Abstract
An increasing number of outbreaks of gastroenteritis recently caused by Escherichia coli O157:H7 have been linked to the consumption of leafy green vegetables. Although it is known that E. coli survives and grows in the phyllosphere of lettuce plants, the molecular mechanisms by which this bacterium associates with plants are largely unknown. The goal of this study was to identify E. coli genes relevant to its interaction, survival, or attachment to lettuce leaf surfaces, comparing E. coli K-12, a model system, and E. coli O157:H7, a pathogen associated with a large number of outbreaks. Using microarrays, we found that upon interaction with intact leaves, 10.1% and 8.7% of the 3,798 shared genes were differentially expressed in K-12 and O157:H7, respectively, whereas 3.1% changed transcript levels in both. The largest group of genes downregulated consisted of those involved in energy metabolism, including tnaA (33-fold change), encoding a tryptophanase that converts tryptophan into indole. Genes involved in biofilm modulation (bhsA and ybiM) and curli production (csgA and csgB) were significantly upregulated in E. coli K-12 and O157:H7. Both csgA and bhsA (ycfR) mutants were impaired in the long-term colonization of the leaf surface, but only csgA mutants had diminished ability in short-term attachment experiments. Our data suggested that the interaction of E. coli K-12 and O157:H7 with undamaged lettuce leaves likely is initiated via attachment to the leaf surface using curli fibers, a downward shift in their metabolism, and the suppression of biofilm formation.
INTRODUCTION
In recent years, the frequency of outbreaks of Escherichia coli O157:H7 linked to the consumption of lettuce and spinach has been increasing (8, 9, 42, 45, 54, 62). While this increasing incidence poses a major public health issue, a proven control strategy has yet to be identified. A better understanding of the ecology of this bacterium is critical for developing interventions. Toward this goal, over the past few years the scientific community has advanced our understanding of food-borne pathogenic bacteria in the agricultural and processing environment (11, 15, 24, 44,). Moreover, the source of the contamination and persistence of this pathogen on fresh produce has become a high research priority in food safety.
Initial studies have examined bacterial survival in soil, manure (15, 24, 44), and plants (11), the ability of harvesting and cutting tools to sequentially contaminate produce (58), the effectiveness of some common sanitizers (38, 68), and the possibility of utilizing novel technologies, such as irradiation (19) and ozone (18), to enhance produce safety. These studies indicate that some food-borne pathogens have the ability to survive in secondary habitats and have a sustained presence in the agricultural environment (9). In particular, E. coli O157:H7 has the capacity to survive the harsh conditions imparted by UV irradiation and low nutrient and water availability conditions and appears to adequately compete with epiphytes, commensals, and phytobacteria in plant phyllospheres and rhizospheres (64, 67).
Despite the important information revealed by these studies, the molecular mechanisms allowing for the establishment of food-borne pathogens in the plant environment have yet to be elucidated. In recent years, the advent of modern and high-throughput molecular tools has given researchers an opportunity to examine these microorganisms in their natural environments (14). These techniques could be applied to obtain an understanding of the intimate interactions between food-borne pathogens and vegetable plants that have served as vehicles of transmission.
The development of microarray technologies to study global gene regulation has allowed for whole-transcriptome approaches to elucidate complex biological interactions. As a result, an increasing number of reports on the effect of environmental factors on the bacterial transcriptome have recently emerged. Microarray technologies have recently been applied to study the interaction of food-borne pathogens with food matrices, such as vegetables, meats, and dairy foods (2, 17, 36). However, genomic approaches to identify and monitor the genes involved in bacterial survival in the environment associated with vegetable production have been used in only a few studies (14, 36).
Kyle et al. (36) conducted one of the first genome-wide studies on the interaction of E. coli O157:H7 with lettuce. They focused their efforts on macerated damaged lettuce, where the wound sites on leaves allowed bacteria to thrive. Their microarray experiments compared E. coli O157:H7 grown in leaf lysates to that grown in minimal medium and revealed the upregulation of genes involved in carbohydrate metabolism, motility and attachment, and stress responses. That study provided some indication of the response of E. coli to the nutrients from a lettuce homogenate (36). Fratamico et al. (17) contributed one of the few reports that employed microarrays to study the molecular responses of E. coli O157:H7 with a food matrix. In that case, transcription was compared between ground beef extract (GBE) and liquid medium (TSB). They found that because of the induction upon GBE exposure of acid-shock-related genes, E. coli O157:H7 detected in ground beef might be primed for acid tolerance and therefore more resilient to the host stomach acidity.
To our knowledge, the present study is the first considering the overall response of E. coli to lettuce leaves over several days. The overall objective of this research was the identification of genes potentially important in the intimate and initial interactions of the E. coli K-12 and O157:H7 strains with lettuce leaves by employing microarray technology in parallel with traditional microbiological methods.
MATERIALS AND METHODS
Strains and culture conditions.
E. coli K-12 strain MG1655 and E. coli O157:H7 strain EDL933 (ATCC 43895) were used for all microarray experiments. Gene knockout mutants of E. coli strain O157:H7 and K-12 and parent strains used in attachment and colonization experiments are listed in Table 1. All mutants possessed a kanamycin resistance cassette as a marker gene. The strains were grown overnight in either Luria-Bertani broth (LB broth) or M9 minimal medium (Na2HPO4, 6.8 g/liter; KH2PO4, 3 g/liter; NaCl, 0.5 g/liter; NH4Cl, 1 g/liter; CaCl2, 3 mg/liter; MgSO4, 1 mM, pH 7.0) supplemented with 0.2% glucose and 50 μg/ml kanamycin when needed. Depending on the concentration of the inoculum needed for individual experiments, overnight cultures were either centrifuged at 1,900 × g for 10 min and the pellet washed twice with sterile deionized water (SDW) and resuspended in SDW or directly diluted in SDW.
Table 1.
Strains used in this study
| Strain | Source or reference |
|---|---|
| E. coli K-12 MG1655 (ATCC 700926) | ATCC |
| E. coli K-12 BW25113 | Keio Collectiona |
| E. coli K-12 JW1025-1 ΔcsgA:Kanr | Keio Collection |
| E. coli K-12 JW1025-1 ΔcsgB:Kanr | Keio Collection |
| E. coli K-12 JW1025-1 ΔycfR::Kanr | Keio Collection |
| E. coli K-12 JW1025-1 ΔybiM::Kanr | Keio Collection |
| E. coli O157:H7 EDL933 (ATCC 43895) | ATCC |
| E. coli O157:H7 EDL933 ΔcsgA::Kanr | Saldana et al., 2009 (51) |
| E. coli O157:H7 EDL933 ΔycfR::Kanr | Kyle et al., 2010 (36) |
Keio Collection at the Coli Genetic Stock Center at Yale University.
Lettuce leaf preparation and inoculation.
Green leaf lettuce (Lactuca sativa) was purchased from a local grocery store and kept at 4°C for a maximum of 2 days before initiation of experiments. To avoid including soiled leaves, the three outermost layers of leaves of the lettuce plants were carefully removed and only inner leaves were used subsequently; no wilted or damage leaves were included in the experiment. The leaves were surface sterilized by immersion for 3 min in a 300-ppm sodium hypochlorite solution, followed by 3 washes in sterile distilled water, as described by Niemira and Cooke (48). For microarray experiments, leaves were allowed to dry for 30 min in a biosafety cabinet and were spray inoculated with E. coli K-12 MG1655 or E. coli O157:H7 to yield a final cell concentration of approximately 1 × 107 CFU/cm2. The microorganisms were allowed to adsorb for 30 min, and the leaves were incubated in a plant growth chamber at 100% relative humidity (RH) at 25°C with a photoperiod of 16 h for 3 days. These conditions ensured maintenance of the integrity and turgidity of the leaves over the 3- or 5-day experimental period.
Microarray analysis.
Lettuce leaves (10 to 15 per sample, average surface of 25 cm2) were spray inoculated with E. coli, incubated for 1 and 3 days, added to 150 ml ice-cold plant extraction buffer (0.1% sodium pyrophosphate and 0.01% Tween 80) containing 4 ml phenol-ethanol (5:95) in an ice-cold sterile conical flask, and shaken for 15 min at 200 rpm. The washings from each set of leaves were pooled, filtered through Miracloth (Merck, Darmstadt, Germany) to remove plant debris, and centrifuged for 10 min at 4°C at 6,000 × g, and the pellet was stored at −80°C.
Total RNA was extracted from pellets using the hot phenol method (5). RNA in samples was quantified by UV spectrophotometry, and the quality of RNA was verified by agarose gel electrophoresis. The cDNA was synthesized from total RNA using the Superscript III reverse transcriptase cDNA labeling kit (Invitrogen) and indirectly labeled with Cy3 or Cy5 dyes prior to hybridization as previously described. cDNA was hybridized to OciChip O157 arrays (Ocimum Biosolutions, Gaithersburg, MD) for 18 to 24 h at 42°C and scanned as previously described. Signal intensities for each spot on scanned image files were determined with the software program GenePix Pro, version 6.0 (Molecular Devices Corp., Sunnyvale, CA). E. coli K-12 data were normalized for spot and slide abnormalities with the spatial Lowess algorithm and analyzed by mixed-effect analysis of variance (ANOVA) (MAANOVA). Lowess and MAANOVA are part of the R/MAANOVA microarray statistical package, available at http://churchill.jax.org/software/rmaanova.shtml.
Values representing the variety by gene interaction between log2-transformed values of the control and experimental spot intensities were combined with the residual noise from each spot to obtain filtered and adjusted expression values (31). Spots from each array were detected, replicates were averaged, and values from MAANOVA were subsequently subjected to significance analysis of microarray (SAM) data with the SAM software package (59). Significantly up- and downregulated genes were identified based on a 1.5- and 2.0-fold cutoff threshold, a global false discovery rate lower than 5%, and an α value of 0.05.
For the E. coli O157:H7 time course experiment, the background-subtracted spot intensities of the six replicates for each day (12 replicates for day 0) were compared using a two-tailed paired t test. Only genes with a significant change in expression levels between day 0 and day 1 and/or 3 (e.g., P < 0.01) were considered for further analysis. For the successive analyses, the median values of the replicates for each day were calculated and considered. The housekeeping gene gyrB was used as a reference for both microarray and quantitative reverse transcription-PCR (qRT-PCR) experiments. Therefore, for each day, the medians of each gene were divided by the corresponding value detected for gyrB. The fold changes were calculated by dividing the normalized values of days 1 and 2 by the corresponding values of day 0. The genes showing at least 2-fold variations for days 1 and/or 3 were included in the subsequent principal component (PCA) hierarchical clustering analyses.
PCA and hierarchical clustering were performed using the JMP 8.0 statistical software package (SAS Inc., Cary, NC). The first component of the PCA was used to sort genes within clusters in a hierarchical cluster analysis. The hierarchical clustering was performed using Ward's minimum variance method and represented through a dendrogram and a two-dimensional (2D) clustering diagram.
Quantitative reverse transcription-PCR.
Microarray expression data for a selected set of genes were confirmed by using qRT-PCR. RNA was extracted from E. coli K-12 and E. coli O157:H7 cells after 0, 1, or 3 days of incubation on lettuce leaves and converted to cDNA using Superscript III reverse transcriptase (Invitrogen) as described above. Quantitative RT-PCR was done using an Applied Biosystems (Foster City, CA) 7500 real-time PCR system using Sequence Detection System version 1.3 software and SYBR green PCR master mix. The PCR program consisted of an initial denaturation at 95°C for 10 min, followed by 45 cycles at 95°C for 15 s and annealing and extension at 60°C for 1 min. Expression values for three biological replicates for each treatment were normalized to the expression level of gyrB, a housekeeping control gene in the E. coli genome. PCR primers are listed in Table 2.
Table 2.
PCR primers used in this study
| Target | Sequence (5′–3′) |
|---|---|
| Gyrase B (gyrB) | Forward: GCAAGCCACGCAGTTTCTC |
| Reverse: GGAAGCCGACCTCTCTGATG | |
| ycfR | Forward: TAAGCTCCATGTCATTTGCC |
| Reverse: TTCCATGGAGGGTATTCGG | |
| ybiM | Forward: GCTGACCGCTGTCCAGGTTA |
| Reverse: CGTCCTGAGCGGAATCTCTCT | |
| Curlin major subunit (csgA) | Forward: GCGGTAATGGTGCAGATGTTG |
| Reverse: GAAGCCACGTTGGGTCAGA | |
| Curlin minor subunit (csgB) | Forward: CATAATTGGTCAACGTGGGACTAA |
| Reverse: GCAACAACCGCCAAAAGTTT |
Attachment and colonization assays.
Lettuce leaf squares (1 cm2) were prepared and sterilized for attachment or colonization experiments as described above. Lettuce leaf sections were spot inoculated with 20 μl of 1 × 105 CFU/ml of E. coli culture or gene knockout mutants and incubated under the same conditions as full leaves. Incubation times were 0.25, 0.5, 1, and 2 h for attachment assays and 0, 1, 3, and 5 days for colonization assays. Enumeration of bacteria on leaf surfaces was done by first rinsing leaves twice by gentle immersion for 1 min in 10 ml of SDW to remove unattached bacteria. Each leaf piece was transferred into a 10- by 10-cm polyethylene bag containing 2 ml SDW and stomached for 2 min, followed by plating on Luria-Bertani agar (LBA) or LBA supplemented with 25 μg per ml kanamycin and incubated at 37°C for 24 h.
RESULTS
Transcriptional responses of E. coli K-12 to growth on lettuce leaves.
The global transcriptional responses of E. coli K-12 while interacting with lettuce leaves were compared to those in cells in stationary phase grown in LB broth. A total of 391 out of 4,288 genes (9.1% of the total genes screened) were significantly differentially regulated on the lettuce leaf surface compared to results for stationary-phase cells (see Table S1 in the supplemental material). About 3.9% (167 of 4,288) of the genes were upregulated, whereas 224 of 4,288 (5.2%) of the genes were downregulated more than 2-fold.
E. coli genes that were induced by the interaction with the leaf surface included those involved in biofilm regulation, phosphate and sulfur starvation responses, and stress-related changes to the cell envelope (Table 3). Specifically, in the case of cell envelope integrity genes, the phage shock operon (pspABCDE), as well as osmotic stress-induced (osmBY, otsAB) and heat shock protein (hspQ) genes, was highly induced, about 23-fold, following incubation of cells on the leaf surface. Genes encoding cell envelope proteins and structures, such as curlin fibers, were also highly upregulated in response to association with lettuce leaves. The major and minor curlin genes csgA and csgB were induced 20.0- and 14.3-fold, respectively, and the regulatory csgD gene was induced 2.6-fold. While these curlin-related genes were induced, other fimbria-related genes were repressed. For example, fimI and fimA were repressed 2.3- and 13.2-fold, respectively (Table 4). Furthermore, a notable group of ribosomal genes was downregulated, and their major regulators (rpoE and rseA) were upregulated (see Table S1 in the supplemental material).
Table 3.
Selected upregulated genes from E. coli K-12 associated with lettuce leaves compared to expression in stationary-phase cells
| Category and gene no. | Fold change in expression | Gene name | Description |
|---|---|---|---|
| Biofilm | |||
| B0806 | 38.1 | ybiM (mcbA) | Protein involved in colanic acid production |
| B1112 | 33.2 | ycfR (bhsA) | Protein involved in stress resistance and biofilm formation |
| Cell envelope | |||
| B1042 | 20.0 | csgA | Curlin major subunit, coiled surface structures |
| B1041 | 14.3 | csgB | Minor curlin subunit precursor, nucleator for assembly of adhesive surface organelles |
| B1743 | 8.7 | spy | Periplasmic protein related to spheroblast formation |
| B4045 | 5.3 | yibJ | Putative stress response protein |
| B1040 | 2.6 | csgD | DNA-binding transcriptional regulator CsgD |
| Cellular processes and stress response | |||
| B1305 | 23.3 | pspB | Phage shock protein, inner membrane protein |
| B1304 | 21.5 | pspA | Phage shock protein, inner membrane protein |
| B1306 | 14.8 | pspC | Phage shock protein: activates phage shock protein expression |
| B1307 | 13.3 | pspD | Phage shock protein |
| B3022 | 6.4 | ygiU | mRNA interferase, toxin of MqsR-YgiT toxin-antitoxin system |
| B1283 | 5.6 | osmB | Lipoprotein, osmotically inducible |
| B1308 | 5.1 | pspE | Phage shock protein E |
| B1530 | 4.5 | MarER | Repressor of mar operon |
| B1563 | 4.1 | relE | Toxin of the RelE-RelB toxin-antitoxin system and cofactor to enhance RelB repressor activity |
| B1564 | 3.9 | relB | Antitoxin of the RelE-RelB toxin-antitoxin system and DNA binding repressor |
| B3021 | 3.7 | ygiT | MqsA antitoxin of the MqsRA toxin-antitoxin system and DNA transcriptional repressor |
| B3337 | 3.4 | yheA | Bacterioferritin-associated ferredoxin |
| B1874 | 3.3 | cutC | Copper homeostasis protein |
| B4376 | 3.0 | osmY | Hyperosmotically inducible periplasmic protein |
| B4224 | 2.8 | chpS | Antitoxin ChpS; suppressor of inhibitory function of ChpB |
| B1897 | 2.6 | otsB | Trehalose-6-phosphate phosphatase |
| B0966 | 2.4 | hspQ | Heat shock protein |
| B1531 | 2.4 | marA | Transcriptional activator of defense systems |
| B0226 | 2.3 | dinJ | Damage-inducible protein J |
| B1020 | 2.1 | phoH | Phosphate starvation inducible |
| B1896 | 2.0 | otsA | Trehalose-6-phosphate synthase |
| B0399 | 2.6 | phoB | Phosphorylated transcriptional dual regulator |
Table 4.
Selected downregulated genes from E. coli K-12 associated with lettuce leaves compared to stationary-phase cells
| Gene no. | Fold change in expression | Symbol | Function |
|---|---|---|---|
| Biofilm | |||
| B0836 | −6.1 | bssR | Biofilm formation regulatory protein BssR |
| Cell envelope | |||
| B1256 | −24.5 | ompW | OmpW, outer membrane protein |
| B4314 | −13.2 | fimA | Major type 1 subunit fimbrin (pilin) |
| B3506 | −11.9 | slp | Slp outer membrane lipoprotein |
| B0957 | −4.0 | ompA | Outer membrane protein 3a |
| B0087 | −2.8 | mraY | Phospho-N-acetylmuramoyl-pentapeptide transferase |
| B4315 | −2.3 | fimI | Fimbrial protein |
| B0091 | −2.1 | murC | UDP-N-acetyl-muramate:alanine ligase |
| B0092 | −2.0 | ddlB | d-Alanine-d-alanine ligase B |
| Energy metabolism | |||
| B3708 | −33.0 | tnaA | Tryptophanase |
| Cellular processes and stress response | |||
| B1492 | −14.0 | gadC | Acid sensitivity protein, putative transporter |
| B1656 | −13.5 | sodB | Superoxide dismutase, iron |
| B0880 | −6.9 | cspD | Cold shock protein |
| B3509 | −6.5 | hdeB | Acid stress chaperone |
| B3510 | −6.2 | hdeA | Periplasmic chaperone of denatured proteins |
| B2579 | −6.0 | yfiD | Stress-induced pyruvate formate-lyase subunit |
| B0607 | −5.4 | uspG | Universal stress protein G |
| B1905 | −5.0 | ftn | Cytoplasmic ferritin (an iron storage protein) |
| B3495 | −4.6 | uspA | Universal stress protein |
| B0598 | −4.5 | cstA | Carbon starvation protein |
| B3942 | −4.5 | katG | Catalase |
| B3339 | −4.2 | tufA | Protein chain elongation factor EF-Tu |
| B0990 | −3.4 | cspG | Homolog of Salmonella cold shock protein |
| B3556 | −3.4 | cspA | Cold shock protein 7.4 |
| B0095 | −3.3 | ftsZ | Cell division |
| B3511 | −2.9 | hdeD | Acid resistance membrane protein |
| B1552 | −2.9 | cspI | Qin prophage, cold shock protein |
| B0812 | −2.9 | dps | Global regulator, starvation conditions |
| B3928 | −2.7 | yiiU | Cell division factor ZapB |
| B0094 | −2.5 | ftsA | Cell division protein FtsA |
| B1557 | −2.4 | cspB | Qin prophage; cold shock protein; predicted DNA-binding transcriptional regulator |
| B0093 | −2.4 | ftsQ | Cell division protein FtsQ |
| B1324 | −2.4 | tpx | Thiol peroxidase |
| B3336 | −2.2 | bfr | Bacterioferrin, an iron storage homoprotein |
| B1333 | −2.1 | ydaA | Stress-induced protein; UV resistance; tandem Usp domains |
The largest group of genes downregulated (79 genes) consisted of those involved in energy metabolism, including tnaA, which encodes a tryptophanase that converts tryptophan into indole, with the most repressed genes having a decrease of 33-fold in expression. This group also included genes involved in nitrate reductase (narG and narJ), glycogen synthesis (glgA, glgS, and glgX), and NADH dehydrogenase (nuoACFHM). Fifteen transport genes were also downregulated more than 2-fold, and three genes responsible for galactitol transport (gatABC) were repressed 13.7-, 10.3-, and 13.1-fold, respectively (see Table S1 in the supplemental material). Environmental stress-related genes, such as the acid stress operon, hdeABD, and numerous cold shock genes, cspABDGI, were similarly repressed in response to incubation with lettuce leaves. In addition, genes involved in cell division, minD, yiiU, ftsA, ftsQ, and ftsZ, were repressed in E. coli attached to leaves, relative to expression seen in stationary-phase controls.
Transcriptional response of E. coli O157:H7 associated with lettuce leaves.
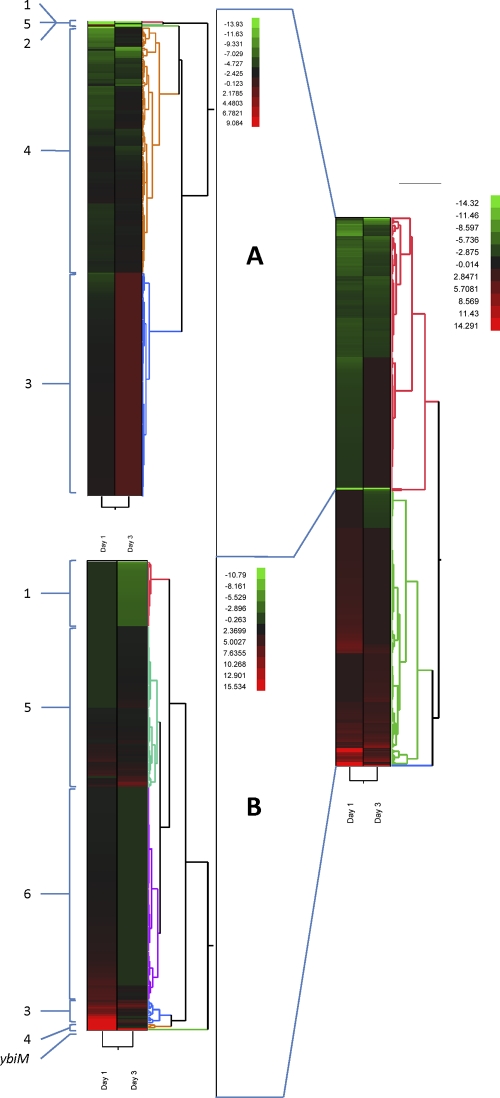
In contrast, statistical analysis revealed that 1,232 E. coli O157:H7 genes (23.1% of the 5,338 screened) displayed significant changes in expression ratios (P < 0.001) between days 1 and 3 compared to expression in cells at inoculation time (time zero). Of these genes, 521 (9.8%) were found to have at least a 2-fold expression change between day 0 and day 1 and/or day 3 (Fig. 1). On day 1, 438 genes (8.2%) were differentially expressed compared to results on day 0, whereas 284 genes (5.3%) were variably expressed between day 0 and day 3. Hierarchical clustering identified 2 major branches (Fig. 1A and B). These two main branches were further analyzed separately. Branch A (Fig. 1A) was comprised of 5 clusters (e.g., clusters 1A to 5A, encompassing 259 genes (4.8%) that had lower expression levels after leaf attachment. Similarly, branch B (Fig. 1B) was comprised of 6 clusters (1B to 6B) representing 262 genes (4.9%). Most genes had markedly higher expression levels following leaf attachment for 1 and 3 days, with the exception of cluster 1B genes. The three genes with the largest difference in expression levels were cspG and cspA, with 52.6- and 34.4-fold decreases, respectively, and ybiM, with a 68.3-fold increase (Table 5). These genes were the sole members of three different clusters (5A, 1A, and 2B, respectively).
Fig 1.
Thermal graph of the hierarchical clustering of significant genes expressed in E. coli O157:H7 associated with lettuce leaves on days 0, 1, and 3. The two main clusters (A and B) were analyzed separately, and the major subclusters are identified by numbers. The colors of the graph change from green (low expression) to red (high expression). The gradient bars show the relation between the color shades and the intensity of gene expression.
Table 5.
Change in levels of gene expression of selected genes from E. coli O157:H7 associated with lettuce leaves, compared over 3 days
| Category and gene ID | Symbol | Description | Fold change in expression |
|
|---|---|---|---|---|
| Day 1 | Day 3 | |||
| Biofilm | ||||
| Z1027 | ybiM | Hypothetical protein | 31.30 | 68.30 |
| Z1697 | yceP (bssS) | Hypothetical protein | 7.90 | 7.90 |
| Cell envelope | ||||
| Z2775 | spy | Periplasmic protein of spheroblast formation | 3.10 | 3.20 |
| Z1037 | ybiP | Putative enzyme | −4.56 | 1.00 |
| Z3189 | wzzB | Regulator of length of O-antigen component of lipopolysaccharide chains | −4.48 | 1.00 |
| Z1676 | csgA | Curlin major subunit, coiled surface structures | 34.60 | 1.00 |
| Z1675 | csgB | Minor curlin subunit precursor, nucleator for assembly of adhesive surface organelles | 13.90 | 1.00 |
| Cellular processes: adaptations to atypical conditions | ||||
| Z0769 | cspE | Cold shock protein | −6.33 | −3.09 |
| Z4981 | cspA | Cold shock protein 7.4, transcriptional activator of hns | −34.39 | −4.62 |
| Z1117 | cspD | Cold shock protein | 2.50 | 1.00 |
| Z1406 | cspG | Homolog of Salmonella cold shock protein | −52.61 | −23.75 |
| Z2330 | hslJ | Heat shock protein hslJ | −2.44 | −3.57 |
| Z5182 | ibpB | Heat shock protein | −8.71 | −2.01 |
| Z3862 | yfiD | Putative formate acetyltransferase | −5.39 | −9.73 |
| Z2216 | xasA | Acid sensitivity protein, putative transporter | 2.20 | 2.20 |
| Z4661 | mscL | Mechano-sensitive channel | 3.30 | 2.70 |
| Z2950 | otsB | Trehalose-6-phosphate phosphatase | 3.00 | 5.10 |
| Z4922 | hdeA | Hypothetical protein | 23.40 | 3.20 |
| Z4921 | hdeB | Hypothetical protein | 3.50 | 1.00 |
| Z4923 | hdeD | Hypothetical protein | 5.70 | 1.00 |
| Z5479 | hslV | Heat shock protein hslVU | −2.47 | 1.00 |
| Z2435 | ydaA | Hypothetical protein | 2.40 | 1.00 |
| Z4895 | uspA | Universal stress protein | 2.60 | 1.00 |
| Z4930 | gadA | Glutamate decarboxylase isozyme | 18.60 | 6.40 |
| Z2216 | gadC | Acid sensitivity protein, putative transporter | 27.20 | 2.20 |
| Z4925 | gadE | GadE transcriptional activator | 9.90 | 5.80 |
| Z4928 | gadW | Putative ARAC-type regulatory protein | 2.40 | 1.00 |
| Cellular processes: detoxification | ||||
| Z2661 | sodC | Superoxide dismutase precursor (Cu-Zn) | 2.60 | 1.00 |
| Z2949 | otsA | Trehalose-6-phosphate synthase | 2.40 | 2.20 |
| Energy metabolism | ||||
| Z2246 | narW | Cryptic nitrate reductase 2, delta subunit, assembly function | 2.20 | 1.00 |
| Z2245 | narY | Cryptic nitrate reductase 2 beta subunit | 2.80 | 1.00 |
| Z2244 | narZ | Cryptic nitrate reductase 2 alpha subunit | 2.30 | 1.00 |
| Energy metabolism: electron transport | ||||
| Z3867 | trxC | Putative thioredoxin-like protein | 2.00 | 1.00 |
| Z1701 | grxB | Glutaredoxin 2 | 2.10 | 1.00 |
| Z3975 | nrdH | Glutaredoxin-like protein | 2.70 | 1.00 |
| Energy metabolism: nitrogen metabolism | ||||
| Z1511 | rutA | Hypothetical protein | 3.00 | 1.00 |
| Z1510 | rutB | Predicted isochorismatase | 3.00 | 1.00 |
| Z1509 | rutC | Predicted endoribonuclease | 4.70 | 1.00 |
| Energy metabolism: pentose phosphate pathway | ||||
| Z3720 | talA | Transaldolase A | 3.80 | 1.00 |
| Z3721 | tktB | Transketolase 2 isozyme | 3.30 | 1.00 |
| Z3191 | gnd | Gluconate-6-phosphate dehydrogenase, decarboxylating | 2.00 | 1.00 |
| Energy metabolism: TCA cycle | ||||
| Z0877 | sdhA | Succinate dehydrogenase, flavoprotein subunit | −4.54 | −2.38 |
| Z0876 | sdhD | Succinate dehydrogenase hydrophobic subunit | −2.54 | 1.00 |
| Z0875 | sdhC | Succinate dehydrogenase | −6.05 | −6.26 |
| Protein synthesis: ribosomal proteins, synthesis and modification | ||||
| Z4531 | rimP | Ribosome maturation protein | −3.30 | 1.00 |
| Z5557 | rplA | 50S ribosomal subunit protein L1, regulates synthesis of L1 and L11 | −3.09 | −2.51 |
| Z4688 | rplB | 50S ribosomal subunit protein L24 | 1.00 | −2.20 |
| Z4690 | rplD | 50S ribosomal subunit protein L11 | 1.00 | −2.70 |
| Z4678 | rplE | 50S ribosomal subunit protein L5 | −4.26 | −5.10 |
| Z4675 | rplF | 50S ribosomal subunit protein L6 | −2.88 | −2.40 |
| Z5556 | rplK | 50S ribosomal subunit protein L11 | −3.28 | −2.69 |
| Z4589 | rplM | 50S ribosomal subunit protein L13 | −3.16 | −2.48 |
| Z4680 | rplN | 50S ribosomal subunit protein L14 | −3.46 | −4.85 |
| Z4674 | rplR | 50S ribosomal subunit protein L4, regulates expression of S10 operon | 1.00 | −2.82 |
| Z4689 | rplW | 50S ribosomal subunit protein L6 | 1.00 | −2.44 |
| Z4679 | rplX | 50S ribosomal subunit protein L24 | −3.90 | −5.41 |
| Z3444 | rplY | 50S ribosomal subunit protein L25 | −5.61 | −3.91 |
| Z5061 | rpmB | 50S ribosomal subunit protein L28 | −3.49 | −2.96 |
| Z5060 | rpmG | 50S ribosomal subunit protein L33 | −3.95 | −2.40 |
| Z5194 | rpmH | 50S ribosomal subunit protein L34 | −2.65 | 1.00 |
| Z0180 | rpsB | 30S ribosomal subunit protein S2 | −2.53 | −3.02 |
| Z4666 | rpsD | 30S ribosomal subunit protein S20 | 1.00 | −3.53 |
| Z4673 | rpsE | 50S ribosomal subunit protein L2 | 1.00 | −2.27 |
| Z5809 | rpsF | 30S ribosomal subunit protein S6 | −3.18 | −2.30 |
| Z4676 | rpsH | 30S ribosomal subunit protein S8 | −4.59 | −4.19 |
| Z4692 | rpsJ | 30S ribosomal subunit protein S10 | −2.33 | −3.00 |
| Z4667 | rpsK | 30S ribosomal subunit protein S11 | −2.36 | −4.16 |
| Z4700 | rpsL | 30S ribosomal subunit protein S12 | −2.26 | −2.13 |
| Z4668 | rpsM | 30S ribosomal subunit protein S13 | −2.37 | −2.15 |
| Z4677 | rpsN | 30S ribosomal subunit protein S14 | −4.56 | −3.76 |
| Z4526 | rpsO | 30S ribosomal subunit protein S15 | −2.25 | 1.00 |
| Z3903 | rpsP | 30S ribosomal subunit protein S16 | −3.24 | −2.46 |
| Z5811 | rpsR | 30S ribosomal subunit protein S18 | −2.29 | 1.00 |
| Z0027 | rpsT | 30S ribosomal subunit protein S20 | −4.93 | −3.51 |
| Z4418 | rpsU | 30S ribosomal subunit protein S21 | −4.05 | 1.00 |
| Z2230 | rpsV | 30S ribosomal subunit protein S22 | 4.00 | 1.00 |
| Z1864 | rluE | Hypothetical protein | −2.03 | 1.00 |
| Regulatory functions: other | ||||
| Z3853 | rseB | Anti-sigma E (sigma 24) factor | −2.29 | 1.00 |
| Z6004 | arcA | Transcriptional dual regulator | 4.80 | 1.00 |
| Z0539 | bolA | Putative regulator of murein genes | 4.20 | 1.00 |
| Z0831 | fur | Negative regulator | −2.12 | −2.02 |
| Z4718 | crp | Cyclic AMP receptor protein | −2.31 | −2.30 |
| Z2741 | himA | Integration host factor (IHF), alpha subunit | 7.80 | 2.80 |
| Z3855 | rpoE | RNA polymerase, sigma E factor | 2.10 | 1.00 |
| Z4565 | rpoN | RNA polymerase sigma (54 or 60) factor RpoN | 2.40 | 1.00 |
| Z4049 | rpoS | RNA polymerase, sigma S (sigma 38) factor | 2.10 | 1.00 |
| Z1034 | dps | Global regulator, starvation conditions | 7.80 | 3.30 |
| Transcription | ||||
| Z1728 | rpmF | RNA polymerase, alpha subunit | 1.00 | −2.61 |
| Z3848 | rnc | RNase III | −2.83 | −3.62 |
| Z5195 | rnpA | RNase P, protein component | −3.66 | −2.17 |
| Z5715 | basR | Response regulator in two-component regulatory system with BasS (OmpR family) | −5.59 | −2.95 |
| Z5714 | basS | Sensor protein for basR | −3.15 | −2.07 |
| Transport and binding proteins | ||||
| Z2243 | narU | Nitrite extrusion protein 2 | 2.50 | 1.00 |
| Z2299 | ydcl | Putative transcriptional regulator, LYSR type | −4.80 | −2.43 |
| Z2287 | ydcL | Hypothetical protein | 3.90 | 1.00 |
The majority of the genes in branch A (131 or 2.5%) were grouped in cluster 4, thus being repressed on day 1 and on day 3, from −13.7- to −2.0-fold and from −10.1- to −9.9-fold, respectively. This group included 20 genes that encode ribosomal proteins (rplAEFKMNXY, rpmBG, and rpsBFHJKLMNPT,), genes encoding heat and cold shock proteins (cspE, hslJ, ibpB, and yfiD) and amino acid and sugar transport systems, and bfd, encoding bactoferritin-associated ferredoxin, a protein involved in iron homeostasis. Among the major transcriptional regulators included in this group, fur, a ferric uptake regulator, and crp, a cyclic-AMP receptor, were downregulated at the same levels on days 1 and 3. The expression of the two-component regulatory system basSR, which is involved in the resistance to high iron concentrations under acidic conditions, was also reduced on both days. Cluster 3 was comprised of 124 genes that as a group were repressed on day 1 only, ranging from −5.5- to −1.9-fold, but returned to expression levels similar to those on day 0 by day 3. Genes encoding ribosomal proteins (rpsEH and rpsORU) were detected as part of this cluster, as well as the gene encoding the ribosomal maturation protein RimP. Thirteen genes in this cluster were involved in cellular envelope synthesis, with the two most repressed being wzzB, the regulator of O-antigen chain-length distribution, and ybiP, a putative phosphoethanolamine transferase.
Cluster 1 in branch B was comprised of 36 genes that had no expression change on day 1, but their relative RNA abundance decreased by day 3, ranging from −4.9- to −2.0-fold relative to that on day 0. Multiple gene subsets involved in amino acid (aspartate, arginine, and methionine) pathways, transport, or binding were placed in this cluster, which also contained a group encoding ribosomal proteins (rpsADE and rplRDWB). The RNA levels of the regulatory gene hns were unchanged on day 1 and were 3.4-fold lower on day 3 compared to that on day 0.
Cluster 5 included 90 genes (1.7%) that had a minor increase in RNA levels on day 1, ranging from 4.6-fold to unchanged or nonsignificant (P < 0.001), and higher expression levels on day 3, ranging from 7.9- to 2.0-fold. Among these genes, ygiB, ycfR (bhsA), and yceP are involved in the regulation of biofilm formation, and xasA, mscL (yhdC), and otsAB are involved in the cellular processes necessary to adapt to new environmental conditions, including desiccation. Many of the genes detected in this cluster did not have an assigned function.
In contrast, cluster 6 contained 119 genes (2.2%), the largest number for this branch. These genes had transcription levels from 2-fold up to 5.9-fold on day 1 that decreased to levels below 2-fold or statistical significance on day 3. The largest functional group was represented by genes involved in energy metabolism, electron transport (trxC, grxB, and nrdH), nitrogen metabolism (rutABC and ydcIH), and the pentose phosphate pathway (gnd, tktB, and talA). Several genes related to adaptation to stress conditions, such as osmotic and acid shock, were also upregulated (ydaA, cspD, uspA, hdeBD, ahpC, and sodC). Several main global regulators (rpoSEN, ArcA, and bolA) were also upregulated on day 1 and returned to basal levels by day 3.
Cluster 3 consisted of a relatively small group of genes that had a transient elevated expression level for day 1 and similar or lower levels by day 3. Three of these genes are involved in adaptation to environmental conditions (gadE, ostB, and psiF). The global regulator for starvation conditions, dps, also grouped in this cluster together with himA and yceP (bssS).
Cluster 4 was comprised of 3 genes with very high expression levels on day 1 and a marked decrease by day 3. Two of these are acid shock-related genes (hdeA and gadC), and one is csgA, a gene coding for the curlin major subunit. Lastly, cluster 2 is represented by only one gene, ybiM, which had an elevated transcriptional level on day 1, increasing further on day 3.
Comparative expression of selected genes of E. coli K-12 and O157:H7 in response to the lettuce leaf environment.
Of the 3,798 genes shared by the E. coli K-12 and O157:H7 genomes and represented on the microarrays we used, 385 (10.1%) were differentially expressed in E. coli K-12, whereas 332 genes (8.7%) changed expression levels in E. coli O157:H7. Of these genes, 119 (3.1%) had differential expression in both the K-12 and O157 strains.
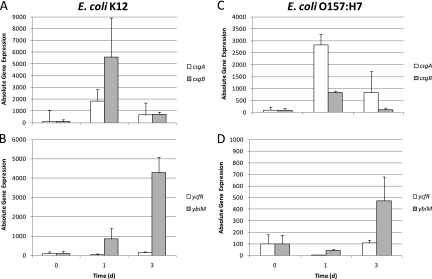
Quantitative RT-PCR was performed on RNA obtained from cells recovered from leaves on days 0, 1, and 3 of incubation. Results of these studies (Fig. 2) confirmed the increased transcription of ybiM, csgA, and csgB for both E. coli K-12 and O157:H7, but the expression of ycfR decreased on day 1 and increased on day 3 to levels either higher than (K-12) or similar to (O157) those on day 0. All expression levels were normalized against the levels detected on day 0, thus assumed to be 100% of the normal expression levels for these genes. Using this method, expression of ycfR in E. coli K-12 decreased 53% on day 1 and increased 280% on day 3. Expression of the same gene in O157:H7 followed a similar trend, decreasing 96% on day 1 and increasing on day 3 to the same levels as those on day 0 (Fig. 2C and D). The patterns of expression of ybiM were similar in both strains, with the greatest increase by day 3. In E. coli K-12, however, gene transcription increased 8-fold from day 0 to day 1 and reached a maximum on day 3 that was 40-fold higher than that on day 0. In O157, the transcriptional levels of this gene decreased on day 1 and increased up to 4-fold by day 3 compared to that on day 0.
Fig 2.
Levels of gene expression of selected genes csgA and csgB in E. coli K-12 (A), csgA and csgB in E. coli O157:H7 (C), ycfR and ybiM in E. coli K-12 (B), or ycfR and ybiM in E. coli O157:H7 (D) after 3 days of incubation on lettuce leaves. Absolute gene expression was normalized to the constitutively expressed gyrB gene. Error bars represent the standard deviations of the means of data from independent triplicate experiments.
The behaviors of the csgA and csgB genes (Fig. 2A and B, respectively) were similar in E. coli K-12 and O157:H7, with a sharp increase in expression on day 1 followed by a decline on day 3. In O157:H7, csgA changed from a 28-fold increase on day 1 to being 8-fold higher at day 3, whereas csgB increased 8-fold by day 1 and decreased to levels similar to those on day 0 by day 3. In K-12, these trends were inverted, with csgB reaching higher expression levels on day 1 (55-fold) and decreased to levels that were 7-fold greater on day 3 than on day 0. Similarly, csgA increased on day 1 to high levels and decreased by day 3 to levels similar to those on day 0.
Attachment of E. coli to lettuce leaves.
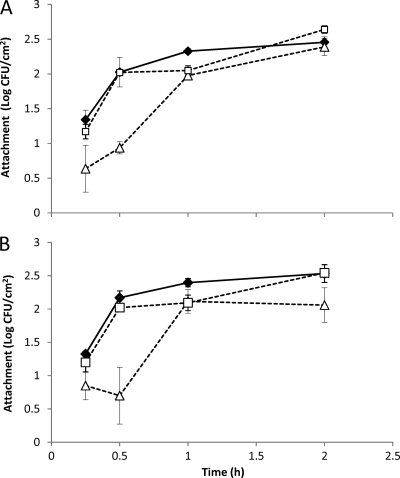
Previous studies suggested that several of the upregulated genes may be involved in bacterial attachment to surfaces. To test whether these genes are involved in adhesion to lettuce leaves, attachment assays were performed using wild-type E. coli K-12 and O157:H7 cells and single gene knockout mutants of the csgA, csgB, ycfR, and ybiM genes of E. coli K-12 and the csgA and ycfR mutants of E. coli O157:H7.
Results shown in Fig. 3A and B showed that the parent or wild-type strains behaved similarly in their levels of leaf attachment over time. With an initial inoculum of 3 log CFU/cm2 of either wild-type E. coli K-12 or O157:H7, more than 1 log CFU/cm2 attached within 15 min (Fig. 3A and B). A further 15 min on the leaf allowed attachment of approximately 2 log CFU/cm2, and further incubation 1 or 2 h post attachment increased an additional 0.5 log CFU/cm2. In contrast, attachment of the csgA mutant of E. coli K-12 was 1 log CFU/cm2 less than that of the wild-type strain. Attachment of the mutant to the leaf remained lower than that of controls for the first 30 min, and after 1 h the mutant lacking csgA was similar to the wild type.
Fig 3.
Attachment of E. coli strains to intact lettuce leaves 2 h after inoculation. (A) Extent of attachment (as bacterial counts) of the ΔcsgA (△) and ΔycfR (bhsA) (□) single knockout mutants and the parental strain E. coli K-12 (♦) on lettuce leaves. (B) Extent of attachment of the ΔcsgA (△) and ΔycfR (bhsA) (□) single knockout mutants and the parental strain E. coli O157:H7 (♦) on lettuce leaves. Error bars represent the standard deviations of the means of data from independent triplicate experiments.
The mutant with the insertion in the ycfR gene, which is thought be involved in biofilm formation, behaved in a manner very similar to that of the wild type, and the mutant lacking ybiM was slower to attach than the wild-type strain, but after 2 h approximately 2 log CFU/cm2 had attached to the leaf. Attachment of the csgA and ycfR mutants of E. coli O157:H7 followed the same trends as did their nonpathogenic counterparts.
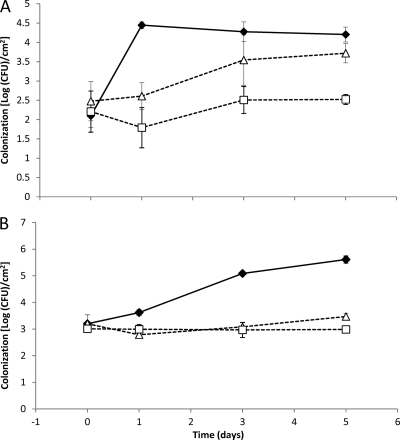
Colonization of lettuce leaves by E. coli K-12 and E. coli O157:H7.
Survival and growth of the same mutants used in the attachment assays were monitored on lettuce leaf pieces during a 5-day period. Wild-type E. coli K-12 cells inoculated at approximately 3 log CFU/cm2 were attached to leaves at a level of about 2 log CFU/cm2 on day 0. By day 1, the numbers of E. coli K-12 increased to more than 4 log CFU/cm2 (Fig. 4A) and remained at this level over the 5-day incubation period. In contrast, wild-type E. coli O157:H7 reached more than 5 CFU/cm2 by day 3 and almost 6 log CFU/cm2 following 5 days of incubation (Fig. 4B). On the other hand, counts of both the csgA and ycfR mutants never attained more than 3.5 log CFU/cm2.
Fig 4.
Colonization of intact lettuce leaves by E. coli strains during 5 days. Counts of the ΔcsgA (△) and ΔycfR (bhsA) (□) single knockout mutants and the parental strain E. coli K-12 (♦) (A) or counts of the ΔcsgA (△) and ΔycfR (bhsA) (□) single knockout mutants and the parental strain E. coli O157:H7 (♦) (B) are shown. Error bars represent the standard deviations of the means of data from independent triplicate experiments.
DISCUSSION
To our knowledge, this is the first report of interaction of E. coli K-12 or O157:H7 with intact surfaces of lettuce leaves that required the activation of biofilm and stress response genes. The transcriptional changes observed following interaction of both partners resulted in the activation or repression of 119 genes in K-12 and O157. However, almost 500 genes belonging to a common genetic pool in these strains had strain-specific expression patterns, which indicated that some of the phenotypic differences observed likely depended on transcriptional rather than structural genomic differences. The interaction of human pathogens with leaves and, more generally, with the phyllosphere has been studied before (16, 23, 25, 35, 36, 48). However, the majority of these studies have focused on the persistence of the pathogens on the leaf surface or on a limited number of virulence genes potentially involved in the adhesion to the plant leaves (51, 66).
Recently, Kyle et al. performed a study focused on the global transcriptional changes in E. coli O157:H7 exposed to leaf lysates (36). Although these data provided valuable information for understanding the physiological changes when this pathogen is exposed to sliced and processed lettuce, their study did not address the molecular mechanisms occurring when E. coli colonizes the surface of undamaged lettuce leaves. Another confounding factor, as stated by the authors of the research, was that the hydrogen peroxide generation from the wounded plant caused a strong oxidative stress response from the pathogen and much of the differential expression observed was caused by such a response more than by the interaction with the leaf. Furthermore, the focus of Kyle et al. was centered mainly on the short-term response (e.g., 15 to 30 min) of the pathogen to exposure to leaf extracts but did not address the mechanisms of the longer-term survival of E. coli O157:H7 on lettuce. As a result, the differences between our study and this previous one were not surprising.
The adaptive response of E. coli K-12 to the leaf surface environment induced direct or indirect changes in the expression of 391 genes, with a majority (75%) decreasing their expression levels (224 versus 167). Although the shift in environmental conditions from a stationary-phase culture grown in a rich medium at 37°C to the leaf surface, scarce in nutrients and colder (e.g., 25°C), likely played a role in many of the transcriptome changes observed, some factors indicate that their impact on the transcriptional levels was not as dramatic as expected.
It has been shown that the genes coding for sigma factors σS and σE, rpoS and rpoE, and their regulons are crucial and are upregulated during stationary phase both in minimal and rich media (13, 28, 29). Under our experimental conditions, we expected that cells surviving in the nutrient-deprived environment represented by the leaf surface would have a slower metabolism than cells in stationary phase in rich medium. Indeed, we observed a decrease in the genes belonging to the rpoE regulon (28), as well as some of the rpoE-regulated genes that have been reported to be induced in stationary phase (e.g., ftsA, fusA, and tktA) (28). The transcripts of tnaA, a tryptophanase that produces indole, and ompW, an outer membrane protein involved in oxidative stress resistance and positively regulated by soxS, had the largest decrease in transcript levels (Table 4). However, we did not observe a corresponding decrease in the level of the rpoE transcript. On the contrary, the transcriptional levels slightly increased, mirrored by an increase in the anti-σE factor, rseA, controlled by rpoS. Nevertheless, the majority of the genes belonging to the rpoE regulon, including rpoH, a gene encoding the heat shock-induced σ32 factor positively regulated by rpoE (60), had no significant change in their expression. Although many of the genes regulated by rpoS were differentially expressed, we did not detect any variation in its transcript. The fact that other genes belonging to this regulon (e.g., hycAH, hypAE, hdeAB, gadABC, eno, aceB, and pfkA) are repressed instead of remaining the same or increasing indicates the action of a regulatory network different from the one governed by rpoS. Considering that the changes in the expression patterns of the stationary-phase-related genes were mostly detected as a small decrease in their transcript abundance, we can assume that the large and positive variations in expression levels observed were most likely caused by adaptations induced specifically in response to the leaf surface environment.
Not surprisingly, highly upregulated genes (those induced more than 4-fold) detected in E. coli K-12 were mainly nutrient starvation-related genes. In particular, the phosphate starvation regulators psiF, phoB, and some genes belonging to its regulon (phoH, phnBC, and pstS) had a marked increase in expression. This result, and the small decrease in tricarboxylic acid (TCA) cycle genes, such as mdh and the sdh operon, indicated that the bacteria on the leaf surface were probably phosphate deprived (43). Cells exposed to the leaf surface also appeared to be starved for sulfur. In fact, the tau and cys operons, responsible for the uptake and utilization of organic and inorganic sulfates, respectively, from the environment, were highly upregulated. We did not observe an increase of CysB transcript, the main regulator of cys and tau, but the inactive protein could have already been present and activated by the redox status of the cell (34). Also, grxA and trxC, encoding glutharedoxin 1 and thioredoxin, respectively, involved in the reduction and cycling of the 3′-phosphoadenylylsulfate reductase encoded by cysH, were upregulated (40), whereas grxB, encoding glutharedoxin 2 and not involved in this process, decreased dramatically (11.7-fold) (39, 40). According to the model proposed by Gyaneshwar et al., sulfur deprivation rather than iron is most likely the cause of the induction of isc, responsible for Fe-S cluster formation and stability, just as we observed (22).
Adaptation of E. coli K-12 to the leaf surface also caused modifications to the cell membrane, possibly as a consequence of damage or desiccation. In fact, psp genes were upregulated. The genes belonging to this operon are responsible for repairing damage to the inner membrane of the cell (33) and maintenance of the proton motive force across the inner membrane (10, 47). The cause of the membrane damage is most likely osmotic or desiccation stress, since the genes otsAB, osmBY, and yciEFG, thought to be involved in the osmotic and desiccation stress responses, were upregulated (27, 37, 56, 61).
While the induction of biofilm genes was observed, we also detected a marked increase in the transcripts of genes shown to repress biofilm formation, by increasing the intracellular indole concentration (e.g., ybiM, ycfR, mtr, hha, and bssR) (65). Indole is produced intracellularly by the breakdown of tryptophan from the tryptophanase encoded by tnaA into indole, pyruvic acid, and ammonia (53). The strong repression of tnaA indicated that if indeed there was a high intracellular indole concentration, it was not generated endogenously but was internalized from the environment, since the compound might be present on the leaf surface in the form of indole-glucosinolates or in its acetylated form, indole 3-acetic acid (63). Interestingly, results shown in Table 3 show that ybiM, ycfR, and yjfY, three genes belonging to the YhcN family (50), were highly upregulated (up to 48-fold).
To better understand the involvement of tnaA, ybiM, ycfR, and yjfY in the survival of E. coli in the leaf environment, we studied the survival of gene-specific knockout mutants for each of these genes. Although all the mutants were somewhat impaired in their initial ability to colonize the leaf surface, ycfR was the only mutant that did not reach colonization levels similar to those of the wild-type strain by the fifth day of incubation (data not shown). These results indicate that although all these genes play some role in the initial colonization of the leaf surface, ycfR is likely important for the subsequent establishment of E. coli K-12.
The role of ycfR in biofilm formation is still unclear, but Zhang et al. suggested that this gene alters the characteristics of the bacterial cell surface, affecting cell aggregation and consequent biofilm formation. In their study, the same group found that a ycfR mutant is not able to form curli (69). Indeed, our microarray experiments detected changes in the expression levels of genes involved in cell envelope stress. Furthermore, the transcriptional levels of csgAB significantly increased, and the csgA mutant was not able to colonize leaves as efficiently as the wild-type strain (Fig. 3A and B). It is important to note that our microarray analyses detected an increase in the levels of csgAB and csgD but not of csgEFG, encoding the assembly and secretion system for the CsgB and CsgA curli subunits (41). This observation suggests that curli were most likely already being formed in the stationary-phase cultures and the interaction with the new environment required the synthesis of new subunits but not of the assembly and secretion machinery. Interestingly, many of the stress-related genes that usually accompany the expression of the csg operons during the formation of biofilm were only minimally activated or repressed, possibly because they were already stimulated by the stationary phase. However, the further induction of csgAB and csgD is a process that is triggered by the leaf colonization process, as underlined by the csgA mutant colonization experiments (Fig. 3A and 4A).
To garner information on the temporal sequence of the interaction of E. coli O157:H7 with intact lettuce leaves, we performed microarrays analyses on a time course experiment over a 3-day period. Hierarchical clustering analysis showed that after 1 day of exposure to the intact leaves, there was a decrease in several rRNA coding genes; some of these returned to preexposure levels at day 3, including rimP, a gene involved in ribosome maturation (49). However, the majority of these genes maintained lower levels for the duration of the experiment. Following this same negative trend, we also observed repression of several genes involved in iron homeostasis. In particular, bfd, fur, and basSR, which are generally induced in response to iron-deprived environments to increase iron uptake, were downregulated, suggesting that the cells might be sensing an iron-rich environment. However, in light of the fact that some of the Fe-S cluster metabolic enzymes also decreased (e.g., encoded by sdh and fdh), it is more likely that the decrease in nutrients slowed down metabolism and lessened the need for the iron as a cofactor.
Most likely, adaptation to new environmental conditions was the cause of the transient increase by day 1 of several genes involved in energy homeostasis, in particular the pentose phosphate shunt (gnd, tktB, and talA), nitrogen metabolism (rutABC and ydcIH), electron transport (trxC, grxB, and nrdH) and acid tolerance (ydaA, cspD, uspA, hdeBD, ahpC, and sodC). In fact, all of these changes could be related to the conversion of ribonucleotides freed by the degradation of rRNA into deoxynucleoside triphosphates (dNTPs). This conversion requires the reduction of ribonucleotides by ribonucleotide reductases (RNRs) (57). In E. coli, NrdEF, a class 1b RNR, is reduced in vivo by a dedicated glutaredoxin-like protein, NrdH. No changes in the transcripts levels of nrdEF were detected in our studies, but these proteins are generally also regulated at the posttranscriptional level, and therefore, constant transcripts do not provide much information on the active enzyme fluctuation. NrdH shares a considerable degree of similarity with glutaredoxins and glutaredoxin-like proteins (26, 55). Despite its similarities to glutaredoxins, this protein is itself reduced by thioredoxin reductase (trxC) in vivo. However, in the absence of thioredoxin reductase and NrdH, glutaredoxin 1 (grxA) can partially replace NrdH (20). This increased need of reducing potential for glutaredoxins and thioredoxins means an increased requirement for NADPH (4, 6, 21, 52) that could be provided by the shunt to the pentose phosphate pathway. Also, the increase in the expression levels of rutABC indicated that nucleotides are being used during this adaptation time as an essential source of nitrogen (32).
Comparisons between results for K-12 and for day 3 O157 showed that the two strains shared a relatively small common pool of differentially expressed genes. Among these genes, some indicated that physiological reactions to the leaf environment are the same for the two strains. Indeed, the translation-related ribosomal genes were repressed, whereas rpoE was slightly induced at day 1. However, we did not detect a corresponding increase in expression of rseA, the anti-σE factor, but we detected a decrease in that of rseB. This gene is a negative modulator of the σE pathway and has been shown to act by binding to the C terminus of RseA, preventing its N terminus cleavage by RseP, a membrane metalloprotease, which allows the release of the RseA/σE complex into the cytosol (1, 7, 30, 46). Likewise, we did not observe changes in the other genes involved in σE (e.g., clpXP, degS, and rseA). This indicates that similar to the case with K-12, RpoE action in O157 is modulated at the protein level, but possibly in a different fashion.
As with K-12, the E. coli O157 cells exposed to the leaf surface showed an increase in some of the genes involved in sulfur and phosphate starvation. However, the response in E. coli O157:H7 was not as clear as those seen in K-12. In fact, the only gene that was strongly activated in the sulfur response was tauD, whereas the other genes were only slightly upregulated or downregulated. Confirming the lack of a sulfur starvation response, we also observed a decrease in the Fe-S cluster-related gene transcripts (e.g., iscARSU). We also observed a slight increase for some of the genes involved in the phosphate starvation response, with psiF being the most induced. The fact that O157 was able to reach higher numbers of CFU/cm2 in our colonization experiment than K-12 suggested that O157 is better able to cope and adapt to the leaf conditions.
Interestingly, csgAB were also highly expressed after the first day of interaction but decreased rapidly to control levels. This observation was confirmed in our attachment and colonization experiments on csgA mutants of K-12 and O157. In fact, our experiment suggested that csgA mutants were slightly impaired in their initial attachment to the leaf surface (within the first 30 min), and although they quickly reached adhesion levels similar to those of the wild type, they were unable to become established on the leaf surface by day 1. More importantly, in the case of O157, colonization never occurred (Fig. 3 and 4).
Curli formation is considered crucial for the initial establishment of biofilms and the adhesion and invasion of E. coli into host cells (3). Our experiments underline their important role in the first stage of lettuce leaf colonization by O157 and K-12. However, as with K-12, it seems that the higher expression of csg genes does not correspond to a switch to biofilm structures. In fact, many genes known to be involved in the steps leading to the establishment of a biofilm are not induced at day 1 and/or day 3 (e.g., fli, flg, and wca) (12). It is possible that the transcriptional changes necessary for the formation of the biofilm have already occurred by the time we collected the cells on day 1 for O157 or day 3 for K-12. However, our expression data indicated a stark increase in expression of several genes that are generally repressed in mature biofilms and that are related to the homeostasis of the intracellular indole levels (e.g., yceP, ybiM, and ycfR).
Based on the microarray analyses performed on E. coli K-12 and O157:H7, the impaired ability of K-12 and O157 csgA mutants to attach to the leaf surface, and the inability of csgA and ycfR mutants to colonize the leaf surface (Fig. 4A and B), we propose that when interacting with lettuce leaves, E. coli K-12 and O157:H7 first attach to the leaf surface using curli fibers, shift down their metabolism to adapt to the nutrient-deprived environment of the leaf, and suppress the formation of a permanent biofilm by increasing their intracellular indole concentration. The fate of these bacteria after this first interaction with the intact leaves is still uncertain; they might be internalized as endophytes, thus finding protection against sanitization and subsequent processing. This would explain their presence in processed ready-to-eat produce. However, further studies would be needed to prove or disprove such an occurrence.
Supplementary Material
ACKNOWLEDGMENT
These studies were funded by a grant from the Healthy Foods, Healthy Life Institute of the University of Minnesota.
Footnotes
Published ahead of print 13 January 2012
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1. Alba BM, Leeds JA, Onufryk C, Lu CZ, Gross CA. 2002. DegS and YaeL participate sequentially in the cleavage of RseA to activate the sigma(E)-dependent extracytoplasmic stress response. Gene Dev. 16:2156–2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bae D, Crowley MR, Wang CL. 2011. Transcriptome analysis of Listeria monocytogenes grown on a ready-to-eat meat matrix. J. Food Prot. 74:1104–1111 [DOI] [PubMed] [Google Scholar]
- 3. Barnhart MM, Chapman MR. 2006. Curli biogenesis and function. Annu. Rev. Microbiol. 60:131–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blanchard JL, Wholey WY, Conlon EM, Pomposiello PJ. 2007. Rapid changes in gene expression dynamics in response to superoxide reveal SoxRS-dependent and independent transcriptional networks. PloS One 2:e1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bowtell D, Sambrook J. 2003. DNA microarrays: a cloning manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 6. Brumaghim JL, Li Y, Henle E, Linn S. 2003. Effects of hydrogen peroxide upon nicotinamide nucleotide metabolism in Escherichia coli—changes in enzyme levels and nicotinamide nucleotide pools and studies of the oxidation of NAD(P)H by Fe(III). J. Biol. Chem. 278:42495–42504 [DOI] [PubMed] [Google Scholar]
- 7. Campbell EA, et al. 2003. Crystal structure of Escherichia coli sigma(E) with the cytoplasmic domain of its anti-sigma RseA. Mol. Cell 11:1067–1078 [DOI] [PubMed] [Google Scholar]
- 8. CDC 2007. Multistate outbreaks of Salmonella infections associated with raw tomatoes eaten in restaurants—United States, 2005–2006. MMWR Morb. Mortal. Wkly. Rep. 56:909–911 [PubMed] [Google Scholar]
- 9. Cooley M, et al. 2007. Incidence and tracking of Escherichia coli O157:H7 in a major produce production region in California. PLoS One 2:e1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Darwin AJ. 2005. The phage-shock-protein response. Mol. Microbiol. 57:621–628 [DOI] [PubMed] [Google Scholar]
- 11. Delaquis P, Bach S, Dinu L-D. 2007. Behavior of Escherichia coli O157:H7 in leafy vegetables. J. Food Prot. 70:1966–1974 [DOI] [PubMed] [Google Scholar]
- 12. Domka J, Lee JT, Bansal T, Wood TK. 2007. Temporal gene-expression in Escherichia coli K-12 biofilms. Environ. Microbiol. 9:332–346 [DOI] [PubMed] [Google Scholar]
- 13. Dong T, Schellhorn HE. 2009. Control of RpoS in global gene expression of Escherichia coli in minimal media. Mol. Genet. Genomics 281:19–33 [DOI] [PubMed] [Google Scholar]
- 14. Duffitt AD, Reber RT, Whipple A, Chauret C. 2011. Gene expression during survival of Escherichia coli O157:H7 in soil and water. Int. J. Microbiol. doi:10.1155/2011/340506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Franz E, Klerks MM, De Vos OJ, Termorshuizen AJ, van Bruggen AH. 2007. Prevalence of Shiga toxin-producing Escherichia coli stx1, stx2, eaeA, and rfbE genes and survival of E. coli O157:H7 in manure from organic and low-input conventional dairy farms. Appl. Environ. Microbiol. 73:2180–2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Franz E, et al. 2007. Quantification of contamination of lettuce by GFP-expressing Escherichia coli O157:H7 and Salmonella enterica serovar Typhimurium. Food Microbiol. 24:106–112 [DOI] [PubMed] [Google Scholar]
- 17. Fratamico PM, Wang SY, Yan XH, Zhang W, Li YS. 2011. Differential gene expression of E. coli O157:H7 in ground beef extract compared to tryptic soy broth. J. Food Sci. 76:M79–M87 [DOI] [PubMed] [Google Scholar]
- 18. Garcia A, Mount JR, Davidson PM. 2003. Ozone and chlorine treatment of minimally processed lettuce. J. Food Sci. 68:2747–2751 [Google Scholar]
- 19. Gomes C, et al. 2008. E-beam irradiation of bagged, ready-to-eat spinach leaves (Spinacea oleracea): an engineering approach. J. Food Sci. 73:E95–E102 [DOI] [PubMed] [Google Scholar]
- 20. Gon S, Faulkner MJ, Beckwith J. 2006. In vivo requirement for glutaredoxins and thioredoxins in the reduction of the ribonucleotide reductases of Escherichia coli. Antioxid. Redox Signal. 8:735–742 [DOI] [PubMed] [Google Scholar]
- 21. Grose JH, Joss L, Velick SF, Roth JR. 2006. Evidence that feedback inhibition of NAD kinase controls responses to oxidative stress. Proc. Natl. Acad. Sci. U. S. A. 103:7601–7606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gyaneshwar P, et al. 2005. Sulfur and nitrogen limitation in Escherichia coli K-12: specific homeostatic responses. J. Bacteriol. 187:1074–1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hassan AN, Frank JF. 2004. Attachment of Escherichia coli O157:H7 grown in tryptic soy broth and nutrient broth to apple and lettuce surfaces as related to cell hydrophobicity, surface charge, and capsule production. Int. J. Food Microbiol. 96:103–109 [DOI] [PubMed] [Google Scholar]
- 24. Himathongkham S, Bahari S, Riemann H, Cliver DO. 1999. Survival of Escherichia coli O157:H7 and Salmonella typhimurium in cow manure and cow manure slurry. FEMS Microbiol. Lett. 178:251–257 [DOI] [PubMed] [Google Scholar]
- 25. Johannessen GS, et al. 2005. Potential uptake of Escherichia coli O157:H7 from organic manure into crisphead lettuce. Appl. Environ. Microbiol. 71:2221–2225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jordan A, et al. 1996. The ribonucleotide reductase system of Lactococcus lactis—characterization of an nrdEF enzyme and a new electron transport protein. J. Biol. Chem. 271:8779–8785 [DOI] [PubMed] [Google Scholar]
- 27. Jung JU, Gutierrez C, Villarejo MR. 1989. Sequence of an osmotically inducible lipoprotein gene. J. Bacteriol. 171:511–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kabir MS, et al. 2005. Cell lysis directed by sigma(E) in early stationary phase and effect of induction of the rpoE gene on global gene expression in Escherichia coli. Microbiology 151:2721–2735 [DOI] [PubMed] [Google Scholar]
- 29. Kabir MS, Yamashita D, Noor R, Yamada M. 2004. Effect of sigma(S) on sigma(E)-directed cell lysis in Escherichia coli early stationary phase. J. Mol. Microbiol. Biotechnol. 8:189–194 [DOI] [PubMed] [Google Scholar]
- 30. Kanehara K, Ito K, Akiyama Y. 2002. YaeL (EcfE) activates the sigma(E) pathway of stress response through a site-2 cleavage of anti-sigma(E), RseA. Genes Dev. 16:2147–2155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kerr M, Martin KM, Churchill GA. 2000. Analysis of variance for gene expression microarray data. J. Comput. Biol. 7:819–837 [DOI] [PubMed] [Google Scholar]
- 32. Kim KS, et al. 2010. The Rut pathway for pyrimidine degradation: novel chemistry and toxicity problems. J. Bacteriol. 192:4089–4102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kobayashi R, Suzuki T, Yoshida M. 2007. Escherichia coli phage-shock protein A (PspA) binds to membrane phospholipids and repairs proton leakage of the damaged membranes. Mol. Microbiol. 66:100–109 [DOI] [PubMed] [Google Scholar]
- 34. Kredich NM. 1992. The molecular-basis for positive regulation of cys promoters in Salmonella typhimurium and Escherichia coli. Mol. Microbiol. 6:2747–2753 [DOI] [PubMed] [Google Scholar]
- 35. Kutter S, Hartmann A, Schmid M. 2006. Colonization of barley (Hordeum vulgare) with Salmonella enterica and Listeria spp. FEMS Microbiol. Ecol. 56:262–271 [DOI] [PubMed] [Google Scholar]
- 36. Kyle JL, Parker CT, Goudeau D, Brandl MT. 2010. Transcriptome analysis of Escherichia coli O157:H7 exposed to lysates of lettuce leaves. Appl. Environ. Microbiol. 76:1375–1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lange R, Barth M, Hengge-Aronis R. 1993. Complex transcriptional control of the σS-dependent stationary-phase-induced and osmotically regulated osmY (csi-5) gene suggests novel roles for lrp, cyclic-AMP (cAMP) receptor protein-cAMP complex, and integration host factor in the stationary-phase response of Escherichia coli. J. Bacteriol. 175:7910–7917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lee SY, Baek SY. 2008. Effect of chemical sanitizer combined with modified atmosphere packaging on inhibiting Escherichia coli O157:H7 in commercial spinach. Food Microbiol. 25:582–587 [DOI] [PubMed] [Google Scholar]
- 39. Lillig CH, Potamitou A, Schwenn JD, Vlamis-Gardikas A, Holmgren A. 2003. Redox regulation of 3′-phosphoadenylylsulfate reductase from Escherichia coli by glutathione and glutaredoxins. J. Biol. Chem. 278:22325–22330 [DOI] [PubMed] [Google Scholar]
- 40. Lillig CH, et al. 1999. New thioredoxins and glutaredoxins as electron donors of 3′-phosphoadenylylsulfate reductase. J. Biol. Chem. 274:7695–7698 [DOI] [PubMed] [Google Scholar]
- 41. Loferer H, Hammar M, Normark S. 1997. Availability of the fibre subunit CsgA and the nucleator protein CsgB during assembly of fibronectin-binding curli is limited by the intracellular concentration of the novel lipoprotein CsgG. Mol. Microbiol. 26:11–23 [DOI] [PubMed] [Google Scholar]
- 42. Lynch MF, Tauxe RV, Hedberg CW. 2009. The growing burden of foodborne outbreaks due to contaminated fresh produce: risks and opportunities. Epidemiol. Infect. 137:307–315 [DOI] [PubMed] [Google Scholar]
- 43. Marzan LW, Shimizu K. 2009. Metabolic regulation of Escherichia coli under phosphate limitation and the effect of phoB gene knockout on the metabolism. J. Biosci. Bioeng. 108:S170–S170 [Google Scholar]
- 44. Maule A. 2000. Survival of verotoxigenic Escherichia coli O157 in soil, water and on surfaces. J. Appl. Microbiol. Symp. Suppl. 88:71S–78S [DOI] [PubMed] [Google Scholar]
- 45. Michino H, et al. 1999. Massive outbreak of Escherichia coli O157:H7 infection in schoolchildren in Sakai City, Japan, associated with consumption of white radish sprouts. Am. J. Epidemiol. 150:787–796 [DOI] [PubMed] [Google Scholar]
- 46. Missiakas D, Mayer MP, Lemaire M, Georgopoulos C, Raina S. 1997. Modulation of the Escherichia coli sigma(E) (RpoE) heat-shock transcription-factor activity by the RseA, RseB and RseC proteins. Mol. Microbiol. 24:355–371 [DOI] [PubMed] [Google Scholar]
- 47. Model P, Jovanovic G, Dworkin J. 1997. The Escherichia coli phage-shock-protein (psp) operon. Mol. Microbiol. 24:255–261 [DOI] [PubMed] [Google Scholar]
- 48. Niemira BA, Cooke PH. 2010. Escherichia coli O157:H7 biofilm formation on romaine lettuce and spinach leaf surfaces reduces efficacy of irradiation and sodium hypochlorite washes. J. Food Sci. 75:M270–M277 [DOI] [PubMed] [Google Scholar]
- 49. Nord S, Bylund GO, Lovgren JM, Wikstrom PM. 2009. The RimP protein is important for maturation of the 30s ribosomal subunit. J. Mol. Biol. 386:742–753 [DOI] [PubMed] [Google Scholar]
- 50. Rudd KE, Humphery-Smith I, Wasinger VC, Bairoch A. 1998. Low molecular weight proteins: a challenge for post-genomic research. Electrophoresis 19:536–544 [DOI] [PubMed] [Google Scholar]
- 51. Saldana Z, et al. 2009. Synergistic role of curli and cellulose in cell adherence and biofilm formation of attaching and effacing Escherichia coli and identification of Fis as a negative regulator of curli. Environ. Microbiol. 11:992–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Singh R, Mailloux RJ, Puiseux-Dao S, Appanna VD. 2007. Oxidative stress evokes a metabolic adaptation that favors increased NADPH synthesis and decreased NADH production in Pseudomonas fluorescens. J. Bacteriol. 189:6665–6675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Snell EE. 1975. Tryptophanase—structure, catalytic activities, and mechanism of action. Adv. Enzymol. Relat. Areas Mol. Biol. 42:287–333 [DOI] [PubMed] [Google Scholar]
- 54. Soderstrom A, et al. 2008. A large Escherichia coli O157 outbreak in Sweden associated with locally produced lettuce. Foodborne Pathog. Dis. 5:339–349 [DOI] [PubMed] [Google Scholar]
- 55. Stehr M, Schneider G, Aslund F, Holmgren A, Lindqvist Y. 2001. Structural basis for the thioredoxin-like activity profile of the glutaredoxin-like NrdH-redoxin from Escherichia coli. J. Biol. Chem. 276:35836–35841 [DOI] [PubMed] [Google Scholar]
- 56. Strøm AR, Kaasen I. 1993. Trehalose metabolism in Escherichia coli: stress protection and stress regulation of gene expression. Mol. Microbiol. 8:205–210 [DOI] [PubMed] [Google Scholar]
- 57. Stubbe J, van der Donk WA. 1998. Protein radicals in enzyme catalysis. Chem. Rev. 98:705–762 [DOI] [PubMed] [Google Scholar]
- 58. Taormina PJ, et al. 2009. Transfer of Escherichia coli O157:H7 to iceberg lettuce via simulated field coring. J. Food Prot. 72:465–472 [DOI] [PubMed] [Google Scholar]
- 59. Tusher VG, Tibshirani R, Chu GU. 2001. Significance analysis of microarrays applied to the ionizing radiation response. Proc. Natl. Acad. Sci. U. S. A. 98:5116–5121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wang QP, Kaguni JM. 1989. A novel sigma-factor is involved in expression of the rpoH gene of Escherichia coli. J. Bacteriol. 171:4248–4253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Weber A, Kogl SA, Jung K. 2006. Time-dependent proteome alterations under osmotic stress during aerobic and anaerobic growth in Escherichia coli. J. Bacteriol. 188:7165–7175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wendel AM, et al. 2009. Multistate outbreak of Escherichia coli O157:H7 infection associated with consumption of packaged spinach, August-September 2006: the Wisconsin investigation. Clin. Infect. Dis. 48:1079–1086 [DOI] [PubMed] [Google Scholar]
- 63. Wittstock U, Kliebenstein DJ, Lambrix V, Reichelt M, Gershenzon J. 2003. Glucosinolate hydrolysis and its impact on generalist and specialist insect herbivores, p 101–125 In John TR. (ed), Recent advances in phytochemistry, vol 37 Elsevier, Philadelphia, PA [Google Scholar]
- 64. Wood JD, Bezanson GS, Gordon RJ, Jamieson R. 2010. Population dynamics of Escherichia coli inoculated by irrigation into the phyllosphere of spinach grown under commercial production conditions. Int. J. Food Microbiol. 143:198–204 [DOI] [PubMed] [Google Scholar]
- 65. Wood TK. 2009. Insights on Escherichia coli biofilm formation and inhibition from whole-transcriptome profiling. Environ. Microbiol. 11:1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Xicohtencatl-Cortes J, Chacon ES, Saldana Z, Freer E, Giron JA. 2009. Interaction of Escherichia coli O157:H7 with leafy green produce. J. Food Prot. 72:1531–1537 [DOI] [PubMed] [Google Scholar]
- 67. Yamazaki A, et al. 2011. Commensal effect of pectate lyases secreted from Dickeya dadantii on proliferation of Escherichia coli O157:H7 EDL933 on lettuce leaves. Appl. Environ. Microbiol. 77:156–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zhang GD, Ma L, Phelan VH, Doyle MP. 2009. Efficacy of antimicrobial agents in lettuce leaf processing water for control of Escherichia coli O157:H7. J. Food Prot. 72:1392–1397 [DOI] [PubMed] [Google Scholar]
- 69. Zhang XS, Garcia-Contreras R, Wood TK. 2007. YcfR (BhsA) influences Escherichia coli biofilm formation through stress response and surface hydrophobicity. J. Bacteriol. 189:3051–3062 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.