h to allow phenotypic expression and were then spread onto the appropriate selective medium. Lysogenic conversion frequency was measured by colony formation on selective medium.
Nucleotide sequence accession number.
The ELPhiS genome sequence has been deposited into GenBank under accession number HM770079.
RESULTS
Genome analysis.
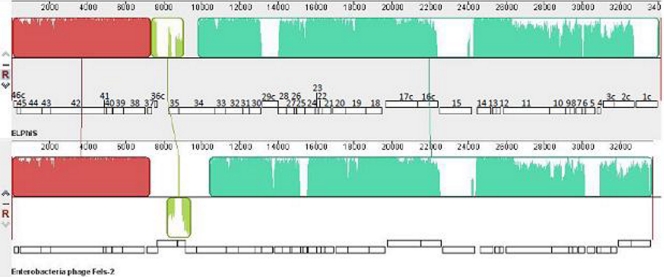
Five phage-encoding regions, including the ELPhiS prophage, were identified from our analysis of the S. Enteritidis strain LK5 genome. While four prophages were identical to the prophages ΦSE10, ΦSE12, ΦSE12A, and ΦSE14 found in S. Enteritidis P125109 (47), the LK5 genome lacked the ΦSE20 prophage and contained the ELPhiS prophage. The ELPhiS prophage region is 34,116 bp flanked on either end by an almost perfect 84-nucleotide direct repeat [5′-AAAAAGGGGCTACGTTTTCACGTAACCCCTTGTT(T/A)TATTTGGTGGAGCTGGCGGGAGTTGAACCCGCGTCCGAAATTCCTACAT-3′]. The ELPhiS attB site overlaps the attB sites of the SopEΦ and Fels-2 phages within the tmRNA gene ssrA (37) located in the homologous region between SEN2612 and SEN2613 of P125109 (47). The sequence analysis suggested that ELPhiS is a P2-like phage closely related to Fels-2 (63.2% DNA identity). The genomes of the two phages differed in their cargo gene content (all predicted to encode hypothetical proteins), as well as an inversion in the ∼2.5- to 3.0-kbp region containing genes 34 to 36c next to the annotated DNA invertase gene (gene no. 37 [Fig. 1]).
Fig 1.
Alignment of ELPhiS and Fels-2 genomes using progressiveMauve showed variation in gene content. The alignment identified three locally colinear blocks (LCBs), colored red, olive, and green, with a minimum LCB weight of 1,990. The olive LCB, containing genes encoding tail fiber proteins, was inverted. White regions within LCBs represent nonidentical sequences. Predicted ELPhiS genes are numbered as described in Table 2.
The ELPhiS prophage genome encodes 46 annotated proteins predicted by RAST (Table 2 and Fig. 2), including structural genes encoding capsid, baseplate, and tail proteins, genes that enable lysis of the host cell, and genes that regulate lysogeny. The prophage genome contained 10 predicted hypothetical proteins of unknown function, three of these (genes 1c, 15, and 29c) being unique to ELPhiS (Fig. 2).
Table 2.
Predicted functions and locations of annotated ELPhiS genes
| Gene no. | Predicted gene function | Size (bp) | Coordinates |
|---|---|---|---|
| 1c | Hypothetical protein | 1,101 | 32,827–33,927 |
| 2c | Integrase | 1,017 | 31,697–32,713 |
| 3c | cI repressor | 633 | 31,062–31,694 |
| 4 | Regulatory protein Ap1 | 159 | 30,782–30,940 |
| 5 | cII transcriptional activator | 510 | 30,156–30,655 |
| 6 | Hypothetical protein | 201 | 29,948–30,148 |
| 7 | Hypothetical protein | 342 | 29,643–29,984 |
| 8 | Hypothetical protein | 234 | 29,342–29,575 |
| 9 | Zn finger-containing protein | 228 | 29,115–29,342 |
| 10 | DNA adenine methylase | 858 | 28,261–29,118 |
| 11 | P2 gpA-like replication protein | 2,400 | 25,850–28,249 |
| 12 | Hypothetical protein | 189 | 25,509–25,697 |
| 13 | Hypothetical protein | 234 | 25,265–25,498 |
| 14 | Hypothetical protein | 732 | 24,474–25,205 |
| 15 | Hypothetical protein | 1,665 | 22,496–24,160 |
| 16c | Capsid packaging protein (Q) | 1,041 | 21,352–22,392 |
| 17c | Terminase, ATPase subunit (P) | 1,719 | 19,634–21,352 |
| 18 | Capsid scaffolding protein (O) | 834 | 18,610–19,443 |
| 19 | Major capsid protein (N) | 1,062 | 17,532–18,593 |
| 20 | Terminase, endonuclease subunit (M) | 651 | 16,878–17,528 |
| 21 | Head completion-stabilization protein (L) | 465 | 16,320–16,784 |
| 22 | Tail component protein (X) | 204 | 16,117–16,320 |
| 23 | Holin lysis protein (Y) | 216 | 15,898–16,113 |
| 24 | Endolysis (lysozyme) (K) | 516 | 15,402–15,917 |
| 25 | Lysozyme subunit B (LysB) | 429 | 14,977–15,405 |
| 26 | Lysozyme subunit C (LysC) | 117 | 14,844–14,960 |
| 27 | Tail completion protein (R) | 432 | 14,450–14,881 |
| 28 | Tail completion protein (S) | 429 | 14,011–14,439 |
| 29c | Hypothetical protein | 852 | 13,158–14,009 |
| 30 | Baseplate assembly protein (V) | 579 | 12,502–13,080 |
| 31 | Baseplate assembly protein (W) | 360 | 12,146–12,505 |
| 32 | Baseplate assembly protein (J) | 909 | 11,251–12,159 |
| 33 | Tail protein (I) | 606 | 10,653–11,258 |
| 34 | Tail fiber protein (H) | 1,854 | 8,803–10,656 |
| 35 | Tail fiber protein (G) | 576 | 8,228–8,803 |
| 36c | Tail fiber protein (G′) | 144 | 7,493–7,636 |
| 37 | DNA invertase | 255 | 7,134–7,358 |
| 38 | Tail sheath protein (FI) | 1,173 | 5,839–7,031 |
| 39 | Major tail tube protein (FII) | 516 | 5,534–5,849 |
| 40 | Tail protein (E) | 303 | 4,977–5,279 |
| 41 | Tail protein (E′) | 120 | 4,843–4,962 |
| 42 | Tail tape measure protein (T) | 2,808 | 2,043–4,850 |
| 43 | Tail protein (U) | 486 | 1,561–2,046 |
| 44 | Tail protein (D) | 1,101 | 464–1,564 |
| 45 | Late promoter activator (ogr) | 183 | 268–450 |
| 46c | Hypothetical protein | 144 | 107–250 |
Fig 2.
Genome organization of the ELPhiS prophage. The phage inserted into the ssrA gene (located between bases 2,755,078 and 2,755,440 of the S. Enteritidis LK5 genome sequence), resulting in an 84-nucleotide direct repeat (dark blue arrows) that flanks the phage. The intact ssrA gene is located next to ELPhiS gene 46c. The prophage genome is organized in modules of genes encoding structural proteins (blue arrows), regulatory proteins (dark green arrows), recombination (green arrows), other DNA replicating, binding, or modifying proteins (orange arrows), lysis proteins (red arrows), and hypothetical proteins (yellow arrows). Black lines show the deleted ELPhiS regions in tagged strains with deletions. Black arrows show insertion sites of Kanr and Camr genes in tagged strains without deletions.
Genetic tagging of prophages allows surveillance of phage viability.
Genetically tagged strains were constructed to identify whether ELPhiS was inducible and able to infect naïve hosts, thus confirming that the annotated ELPhiS genes were functional. A total of six independent insertions were made in the ELPhiS prophage (see Table S2 in the supplemental material) using the λ-Red system (13); three were point insertions (no deletion of prophage DNA), while three had either 7 bp, 468 bp, or 580 bp of phage DNA deleted. Insertion sites were chosen that were least likely to affect phage excision, genome packaging, and infectivity (i.e., within genes of unknown function or between open reading frames).
Detection of packaged DNA by PCR.
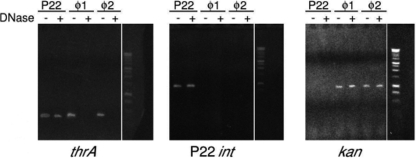
PCR analysis was used to specifically detect the DNA packaged by induced phage (Fig. 3). Lysates were collected from the Kanr S. Enteritidis donor strains, and a fresh lysate of phage P22 HT was grown on wild-type S. Enteritidis LK5 as a control. DNase I-treated and untreated aliquots of these lysates served as the templates in the PCR amplifications. An appropriately sized PCR product corresponding to the Salmonella housekeeping gene thrA was present in each reaction in which samples were not treated with DNase I and indicated the presence of chromosomal DNA in the lysate. A thrA product was also observed in the reaction in which a DNase I-treated aliquot of P22 lysate was the template. The presence of the product was due to packaged chromosomal DNA being protected from DNase I degradation. P22 HT105/1 is a generalized transducing phage, and up to 50% of the phage particles in a lysate will contain chromosomal DNA. The P22 int gene served as a positive control for packaged phage DNA. The correct sized 920-bp product was observed in both the treated and untreated P22 samples, demonstrating that P22 phage particles were protecting the int gene from enzymatic degradation.
Fig 3.
Detection of phage-encapsidated DNA using thrA primers. P22 eae/int primers amplified regions specific to P22. The samples in lanes marked “+” were treated with DNase. A PCR product using primers to amplify the kanamycin gene proved that phage were viable and packaged its own genome. ϕ1 corresponds to phage donor strain RE522. ϕ2 corresponds to phage donor RE579.
A PCR product of the appropriate size for the Kanr marker was observed in lysates prepared from the two S. Enteritidis phage donor strains RE522 and RE579 (Fig. 3) as well as RE523. This product was present in both the DNase I-treated and untreated samples, indicating that the kanamycin resistance gene was packaged into a phage head and protected from degradation. However, no Kanr product was detected in lysates prepared from RE548, RE574, or RE581. No thrA product was detected in the DNase I-treated samples; this showed that packaging of DNA was specific for ELPhiS DNA.
Protection from DNase degradation and PCR amplification of the Kanr marker located in the ELPhiS prophage provided evidence that ELPhiS DNA was packaged upon induction. Furthermore, phage genome-specific packaging was shown by the exclusion of the thrA gene from the phage head.
Rapid screening assay to identify susceptible hosts.
Most studies of genetic exchange by phages in Salmonella have relied upon the serovar Typhimurium laboratory strain LT2 as both a phage donor and recipient (1, 20–22, 31, 37). Mitomycin C-induced lysates were collected from each of the Kanr and Camr S. Enteritidis phage donor strains and were used to infect LT2. However, none of the S. Enteritidis lysates converted LT2 to antibiotic resistance. In addition, plaque assays revealed that none of the phages present in any of the S. Enteritidis donor strains formed plaques on a LT2 lawn. Therefore, a rapid screening assay for lysogenic conversion to antibiotic resistance was developed using microtiter plates inoculated with 72 strains from the SARB collection (4) and additional strains from the laboratory collection which served as positive or negative controls (see Table S1 in the supplemental material).
Lysates prepared from the Kanr donor strains RE522 and RE579 were capable of converting the same 11 strains to Kanr in a reproducible manner (Table 3). The assays were repeated using lysates collected from the Camr phage donor strains RE641 and RE648. Results of this second group of assays were identical to those seen with the Kanr donor strains. Taken together, these results allowed us to qualitatively identify suitable hosts for ELPhiS from a large pool of Salmonella strains.
Table 3.
Recipient strains identified by rapid lysogenic conversion assays
| Strain | S. enterica serovar | ETa | Animal source | Location |
|---|---|---|---|---|
| SARB 4 | Choleraesuis | Cs 1 | Swine | Minnesota |
| SARB 6 | Choleraesuis | Cs 11 | Thailand | |
| SARB 24 | Heidelberg | He 3 | Thailand | |
| SARB 27 | Infantis | In 3 | Senegal | |
| SARB 36 | Newport | Np 8 | Human | North Carolina |
| SARB 40 | Panama | Pn 2 | Human | North Carolina |
| SARB 41 | Panama | Pn 12 | Human | North Carolina |
| SARB 42 | Paratyphi A | Pa 1 | Laboratory strain | |
| SARB 48 | Paratyphi C | Pc 1 | France | |
| SARB 49 | Paratyphi C | Pc 2 | Human | France |
| SARB 66 | Typhimurium | Tm 7 | Parrot | California |
ET, electrophoretic type.
Evaluation of plaque formation by S. Enteritidis phage.
We compared the ability of the phage lysates from RE522 and RE579 to form plaques on each of the susceptible host strains. Lysates were collected from mitomycin C-induced cultures of RE522 and RE579 and spotted onto EBU top agar plates prepared with each of the susceptible host strains. Although all 11 host strains were susceptible to lysogenic conversion, only two strains yielded plaques (2.4% of all the strains tested). SARB40 (S. enterica serovar Panama) yielded faint plaques, while SARB49 (S. enterica serovar Paratyphi C) allowed the development of larger, well-defined plaques. SARB49 was therefore selected as the host to generate high-titer lysates used in subsequent assays.
The high-titer lysates of phage originally obtained from RE522 and RE579 but grown in SARB49 were used to repeat the plaque assay. As a result, plaques were observed on two additional strains, SARB4 (S. enterica serovar Choleraesuis) and SARB36 (S. enterica serovar Newport). The other seven recipient strains capable of lysogeny remained resistant to plaque formation.
Quantitative lysogenic conversion assays reveal differences in lysogeny frequency.
The rapid screening assays were instrumental in identifying which of the marked S. Enteritidis strains produced functional phages and which Salmonella strains were able to serve as hosts for these phages. However, these assays were not quantitative and further assays were necessary to quantify the frequency of lysogenic conversion. High-titer lysates prepared from both Kanr and Camr strains (RE522/RE641, RE523/RE642, and RE579/RE648) were used to infect the recipients identified in the screening assays. Lysogenic conversion to antibiotic resistance was observed in each lysate and host mixture, confirming the results seen with the screening assays. The frequency of lysogenic conversion, as measured by the ratio of the number of antibiotic resistant lysogens to the total number of host cells, varied greatly (Fig. 4). The variance was not due to differences in spontaneous resistance to kanamycin or chloramphenicol, as transfer of the resistance genes was demonstrated by PCR and no growth was observed on control plates containing cells or lysate only, but could be the result of the recipient bacteria's barriers to horizontal gene transfer or the inefficient packaging due to the size and positioning of the antibiotic resistance markers in the prophage.
Fig 4.
Variations in the frequency of lysogenic conversion by ELPhiS. Lysates prepared from RE522 (vertical lines), RE523 (horizontal lines), RE579 (diagonal lines), RE641 (solid black bars), RE642 (white shading), and RE648 (gray shading) were used in quantitative lysogenic conversion assays.
Confirmation that ELPhiS is a single inducible phage.
Experimental data showed that two independent insertions in the same region of the S. Enteritidis LK5 chromosome appear to be in a viable prophage. Our hypothesis was that the two insertions were within the same phage, but we could not demonstrate simultaneous transfer of two markers between strains (i.e., simultaneous coinheritance of Kanr and Camr) even though we could demonstrate that with transducing phage P22 (see Fig. S1 in the supplemental material). The combined size of the insertions probably adversely affected phage DNA packaging and/or recircularization. We therefore used a PCR-based approach to detect cotransfer of independent regions. Host strains lysogenized by phages released from RE522 always acquired both the region harboring the selected insertion and the locus where the alternative insertion is located in RE579. Reciprocal experiments demonstrated linkage between transfer of RE579 and RE522. Together, these data imply we have identified a single inducible phage, which we named ELPhiS (Enteritidis lysogenic phage S).
Isolation of viable phages from ELPhiS lysogens.
The genome sequence suggests that ELPhiS is intact and able to express all proteins necessary for phage production. To confirm this hypothesis and determine whether ELPhiS was capable of autonomous induction, the Salmonella host strains identified by the rapid screening assay were used in the larger scale assays described previously. Lysogens of the 11 host strains formed by infection with ELPhiS lysate derived from RE522 and RE579 were selected for by demanding growth on kanamycin. Once the presence of the prophage in the recipients was confirmed by PCR, mitomycin C was used to induce the prophages again. Lysates were prepared and an aliquot was spotted onto a fresh lawn of SARB49, chosen because of its ability to support well-defined plaques. Lysates collected from all of the lysogens produced plaques on this strain. Furthermore, antibiotic-resistant lysogens were recovered from each of the plaques. These strains could again be induced to release phage particles capable of antibiotic resistance lysogenic conversion. This serial induction and passage of the phage implied that ELPhiS does not require a helper phage for mobilization unless it is present in all 11 SARB members.
DISCUSSION
The salmonellae have been instrumental tools in the development and understanding of phage biology. The temperate phage P22, whose normal host is serovar Typhimurium, was identified more than half a century ago when Zinder and Lederberg discovered the generalized transduction of genetic material between different Salmonella isolates (serovar Typhimurium isolates LT2 and LT22) (50). Since that seminal work, several more phages from S. Typhimurium have been identified, including SopEΦ, two Fels phages, and three Gifsy phages. Similar to other temperate phages, these phages confer new or advantageous qualities on their host, including virulence determinants encoded by SopEΦ and the Gifsy phages, and factors affecting bacterial survival that are encoded by the Fels-2 prophage. Furthermore, these phages that were long considered cryptic are capable of converting from a lysogenic to a lytic lifestyle and producing infective particles when exposed to the appropriate environmental signals. In each case, transfer has only been compared between parental strains and certain genetically manipulated Typhimurium strains cured of the prophage. The presence of viable phage in other Salmonella serovars and their role in the transfer of genetic information between the serovars remain outstanding questions.
Despite extensive studies using serovar Typhimurium strains, there have been few studies on viable prophage in other Salmonella serovars. Genome sequencing of different serovar S. Enteritidis isolates has revealed regions on the chromosome with homology to known phage genes. Thomson et al. identified five prophages in S. Enteritidis PT4 (47), and we identified five prophages in S. Enteritidis PT8 (Matthews et al., unpublished). Only ΦSE20 appeared intact in S. Enteritidis PT4; the other four prophages, also present in PT8 strain LK5, contained numerous pseudogenes. However, genome sequence analysis alone cannot ascertain whether these elements are inducible. Therefore, we developed a rapid assay to test whether the ELPhiS prophage in the S. Enteritidis PT8 strain LK5 genome is inducible and capable of lysogenizing a range of hosts.
Electron microscopic studies provided compelling evidence for S. Enteritidis prophage viability: several apparently intact phages were seen in a lysate prepared from wild-type S. Enteritidis LK5 (see Fig. S2 in the supplemental material). However, phage particles cannot be correlated to the prophages from images. Similarly, plaque assays, historically used to detect viable phages, have limitations in their ability to assess prophage viability. The relationship between prophages and intact virions cannot be determined solely by plaque formation. In addition, the plaque assay will indicate only bacterial lysis by phages, an event dependent on host susceptibility and the nature of the infective particle (i.e., lytic versus temperate phage). Indeed, phage ecologists have shown that viable phages are present in the environment in substantially larger numbers than predicted plaque counts (2).
Several lines of evidence indicate that a viable prophage was identified in the S. Enteritidis strain LK5 genome: (i) packaged DNA could be detected in cell-free lysates and was protected from DNase I treatment; (ii) markers could be transferred between strains using cell-free lysates, and the transfer could be repeated ad infinitum; and (iii) individual plaques were identified following serial dilutions, and bacteria isolated from the center of the plaques contained antibiotic-resistant lysogens.
While phage-mediated gene transfer occurs frequently in bacteria (34, 35), many mechanisms have evolved to limit such exchanges. The phage must find a suitable receptor on the host surface that is not masked by capsule or other surface components. Subsequently, phage DNA must be introduced into the host cell, a process that may require host factors. Once inside the host cytoplasm, the phage DNA is susceptible to restriction modification, CRISPR (clusters of regularly interspaced short palindromic repeats), and superinfection exclusion systems. The phage DNA must be faithfully replicated and expressed, often requiring the interaction of host and phage proteins. The low number of Salmonella strains susceptible to lysogenic conversion and lysis when exposed to ELPhiS shows the effectiveness of these barriers. Our results with the high-titer lysate suggest that alternate modification systems may extend the phage host range and are in agreement with previous studies that showed that inactivation of restriction/modification systems increases phage transfer efficiency (17).
We cannot fully explain why some strains appear to support only lysogenic growth of the virus particles and not lytic growth. Formation of a plaque requires multiple infection-replication-lysis cycles, and the phages may not be sufficiently adept at completing this cycle in many of the potential hosts. Other factors, such as host restriction systems and the presence of other prophages, may affect this process.
Our observations eliminate the possibility that the conversion of the 11 SARB recipients to antibiotic resistance was due to recombination between homologous regions transferred by generalized transduction and support the hypothesis of a functional S. Enteritidis phage capable of infecting and lysogenizing a naïve host. Furthermore, ELPhiS induced from non-S. Enteritidis lysogenic hosts could infect the same range of naïve hosts. ELPhiS is also capable of transferring genes horizontally through lysogenic conversion. Many phages contain cargo genes in their genomes that affect the virulence or fitness of the lysogen (reviewed in reference 5). ELPhiS contains three hypothetical cargo genes of unknown function, and these genes may also contribute to the virulence or fitness of LK5.
In addition to host-mediated factors, the size and location of the insertions in the donor chromosome affected the ability to detect the presence of the Kanr gene via PCR in a lysate as well as the frequency of lysogenic conversion. Only strains having deletions that compensate for the size of the inserted Kanr gene released phage particles detectable by PCR. Furthermore, the size of the inserted Camr gene was 463 bp smaller than the Kanr insert and the analogous Camr strains had a higher lysogenic conversion frequency (Fig. 4). These data suggest that packaging of the ELPhiS genome is limited by its size. Although we speculate this is due to the constraints of headful packaging, we cannot eliminate the possibility of unforeseen effects on neighboring genes.
Induction of phage from bacterial genomes has been observed for more than 50 years. However, a reliable system to assess the viability of potential mobile genetic elements has not been developed. Assaying plaque formation on a single host, however useful for the isolation of lytic phage, will most likely miss a great number of viable phages. The rapid screening assay described here exploits the natural variations among bacteria to circumvent barriers to phage infection and permits identification of viable nonlytic phage by screening for lysogenic conversion. Using this assay, we have identified the inducible prophage ELPhiS within the S. Enteritidis LK5 genome and showed that it can transfer its own genetic material to naïve strains. With these genetic tools in hand, the role of the individual phage proteins in the infection process can be dissected. The rapid screening assay is not restricted to the salmonellae; it can easily be adapted for use in other bacterial species where multiple isolates are available. Since phage play an important role in the transfer of genes encoding toxins and other virulence factors, this assay will further our ability to track the emergence of new bacterial pathogens by identifying suitable hosts.
Supplementary Material
ACKNOWLEDGMENTS
We thank Theresa Ho for experimental advice. We thank Pat Ryan, Anca Segall, Forest Rohwer, and Stanley Maloy for helpful discussions and reading the manuscript. We also thank the Ecological Metagenomics undergraduates at San Diego State University for sequencing of S. Enteritidis LK5 (supported by NSF TUES grant 1044453).
This work was supported by grants from the University of Tennessee Center of Excellence in Genomics and Bioinformatics, St. Jude Children's Research Hospital, and NSF grant DBI:0850356 from the Division of Biological Infrastructure.
Footnotes
Published ahead of print 13 January 2012
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1. Affolter M, Parent-Vaugeois C, Anderson A. 1983. Curing and induction of the Fels 1 and Fels 2 prophages in the Ames mutagen tester strains of Salmonella typhimurium. Mutat. Res. 110:243–262 [DOI] [PubMed] [Google Scholar]
- 2. Ashelford KE, Day MJ, Fry JC. 2003. Elevated abundance of bacteriophage infecting bacteria in soil. Appl. Environ. Microbiol. 69:285–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aziz RK, et al. 2008. The RAST Server: rapid annotations using subsystems technology. BMC Genomics 9:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boyd EF, et al. 1993. Salmonella reference collection B (SARB): strains of 37 serovars of subspecies I. J. Gen. Microbiol. 139(Pt. 6):1125–1132 [DOI] [PubMed] [Google Scholar]
- 5. Brussow H, Canchaya C, Hardt WD. 2004. Phages and the evolution of bacterial pathogens: from genomic rearrangements to lysogenic conversion. Microbiol. Mol. Biol. Rev. 68:560–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Callaway TR, Edrington TS, Anderson RC, Byrd JA, Nisbet DJ. 2008. Gastrointestinal microbial ecology and the safety of our food supply as related to Salmonella. J. Anim. Sci. 86:E163–E172 [DOI] [PubMed] [Google Scholar]
- 7. Campbell A. 1996. Bacteriophages, p 2325–2338 In Neidhardt FC, et al. (ed), Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, DC [Google Scholar]
- 8. Carver T, et al. 2008. Artemis and ACT: viewing, annotating and comparing sequences stored in a relational database. Bioinformatics 24:2672–2676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Casjens S. 2003. Prophages and bacterial genomics: what have we learned so far? Mol. Microbiol. 49:277–300 [DOI] [PubMed] [Google Scholar]
- 10. Casjens S, et al. 2000. A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs in an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol. Microbiol. 35:490–516 [DOI] [PubMed] [Google Scholar]
- 10a. Centers for Disease Control and Prevention 2010. Surveillance for foodborne disease outbreaks—United States, 2006. Ann. Emerg. Med. 55:47–49 [DOI] [PubMed] [Google Scholar]
- 11. Darling AC, Mau B, Blattner FR, Perna NT. 2004. Mauve: multiple alignment of conserved genomic sequence with rearrangements. Genome Res. 14:1394–1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Darling AE, Mau B, Perna NT. 2010. progressiveMauve: multiple genome alignment with gene gain, loss and rearrangement. PLoS One 5:e11147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 97:6640–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Davis BM, Waldor MK. 2002. Mobile genetic elements and bacterial pathogenesis, p 1040–1059 In Craig NL, Cragie R, Gellert M, Lambowitz AM. (ed), Mobile DNA II. ASM Press, Washington, DC [Google Scholar]
- 15. De Lappe N, Doran G, O'Connor J, O'Hare C, Cormican M. 2009. Characterization of bacteriophages used in the Salmonella enterica serovar Enteritidis phage-typing scheme. J. Med. Microbiol. 58:86–93 [DOI] [PubMed] [Google Scholar]
- 16. Delcher AL, Phillippy A, Carlton J, Salzberg SL. 2002. Fast algorithms for large-scale genome alignment and comparison. Nucleic Acids Res. 30:2478–2483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Edwards RA, Helm RA, Maloy SR. 1999. Increasing DNA transfer efficiency by temporary inactivation of host restriction. Biotechniques 26:892–894, 896,, 898 passim [DOI] [PubMed] [Google Scholar]
- 18. Edwards RA, Olsen GJ, Maloy SR. 2002. Comparative genomics of closely related salmonellae. Trends Microbiol. 10:94–99 [DOI] [PubMed] [Google Scholar]
- 19. Edwards RA, Rohwer F. 2005. Viral metagenomics. Nat. Rev. Microbiol. 3:504–510 [DOI] [PubMed] [Google Scholar]
- 20. Figueroa-Bossi N, Bossi L. 1999. Inducible prophages contribute to Salmonella virulence in mice. Mol. Microbiol. 33:167–176 [DOI] [PubMed] [Google Scholar]
- 21. Figueroa-Bossi N, Coissac E, Netter P, Bossi L. 1997. Unsuspected prophage-like elements in Salmonella typhimurium. Mol. Microbiol. 25:161–173 [DOI] [PubMed] [Google Scholar]
- 22. Figueroa-Bossi N, Uzzau S, Maloriol D, Bossi L. 2001. Variable assortment of prophages provides a transferable repertoire of pathogenic determinants in Salmonella. Mol. Microbiol. 39:260–271 [DOI] [PubMed] [Google Scholar]
- 23. Fouts DE. 2006. Phage_Finder: automated identification and classification of prophage regions in complete bacterial genome sequences. Nucleic Acids Res. 34:5839–5851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guibourdenche M, et al. 2010. Supplement 2003–2007 (no. 47) to the White-Kauffmann-Le Minor scheme. Res. Microbiol. 161:26–29 [DOI] [PubMed] [Google Scholar]
- 25. Hardt WD, Urlaub H, Galan JE. 1998. A substrate of the centisome 63 type III protein secretion system of Salmonella typhimurium is encoded by a cryptic bacteriophage. Proc. Natl. Acad. Sci. U. S. A. 95:2574–2579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ho TD, et al. 2002. Identification of GtgE, a novel virulence factor encoded on the Gifsy-2 bacteriophage of Salmonella enterica serovar Typhimurium. J. Bacteriol. 184:5234–5239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jiang SC, Paul JH. 1998. Gene transfer by transduction in the marine environment. Appl. Environ. Microbiol. 64:2780–2787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kurtz S, et al. 2004. Versatile and open software for comparing large genomes. Genome Biol. 5:R12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Maloy SR. 1990. Experimental techniques in bacterial genetics. Jones and Bartlett Publishers, Boston, MA [Google Scholar]
- 30. Maloy SR, Stewart VJ, Taylor RK. 1996. Genetic analysis of pathogenic bacteria. Cold Spring Harbor Laboratory Press, Plainview, N. Y [Google Scholar]
- 31. Mirold S, et al. 1999. Isolation of a temperate bacteriophage encoding the type III effector protein SopE from an epidemic Salmonella typhimurium strain. Proc. Natl. Acad. Sci. U. S. A. 96:9845–9850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Morschhauser J, et al. 2000. Evolution of microbial pathogens. Philos. Trans. R. Soc. Lond. B Biol. Sci. 355:695–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Paul JH. 1999. Microbial gene transfer: an ecological perspective. J. Mol. Microbiol. Biotechnol. 1:45–50 [PubMed] [Google Scholar]
- 34. Paul JH, Sullivan MB. 2005. Marine phage genomics: what have we learned? Curr. Opin. Biotechnol. 16:299–307 [DOI] [PubMed] [Google Scholar]
- 35. Paul JH, Sullivan MB, Segall AM, Rohwer F. 2002. Marine phage genomics. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 133:463–476 [DOI] [PubMed] [Google Scholar]
- 36. Pedulla ML, et al. 2003. Origins of highly mosaic mycobacteriophage genomes. Cell 113:171–182 [DOI] [PubMed] [Google Scholar]
- 37. Pelludat C, Mirold S, Hardt WD. 2003. The SopEΦ phage integrates into the ssrA gene of Salmonella enterica serovar Typhimurium A36 and is closely related to the Fels-2 prophage. J. Bacteriol. 185:5182–5191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Popoff MY, LE Minor LE. 2005. Salmonella, p 764–799 In Garrity G. M. (ed), Bergey's manual of systematic bacteriology, 2nd ed, vol 2 Springer Science + Business Media Inc., New York, NY [Google Scholar]
- 39. Reference deleted.
- 40. Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 41. Santiviago CA, et al. 2010. Spontaneous excision of the Salmonella enterica serovar Enteritidis-specific defective prophage-like element ϕSE14. J. Bacteriol. 192:2246–2254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schicklmaier P, Moser E, Wieland T, Rabsch W, Schmieger H. 1998. A comparative study on the frequency of prophages among natural isolates of Salmonella and Escherichia coli with emphasis on generalized transducers. Antonie Van Leeuwenhoek 73:49–54 [DOI] [PubMed] [Google Scholar]
- 43. Silhavy TJ, Berman ML, Enquist LW. 1984. Experiments with gene fusions. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 44. Sillankorva S, et al. 2010. Salmonella Enteritidis bacteriophage candidates for phage therapy of poultry. J. Appl. Microbiol. 108:1175–1186 [DOI] [PubMed] [Google Scholar]
- 45. Sokurenko EV, Hasty DL, Dykhuizen DE. 1999. Pathoadaptive mutations: gene loss and variation in bacterial pathogens. Trends Microbiol. 7:191–195 [DOI] [PubMed] [Google Scholar]
- 46. Srividhya KV, et al. 2007. Identification of prophages in bacterial genomes by dinucleotide relative abundance difference. PLoS One 2:e1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Thomson NR, et al. 2008. Comparative genome analysis of Salmonella Enteritidis PT4 and Salmonella Gallinarum 287/91 provides insights into evolutionary and host adaptation pathways. Genome Res. 18:1624–1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Woese CR. 2000. Interpreting the universal phylogenetic tree. Proc. Natl. Acad. Sci. U. S. A. 97:8392–8396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhang S, et al. 2002. Phage mediated horizontal transfer of the sopE1 gene increases enteropathogenicity of Salmonella enterica serotype Typhimurium for calves. FEMS Microbiol. Lett. 217:243–247 [DOI] [PubMed] [Google Scholar]
- 50. Zinder ND, Lederberg J. 1952. Genetic exchange in Salmonella. J. Bacteriol. 64:679–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.