Summary
Understanding the mechanisms underlying the induction of immunity in the gastrointestinal mucosa following oral immunization and the cross-talk between mucosal and systemic immunity should expedite the development of vaccines to diminish the global burden caused by enteric pathogens. Identifying an immunological correlate of protection in the course of field trials of efficacy, animal models (when available), or human challenge studies is also invaluable. In industrialized country populations, live attenuated vaccines (e.g. polio, typhoid, and rotavirus) mimic natural infection and generate robust protective immune responses. In contrast, a major challenge is to understand and overcome the barriers responsible for the diminished immunogenicity and efficacy of the same enteric vaccines in underprivileged populations in developing countries. Success in developing vaccines against some enteric pathogens has heretofore been elusive (e.g. Shigella). Different types of oral vaccines can selectively or inclusively elicit mucosal secretory immunoglobulin A and serum immunoglobulin G antibodies and a variety of cell-mediated immune responses. Areas of research that require acceleration include interaction between the gut innate immune system and the stimulation of adaptive immunity, development of safe yet effective mucosal adjuvants, better understanding of homing to the mucosa of immunologically relevant cells, and elicitation of mucosal immunologic memory. This review dissects the immune responses elicited in humans by enteric vaccines.
Keywords: vaccination, mucosa, infectious diseases
Introduction
Gut mucosal vaccines constitute a broad strategy to prevent clinical illness by an array of enteric pathogens, including those that (i) colonize the intestinal mucosa without invasion or morphological damage and elicit watery diarrhea by the effects of powerful enterotoxins [e.g. Vibrio cholerae O1 and O139 and enterotoxigenic Escherichia coli (ETEC)], (ii) intimately attach to the mucosa by translocating bacterial proteins and induce enterocyte effacement (e.g. enteropathogenic E. coli), (iii) induce enterocyte effacement and elaborate the powerful exotoxins Shiga toxin 1 and 2 (enterohemorrhagic E. coli such as prototype serotype O157:H7), (iv) locally invade and destroy the mucosa (e.g. Shigella and rotavirus), (v) locally invade the mucosa and drain to mesenteric lymph nodes (MLNs) (e.g. non-typhoidal Salmonella), and (vi) translocate the mucosa, invade systemically and disseminate to distal organs (e.g. Salmonella Typhi and Salmonella Paratyphi A and B and polioviruses).
Vaccines to prevent disease caused by some of these pathogens can be administered parenterally or transcutaneously (1). However, administering vaccine directly via the gut mucosa by oral immunization has immunologic and practical advantages. There is no vaccine that is more amenable to mass immunization in field settings than oral polio vaccine (OPV), which requires only the installation of a few drops into the subject’s mouth, at whatever age. Although rectal administration of vaccines is effective, this mode of immunization is less practical and often has cultural barriers. Therefore, this review focuses exclusively on oral vaccines.
Oral vaccines, depending on their nature, can activate every effector arm of the immune system. Thus, oral vaccines can elicit mucosal secretory IgA (sIgA) antibodies that prevent attachment and invasion and neutralize enterotoxins, serum IgG antibodies that control mucosally invasive and systemically invasive pathogens, an array of cell-mediated immune responses (CMI) against intracellular bacteria (e.g. S. Typhi) and viruses (e.g. rotavirus), as well as antibody-dependent cellular cytotoxicity responses.
Licensed oral vaccines against polio, rotavirus, S. Typhi and V. cholerae O1 are based on robust technologies including attenuation of viruses by repeated passage in tissue culture (polio and Rotarix® rotavirus) (2), attenuation of bacteria by chemical mutagenesis (typhoid) (3), inactivated V. cholerae alone or in combination with recombinant B subunit (cholera toxin, CT) (4), reassortant rotaviruses based on expressing human rotavirus glycoproteins in a porcine rotavirus background (Rotateq® rotavirus) (5), and attenuation of bacterial strains by recombinant deletion of virulence genes (cholera) (6). These vaccines have achieved considerable success in disease prevention and control among target populations that include infants in the industrialized world (rotavirus vaccines), infants in developing countries (e.g. polio vaccine), school age children in developing countries (typhoid and cholera vaccines), and adult and pediatric travelers from industrialized countries who visit endemic regions in developing countries (typhoid vaccines). Despite these successes, important challenges remain to be overcome. One is that with existing oral vaccines, the immune responses and efficacy are often diminished in certain sub-populations in developing countries (7–10). Understanding the biological basis of this phenomenon can lead to interventions to enhance immunogenicity and protection. Also, whereas impressive strides have been made in the biotechnological approaches to construct new vaccine candidates, practical progress in developing vaccines against certain high priority pathogens such as Shigella and ETEC has been frustratingly erratic (11). One barrier to progress has been devising a strategy to achieve broad spectrum protection against pathogens that exhibit diverse serotypes (e.g. Shigella) and antigenic types (e.g. ETEC, noroviruses) (11). However, these more complicated vaccine development projects are also hampered by insufficient knowledge in our understanding of the intricacies of the gut immune system, which continually grapples to achieve a delicate balance between muting the immunologic responses to food and environmental antigens, despite constant exposure, versus recognizing and activating to defend against pathogens.
This review addresses current knowledge on human immune responses to licensed and candidate oral vaccines. Despite the many challenges, experience shows that successful oral immunization is a feasible goal. To advance in this area, a better understanding of the processes that regulate vaccine-induced immune responses and their association with other host-specific factors is essential.
Mucosal defenses in the gut: activation of the adaptive immune system following oral immunization
The mucosal surface of the gastrointestinal tract, the largest immunologic organ in the human body, is protected by specialized components of the innate and adaptive immune system; the former provides cells that take up and process antigens and secrete pro-inflammatory signals, whereas the latter is responsible for specific antigen recognition, specific effector functions, and immunologic memory. These mucosal defenses protect the host against microbial pathogens, control responses to food components, and maintain tolerance to harmful external antigens.
Within the gut mucosa, specialized cells that overlie organized lymphoid follicles play key roles in these processes. Microfold (M) cells and dendritic cells (DCs) are largely responsible for antigen capture, transport, processing, and presentation, whereas B and T lymphocytes perform specialized immune effector functions (i.e. secretion of antibodies, cytokines, and cytotoxic function). DCs in the gastrointestinal tissue have a unique morphology that includes extended transepithelial dendrites that allows them to sample antigen directly from the lumen (12). Depending on their phenotype (i.e. conventional CD11chi or plasmacytoid DCs) and the microenvironment, gut mucosal DCs mount distinct but appropriate immune responses to commensal and pathogenic organisms, ultimately resulting in the protection of intestinal tissue from damage (12). While en route from the mucosa to the lymph node, DCs acquire an activated and mature phenotype. These cells exhibit a high degree of specialization and plasticity and have the capacity to imprint specific homing patterns on lymphocytes. DCs from the Peyer's patches, small-intestine, and MLNs promote the expression of the gut-homing receptors integrin α4/β7 and CCR9, specific for gut-associated mucosal addressing cell adhesion molecule-1 (MAdCAM-1) and TECK/CCL5, respectively, on T and B cells (12,13). In contrast, conventional DCs from spleen and cervical lymph nodes appear to be incapable of inducing α4/β7 and CCR9 expression on lymphocyte populations (12). Consistent with this observation, DCs could also promote the expression of gut-homing receptors by thymically derived CD4+CD25+forkhead box protein 3 (FOXP3)+ regulatory T cells (Tregs) (14) and B cells (15). Thus, B and T cells primed in the mucosa acquire specific migration patterns that allow them to enter the systemic circulation and eventually migrate back to the mucosal priming site or distant effector sites.
Secretory IgAinduced by oral immunization or exposure to intestinal pathogens
sIgA, representing at least 70% of all Ig produced in mammals, plays a major role as the first line of defense against adherence and invasion by enteric pathogens and neutralization of the toxins they elaborate. sIgA is produced by IgA+ plasma cells residing in the intestinal lamina propria (16). Following oral immunization, naive B cells primed by vaccine antigens (soluble or associated with DCs) cluster into the mucosal-associated lymphoid follicles forming germinal centers (GCs) or migrate to peripheral lymph node GCs, where they clonally expand, differentiate, and undergo affinity maturation. IgA+ plasmablasts, which secrete large amounts of high affinity IgA, can remain in the lymphoid tissue of origin or they can traffic though the efferent lymph to the blood to populate distant sites. Terminal differentiation into polymeric IgA (pIgA)-secreting plasma cells occurs in the lamina propria in a process regulated by cytokines and mediators secreted by activated CD4+ T helper 2 (Th2) cells [interleukin-2 (IL-2), IL-5, and IL-10], DCs [retinoic acid, IL-10, transforming growth factor-β (TGF-β), and IL-6] and intestinal epithelial cells (IECs) (TGF-β, IL-6) (12,16). A T-cell-independent mechanism of sIgA production by B-1 cells upon interaction with DCs loaded with commensal bacteria has also been described (17), although the relevance of this process in humans remains unclear. sIgA reaches the lumen through active transcytosis, binding to a pIgA receptor expressed constitutively by mucosal IECs. sIgA is typically secreted as a dimer, and as such, it has enhanced binding capacity and stability.
Two IgA subclasses have been described in humans. IgA1 predominates in the nasal and bronchial mucosa, saliva, and proximal small intestine (and is also the main subclass produced by the systemic immune system), whereas IgA2 predominates in the distal small intestine and colon (18,19). Human IgA2 is resistant to proteases synthesized by a variety of potentially pathogenic bacteria and is better suited than IgA1 to deal with the dense microbial community in the large intestine (16,18).
One of the main roles of sIgA, in combination with gut innate immune defense mechanisms, is to serve as an immunological barrier, preventing the attachment of pathogens to the mucosal surfaces (18). IgA binds surface proteins that mediate cell attachment of intestinal microbial pathogens thereby preventing colonization and cell invasion. The large complexes formed by sIgA bound to intestinal pathogens are easily entrapped in the mucus and subject to peristaltic or ciliary clearance. Neutralizing antibodies induced by viral vaccines block infection by preventing the attachment of virus particles to the host cell and subsequent internalization; sIgA also neutralizes bacterial enterotoxins (20).
IgA can trap incoming organisms within the epithelial cell vesicular compartments, exporting them back into the lumen (21). It can also neutralize viruses intracellularly, and this mechanism of defense is particularly relevant for intestinal viruses (21–24). An additional adaptive clearance function includes the ability of sIgA to shuttle antigens that have crossed the mucosal epithelium from the basolateral surface back to the lumen through the IgA export system (18). IgA can also enhance immune responses by favoring uptake of luminal antigens via IgA binding to M cells (18). When Shigella bound by specific sIgA are taken up by M cells overlying Peyer’s patches, there is a significant diminution in the propensity of these invasive pathogens to cause inflammation and mucosal destruction compared with Shigella taken up in the absence of specific sIgA (25).
IgG and other antibodies in intestinal secretions
In the mucosal environment, all plasma cells, irrespective of their Ig isotype, express J chain, the small polypeptide required for IgA polymerization. Although IgA is the main isotype in the mucosal secretions, IgG is also present and may contribute to the adaptive immune defenses in the gut. IgG reaches luminal secretions mainly by transudation of systemic antibodies, although small amounts are also synthesized locally (18). The intestine of suckling rodents and the human placenta express the neonatal Fc-receptor (FcRn), which binds and transport IgG molecules across epithelial barriers into the lumen by transcytosis.Expression of FcRn is downregulated to nearly undetectable levels after weaning in rodents. In contrast, absorptive epithelial cells lining the human intestine continue to express FcRn in adult life (26) and exhibit in vitro FcRn-dependent transcytosis of IgG in both directions acrossthe epithelial monolayer (27). The FcRn can also recycle IgG-antigen complexes back across the intestinal barrier into the lamina propria for antigen processing and presentation to CD4+ T cells in regional organized lymphoid structures (28). Mucosal sIgM is less abundant than sIgA but is also present in secretions (18); likewise sIgA, J-chain-containing pentameric IgM is exported by secretory IECs. In the perinatal period, secretions contain only traces of sIgA and sIgM with some maternal IgG leaked from mucosal lamina propria. Therefore, newborns depend on maternal breastmilk sIgA for an adequate mucosal barrier function. IgE is also produced by lamina propria plasma cells and is believed to mediate protection against certain intestinal helminths through the activation of local mast cells, which in turn release tumor necrosis factor-α (TNF-α); collectively, this creates a mucosal environment inhospitable to attachment by the parasites.
Mucosally primed antibody-secreting cells with gut-homing potential
B and T cells primed by vaccine antigens in mucosal inductive sites acquire a distinct homing program. They lose their adhesion to stromal cells, leave organized mucosal lymphoid structures, and disseminate via draining lymph and blood circulation to mucosal effector sites or return to the priming site. The tissue specificity of homing IgA antibody-secreting cells (ASCs) is determined by complex interactions between lymphocyte homing receptors (differentially expressed depending on the priming site) and ligands expressed on the vascular endothelium of target tissues. Virtually all IgA and even some IgG ASCs detected after peroral and rectal immunization express integrin α4/β7, while only a minority express peripheral lymph node CD62L (L-selectin) receptor, which is mainly induced after systemic immunization (29). Lymphocytes expressing integrin α4/β7 interact with MAdCAM-1, which mediates selective lymphocyte binding to post-capillary blood vessels of the lamina propria endothelial cells at mucosal effector sites. Thus, cells expressing integrin α4/β7 but not CD62L are destined to home to the gut mucosa, whereas cells expressing CD62L but not integrin α4/β7 are destined to home to peripheral and MLNs.
Other routes of mucosal immunization (e.g. intranasal) and transcutaneous immunization (30) are able to elicit a ‘mixed’ B-cell migration pattern, which includes stimulation of vaccine-specific ASCs expressing α4/β7+ or CCR9 with the capacity to home to the gut as well CD62L+ cells that home to systemic lymphoid tissue. We have recently described the induction of virus-like particle (VLP)-specific IgA and IgG ASCs with different homing potentials in humans following intranasal immunization with adjuvanted Norwalk VLPs. While Norwalk-specific IgA ASCs displayed receptors that would allow them to home to the gut mucosa (CD19+CD27+integrin α4/β7+CD62L−) and peripheral lymphoid tissues (CD19+CD27+integrin α4/β7+CD62L+), IgG ASCs exhibited homing potential almost exclusively to peripheral lymphoid tissues (CD19+CD27+integrin α4/β7+CD62L+). It is particularly interesting that intranasal immunization was able to elicit ASCs that can home to the gut mucosa, although it failed to induce substantial levels of IgG or IgA ASCs with the capacity to home exclusively to peripheral lymphoid tissues (31).
Extravasation of lymphocytes into effector sites requires chemoattractant signals. IgA ASCs in the Peyer’s patches and MLNs express chemokine receptor CCR9 and migrate efficiently to its ligand CCL25 (expressed mainly by IECs). In contrast, IgG ASCs respond to other chemokines such as CXCL12 and CXCL9, ligands for CXCR4 and CXCR3, respectively, and most express integrin α4β1 (32). Selectivity in isotype-specific ASC chemokine receptor expression is likely a major determinant of IgA ASC localization to the gut mucosa (32).
Systemic antibodies and memory B cells elicited by oral immunization or exposure to enteric pathogens
As described above, B cells primed in the gut mucosa form GC structures in the intestinal lymphoid follicles and Peyer’s patches, where they undergo class switching, selection of high avidity variants, and differentiation into long-lived plasma cells or memory (BM) cells. The latter do not actively secrete antibody but can rapidly turn into ASCs upon re-exposure to antigen (33).
Vaccine-specific plasma cells residing in the spleen or in circulation maintain a sustained level of systemic IgG that neutralizes virus particles and disarms bacteria that have invaded the blood, thereby abrogating massive dissemination; serum IgG can also can neutralize the effect of toxins. Antigen-specific BM cells in circulation can be quantified through expansion with mitogens and differentiation into ASCs (33). Despite substantial subset heterogeneity, based on their cell membrane surface expression, it is generally accepted that most BM cells are CD19+, CD20+, CD27+,CD38+, IgD−, IgG+ or IgA+, while plasmablasts/plasma cells are CD19+, CD20−/low, CD27hi, CD38hi, IgD−, IgG−, IgA− (33, 34). Homing markers such as integrin α4/β7, CCR9, and CCR10 have been used to identify populations that migrate to the gut. Antigen-specific BM cells have been described in humans after vaccination or infection with enteric pathogens including rotavirus (35), V. cholerae (36), and Shigella (37). In a study that examined the role of BM in protection, peripheral blood mononuclear cells (PBMCs) from children vaccinated with live-attenuated rotavirus vaccine were incubated with fluorochrome-labeled VLPs and examined for expression of CD19, CD27, CD38, IgD, integrin α4/β7, and CCR9; CD27hi and CD38hi cells were excluded from analysis. Fewer than 0.01% of total B cells were antigen-specific before or after vaccination, and no statistical differences were found between 159 vaccine and 160 placebo recipients, despite 75% protection against any rotavirus gastroenteritis. A weak correlation with protection (Rho 0.17, p=0.034 by Spearman one-tailed) was found between rotavirus-specific IgD−CD27+α4β7+CCR9+ cells after dose 1. The authors concluded that factors other than BM cells may play a role in protection (35).
In another study, BM cell responses against the lipopolysaccharide (LPS) as well as the B-subunit of CT (CTB), and toxin coregulated pilus major subunit A (TcpA) were examined in 39 patients with culture-confirmed cholera. IgA BM cell responses in this study were on the order of 0.4% of total for anti-LPS and 0.2% of total for anti-CTB and anti-TcpA IgA, with slightly lower IgG BM responses (36). Our group at the Center for Vaccine Development (CVD) has recently measured anti-LPS and anti-invasion plasmid antigen (Ipa)B BM cell responses in 12 LPS seroresponder and 12 non-responder subjects, all of whom were immunized with a single dose of a live-attenuated Shigella candidate vaccine. Among the seroresponders, the LPS IgG BM cells increased from a median of 0 to 0.02% of total IgG (p=0.008 by Wilcoxon signed rank test); anti-IpaB responses were less pronounced. Unlike others, we found relatively strong correlations between antigen-specific serum IgG and IgG BM cells (Rho=0.95, p=0.0003 by Spearman two-tailed for LPS) (37), as well as between serum and stool IgA and IgA BM cells (Simon J.K. & Sztein M.B., unpublished data.
Cell-mediated immunity elicited by oral immunization or exposure to enteric pathogens
Microbes transported by M cells are endocytosed by immature DCs in the M cell pocket and ferried to adjacent interfollicular T-cell zones and/or regional lymph nodes, where DC maturation and antigen presentation to naive T cells occurs. T cells primed in the intestinal mucosal (e.g. Peyer’s patches) rapidly migrate to MLNs, where they differentiate into effector or memory T cells; from there, these cells enter the systemic circulation via the efferent lymph and home back to the lamina propria, where they reside (18). Two main memory T-cell subsets have been described, i.e. central/memory T cells (TCM) and effector memory T cells (TEM). Most TCM cells express the chemokine receptor CCR7, the peripheral lymph node CD62L (L-selectin) receptor, and CD45RO in the absence CD45RA, while classical TEM cells express CD45RO in the absence of CCR7, CD62L, and CD45RA. An important subset of TEM which co-expresses CD45RA (TEMRA) appears to encompass the most active effectors (38;39). It is widely accepted that TEM populations are ready for immediate effector action, while TCM are important in the generation of a new wave of effector T cells (39, 40). The memory T cells that accumulate in the lamina propria are mostly TEM cells (39). Selective homing of effector memory cells to the lamina propria of the gut is driven, to a large extent, by the expression of the integrin α4β7, which binds to its ligand MAdCAM-1, expressed in the high endothelial venules (41). Another molecule that participates in gut homing of integrin α4β7-bearing cells is CCR9 (42), which is expressed by virtually all T cells in this tissue (43). TECK/CCL25, present on the crypt epithelium in the jejunum and ileum, mediates chemotaxis of memory α4β7hi CD4+ and CD8+ T lymphocytes expressing CCR9 receptors into the lamina propria (43).
Effector immune responses mediated by CD8+ cells in mucosal tissues include immunoregulation and activation of innate immunity, largely dependent on cytokine production, as well as cell-mediated cytotoxicity. Of the various CD8+ populations found in mucosal tissues, TEM T-cell receptor (TCR)αβ expressing CD8αβ or CD8αβ and CD8αα have mostly effector/cytotoxic T-lymphocyte (CTL) activity, whereas T cells double negative (DN) for TCRαβ or expressing only CD8αα have predominantly effector/immunoregulatory activity. On the other hand, TCRγ δ DN or expressing CD8αα have predominantly immunoregulatory and tissue repair activities (44). Protection against pathogens that enter the host via mucosal surfaces has been shown to be mostly mediated by intraepithelial (IEL) and lamina propria lymphocytes. TEM CD8αβ+ TCRαβ+ exhibit potent CTL activity against specific-pathogen-infected target cells, e.g. during lymphocytic choriomeningitis virus infection (44, 45). As in other tissues, IFN-γ and TNF-α secreted by CD8+ T cells play key roles in amplifying inflammatory responses in the lamina propria, enabling phagocytic cells to effectively kill microorganisms that have breached the mucosal barrier.
A sizable proportion of the intraepithelial CD8+ T cells express TCRγ δ. However, the proportion of TCRγ δ+ IELs varies among species. Although in mice 10–50% of IELs are TCRγ δ+, in humans they constitute ~10% of IELs. This is in contrast to other tissues, including peripheral blood, where <5% of T cells are TCRγ δ+ (46). These cells have been implicated in regulating the development of IECs (47) and in killing both infected and malignant cells (48). Human TCRγ δ T cells have been shown to recognize non-classical human leukocyte antigen (HLA) class I molecules; these molecules are upregulated in epithelial cells following adhesion of pathogens such as E. coli (49).
Tregs constitute another key population of T cells that play a fundamental role maintaining immune homeostasis in the gut microenvironment by controlling responses to pathogens and maintaining tolerance against commensal flora and environmental antigens (50, 51). The immunosuppressive properties of naturally occurring Tregs have been linked to an intrinsic state of hyporesponsiveness. IL-10-secreting CD4+Tr1 cells were shown to prevent chronic intestinal inflammation, and TGF-β-secreting Th3 cells are believed to participate in the maintenance of tolerance to dietary antigens (51). An important marker of the suppressive capacity of Tregs is the forkhead-winged-helix transcription factor Foxp3,and Foxp3+CD4+ T cells are crucial for the control of autoimmune responses (52). In contrast to murine systems, the Foxp3 expression in humans is not exclusive for Tregs, and a transient expression might be seen in activated CD4+CD25+ effector T cells (52). Further studies on mucosal Tregs are necessary to increase our understanding on the role of this cell population in response to oral vaccination. Interaction with IECs has been shown to result in the expansion of CD8+ T cells with regulatory function (53). Suppressive activity of these IEC-activated CD8+ lymphocytes was found to be mediated by a CD101+CD103+ subset and appears to require cell contact. In mice, tolerance to oral antigen requires CD8+ T cells for local suppression of IgA responses (54), although the mechanisms are unknown. It has been suggested that a subpopulation of CD8+ T cells might suppress CD4+ T and B-cell responses through an interaction that requires expression of the major histocompatibility complex (MHC) class Ib molecule Qa-1 on activated target cells (55). Preliminary evidence for the existence of this mechanism in humans is supported by experiments in vitro showing that human CD8+ T cells can be induced to differentiate into regulatory cells whose function is dependent on HLA-E, the human homolog of Qa-1 (56).
Th17 cells are present at high frequencies in the lamina propria, in all segments of the intestine (57). Certain intestinal commensal organisms provide the major signal for such enrichment of Th17 cells (58). Although they are believed to be major effector T cells against pathogens, they have been shown to have pleiotropic functions. The exact roles of Th17 cells in the gut mucosa are not totally understood, and intensive research is ongoing in this area (57). Their involvement in immune responses to oral vaccines also remains unknown.
Licensed oral vaccines
Live oral typhoid vaccine (Ty21a)
Typhoid fever caused by Salmonella enterica serovar Typhi (S. Typhi) is an acute generalized infection of the reticuloendothelial system, intestinal lymphoid tissue, and gallbladder characterized by persisting high fever. The disease is restricted to human hosts, who also constitute the reservoir of infection. In most endemic areas, typhoid accounts for ~75–80% of enteric fever, with S. Paratyphi A and B causing the remaining cases. Typhoid infection, which is acquired by ingestion of contaminated food or water, is uncommon in modern industrialized countries, but it is endemic in many populations in less-developed countries who lack access to treated water supplies and live in poor sanitary conditions. Systematic passive surveillance methods (that typically detect more severe cases) document that the peak incidence of typhoid fever is seen among school age children (59, 60). In urban slum environments in South Asia, systematic household and health center-based active surveillance demonstrated a high incidence of bacteremic typhoid infection among febrile toddlers and pre-school children (60–62), corroborating the initial observation of such infections that came from South America (63). Travelers from industrialized countries to developing countries are also at increased risk of developing typhoid fever (64).
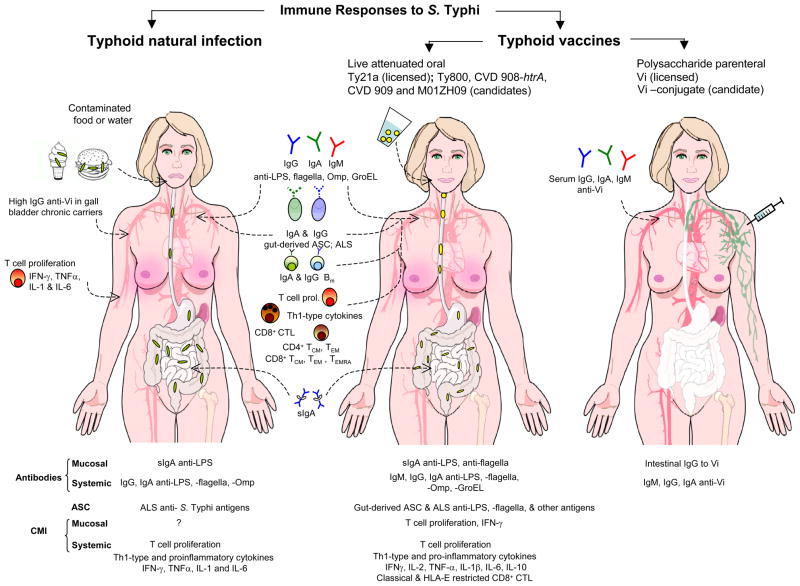
Natural S. Typhi infection induces relatively high titers of serum antibody to bacterial antigens (e.g. LPS, flagella, Omp, GroEL, HlyE), gut-derived ASC/antibody in lymphocyte supernatant (ALS), and sIgA intestinal antibody (65–67) (Fig. 1). CMI responses also occur (68). High titers of serum IgG against the capsular Vi antigen are found in approximately 80–90% of chronic biliary S. Typhi carriers, in 12–38% of acute typhoid fever patients who do not become chronic carriers, but only rarely in healthy individuals in endemic areas (69–71). Proliferative responses to S. Typhi antigens were observed in healthy adults living in typhoid-endemic areas who have no known history of clinically overt typhoid fever (72, 73). Elevated serum levels of IFN-γ, TNF-α, IL-1, IL-6, and TNF receptor (TNF-R) p55 and TNF-R p75, indicative of a dominant Th1-type response, were seen in patients with culture-positive typhoid fever (74). We recently examined the role of DCs in induction of immunity to S. Typhi infection and observed that upon in vitro infection with S. Typhi, human primary DCs produced high levels of pro-inflammatory cytokines IL-6, IL-8, and TNF-α, but low levels of IL-12 p70 and IFN-γ . In contrast, DCs co-cultured with S. Typhi-infected cells, through suicide cross-presentation, were able to uptake these infected cells and release high levels of IFN-γ and IL- 12p70, leading to the subsequent presentation of bacterial antigens and induction of memory T cells, mostly CD3+CD8+CD45RA−CD62L−effector/memory T cells (75).
Fig. 1. Immune responses to S. Typhi following natural infection and vaccination.
Immune responses following natural infection include: 1) serum antibodies to the O-antigen [lipopolysaccharide (LPS)], the H antigen (flagellar component), polysaccharide capsular Vi antigen, bacterial heat shock proteins (e.g. GroEL) and outer membrane proteins (Omp); 2) gut-derived antibody-secreting cells (ASCs) specific for LPS and Typhoid antigens; 3) secretory IgA to some of these antigens in intestinal fluids and bile, and 4) cell-mediated immune responses including T-cell proliferation and increased levels of pro-inflammatory and Th1-type cytokines. Chronic S. Typhi biliary carriers (80%) have high levels of serum IgG anti-Vi capsular polysaccharide; in contrast elevated serum IgG following acute typhoid fever is seen in only ~ 20% of subjects who do not become chronic carriers.
Ty21a is the only licensed live oral typhoid vaccine. The most promising vaccine candidates to date include: CVD 908-htrA, CVD 909 (a further derivative of CVD 908-htrA that constitutively expresses Vi), M01ZH09 and Ty800. Oral immunization of adult volunteers with these vaccines showed the presence of mucosal and systemic immune responses.Mucosal immune responses include sIgA against bacterial antigens (e.g. LPS, flagella, Omp) and mucosally primed ASCs detected in peripheral blood mononuclear cells (PBMCs). Systemic responses include serum antibodies to different bacterial antigens (e.g. LPS, flagella, Omp, GroEL, HlyE). Cell-mediated immunity (CMI) (studied in depth for Ty21a, CVD 908-htrA and CVD 909) include proliferative responses by PBMCs, production of Th1-type cytokines (i.e. IFN-γ and TNF-α) in the absence of IL-4 and IL-5, and classical (HLA-Ia)- and non-classical (HLA-E)- restricted CD8+ cytotoxic lymphocytes (CTLs). Oral live vaccines also generate memory B and T cells; the latter include IFN-γ-secreting CD4+ and CD8+ T-central memory (TCM)andT-effector memory (TEM) subsets expressing the α4/β7 and/or CD62L. Immunological correlates of protection remain undefined. The relative contribution CMI responses and antibodies and their interplay in vaccine efficacy remains unclear.
For purposes of comparison, the far right panel summarizes the immune responses to the licensed parenteral Vi polysaccharide typhoid vaccine. In contrast to the broad responses elicited by live attenuated strains, responses to parenteral Vi polysaccharide are restricted to systemic Vi antibodies (mainly IgG and IgA). A Vi polysaccharide conjugated to the recombinant exoprotein A from Pseudomonas aeruginosa elicited high levels of serum Vi IgG (compared with unconjugated Vi) in children of school age and pre-school age from highly endemic areas and these responses were long-lasting (248). Gradients of colors reflect the location of immune responses induced by natural infection and oral or parenteral immunization.
A live attenuated oral typhoid vaccine, strain Ty21a, is licensed for use in the U.S. and many countries worldwide. It was developed in the early 1970s by chemical mutagenesis of pathogenic S. Typhi strain Ty2 (3). Putatively relevant mutations in this strain include an inactivation of the galE gene that encodes an epimerase involved in LPS biosynthesis and an inability to express Vi polysaccharide (3). However, Ty21a also harbors more than two dozen additional mutations compared to its wildtype parent (76).
Ty21a provides significant protection without causing adverse reactions. Results of three double-blind, placebo-controlled studies that utilized active surveillance to assess the reactogenicity of Ty21a in adults and children show that adverse reactions were not observed significantly more often in vaccinees than placebo recipients for any symptom or sign (77, 78). In large-scale efficacy field trials with Ty21a, involving approximately 514,000 schoolchildren in Chile (79–81), 32,388 in Egypt (82), and 20,543 subjects from three years of age to adults in Indonesia (77), passive surveillance failed to identify vaccine-attributable adverse reactions over placebo background (79–81;83)
Results of controlled field trials of Ty21a revealed that the formulation of the vaccine, the number of doses administered, and the spacing between the doses markedly influence the level of protection that can be achieved (77, 80, 83, 84). Two formulations are licensed, including enteric-coated capsules and a ‘liquid’ formulation in which lyophilized vaccine is reconstituted along with buffer powder into a vaccine cocktail. However, in recent years, only the enteric coated capsule formulation has been manufactured and available commercially. Based on a field trial in Santiago, Chile that demonstrated that three doses of Ty21a in enteric-coated capsules given on an every other day schedule conferred 67% efficacy over three years of follow-up (83, 85), this formulation and immunization schedule are used throughout the world, except for the U.S. and Canada where a four-dose regimen is used. The four-dose North American regimen is based on results of another large-scale, randomized comparative trial carried out in Chile, where four doses of Ty21a in enteric-coated capsules were significantly more protective than two or three doses (84).
The first field trial of Ty21a carried out in Alexandria, Egypt demonstrated 96% vaccine efficacy over three years of follow-up (82). However, the prototoype liquid formulation used in this field trial was not amenable to consistent manufacture. In the mid-1980s, the Swiss Serum and Vaccine Institute (currently Berna Biotech, a Crucell Company) succeeded in preparing a new ‘liquid suspension’ formulation of Ty21a for large-scale field trials that was amenable to large-scale manufacture. The new formulation consisted of two packets, one containing a dose of lyophilized vaccine and the other containing buffer. To prepare a ‘vaccine cocktail’; contents of the two packets are mixed in a cup containing 100 ml of water and the suspension is then ingested by the subject to be vaccinated. Randomized placebo-controlled field trials were carried out in Santiago, Chile (81) and Plaju, Indonesia (77) to directly compare this new liquid formulation of Ty21a with the enteric coated capsule formulation. In both trials, the vaccine administered as a liquid suspension was superior to the vaccine in enteric-coated capsules. Moreover, Ty21a given as a liquid suspension protected young children as well as older children (77, 81). The liquid formulation was more practical for giving Ty21a to children < 7 years of age and was strongly immunogenic in toddlers and pre-school children (81, 86).
Ty21a serves as a paradigm among oral bacterial enteric vaccines because of the impressive long-lived protection that it confers (85). Follow-up of cohorts in two Chilean field trials were continued for a total of seven years and five years, respectively. Over seven years, the enteric-coated capsule formulation of Ty21a conferred 62% vaccine efficacy (CI, 48–73%; p<0.0001) (85), while the liquid formulation demonstrated a point estimate of vaccine efficacy of 78% (CI, 65–87%; p<0.0001) over 5 years of follow-up (with no hint of fall-off when surveillance was discontinued for fiscal reasons). One of the challenges that investigators at the CVD undertook subsequently was to explore the immune responses to Ty21a and their longevity to be able to account for the extended protection (87–91).
Immune responses recorded in Ty21a recipients include significant rises in serum IgG against the O polysaccharide (78, 79) (Fig. 1), which increased with additional doses (79). Approximately 7–10 days following oral immunization with Ty21a, mucosally primed O-specific ASCs can be readily detected among PBMCs (92). Most of these ASCs carry the integrin α4β7 homing marker that direct the cells back to the intestinal mucosa (92, 93). Two immunologic measurements were found to correlate with the protection conferred by different formulations and immunization schedules of Ty21a in field trials. These include serum IgG O antibody (seroconversions) (79) and gut-derived IgA O ASCs. Intestinal (jejunal fluid or fecal) sIgA antibody responses have been reported following oral immunization with various formulations and regimens of Ty21a, including several known to be protective (94). However, it remains unclear whether the antibody responses elicited by this attenuated strain are the operative immune mechanism responsible for protection. Antibody-dependent cellular cytotoxicity has also been described following oral immunization with Ty21a (95, 96).
Oral Ty21a vaccine stimulates strong CMI responses, including T-cell proliferation, secretion of Th1-type cytokines (e.g. IFN-γ , TNF-α) (88, 90, 95, 97) and classical class Ia-restricted as well as non-classical class-Ib HLA-E-restricted CD8+ CTLs (87–90). Previous studies in humans have demonstrated a relationship between the presence of multifunctional T cells (capable of producing multiple cytokines) and the quality of the immune responses (98, 99). We have recently completed studies demonstrating that the majority of HLA-E-restricted CD8+ cells 28 to 56 days after immunization were multifunctional T cells co-expressing IFN-γ , TNF-α, and CD107 (87). CD107 was used as a measurement of degranulation, a mechanism essential for the killing of Salmonella infected targets by cytotoxic CD8+ cells (100). We were also able to derive from PBMC isolated from Ty21a vaccinees (up to 3 years after immunization), T-cell clones with TEM phenotype (i.e. CCR7− CD27−CD45RO+CD62L−) co-expressing gut homing molecules (i.e.α4β7highCCR9intCD103low)(91). These long-term T-cell responses to Ty21a were oligoclonal and involved multiple TCR Vβ families (91). It is reasonable to assume that T-cell responses contribute to and correlate with protection. However, this cannot be proven, as these assays were not available at the time of the field trials of Ty21a. Of note, no correlations were observed between CMI responses and serum antibody responses in multiple studies involving immunization with experimental attenuated oral typhoid vaccinees (88, 101, 102).
These observations suggest that while serum antibodies to S. Typhi antigens, including O, H, and Vi, as well as other S. Typhi antigens elicited by Ty21a, might play a role in protection against S. Typhi, they do not appear to represent the dominant immune responses associated with long-term vaccine efficacy.
Cholera vaccines
V. cholera does not invade the gut epithelium but rather exerts its powerful diarrheagenic effect via the intoxication of enterocytes with CT. Of the > 200 V. cholera serogroups, only O1 and O139 routinely express CT and cause epidemic cholera. Within the O1 serogroup, there are two distinct serotypes, Inaba and Ogawa, and two biotypes, classical or El Tor. Globally, cholera is at present overwhelmingly caused by V. cholerae O1 El Tor, with the exception of some O139 cases in Bangladesh and India.
Cholera is acquired through contaminated food and water. Once ingested, the vibrios must withstand gastric acid and reach the proximal small intestine where, without invading mucosal cells, they colonize with the help of Tcp and produce CT. CT is composed of five ‘binding’ B subunits, which bind to GM1 ganglioside receptors, and one ‘active’ A subunit, which is responsible for intracellular toxicity. After binding of the B subunits, the A subunit is internalized into the epithelial cell where it activates adenylate cyclase, resulting in cyclic adenosine monophosphate production and profuse secretion of water and electrolytes (103). Ingestion of a few micrograms of purified CT alone is sufficient to cause voluminous watery diarrhea (103), and deletion ctx or ctxA from wildtype V. cholerae O1 disarms this enteropathogen, making it no longer capable of causing severe diarrhea (6).
Prior natural clinical infection with V. cholerae can protect subjects from illness upon subsequent re-exposure (104, 105). Most volunteers challenged with wildtype V. cholerae O1 in clinical studies were protected upon subsequent challenge (106, 107). Although there is no appreciable cross-protective immunity between O1 and O139 strains, there is substantial cross-protection between biotypes and serotypes within the O1 serogroup (106;108).
There are three licensed oral cholera vaccines, two of which are currently being manufactured; no oral cholera vaccine is currently licensed in the U.S. An rBS-WC (recombinant B sub-unit-inactivated whole cell) cholera vaccine is manufactured by Crucell (Dukoral®; Crucell, Leiden, The Netherlands). Each dose contains approximately 2.5×1010 inactivated V. cholerae O1 of classical Inaba (heat inactivated), El Tor Inaba (formalin inactivated), classical Ogawa (heat inactivated) and classical Ogawa (formalin inactivated), as well as 1 mg of recombinant CT; this vaccine is given with an alkaline buffer. The World Health Organization (WHO) recommends the administration of 2 doses 10–14 days apart for all ages (109), whereas the manufacturer recommends 3 doses for children 2 to 6 years old and an interval between doses of at least 1 week (110). A booster dose is recommended after 2 years in those over the age of 6 years and after 6 months in those aged 2 to 6 years (111). A second non-living cholera vaccine (Shanchol®; Shantha Biotech, Hyderabad, India) was recently licensed in India (112, 113). It contains inactivated V. cholerae O139 as well as O1 bacteria and is not combined with B subunit; it also utilizes a two-dose immunization schedule.
A single-dose recombinant live oral cholera vaccine CVD 103-HgR was engineered at the CVD by deleting 94% of the gene encoding the CT A1 subunit from a wildtype V. cholerae O1 classical Inaba strain and inserting into the hemolysin A (hlyA) locus a gene encoding resistance to mercury (114). CVD 103-HgR was originally manufactured for a number of years by the Swiss Serum Vaccine and Institute (Berna, Switzerland) and commercialized as a single-dose oral cholera vaccine under the trade names Orochol® and Mutacol®.
CVD 103-HgR was a licensed cholera vaccine in Switzerland, Finland, Canada, Australia, New Zealand, Argentina, Colombia, Guatemala, Peru, Venezuela, the Philippines, and Sri Lanka. A U.S. company, PaxVax, has undertaken the re-commercialization of CVD 103-HgR. Two other live vaccines (strains Peru 15 and 638) are also under development, although they have not advanced beyond clinical trials (115, 116).
The immune response to cholera is directed against bacterial surface structures including LPS and TcP as well as against CT. Infection results in (i) humoral immune responses including mucosal sIgA, serum IgA, and IgG, and serum vibriocidal antibodies (117); (ii) ASCs (118–120); (iii) T-cell responses including memory (121,122); and (iv) BM cells (36). Recipients of the oral whole-cell vaccine combined with B subunit as well as naturally infected individuals develop serum antibodies to CT and LPS and sIgA in intestinal lavage fluid. Vibriocidal responses peak at day 9 and are higher in naturally infected individuals than those vaccinated orally (117). Vibriocidal antibodies have been used as surrogate of protection when the inactivated or live cholera vaccine is given orally.
Anti-LPS, anti-CT (119), and anti-Tcp (120) IgA ASCs as well as antibody in lymphocyte supernatant (ALS) have been reported after natural cholera infection. LPS and CT ASC responses were examined in volunteers who received CVD 103-HgR and were subsequently challenged on days 8, 30, and 180 after vaccination with homologous V. cholerae classical Inaba (118). The ASC responses peaked on day 7 after antigen exposure in vaccinees as well as controls, similar to natural infection. The majority of ASC responses were IgA. The vaccinees had similar peak ASC responses as challenge strain recipients after initial antigen exposure and 180 days after challenge. Interestingly, significantly fewer ASCs were present after challenge on days 8 and 30 in vaccinees, with 100% vaccine efficacy in spite of a low secondary ASC response. Similar decreased ASC responses on re-exposure to antigen have been documented among recipients of attenuated S. Typhi oral vaccine (123) and re-challenge with Shigella (124). This is thought to represent the presence of an adequate number of antigen-specific ASCs at the level of the gut upon challenge.
T-cell responses to cholera have not been as well characterized as the B-cell responses. CMI responses have been described with increased proliferation of CD4+ and CD8+ T cells 2 days after natural infection, followed by an increase in CD4+, CD8+, and CD19+ cells expressing gut homing marker β7. Th1 as well as Th2 cytokine production was elevated in cholera patients when compared to healthy controls (121). T memory cells 7 days after infection responded to T-dependent antigens including membrane proteins, TcP, and a recently described antigen V. cholerae cytolysin/hemolysin (VCC). T memory cells were not increased in response to T-independent antigen LPS (122).
The vibriocidal antibody is the most widely accepted immune correlate of protection against cholera, despite some shortcomings of this assay. For example, vibriocidal antibody titers decreased 1 year after vaccination with oral inactivated cholera vaccine, whereas protection persisted for three years (125). Protective immunity against cholera appears to involve multiple factors, starting with sIgA at the mucosa and followed by systemic immunity.
Sabin oral live polio vaccine
Polioviruses are small single stranded RNA viruses in the genus Enterovirus that are restricted to human hosts; three serotypes (1, 2, and 3) exist. Polioviruses cross the intestinal epithelial lining through M cells and adjacent enterocytes and replicate in the underlying lymphoid tissues (126). Most poliovirus infections are either asymptomatic or clinically mild (manifested by low grade fever and sore throat). However, in ~0.1–2% of infected individuals, viremia is followed by dissemination to the motor neurons, resulting in flaccid paralysis.
A live attenuated trivalent OPV was developed by Albert Sabin (127). Attenuation was achieved through serial passage to identify viruses that had lost their neurotropic character. The Sabin live OPV is the basis of the Global Polio Eradication Initiative that has eliminated transmission of wildtype polio virus in three WHO regions, including the Americas, Western Pacific, and Europe. Moreover, since 1999, transmission of type 2 poliovirus has been eliminated globally (128). Despite the impressive success in eliminating wildtype poliomyelitis in most of the world, pockets of polio still remain, in particular in the states of Uttar Pradesh and Bihar in India. In these residual pockets, OPV appears to be less immunogenic than in other populations. A drawback of OPV is the rare reversion to neurovirulence of attenuated strains, which results in vaccine-associated paralytic poliomyelitis (VAPP) in approximately 1 out of 2.5 million OPV doses (129). Revertant strains can circulate fairly widely in some settings.
Serum neutralizing antibodies (1:4–1:8 titer) are considered protective for OPV as well as for the inactivated polio vaccine (IPV) (130). The immune response induced by OPV is systemic, mucosal, and long lived (131). Comparisons of immune response to enhanced potency (EP)-IPV and OPV were undertaken when the decisions to stop using OPV and only use IPV in developed countries were being made. One study conducted at our center compared children that have received three doses of licensed OPV to three doses of licensed EP-IPV. The children were ‘challenged’ with monovalent type 1 OPV (same as that received in the OPV vaccine). Pre-challenge serum neutralizing antibodies were much higher in the EP-IPV vaccine recipients but the shedding of challenge OVP strain was higher in EP-IPV vaccinees than OPV recipients, despite their higher serum response. Polio-specific IgA in stool correlated with viral excretion in OPV recipients only (132). To assess the effect of vaccinating a child with IPV initially for safety followed by OPV for enhanced immunogenicity, vaccination schedules that incorporated IPV and OPV were compared. Serum immune responses are higher after two doses of EP-IPV than after two doses of OPV but highest in EP-IPV followed by OPV. Secretory IgA immune responses were higher in OVP recipients, whether OPV was received alone or preceded by EP-IPV; OPV followed by EP-IPV was not assessed. Shedding was reduced in OPV recipients compared with EP-IPV followed by OPV. The authors concluded that both EP-IPV and OPV when used alone are immunogenic and effective and that EP-IPV followed by OPV may reduce the incidence of VAPP in OPV recipients. Due to the lower impact on vaccine strain shedding, however, EP-IPV followed by OPV may not reduce the incidence of VAPP in susceptible contacts (133).
Live oral rotavirus vaccine
Rotavirus infection is the most common cause of severe gastroenteritis globally. It is estimated that by 5 years of age, every child has experienced at least 1 rotavirus infection, and every minute four children are hospitalized and one dies (134). Rotaviruses are non-enveloped segmented double-stranded RNA viruses in the family Reoviridae. The vast majority of human rotavirus infections are caused by group A viruses. Serotyping is based on VP4 (protease sensitive, P types) and VP7 (glycoprotein G types) surface proteins (135), and multiple human serotypes exist (136). Rotavirus infects mature enterocytes of the villus tip in the small intestine leading to an increase in water and electrolyte secretion (137). Non-structural protein 4 has been implicated as a novel enterotoxin and secretagogue (138). Additionally, the virus inhibits the activities of digestive enzymes at the apical brush border membrane resulting in malabsorbtive diarrhea (139).
The live attenuated tetravalent rhesus reassortant rotavirus vaccine (RRV-TV), Rotashield®, was licensed in the U.S. in 1998 but was withdrawn from the market by the manufacturer after a year because of the association of vaccination with intussusception (140, 141). RRV-TV was comprised of a rhesus rotavirus serotype 3 (which cross-reacts with human G protein 3) and three rhesus-human reassortant viruses encoding VP7 antigens for human serotypes 1, 2, and 4. Each vial of Rotashield® contained 4×105 plaque forming units (PFU), and three doses were administered orally to infants at 2, 4, and 6 months of age. The efficacy in three randomized controlled trials was 49–83% for any rotavirus gastroenteritis and 65–82% for severe rotavirus gastroenteritis (142–144). Because of the association with increased risk of intussusception, RRV-TV use was permanently halted.
The second generation of live attenuated rotavirus vaccines includes RotaTeq (Merck & Co., Inc., Whitehouse Station, NJ, USA) and Rotarix (GlaxoSmithKline plc., London, UK). RotaTeq® is a pentavalent vaccine containing five bovine reassortant strains expressing G proteins 1–4 and the fifth expresses P1A (8) from the human rotavirus donor strain. Each dose of RotaTeq® contains 2.0–2.8×108 infectious units (IU) per reassortant, and infants are given three doses at 2, 4, and 6 months of age. A multi-site global field trial showed that protection from any rotavirus gastroenteritis was 74% (95%CI 67–80) and from severe rotavirus gastroenteritis was 98% (95%CI 88–100) through the first rotavirus season (5). Rotarix® is a live attenuated human monovalent rotavirus strain G1P1A(8) licensed for the prevention of G1 and non-G1 (G3, G4, and G9) serotypes due to its cross-reactivity. Rotarix® contains a minimum of 1×106 Cell Culture Infective Dose (CCID50) and is administered as two doses, typically at 2 and 4 months of age. A multi-site global efficacy field trial showed an efficacy of 87% (95%CI 80–92) from any rotavirus gastroenteritis and 96% (95%CI 90–99) from severe rotavirus gastroenteritis through the first rotavirus season (145). Based on these results, the current recommendations of the Advisory Committee on Immunization Practices (ACIP) are to routinely vaccinate U.S. infants with either licensed rotavirus vaccine (146). Although efficacy data in developing countries reveal vaccine efficacy approximately 30% lower than described in the developed world (7), the disease burden is so high that inclusion of rotavirus vaccination into all national immunization programs has been recommended by the Strategic Advisory Group of Experts of the WHO (147). The most widely studied immune responses to rotavirus are humoral. Natural infection induces homologous protection as well as heterologous protection (148). The serum IgA anti-rotavirus response following vaccination, more than the IgG response, appears to be a marker of protection, although a surrogate that fully correlates with clinical outcome has not been determined (149).
Multiple attempts have been made to correlate stool, jejunal, and serum humoral immune response with protection. In adults challenged with rotavirus, jejunal and serum neutralizing antibody predicted protection from illness (150). Similarly, in children, systemic rotavirus IgA correlated with protection (151). Interestingly, the latter group measured the secretory component of IgA and hypothesized that IgA in serum is the result of ‘spillover’ from locally produced IgA. Supporting this hypothesis is a correlation of peripheral blood rotavirus IgA ASCs with serum rotavirus IgA (r=0.57 p=0.013) and intestinal rotavirus IgA ASCs as obtained by biopsy (r=0.72 p=0.004) by Pearson’s correlation coefficient (152). When stool sIgA was assessed in adults challenged with wildtype rotavirus, univariate correlations with protection were found for serum neutralizing antibody, rotavirus-specific IgG and IgA, as well as for jejunal neutralizing antibody and rotavirus IgA, but not for stool sIgA/total IgA. Multivariate logistic regression only identified serum rotavirus IgG as independently associated with protection (153). Several day care studies, on the other hand, did report an association of fecal rotavirus IgA and protection from illness (154, 155). Interestingly, the investigators identified an association but did not examine the ratio between rotavirus-specific and total IgA. Dividing antigen-specific IgA by total IgA allows normalizing the data for variable amounts of mucus and water in stool specimens. In our experience, total IgA titers as well as antigen-specific sIgA both increase in a non-specific manner after infection or vaccination. Thus, it is important to report data both as antigen-specific fecal IgA as well antigen-specific over total IgA. Other methods used to minimize the variability among stool specimens include mixing prior to sampling and lyophilization. The large phase 3 trials assessing efficacy of Rotashield®,Rotateq®, and Rotarix® did not report fecal rotavirus sIgA or total IgA. Serum responses were measured for a subset of enrolled subjects and immune surrogates of protection have not been definitively identified. It is logistically difficult and more expensive to assess immunological markers other than serum antibody, and sponsors of large efficacy trials may not benefit from identifying an immune surrogate of protection that will make it easier for competitors to have their products licensed.
Adjuvants to enhance immunogenicity of enteric vaccines
Whereas identifying safe and effective adjuvants to enhance the immunogenicity of parenteral vaccines is challenging, this quest is even more difficult with respect to oral vaccines. Indeed, most adjuvants that are currently licensed in combination with parenteral vaccines function poorly, if at all, as adjuvants when combined with oral vaccines. Striking exceptions are mutants of CT and the E. coli heat-labile enterotoxin (LT). Genetically defined mutant toxins with reduced toxicity are the most promising adjuvants to augment local and systemic immune responses to antigens administered orally. Although the mechanisms of adjuvanticity of these mutant toxins are not completely understood, they appear to involve both the capacity of these molecules to bind distinct ganglioside receptors (present in most nucleated mammalian cells) and their ADP-ribosylating activity (156). Some candidate mutants such as LTR192G retain their NAD binding sites intact but have amino acid substitutions in the portion of the A1 subunit that must be nicked by a protease for full enterotoxigenicity to ensue (157, 158). Other mutant toxin adjuvants, such as LTK63 or LTR72 (159–161), harbor mutations within the NAD binding site that greatly diminish the ADP ribosylating enzymatic activity of the molecule, thereby diminishing enterotoxigenic potential; these molecules nevertheless retain measurable adjuvanticity. Studies in mice showed LT and CT-mediated recruitment of DCs into the follicle-associated epithelium, and DC maturation and migration into interfollicular T-cell areas of the Peyer’s patches (162) and MLNs (163). A mutant LT harboring a single amino acid substitution, LTR192G, has been evaluated in several human clinical studies, one of which was a dose escalation safety analysis with dosage levels up to 100 μg in comparison with native LT (up to 5 μg) (164). Three other clinical trials evaluated the adjuvant activity of LTR192G when given with Campylobacter and Helicobacter pylori whole cell vaccines (165–167) as well as E. coli colonization surface antigen 6 (168). In all these studies, LTR192G was generally well tolerated (in doses up to 50 μg) and elicited vaccine-specific IgA and IgG and ASCs. The vaccine-adjuvant combination did elicit a transient self-limiting diarrhea in some subjects. A significant increase in the proportion of responders to Campylobacter inactivated whole cell antigen was observed when this vaccine was given with LTR192G, although subjects were not protected against experimental challenge with 109 live C. jejuni strain 81–176 (169). Additional vaccine-specific responses observed included IFN-γ production and fecal IgA. In contrast, LTR192 failed to boost the IgG responses to H. pylori whole cell and only a marginal increase in serum IgA was reported (165). A second generation of CT and LT double mutants (e.g. LTR192G/L211A, dmCTE112K/KDGL) have been developed (170, 171) and are being tested in humans.
The use of oral adjuvants carries advantages and disadvantages. On the one hand, they can be powerful tools to enhance immunogenicity for orally delivered antigens by recruiting and activating innate immune cells and triggering pro-inflammatory signals necessary to activate an adaptive response. On the other hand, oral adjuvants can disturb the delicate balance that allows the host to downregulate responses to food and potentially harmful environmental antigens; a disruption of this balance can have major implications including, perhaps, the development of inflammatory bowel disease. This becomes an even greater concern when considering very young infants (including in some instances newborns) as the targets for oral adjuvanted immunization as the mechanisms of tolerance begin to establish early in life. The mucosal epithelial barrier and immunoregulatory network are poorly developed in newborns and the first year of life is critical with regard to the induction of food allergy (172).
Oral live vaccine candidates against diarrheal pathogens in the pipeline
S. Typhi
Despite the pioneering role and many positive attributes of Ty21a (including its excellent safety record, clinical acceptability, and stimulation of a moderate level of long-lived protection), this vaccine has some drawbacks such as its empiric attenuation, modest immunogenicity, and the need to administer three or four spaced doses in order to confer a moderate level of enduring protection (85, 173, 174). Accordingly, a new generation of live oral typhoid vaccines has been developed with candidates that aim to be as well tolerated clinically as Ty21a yet more immunogenic and protective and require administration of just a single oral dose.
The new strains of S. Typhi have been genetically engineered to harbor precise attenuating deletion mutations. Four candidates, all derivatives of wildtype S. Typhi strain Ty2 (which is also the parent of Ty21a), have been successfully tested in Phase 1 and 2 clinical trials, where they were shown to be well tolerated and immunogenic after ingestion of just a single oral dose. These live oral vaccine candidates include strains M01ZH09, a derivative with deletion mutations in aroC (rendering the strain auxotrophic for 2,3 dihydroxybenzoate, a substrate required by Salmonella to sustain growth but that is not available in sufficient quantity in human tissues) and ssaV (a component of the type III secretion system encoded by Salmonella Pathogenicity Island-2) (175–177), Ty800, a derivative deleted in phoP/phoQ (which encodes a global regulatory system involved in survival within macrophages) (178), CVD 908-htrA harboring deletion mutations in aroC, aroD and htrA (encoding a serine protease that functions as a stress protein) (174, 179) and CVD 909, a further derivative of CVD 908-htrA that constitutively expresses Vi (180;181).
Oral immunization of adult volunteers with these vaccine strains elicited gut-derived IgA ASCs and serum IgG and IgA antibodies to S. Typhi LPS O antigen (Fig. 1). CMI responses were intensively studied in clinical trials with CVD 908 (the parent strain of CVD 908-htrA, harboring deletions in aroC and aroD), CVD 908-htrA and CVD 909. CVD 908 and CVD 908-htrA elicited robust CMI responses including S. Typhi-specific CD4+ T cells with the capacity to produce Th1-type cytokines (i.e. IFN-γ and TNF-αin the absence of IL-4 and IL-5), as well as CD8+ CTLs that kill S. Typhi-infected target cells (102, 182, 183). Immunization with CVD 909 also elicited a wide array of CMI responses similar to those induced by CVD 908-htrA (184). We have also demonstrated that PBMCs from Ty21a and CVD 909 vaccinees additionally secreted IL-1β, TNF-α, and IL-10 following in vitro incubation with S. Typhi flagella (184, 185). Further studies revealed that oral immunization with attenuated S. Typhi strains elicits diverse S. Typhi-specific IFN-γ-secreting CD4+ and CD8+ TCM andTEM subsets that express integrin α4/β7 orCD62L, thus having the potential to migrate either to the gut (integrin α4/β7+) or secondary lymphoid tissues (CD62L+) (186). These studies showed for the first time CD3+CD8+ TEMRA subsets induced through oral vaccination with live bacteria. CD3+CD8+ TEMRA subsets are widely regarded as the most active effector cells (38, 187). Of importance, throughout these studies we observed similar antibody and CMI responses (both qualitatively and quantitatively) in subjects immunized with 1 dose of the S. Typhi live vaccine candidates CVD 908, CVD 908-htrA and CVD 909 or with 4 doses of Ty21a, suggesting that these newer recombinant S. Typhi strains might be as protective as the licensed Ty21a vaccine.
Lymphoproliferative responses and IFN-γ production have also been observed in subjects immunized with M01ZH09 (176, 177). In clinical studies conducted in the U.S. and Vietnam, M01Z09 was well tolerated and immunogenic in children 5–14 years of age, as well as in adults. Collectively, these results illustrate the wide range of effector responses that can be elicited by a single immunization with the modern generation of recombinant attenuated S. Typhi vaccine strains in different age groups. It is likely that each of these effector responses contributes to protection against typhoid fever.
S. Paratyphi
Paratyphoid fever is the clinically identical febrile infection caused by Salmonella Paratyphi A or B (or more rarely C) (188). Typhoid and paratyphoid fevers are often referred to collectively as enteric fevers. In Asia, S. Paratyphi A is emerging to contribute an increasingly larger proportion of all enteric fevers (189, 190). During the field trials of Ty21a in Area Occidente and Area Norte in Santiago, Chile there was a considerable amount of paratyphoid fever due to S. Paratyphi B, thereby allowing an estimation of vaccine efficacy against S. Paratyphi B (191). There was a trend in each trial indicating protection against S. Paratyphi B disease (56% efficacy in Norte and 38% efficacy in Occidente). To enhance statistical power, an analysis was performed of pooled data from the two trials. In the pooled analysis, Ty21a conferred significant protection against Paratyphoid B disease, 49% vaccine efficacy (CI, 8%–73%; p=0.019). Given the ability of attenuated strains of S. Typhi to function as live oral vaccines that prevent typhoid fever and in view of the partial protection conferred by oral Ty21a against S. Paratyphi B disease (191), one rational strategy to prevent S. Paratyphi A and B disease is the development of attenuated strains of these serovars to serve as live oral vaccines. Accordingly, a candidate live oral S. Paratyphi A vaccine strain was developed (192). Based on the excellent preclinical and clinical experience with guaBA mutants of S. Typhi (181) and Shigella (193), a deletion in guaBA was introduced into wild type S. Paratyphi 9150 as the primary attenuating mutation. An additional deletion mutation was then made in clpX to derive candidate vaccine strain CVD 1902. In pre-clinical studies, CVD 1902 was markedly attenuated compared to the wildtype parent. A Phase 1 clinical trial has been initiated.
Shigella
Shigella is an intracellular Gram-negative rod that causes approximately 165 million cases of shigellosis each year, of which 1.1 million people are estimated to die. It is an antigenically diverse pathogen containing four species (or groups) and 50 serotypes and subserotypes. Fourteen serotypes of Shigella flexneri, 20 serotypes of Shigella boydii, 15 serotypes of Shigella dysenteriae, and one serotype of Shigella sonnei make development of a vaccine challenging (11). It is generally acknowledged that the protection stimulated by a Shigella vaccine must be broad enough in spectrum to protect against 16 serotypes, including S. dysenteriae 1, all 14 S. flexneri types and S. sonnei. Epidemiologically across the world, these are the most important serotypes from the purview of prevalence and disease severity (11).
When it reaches the mucosa of the terminal ileum and colon, Shigella traverses the follicle-associated epithelium and is taken up by phagocytic cells; the bacteria then invade the neighboring epithelial cells of the mucosa through the basolateral pole, spreading cell to cell. Apoptosis of infected macrophages leading to release of pro-inflammatory cytokines and stimulation of the innate immune system of the gut mucosa elicits a strong inflammatory response involving the influx of polymorphonclear neutrophilic leukocytes and destruction of the intestinal epithelium. Shigella, depending on serotype, also elaborate one or more toxins that contribute to virulence (11).
Natural clinical Shigella infection confers ~75% protection against illness upon subsequent exposure to the homologous Shigella serotype and in some instances against heterologous serotypes. Antibodies (serum or mucosal) directed against the LPS O-antigen appear to play a major role in protection (194, 195). The first line of defense, however, occurs at the mucosa. This has been illustrated by the efficacy of passively transferred type-specific oral immunoglobulin in preventing shigellosis (196) and perhaps the ameliorating effects of breastfeeding on disease severity in infants in developing countries (197). In healthy adult volunteers, gut-derived O-specific IgA ASCs circulating in the bloodstream 7–10 days after oral vaccination is a measure of intestinal priming that has been correlated with vaccine efficacy (198, 199). Cell-mediated immunity may also contribute to the defense against this intracellular pathogen. Upregulation of IFN-γ production and expression of IFN-γ receptor were seen in the epithelium of rectal biopsies from Bangladeshi patients convalescing from shigellosis (200), and elevated levels were found in both serum and stool (201, 202). Moreover, we showed a predominant Th1-type response with production of IFN-γ and IL-10 but not IL-4, IL-5, IL-12, or IL-15, in PBMCs from volunteers challenged with modified virulent S. dysenteriae 1 strain SC595 upon stimulation with Shigella antigens (including Ipa) (203).
A pentavalent strategy developed at the CVD purports that 5 Shigella strains (S. sonnei, S. dysenteriae 1, and S. flexneri 2a, 3a, and 6) can collectively provide the necessary broad spectrum protection needed to achieve a vaccine of global utility (11). This strategy is based on the assumption (from analysis of Shigella O antigens and animal cross protection studies) that inclusion of S. flexneri 2a, 3a, and 6 in the vaccine will provide cross protection against the other 11 S. flexneri serotypes because of shared group antigens (11;204).
Molecular engineering has enabled the development of live oral Shigella vaccine candidates with defined deletion mutations in genes involved in metabolism (guaBA), cell-cell spread (virG), and toxin production (sen, set, and stxA). CVD 1208 is a prototype S. flexneri 2a vaccine that has deletions in guaBA, sen, and set. It proved to be well tolerated in a Phase 1 clinical trial and induces a geometric mean IgA ASC of 62 per 106 PBMCs, with 71% of subjects exhibiting fourfold rises in serum IgA and/or IgG and 86% exhibiting fourfold rises in fecal IgA at 109 CFU (193, 205). For regulatory considerations, CVD 1208 was reconstructed on animal-free media and the new construct was designated as CVD 1208S. A Phase 1 study in which subjects were randomized to receive a single oral dose of either placebo or CVD 1208S at 8 or 9-logs gave similar results to those reported for CVD 1208 (193). Moreover, IFN-γ production by PBMCs in response to Shigella antigens was observed in 57% recipients of 109 CFU (205). Phase 2 studies with CVD 1208S are planned.
Alternative vaccine approaches and immunization regimens
Live vector vaccines
Attenuated strains of S. Typhi, S. Typhimurium, Shigella and other organisms have been utilized as mucosally administered live vectors to deliver protective antigens from unrelated pathogens. A small number of these vaccines have been evaluated in clinical studies (206). S. Typhimurium-based live vectors have been extraordinarily successful stimulating immune responses against a variety of foreign antigens in mice, many of which showed protection against experimental challenge. However, despite this success, when S. Typhi live vector homologues were tested in Phase 1 clinical trials, immune responses were disappointing (206). Several groups are devoting efforts to improve the live vector strategy (206, 207), refining strategies to delay attenuation and antigen expression until certain niches are reached, improving biocontainment, optimizing plasmid stability and chromosomal antigen expression, engineering antigen export systems and using non-antibiotic resistance markers in expression plasmids (208), among others. One strategy that has allowed enhanced immune responses to a foreign antigen expressed in a live vector vaccine is through heterologous prime-boost immunization (209–212). A recent study from our group showed that rhesus macaques primed orally with S. Typhi expressing B. anthracis protective antigen (PA) and subsequently boosted parenterally with a single dose of either recombinant PA or PA-containing licensed Biothrax® vaccine mounted extraordinarily rapid serum anthrax toxin neutralizing antibody levels that exceeded protective levels in < one week (210). The prime-boost strategy has improved efficacy for other vaccines and pathogens (213). The immunological mechanisms responsible for the enhanced responses are not completely understood but likely involve access to different immune cell populations in the mucosal and systemic compartment. This approach is yet to be tested in humans.
VLP vaccines
VLPs consisting of self-assembling virus structural subunits devoid of nucleic acids have been examined as oral vaccines in humans (1). A study conducted at the CVD showed that Norwalk VLP given orally to adult volunteers were able to generate Norwalk-specific serum IgG responses, mucosally primed IgA ASCs and fecal sIgA, as well as systemic T-cell proliferative responses (214).
Adenovirus-based vaccines
Replication-incompetent adenovirus-based vaccines administered parenterally have been shown to induce cytotoxic lymphocytes and CD4+ T-cell responses in humans. These adenovirus-based vaccines are also being investigated to serve as oral vaccines to deliver antigens to the gut to generate systemic and mucosal immunity. This approach has been applied to the development of human immunodeficiency virus vaccines to help establish a local cellular response (in addition to a strong systemic response) that can mitigate the high rate of virus replication and the severe depletion of CD4+ TEM cells in the intestinal and other mucosal tissues that occurs shortly after infection (215, 216). Oral priming with an enteric-coated rAd5-based HIV vaccine followed by intranasal boosting with an envelope peptide cocktail adjuvanted with a mutant CT, induced HIV-specific IFN-γ-producing CD4+ and CD8+ effector memory T cells in the intestine of rhesus macaques (217). In a rodent model, oral priming with rAd41-Env (Ad 41 is a human serotype with gut tropism) followed by intramuscular boost with rAd5-Env induced the highest levels of antigen-specific tetramer-positive CD8+ T-cell responses in the small intestine. In contrast, heterologous intramuscular rAd41-Env followed by intramuscular rAd5-Env boost induced the highest systemic response (218). Oral administration of rAd41-Env does not stimulate neutralizing anti-rAd41 antibodies (i.e. anti-vector antibodies) and was able to prime for an anamnestic anti-Env response to a subsequent rAd-Env boost (218).
Plant-based vaccines
An innovative live vector strategy popularized in the 1990s was the concept of expressing protective vaccine antigens in transgenic plants for use as ‘edible vaccines’ (219). A number of plants such as potatoes, tomatoes, lettuce, bananas, corn, and rice were used to express antigens from various pathogens including V. cholerae, ETEC, Norwalk virus, hepatitis B virus, and rotavirus, among others (220). It was argued that the potential ease of administration and supposed low cost of edible vaccines might make them useful for getting vaccines to impoverished populations in the developing world. In early Phase 1 clinical trials proof-of-principle transgenic plant vaccines worked impressively well (221, 222). CVD investigators evaluated in volunteers an edible ETEC vaccine consisting of the B subunit of the E. coli heat-labile enterotoxin (LT-B) expressed in potato and demonstrated the induction of serum LT-specific (including neutralizing) antibodies and gut-derived ASCs (221). Similarly, potatoes expressing the Norwalk virus capsid protein were also successfully tested in dose-escalation, in human volunteers (222). All these subjects responded with vaccine-specific IgA ASCs, and 90% of those who received the lowest dose (250 μg) developed rises in serum IgG. T-cell proliferation and IFN-γ secretion were observed transiently in the low dose groups. Transgenic corn expressing recombinant E. coli LT-B was also studied in a Phase 1 human clinical study; 78% of the vaccinees developed rises in LTB-specific serum IgG and ASCs, whereas 44% developed stool IgA (223). However, it is now generally accepted that transgenic plant edible vaccines are unlikely to be able to meet rigorous regulatory hurdles that would allow licensure, and this concept has been largely abandoned. However, the sophistication of expression of vaccine antigens in plants, sometimes in conjunction with adjuvants, has increased in recent years. Accordingly, it is now widely viewed that recombinant plants grown in large, carefully regulated green houses may serve as an alternative method for large-scale production of vaccine antigens.
Immune correlates of protection related to oral vaccines
All vaccine developers try to identify immune responses to vaccination that correlate with the protection conferred by the vaccine. Ideally, a serological correlate is identified that is easy to measure, is consistent, and has a threshold level above which protection is likely to be present in the vaccinated subject. Different types of correlates and surrogates of protection for various licensed vaccines have recently been reviewed (130). During vaccine development, such correlates can be derived from (i) animal models that recapitulate disease, (ii) randomized, controlled field trials of efficacy, (iii) experimental challenge models in volunteers, and (iv) seroepidemiologic field studies comparing naturally infected individuals who develop confirmed disease versus other similarly exposed individuals who remain asymptomatic. Similarly, post-licensure correlates of protection can be identified or recalibrated by comparing the titers of specific antibody among exposed vaccinated subjects and exposed unvaccinated subjects in relation to whether or not they develop clinical illness; these opportunities typically come in the course of outbreaks (224). With respect to oral vaccines, volunteer challenge models have been established for cholera (6,118, 225, 226), Shigella (124), E. coli (227–229), and C. jejuni (230).
Defined immunological end-points that can predict vaccine efficacy facilitate the evaluation of vaccines during both pre-licensure development and post-licensure optimization and allow the comparison of formulations, immunization schedules or other modification to the final product. A correlate enables the performance of the vaccine to be predicted in different populations and settings and to bridge old and new vaccine candidates. If the correlate of protection is robust and has a well accepted threshold titer, national regulatory agencies may accept immunological endpoints as the basis for licensure in pivotal clinical studies or bridging studies (e.g. as has been done for serum bactericidal antibody titers and new meningococcal vaccines or anti-PRP antibody and Haemophilus influenzae type b conjugate vaccines). The correlates of protection that exist for current vaccines are in all instances serum antibodies, whether for viral vaccines (e.g. > 120 mIU of plaque reduction inhibition antibody for measles) or bacteria (e.g. ≥ 0.01 IU/ml of tetanus antitoxin). In contrast, although attaining a serum neutralization titers ≥ 1:8 is a strong correlate of protection against paralytic polio following OPV, correlates of immune protection are not well defined for the other licensed oral vaccines such as typhoid cholera or rotavirus. Vibriocidal antibodies have been correlated with protection from cholera, but the correlation is relative rather than absolute and some vaccinated subjects with elevated serum vibriocidal titers may still experience clinical cholera diarrhea (231). With increasing age in a cholera-endemic area of Bangladesh, the geometric mean titer (GMT) of vibriocidal antibody increased. For every doubling of the vibriocidal GMT, there was a halving of the incidence of cholera (232, 233). Family contacts of index cholera patients had lower secondary attack rates for cholera if they had elevated baseline vibriocidal titers (104). Since is unclear how complement-binding vibriocidal antibodies protect against a mucosally colonizing non-invasive organism, we have hypothesized that these serum antibodies are more likely a surrogate for specific mucosal responses, which are the ones that actually mediate protection.
Measurement of mucosal sIgA in stool or intestinal lavage specimens in vaccinees has also not correlated absolutely with protection against most enteric pathogens where this has been studied. This may be because the immune response to most enteric pathogens is complex and redundant and may require combinations of several immune effectors to better predict protection (130). Improved assays and statistical approaches are providing an opportunity to assess the immune response to better quantify protection; novel statistical approaches such as classification and regression tree (CART) analysis and multivariate logistic regression (LR) have been applied to define immune correlates of protection in pertussis (234) and may be applied to enteric pathogens. Systems biology approaches incorporating genomics (235), transcriptomics (236), immune phenotype, and mathematical modeling (237) may assist in understanding the mechanisms of protection and more accurately predicting optimal protection from combinations of immune markers.
Challenges of effective oral immunization
Whereas oral vaccines offer many advantages including practicality, they also face challenges to achieve their full potential. At the global level, perhaps the greatest challenge is to understand why many oral vaccines, e.g. Sabin polio (8, 238), rotavirus (7,239,240), and cholera (241–244), exhibit diminished immunogenicity or lower levels of protection in poor populations in developing countries compared to the performance of those vaccines in industrialized country populations. This effect correlates with the socioeconomic level of the population immunized; the more impoverished the subjects, the lower the immune response or efficacy level. In very young infants, elevated levels of maternally transferred antibodies and high titers of antibodies in breast milk may in part explain diminished responses to the oral vaccines. In addition, human breast milk, which is known to promote the development and maturation of the mucosa-associated lymphoid tissue, contains numerous immunomodulatory components that influence immunological competency, some of which have tolerogenic functions such as TGFβ, IL-10, and vitamin A (245). On the other hand, diminished responses have also been observed in older children and adults. Among the factors that have been associated with diminished immune responses to oral vaccines in older children in developing countries are small bowel bacterial overgrowth (indicative of environmental enteropathy) (246) and heavy intestinal helminth infestation (247). Before this barrier can be overcome, research will have to unravel the pathophysiologic basis of the diminished immunogenicity of oral vaccines in impoverished populations. It will then be possible to rationally design counter measures.
Conclusions and future prospects
Oral vaccines hold great promise to reduce the burden of disease and mortality caused by enteric pathogens (e.g. rotavirus, ETEC, V. cholerae O1 and O139, Shigella), as well as by pathogens that invade via the intestinal mucosa as a preliminary to causing systemic disease (e.g. S. Typhi and S. Paratyphi A and B) or those that disseminate to affect distal organs (e.g. polioviruses). In industrialized country populations, live attenuated organisms that mimic natural infection stimulate the gut immune system in a powerful manner, eliciting broad and robust immune responses that encompass serum and mucosal antibodies, gut-derived ASCs, and effector and memory T cells that act synergistically to protect against disease. Some live oral vaccines also elicit B and T-memory responses that confer long-term efficacy. The downside of live vaccines is the ever present concern of unacceptable reactogenicity, especially in young children and immunocompromised hosts. Non-living vaccines offer the advantage of being better tolerated but have sometimes been inadequately immunogenic. Several licensed viral (e.g. Sabin polio) and bacterial (e.g. Ty21a) oral vaccines have been used for decades, attesting to the feasibility of this approach. Over the years, scientists have tried a number of strategies to deliver vaccine antigens via the gut, such as recombinant live vectors, non-living particles, replication-deficient viruses, and antigen-expressing plants, among others. Despite the great promise shown in animal models, most of these approaches have been disappointing when tested in Phase 1 proof-of-principle clinical trials in humans. Experience suggests that the major impediment to creating successful future oral vaccines for global use will be to achieve adequate safety for industrialized country populations, while retaining immunogenicity and efficacy in developing country populations.
The mechanisms that underlie the generation of effective immune responses in the gastrointestinal mucosal following oral immunization are complex and involve both the mucosal and systemic immune systems. Areas of research that require more intensive study include the following: the interaction of vaccine antigens with innate immune cells in the gut and the modulation of adaptive immunity; development of safe yet effective mucosal adjuvants that can be used with oral vaccines; better understanding of the processes of lymphocyte trafficking and homing to mucosal effector sites, and ways to more effectively induce robust and persistent immunological memory.
Acknowledgments
The authors thank Dr. Lou Bourgeois and Richard Walker (PATH) for reviewing the adjuvant section and providing clinical data, and Dr. Theresa Huwar (CVD) for editorial assistance. This paper includes work funded, in part, by NIAID, NIH, DHHS federal research grants and contracts R01 AI065760-01 & R01 AI089519 (to M. F. Pasetti), K23AI065759 to J.S.; R01 AI036525 & U19 AI082655 (CCHI) (to M. B. Sztein) and R01 AI029471 & U54 AI57168 (to M. Levine).
Reference List
- 1.Plotkin SA. Vaccines: the fourth century. Clin Vaccine Immunol. 2009;16:1709–1719. doi: 10.1128/CVI.00290-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruiz-Palacios GM, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354:11–22. doi: 10.1056/NEJMoa052434. [DOI] [PubMed] [Google Scholar]
- 3.Germanier R, Fuer E. Isolation and characterization of Gal E mutant Ty 21a of Salmonella typhi: a candidate strain for a live, oral typhoid vaccine. J Infect Dis. 1975;131:553–558. doi: 10.1093/infdis/131.5.553. [DOI] [PubMed] [Google Scholar]
- 4.Clemens JD, et al. Field trial of oral cholera vaccines in Bangladesh: results from three-year follow-up. Lancet. 1990;335:270–273. doi: 10.1016/0140-6736(90)90080-o. [DOI] [PubMed] [Google Scholar]
- 5.Vesikari T, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;354:23–33. doi: 10.1056/NEJMoa052664. [DOI] [PubMed] [Google Scholar]
- 6.Levine MM, et al. Safety, immunogenicity, and efficacy of recombinant live oral cholera vaccines, CVD 103 and CVD 103-HgR. Lancet. 1988;2:467–470. doi: 10.1016/s0140-6736(88)90120-1. [DOI] [PubMed] [Google Scholar]
- 7.Madhi SA, et al. Effect of human rotavirus vaccine on severe diarrhea in African infants. N Engl J Med. 2010;362:289–298. doi: 10.1056/NEJMoa0904797. [DOI] [PubMed] [Google Scholar]
- 8.Patriarca PA, Wright PF, John TJ. Factors affecting the immunogenicity of oral poliovirus vaccine in developing countries: review. Rev Infect Dis. 1991;13:926–939. doi: 10.1093/clinids/13.5.926. [DOI] [PubMed] [Google Scholar]
- 9.Zaman K, Sack DA, Yunus M, et al. Successful co-administration of a human rotavirus and oral poliovirus vaccines in Bangladeshi infants in a 2-dose schedule at 12 and 16 weeks of age. Vaccine. 2009;27:1333–1339. doi: 10.1016/j.vaccine.2008.12.059. [DOI] [PubMed] [Google Scholar]
- 10.Armah GE, et al. Efficacy of pentavalent human-bovine reassortant rotavirus vaccine against severe rotavirus gastroenteritis in sub-Saharan Africa: a randomized, double-blind, placebo-controlled trial. Lancet. 2010;376:606–614. doi: 10.1016/S0140-6736(10)60889-6. [DOI] [PubMed] [Google Scholar]
- 11.Levine MM, Kotloff KL, Barry EM, Pasetti MF, Sztein MB. Clinical trials of Shigella vaccines: two steps forward and one step back on a long, hard road. Nat Rev Microbiol. 2007;5:540–553. doi: 10.1038/nrmicro1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soloff AC, Barratt-Boyes SM. Enemy at the gates: dendritic cells and immunity to mucosal pathogens. Cell Res. 2010;20:872–885. doi: 10.1038/cr.2010.94. [DOI] [PubMed] [Google Scholar]
- 13.Johansson-Lindbom B, Agace WW. Generation of gut-homing T cells and their localization to the small intestinal mucosa. Immunol Rev. 2007;215:226–242. doi: 10.1111/j.1600-065X.2006.00482.x. [DOI] [PubMed] [Google Scholar]
- 14.Siewert C, et al. Induction of organ-selective CD4+ regulatory T cell homing. Eur J Immunol. 2007;37:978–989. doi: 10.1002/eji.200636575. [DOI] [PubMed] [Google Scholar]
- 15.Mora JR, et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314:1157–1160. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- 16.Cerutti A. Location, location, location: B-cell differentiation in the gut lamina propria. Mucosal Immunol. 2008;1:8–10. doi: 10.1038/mi.2007.8. [DOI] [PubMed] [Google Scholar]
- 17.Macpherson AJ, Gatto D, Sainsbury E, Harriman GR, Hengartner H, Zinkernagel RM. A primitive T cell-independent mechanism of intestinal mucosal IgA responses to commensal bacteria. Science. 2000;288:2222–2226. doi: 10.1126/science.288.5474.2222. [DOI] [PubMed] [Google Scholar]
- 18.Brandtzaeg P. Induction of secretory immunity and memory at mucosal surfaces. Vaccine. 2007;25:5467–5484. doi: 10.1016/j.vaccine.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Kett K, Brandtzaeg P, Radl J, Haaijman JJ. Different subclass distribution of IgA-producing cells in human lymphoid organs and various secretory tissues. J Immunol. 1986;136:3631–3635. [PubMed] [Google Scholar]
- 20.Macpherson AJ, McCoy KD, Johansen FE, Brandtzaeg P. The immune geography of IgA induction and function. Mucosal Immunol. 2008;1:11–22. doi: 10.1038/mi.2007.6. [DOI] [PubMed] [Google Scholar]
- 21.Hutchings AB, et al. Secretory immunoglobulin A antibodies against the sigma1 outer capsid protein of reovirus type 1 Lang prevent infection of mouse Peyer's patches. J Virol. 2004;78:947–957. doi: 10.1128/JVI.78.2.947-957.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwartz-Cornil I, Benureau Y, Greenberg H, Hendrickson BA, Cohen J. Heterologous protection induced by the inner capsid proteins of rotavirus requires transcytosis of mucosal immunoglobulins. J Virol. 2002;76:8110–8117. doi: 10.1128/JVI.76.16.8110-8117.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mazanec MB, Kaetzel CS, Lamm ME, Fletcher D, Nedrud JG. Intracellular neutralization of virus by immunoglobulin A antibodies. Proc Natl Acad Sci USA. 1992;89:6901–6905. doi: 10.1073/pnas.89.15.6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mazanec MB, Nedrud JG, Kaetzel CS, Lamm ME. A three-tiered view of the role of IgA in mucosal defense. Immunol Today. 1993;14:430–435. doi: 10.1016/0167-5699(93)90245-G. [DOI] [PubMed] [Google Scholar]
- 25.Boullier S, et al. Secretory IgA-mediated neutralization of Shigella flexneri prevents intestinal tissue destruction by down-regulating inflammatory circuits. J Immunol. 2009;183:5879–5885. doi: 10.4049/jimmunol.0901838. [DOI] [PubMed] [Google Scholar]
- 26.Israel EJ, et al. Expression of the neonatal Fc receptor, FcRn, on human intestinal epithelial cells. Immunology. 1997;92:69–74. doi: 10.1046/j.1365-2567.1997.00326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dickinson BL, et al. Bidirectional FcRn-dependent IgG transport in a polarized human intestinal epithelial cell line. J Clin Invest. 1999;104:903–911. doi: 10.1172/JCI6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshida M, et al. Human neonatal fc receptor mediates transport of IgG into luminal secretions for delivery of antigens to mucosal dendritic cells. Immunity. 2004;20:769–783. doi: 10.1016/j.immuni.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 29.Quiding-Jarbrink M, et al. Differential expression of tissue-specific adhesion molecules on human circulating antibody-forming cells after systemic, enteric, and nasal immunizations. A molecular basis for the compartmentalization of effector B cell responses. J Clin Invest. 1997;99:1281–1286. doi: 10.1172/JCI119286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang SY, et al. Cutting edge: Langerin+ dendritic cells in the mesenteric lymph node set the stage for skin and gut immune system cross-talk. J Immunol. 2008;180:4361–4365. doi: 10.4049/jimmunol.180.7.4361. [DOI] [PubMed] [Google Scholar]
- 31.el-Kamary SS, et al. Adjuvanted intranasal Norwalk VLP vaccine elicits antibodies and antibody secreting cells expressing homing receptors in mucosal and peripheral lymphoid tissues. J Infect Dis. 2010 doi: 10.1086/657087. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kunkel EJ, Butcher EC. Plasma-cell homing. Nat Rev Immunol. 2003;3:822–829. doi: 10.1038/nri1203. [DOI] [PubMed] [Google Scholar]
- 33.Crotty S, Aubert RD, Glidewell J, Ahmed R. Tracking human antigen-specific memory B cells: a sensitive and generalized ELISPOT system. J Immunol Methods. 2004;286:111–122. doi: 10.1016/j.jim.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 34.Avery DT, Ellyard JI, Mackay F, Corcoran LM, Hodgkin PD, Tangye SG. Increased expression of CD27 on activated human memory B cells correlates with their commitment to the plasma cell lineage. J Immunol. 2005;174:4034–4042. doi: 10.4049/jimmunol.174.7.4034. [DOI] [PubMed] [Google Scholar]
- 35.Rojas OL, et al. Evaluation of circulating intestinally committed memory B cells in children vaccinated with attenuated human rotavirus vaccine. Viral Immunol. 2007;20:300–311. doi: 10.1089/vim.2006.0105. [DOI] [PubMed] [Google Scholar]
- 36.Harris AM, et al. Antigen-specific memory B-cell responses to Vibrio cholerae O1 infection in Bangladesh. Infect Immun. 2009;77:3850–3856. doi: 10.1128/IAI.00369-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simon JK, et al. Antigen-specific B memory cell responses to lipopolysaccharide (LPS) and invasion plasmid antigen (Ipa) B elicited in volunteers vaccinated with live-attenuated Shigella flexneri 2a vaccine candidates. Vaccine. 2009;27:565–572. doi: 10.1016/j.vaccine.2008.10.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oswald-Richter K, et al. Identification of a CCR5-expressing T cell subset that is resistant to R5-tropic HIV infection. PLoS Pathog. 2007;3:e58. doi: 10.1371/journal.ppat.0030058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 40.Lefrancois L. Development, trafficking, and function of memory T-cell subsets. Immunol Rev. 2006;211:93–103. doi: 10.1111/j.0105-2896.2006.00393.x. [DOI] [PubMed] [Google Scholar]
- 41.Butcher EC, Williams M, Youngman K, Rott L, Briskin M. Lymphocyte trafficking and regional immunity. Adv Immunol. 1999;72:209–253. doi: 10.1016/s0065-2776(08)60022-x. [DOI] [PubMed] [Google Scholar]
- 42.Salmi M, Jalkanen S. Lymphocyte homing to the gut: attraction, adhesion, and commitment. Immunol Rev. 2005;206:100–113. doi: 10.1111/j.0105-2896.2005.00285.x. [DOI] [PubMed] [Google Scholar]
- 43.Kunkel EJ, et al. Lymphocyte CC chemokine receptor 9 and epithelial thymus-expressed chemokine (TECK) expression distinguish the small intestinal immune compartment: Epithelial expression of tissue-specific chemokines as an organizing principle in regional immunity. J Exp Med. 2000;192:761–768. doi: 10.1084/jem.192.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheroutre H, Madakamutil L. Acquired and natural memory T cells join forces at the mucosal front line. Nat Rev Immunol. 2004;4:290–300. doi: 10.1038/nri1333. [DOI] [PubMed] [Google Scholar]
- 45.Muller S, Buhler-Jungo M, Mueller C. Intestinal intraepithelial lymphocytes exert potent protective cytotoxic activity during an acute virus infection. J Immunol. 2000;164:1986–1994. doi: 10.4049/jimmunol.164.4.1986. [DOI] [PubMed] [Google Scholar]
- 46.Fahrer AM, Konigshofer Y, Kerr EM, et al. Attributes of gammadelta intraepithelial lymphocytes as suggested by their transcriptional profile. Proc Natl Acad Sci USA. 2001;98:10261–10266. doi: 10.1073/pnas.171320798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Komano H, et al. Homeostatic regulation of intestinal epithelia by intraepithelial gamma delta T cells. Proc Natl Acad Sci USA. 1995;92:6147–6151. doi: 10.1073/pnas.92.13.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hayday A, Theodoridis E, Ramsburg E, Shires J. Intraepithelial lymphocytes: exploring the Third Way in immunology. Nat Immunol. 2001;2:997–1003. doi: 10.1038/ni1101-997. [DOI] [PubMed] [Google Scholar]
- 49.Groh V, Steinle A, Bauer S, Spies T. Recognition of stress-induced MHC molecules by intestinal epithelial gammadelta T cells. Science. 1998;279:1737–1740. doi: 10.1126/science.279.5357.1737. [DOI] [PubMed] [Google Scholar]
- 50.Saurer L, Mueller C. T cell-mediated immunoregulation in the gastrointestinal tract. Allergy. 2009;64:505–519. doi: 10.1111/j.1398-9995.2009.01965.x. [DOI] [PubMed] [Google Scholar]
- 51.Nagler-Anderson C, Bhan AK, Podolsky DK, Terhorst C. Control freaks: immune regulatory cells. Nat Immunol. 2004;5:119–122. doi: 10.1038/ni0204-119. [DOI] [PubMed] [Google Scholar]
- 52.Roncarolo MG, Gregori S. Is FOXP3 a bona fide marker for human regulatory T cells? Eur J Immunol. 2008;38:925–927. doi: 10.1002/eji.200838168. [DOI] [PubMed] [Google Scholar]
- 53.Allez M, Brimnes J, Dotan I, Mayer L. Expansion of CD8+ T cells with regulatory function after interaction with intestinal epithelial cells. Gastroenterology. 2002;123:1516–1526. doi: 10.1053/gast.2002.36588. [DOI] [PubMed] [Google Scholar]
- 54.Grdic D, Hornquist E, Kjerrulf M, Lycke NY. Lack of local suppression in orally tolerant CD8-deficient mice reveals a critical regulatory role of CD8+ T cells in the normal gut mucosa. J Immunol. 1998;160:754–762. [PubMed] [Google Scholar]
- 55.Hu D, Ikizawa K, Lu L, Sanchirico ME, Shinohara ML, Cantor H. Analysis of regulatory CD8 T cells in Qa-1-deficient mice. Nat Immunol. 2004;5:516–523. doi: 10.1038/ni1063. [DOI] [PubMed] [Google Scholar]
- 56.Chess L, Jiang H. Resurrecting CD8+ suppressor T cells. Nat Immunol. 2004;5:469–471. doi: 10.1038/ni0504-469. [DOI] [PubMed] [Google Scholar]
- 57.Mucida D, Salek-Ardakani S. Regulation of TH17 cells in the mucosal surfaces. J Allergy Clin Immunol. 2009;123:997–1003. doi: 10.1016/j.jaci.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ivanov II, et al. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4:337–349. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ochiai RL, et al. A study of typhoid fever in five Asian countries: disease burden and implications for controls. Bull World Health Organ. 2008;86:260–268. doi: 10.2471/BLT.06.039818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lin FY, et al. The epidemiology of typhoid fever in the Dong Thap Province, Mekong Delta region of Vietnam. Am J Trop Med Hyg. 2000;62:644–648. doi: 10.4269/ajtmh.2000.62.644. [DOI] [PubMed] [Google Scholar]
- 61.Sinha A, et al. Typhoid fever in children aged less than 5 years. Lancet. 1999;354:734–737. doi: 10.1016/S0140-6736(98)09001-1. [DOI] [PubMed] [Google Scholar]
- 62.Brooks WA, et al. Bacteremic typhoid fever in children in an urban slum, Bangladesh. Emerg Infect Dis. 2005;11:326–329. doi: 10.3201/eid1102.040422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ferreccio C, et al. Benign bacteremia caused by Salmonella typhi and paratyphi in children younger than 2 years. J Pediatr. 1984;104:899–901. doi: 10.1016/s0022-3476(84)80492-8. [DOI] [PubMed] [Google Scholar]
- 64.Connor BA, Schwartz E. Typhoid and paratyphoid fever in travellers. Lancet Infect Dis. 2005;5:623–628. doi: 10.1016/S1473-3099(05)70239-5. [DOI] [PubMed] [Google Scholar]
- 65.Charles RC, et al. Characterization of anti-Salmonella enterica serotype Typhi antibody responses in bacteremic Bangladeshi patients using Immuno-affinity Proteomic-based Technology (IPT) Clin Vaccine Immunol. 2010;17:1188–1195. doi: 10.1128/CVI.00104-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ortiz V, Isibasi A, Garcia Ortigoza E, Kumate J. Immunoblot detection of class-specific humoral immune response to outer membrane proteins isolated from Salmonella typhi in humans with typhoid fever. J Clin Microbiol. 1989;27:1640–1645. doi: 10.1128/jcm.27.7.1640-1645.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sheikh A, et al. Salmonella enterica serovar Typhi-specific immunoglobulin A antibody responses in plasma and antibody in lymphocyte supernatant specimens in Bangladeshi patients with suspected typhoid fever. Clin Vaccine Immunol. 2009;16:1587–1594. doi: 10.1128/CVI.00311-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rajagopalan P, Kumar R, Malaviya AN. Immunological studies in typhoid fever. II. Cell-mediated immune responses and lymphocyte subpopulations in patients with typhoid fever. Clin Exp Immunol. 1982;47:269–274. [PMC free article] [PubMed] [Google Scholar]
- 69.Lanata CF, et al. Vi serology in detection of chronic Salmonella typhi carriers in an endemic area. Lancet. 1983;2:441–443. doi: 10.1016/s0140-6736(83)90401-4. [DOI] [PubMed] [Google Scholar]
- 70.Losonsky GA, Ferreccio C, Kotloff KL, Kaintuck S, Robbins JB, Levine MM. Development and evaluation of an enzyme-linked immunosorbent assay for serum Vi antibodies for detection of chronic Salmonella typhi carriers. J Clin Microbiol. 1987;25:2266–2269. doi: 10.1128/jcm.25.12.2266-2269.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mirza NB, Wamola IA, Estambale BA, Mbithi E, Poillet M. Typhim Vi vaccine against typhoid fever: a clinical trial in Kenya. East Afr Med J. 1995;72:162–164. [PubMed] [Google Scholar]
- 72.Murphy JR, et al. Immunity to Salmonella typhi: considerations relevant to measurement of cellular immunity in typhoid-endemic regions. Clin Exp Immunol. 1989;75:228–233. [PMC free article] [PubMed] [Google Scholar]
- 73.Murphy JR, et al. Characteristics of humoral and cellular immunity to Salmonella typhi in residents of typhoid-endemic and typhoid-free regions. J Infect Dis. 1987;156:1005–109. doi: 10.1093/infdis/156.6.1005. [DOI] [PubMed] [Google Scholar]
- 74.Butler T, Ho M, Acharya G, Tiwari M, Gallati H. Interleukin-6, gamma interferon, and tumor necrosis factor receptors in typhoid fever related to outcome of antimicrobial therapy. Antimicrob Agents Chemother. 1993;37:2418–2421. doi: 10.1128/aac.37.11.2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Salerno-Goncalves R, Sztein MB. Priming of Salmonella enterica serovar typhi-specific CD8(+) T cells by suicide dendritic cell cross-presentation in humans. PLoS ONE. 2009;4:e5879. doi: 10.1371/journal.pone.0005879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kopecko DJ, et al. Genetic stability of vaccine strain Salmonella Typhi Ty21a over 25 years. Int J Med Microbiol. 2009;299:233–246. doi: 10.1016/j.ijmm.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 77.Simanjuntak C, et al. Oral immunisation against typhoid fever in Indonesia with Ty21a vaccine. Lancet. 1991;338:1055–1059. doi: 10.1016/0140-6736(91)91910-m. [DOI] [PubMed] [Google Scholar]
- 78.Black R, et al. Immunogenicity of Ty21a attenuated “Salmonella typhi” given with sodium bicarbonate or in enteric-coated capsules. Dev Biol Stand. 1983;53:9–14. [PubMed] [Google Scholar]
- 79.Levine MM, Ferreccio C, Black RE, Tacket CO, Germanier R Chilean Typhoid Committee. Progress in vaccines to prevent typhoid fever. Rev Infect Dis. 1989;11:S552–S567. doi: 10.1093/clinids/11.supplement_3.s552. [DOI] [PubMed] [Google Scholar]
- 80.Black RE, Levine MM, Ferreccio C, et al. Efficacy of one or two doses of Ty21a Salmonella typhi vaccine in enteric-coated capsules in a controlled field trial. Chilean Typhoid Committee Vaccine. 1990;8:81–84. doi: 10.1016/0264-410x(90)90183-m. [DOI] [PubMed] [Google Scholar]
- 81.Levine MM, Ferreccio C, Cryz S, Ortiz E. Comparison of enteric-coated capsules and liquid formulation of Ty21a typhoid vaccine in randomised controlled field trial. Lancet. 1990;336:891–894. doi: 10.1016/0140-6736(90)92266-k. [DOI] [PubMed] [Google Scholar]
- 82.Wahdan MH, Serie C, Cerisier Y, Sallam S, Germanier R. A controlled field trial of live Salmonella Typhi strain Ty 21a oral vaccine against typhoid: three-year results. J Infect Dis. 1982;145:292–295. doi: 10.1093/infdis/145.3.292. [DOI] [PubMed] [Google Scholar]
- 83.Levine MM, Ferreccio C, Black RE, Germanier R. Large-scale field trial of Ty21a live oral typhoid vaccine in enteric-coated capsule formulation. Lancet. 1987;1:1049–1052. doi: 10.1016/s0140-6736(87)90480-6. [DOI] [PubMed] [Google Scholar]
- 84.Ferreccio C, Levine MM, Rodriguez H, Contreras R. Comparative efficacy of two, three, or four doses of TY21a live oral typhoid vaccine in enteric-coated capsules: a field trial in an endemic area. JID. 1989;159:766–769. doi: 10.1093/infdis/159.4.766. [DOI] [PubMed] [Google Scholar]
- 85.Levine MM, Ferreccio C, Abrego P, Martin OS, Ortiz E, Cryz S. Duration of efficacy of Ty21a, attenuated Salmonella Typhi live oral vaccine. Vaccine. 1999;17 (Suppl):S22–S27. doi: 10.1016/s0264-410x(99)00231-5. [DOI] [PubMed] [Google Scholar]
- 86.Cryz SJ, Jr, Vanprapar N, Thisyakorn U, et al. Safety and immunogenicity of Salmonella typhi Ty21a vaccine in young Thai children. Infect Immun. 1993;61:1149–1151. doi: 10.1128/iai.61.3.1149-1151.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Salerno-Goncalves R, Wahid R, Sztein MB. Ex vivo kinetics of early and long-term multifunctional HLA-E specific CD8+ cells in volunteers immunized with Ty21a typhoid vaccine. Clin Vaccine Immunol. 2010;17:1305–1314. doi: 10.1128/CVI.00234-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sztein MB. Cell-mediated immunity and antibody responses elicited by attenuated Salmonella enterica Serovar Typhi strains used as live oral vaccines in humans. Clin Infect Dis. 2007;45 (Suppl):S15–S19. doi: 10.1086/518140. [DOI] [PubMed] [Google Scholar]
- 89.Salerno-Goncalves R, Fernandez-Vina M, Lewinsohn DM, Sztein MB. Identification of a human HLA-E-restricted CD8+ T cell subset in volunteers immunized with Salmonella enterica serovar Typhi strain Ty21a typhoid vaccine. J Immunol. 2004;173:5852–5862. doi: 10.4049/jimmunol.173.9.5852. [DOI] [PubMed] [Google Scholar]
- 90.Salerno-Goncalves R, Pasetti MF, Sztein MB. Characterization of CD8+ effector T cell responses in volunteers immunized with Salmonella enterica serovar Typhi strain Ty21a typhoid vaccine. J Immunol. 2002;169:2196–2203. doi: 10.4049/jimmunol.169.4.2196. [DOI] [PubMed] [Google Scholar]
- 91.Salerno-Goncalves R, Wahid R, Sztein MB. Immunization of volunteers with Salmonella enterica serovar Typhi strain Ty21a elicits the oligoclonal expansion of CD8+ T cells with predominant Vbeta repertoires. Infect Immun. 2005;73:3521–3530. doi: 10.1128/IAI.73.6.3521-3530.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kantele A. Peripheral blood antibody-secreting cells in the evaluation of the immune response to an oral vaccine. J Biotechnol. 1996;44:217–224. doi: 10.1016/0168-1656(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 93.Kantele A, et al. Homing potentials of circulating lymphocytes in humans depend on the site of activation: oral, but not parenteral, typhoid vaccination induces circulating antibody-secreting cells that all bear homing receptors directing them to the gut. J Immunol. 1997;158:574–579. [PubMed] [Google Scholar]
- 94.Kantele A. Antibody-secreting cells in the evaluation of the immunogenicity of an oral vaccine. Vaccine. 1990;8:321–326. doi: 10.1016/0264-410x(90)90088-4. [DOI] [PubMed] [Google Scholar]
- 95.D'Amelio R, Tagliabue A, Nencioni L, et al. Comparative analysis of immunological responses to oral (Ty21a) and parenteral (TAB) typhoid vaccines. Infect Immun. 1988;56:2731–2735. doi: 10.1128/iai.56.10.2731-2735.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tagliabue A, Nencioni L, Caffarena A, et al. Cellular immunity against Salmonella typhi after live oral vaccine. Clin Exp Immunol. 1985;62:242–247. [PMC free article] [PubMed] [Google Scholar]
- 97.Lundin BS, Johansson C, Svennerholm AM. Oral immunization with a Salmonella enterica serovar typhi vaccine induces specific circulating mucosa-homing CD4+ and CD8+ T cells in humans. Infect Immun. 2002;70:5622–5627. doi: 10.1128/IAI.70.10.5622-5627.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Precopio ML, et al. Immunization with vaccinia virus induces polyfunctional and phenotypically distinctive CD8+ T cell responses. J Exp Med. 2007;204:1405–1416. doi: 10.1084/jem.20062363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Betts MR, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107:4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Betts MR, et al. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281:65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 101.Tacket CO, et al. Phase 2 clinical trial of attenuated Salmonella enterica serovar Typhi oral live vector vaccine CVD 908-htrA in U.S. volunteers. Infect Immun. 2000;68:1196–1201. doi: 10.1128/iai.68.3.1196-1201.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Salerno-Goncalves R, Wyant TL, Pasetti MF, et al. Concomitant induction of CD4+ and CD8+ T cell responses in volunteers immunized with Salmonella enterica serovar Typhi strain CVD 908-htrA. J Immunol. 2003;170:2734–2741. doi: 10.4049/jimmunol.170.5.2734. [DOI] [PubMed] [Google Scholar]
- 103.Levine MM, Kaper JB, Black RE, Clements ML. New knowledge on pathogenesis of bacterial enteric infections as applied to vaccine development. Microbiol Rev. 1983;47:510–550. doi: 10.1128/mr.47.4.510-550.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Glass RI, Svennerholm AM, Khan MR, Huda S, Huq MI, Holmgren J. Seroepidemiological studies of El Tor cholera in Bangladesh: association of serum antibody levels with protection. J Infect Dis. 1985;151:236–242. doi: 10.1093/infdis/151.2.236. [DOI] [PubMed] [Google Scholar]
- 105.Clemens JD, et al. Biotype as determinant of natural immunising effect of cholera. Lancet. 1991;337:883–884. doi: 10.1016/0140-6736(91)90207-6. [DOI] [PubMed] [Google Scholar]
- 106.Levine MM, et al. Immunity of cholera in man: relative role of antibacterial versus antitoxic immunity. Trans R Soc Trop Med Hyg. 1979;73:3–9. doi: 10.1016/0035-9203(79)90119-6. [DOI] [PubMed] [Google Scholar]
- 107.Levine MM, Black RE, Clements ML, Cisneros L, Nalin DR, Young CR. Duration of infection-derived immunity to cholera. J Infect Dis. 1981;143:818–820. doi: 10.1093/infdis/143.6.818. [DOI] [PubMed] [Google Scholar]
- 108.Kaper JB, Morris JG, Jr, Levine MM. Cholera. Clin Microbiol Rev. 1995;8:48–86. doi: 10.1128/cmr.8.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.World Health Organization. Cholera vaccines. Wkly Epidemiol Rec. 2001;76:117–124. [PubMed] [Google Scholar]
- 110.Dukoral (package insert) Stockholm, Sweden: Crucell/SBL Vaccines; 2007. [Google Scholar]
- 111.Ryan ET, Calderwood SB. Cholera vaccines. J Travel Med. 2001;8:82–91. doi: 10.2310/7060.2001.24300. [DOI] [PubMed] [Google Scholar]
- 112.Sur D, Lopez AL, Kanungo S, et al. Efficacy and safety of a modified killed-whole-cell oral cholera vaccine in India: an interim analysis of a cluster-randomised, double-blind, placebo-controlled trial. Lancet. 2009;374:1694–1702. doi: 10.1016/S0140-6736(09)61297-6. [DOI] [PubMed] [Google Scholar]
- 113.Kanungo S, Paisley A, Lopez AL, et al. Immune responses following one and two doses of the reformulated, bivalent, killed, whole-cell, oral cholera vaccine among adults and children in Kolkata, India: a randomized, placebo-controlled trial. Vaccine. 2009;27:6887–6893. doi: 10.1016/j.vaccine.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 114.Ketley JM, Michalski J, Galen J, Levine MM, Kaper JB. Construction of genetically marked Vibrio cholerae O1 vaccine strains. FEMS Microbiol Lett. 1993;111:15–21. doi: 10.1111/j.1574-6968.1993.tb06355.x. [DOI] [PubMed] [Google Scholar]
- 115.Qadri F, et al. Peru-15, a live attenuated oral cholera vaccine, is safe and immunogenic in Bangladeshi toddlers and infants. Vaccine. 2007;25:231–238. doi: 10.1016/j.vaccine.2006.08.031. [DOI] [PubMed] [Google Scholar]
- 116.Garcia L, et al. The vaccine candidate Vibrio cholerae 638 is protective against cholera in healthy volunteers. Infect Immun. 2005;73:3018–3024. doi: 10.1128/IAI.73.5.3018-3024.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Svennerholm AM, Gothefors L, Sack DA, Bardhan PK, Holmgren J. Local and systemic antibody responses and immunological memory in humans after immunization with cholera B subunit by different routes. Bull World Health Organ. 1984;62:909–918. [PMC free article] [PubMed] [Google Scholar]
- 118.Losonsky GA, Tacket CO, Wasserman SS, Kaper JB, Levine MM. Secondary Vibrio cholerae-specific cellular antibody responses following wild-type homologous challenge in people vaccinated with CVD 103-HgR live oral cholera vaccine: changes with time and lack of correlation with protection. Infect Immun. 1993;61:729–733. doi: 10.1128/iai.61.2.729-733.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Qadri F, et al. Antigen-specific immunoglobulin A antibodies secreted from circulating B cells are an effective marker for recent local immune responses in patients with cholera: comparison to antibody-secreting cell responses and other immunological markers. Infect Immun. 2003;71:4808–4814. doi: 10.1128/IAI.71.8.4808-4814.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Asaduzzaman M, et al. The major subunit of the toxin-coregulated pilus TcpA induces mucosal and systemic immunoglobulin A immune responses in patients with cholera caused by Vibrio cholerae O1 and O139. Infect Immun. 2004;72:4448–4454. doi: 10.1128/IAI.72.8.4448-4454.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bhuiyan TR, et al. Cholera caused by Vibrio cholerae O1 induces T-cell responses in the circulation. Infect Immun. 2009;77:1888–1893. doi: 10.1128/IAI.01101-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Weil AA, et al. Memory T-cell responses to Vibrio cholerae O1 infection. Infect Immun. 2009;77:5090–5096. doi: 10.1128/IAI.00793-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kantele A, Kantele JM, Arvilommi H, Makela PH. Active immunity is seen as a reduction in the cell response to oral live vaccine. Vaccine. 1991;9:428–431. doi: 10.1016/0264-410x(91)90130-x. [DOI] [PubMed] [Google Scholar]
- 124.Kotloff KL, et al. A modified Shigella volunteer challenge model in which the inoculum is administered with bicarbonate buffer: clinical experience and implications for Shigella infectivity. Vaccine. 1995;13:1488–1494. doi: 10.1016/0264-410x(95)00102-7. [DOI] [PubMed] [Google Scholar]
- 125.Sack DA, et al. Antibody responses after immunization with killed oral cholera vaccines during the 1985 vaccine field trial in Bangladesh. J Infect Dis. 1991;164:407–411. doi: 10.1093/infdis/164.2.407. [DOI] [PubMed] [Google Scholar]
- 126.Iwasaki A, Welker R, Mueller S, Linehan M, Nomoto A, Wimmer E. Immunofluorescence analysis of poliovirus receptor expression in Peyer's patches of humans, primates, and CD155 transgenic mice: implications for poliovirus infection. J Infect Dis. 2002;186:585–592. doi: 10.1086/342682. [DOI] [PubMed] [Google Scholar]
- 127.Sabin AB. Oral poliovirus vaccine: history of its development and use and current challenge to eliminate poliomyelitis from the world. J Infect Dis. 1985;151:420–36. doi: 10.1093/infdis/151.3.420. [DOI] [PubMed] [Google Scholar]
- 128.Aylward RB. Eradicating polio: today's challenges and tomorrow's legacy. Ann Trop Med Parasitol. 2006;100:401–413. doi: 10.1179/136485906X97354. [DOI] [PubMed] [Google Scholar]
- 129.Strebel PM, et al. Epidemiology of poliomyelitis in the United States one decade after the last reported case of indigenous wild virus-associated disease. Clin Infect Dis. 1992;14:568–579. doi: 10.1093/clinids/14.2.568. [DOI] [PubMed] [Google Scholar]
- 130.Plotkin SA. Correlates of protection induced by vaccination. Clin Vaccine Immunol. 2010;17:1055–1065. doi: 10.1128/CVI.00131-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ogra PL, Fishaut M, Gallagher MR. Viral vaccination via the mucosal routes. Rev Infect Dis. 1980;2:352–369. doi: 10.1093/clinids/2.3.352. [DOI] [PubMed] [Google Scholar]
- 132.Onorato IM, Modlin JF, McBean AM, Thoms ML, Losonsky GA, Bernier RH. Mucosal immunity induced by enhance-potency inactivated and oral polio vaccines. J Infect Dis. 1991;163:1–6. doi: 10.1093/infdis/163.1.1. [DOI] [PubMed] [Google Scholar]
- 133.Ogra PL. Comparative evaluation of immunization with live attenuated and inactivated poliovirus vaccines. Ann N Y Acad Sci. 1995;754:97–107. doi: 10.1111/j.1749-6632.1995.tb44442.x. [DOI] [PubMed] [Google Scholar]
- 134.Grimwood K, Lambert SB, Milne RJ. Rotavirus infections and vaccines: burden of illness and potential impact of vaccination. Paediatr Drugs. 2010;12:235–256. doi: 10.2165/11537200-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 135.Clark B, McKendrick M. A review of viral gastroenteritis. Curr Opin Infect Dis. 2004;17:461–469. doi: 10.1097/00001432-200410000-00011. [DOI] [PubMed] [Google Scholar]
- 136.Parashar UD, Alexander JP, Glass RI. Prevention of rotavirus gastroenteritis among infants and children. Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2006;55:1–13. [PubMed] [Google Scholar]
- 137.Wilhelmi I, Roman E, Sanchez-Fauquier A. Viruses causing gastroenteritis. Clin Microbiol Infect. 2003;9:247–262. doi: 10.1046/j.1469-0691.2003.00560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ball JM, Tian P, Zeng CQ, Morris AP, Estes MK. Age-dependent diarrhea induced by a rotaviral nonstructural glycoprotein. Science. 1996;272:101–104. doi: 10.1126/science.272.5258.101. [DOI] [PubMed] [Google Scholar]
- 139.Lorrot M, Vasseur M. Physiopathology of Rotavirus diarrhea. Arch Pediatr. 2007;14 (Suppl):S145–S151. doi: 10.1016/s0929-693x(07)80018-2. [DOI] [PubMed] [Google Scholar]
- 140.Murphy TV, et al. Intussusception among infants given an oral rotavirus vaccine. N Engl J Med. 2001;344:564–572. doi: 10.1056/NEJM200102223440804. [DOI] [PubMed] [Google Scholar]
- 141.Murphy TV, Smith PJ, Gargiullo PM, Schwartz B. The first rotavirus vaccine and intussusception: epidemiological studies and policy decisions. J Infect Dis. 2003;187:1309–1313. doi: 10.1086/374420. [DOI] [PubMed] [Google Scholar]
- 142.Rennels MB, Glass RI, Dennehy PH, et al. Safety and efficacy of high-dose rhesus-human reassortant rotavirus vaccines--report of the National Multicenter Trial. United States Rotavirus Vaccine Efficacy Group. Pediatrics. 1996;97:7–13. [PubMed] [Google Scholar]
- 143.Santosham M, et al. Efficacy and safety of high-dose rhesus-human reassortant rotavirus vaccine in Native American populations. J Pediatr. 1997;131:632–638. doi: 10.1016/s0022-3476(97)70076-3. [DOI] [PubMed] [Google Scholar]
- 144.Joensuu J, Koskenniemi E, Pang XL, Vesikari T. Randomised placebo-controlled trial of rhesus-human reassortant rotavirus vaccine for prevention of severe rotavirus gastroenteritis. Lancet. 1997;350:1205–1209. doi: 10.1016/S0140-6736(97)05118-0. [DOI] [PubMed] [Google Scholar]
- 145.Vesikari T, et al. Efficacy of human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in European infants: randomised, double-blind controlled study. Lancet. 2007;370:1757–1763. doi: 10.1016/S0140-6736(07)61744-9. [DOI] [PubMed] [Google Scholar]
- 146.Cortese MM, Parashar UD. Prevention of rotavirus gastroenteritis among infants and children: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2009;58:1–25. [PubMed] [Google Scholar]
- 147.World Health Organization. Meeting of the immunization Strategic Advisory Group of Experts, April 2009--conclusions and recommendations. Wkly Epidemiol Rec. 2009;84:220–236. [PubMed] [Google Scholar]
- 148.Velazquez FR, et al. Rotavirus infections in infants as protection against subsequent infections. N Engl J Med. 1996;335:1022–1028. doi: 10.1056/NEJM199610033351404. [DOI] [PubMed] [Google Scholar]
- 149.Franco MA, Angel J, Greenberg HB. Immunity and correlates of protection for rotavirus vaccines. Vaccine. 2006;24:2718–2731. doi: 10.1016/j.vaccine.2005.12.048. [DOI] [PubMed] [Google Scholar]
- 150.Kapikian AZ, et al. Oral administration of human rotavirus to volunteers: induction of illness and correlates of resistance. J Infect Dis. 1983;147:95–106. doi: 10.1093/infdis/147.1.95. [DOI] [PubMed] [Google Scholar]
- 151.Hjelt K, Grauballe PC, Paerregaard A, Nielsen OH, Krasilnikoff PA. Protective effect of preexisting rotavirus-specific immunoglobulin A against naturally acquired rotavirus infection in children. J Med Virol. 1987;21:39–47. doi: 10.1002/jmv.1890210106. [DOI] [PubMed] [Google Scholar]
- 152.Brown KA, Kriss JA, Moser CA, Wenner WJ, Offit PA. Circulating rotavirus-specific antibody-secreting cells (ASCs) predict the presence of rotavirus-specific ASCs in the human small intestinal lamina propria. J Infect Dis. 2000;182:1039–1043. doi: 10.1086/315808. [DOI] [PubMed] [Google Scholar]
- 153.Ward RL, et al. Effects of antibody to rotavirus on protection of adults challenged with a human rotavirus. J Infect Dis. 1989;159:79–88. doi: 10.1093/infdis/159.1.79. [DOI] [PubMed] [Google Scholar]
- 154.Matson DO, O'Ryan ML, Herrera I, Pickering LK, Estes MK. Fecal antibody responses to symptomatic and asymptomatic rotavirus infections. J Infect Dis. 1993;167:577–583. doi: 10.1093/infdis/167.3.577. [DOI] [PubMed] [Google Scholar]
- 155.Coulson BS, Grimwood K, Hudson IL, Barnes GL, Bishop RF. Role of coproantibody in clinical protection of children during reinfection with rotavirus. J Clin Microbiol. 1992;30:1678–1684. doi: 10.1128/jcm.30.7.1678-1684.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Freytag LC, Clements JD. Mucosal adjuvants. Vaccine. 2005;23:1804–1813. doi: 10.1016/j.vaccine.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 157.Dickinson BL, Clements JD. Dissociation of Escherichia coli heat-labile enterotoxin adjuvanticity from ADP-ribosyltransferase activity. Infect Immun. 1995;63:1617–1623. doi: 10.1128/iai.63.5.1617-1623.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Freytag LC, Clements JD, Eliasson D, Lycke N. Use of genetically or chemically detoxified mutants of cholera and Escherichia coli heat-labile enterotoxins as mucosal adjuvants. In: Levine MM, Dougan G, Good MF, Liu MA, Nabel GJ, Nataro JPRR, editors. New Generation Vaccines. 4. New York: Informa Healthcare USA, Inc; 2010. pp. 273–283. [Google Scholar]
- 159.Giuliani MM, et al. Mucosal adjuvanticity and immunogenicity of LTR72, a novel mutant of Escherichia coli heat-labile enterotoxin with partial knockout of ADP-ribosyltransferase activity. J Exp Med. 1998;187:1123–1132. doi: 10.1084/jem.187.7.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Douce G, Giuliani MM, Giannelli V, Pizza MG, Rappuoli R, Dougan G. Mucosal immunogenicity of genetically detoxified derivatives of heat labile toxin from Escherichia coli. Vaccine. 1998;16:1065–1073. doi: 10.1016/s0264-410x(98)80100-x. [DOI] [PubMed] [Google Scholar]
- 161.Marchetti M, et al. Protection against Helicobacter pylori infection in mice by intragastric vaccination with H. pylori antigens is achieved using a non-toxic mutant of E. coli heat-labile enterotoxin (LT) as adjuvant. Vaccine. 1998;16:33–37. doi: 10.1016/s0264-410x(97)00153-9. [DOI] [PubMed] [Google Scholar]
- 162.Anosova NG, Chabot S, Shreedhar V, Borawski JA, Dickinson BL, Neutra MR. Cholera toxin, E. coli heat-labile toxin, and non-toxic derivatives induce dendritic cell migration into the follicle-associated epithelium of Peyer's patches. Mucosal Immunol. 2008;1:59–67. doi: 10.1038/mi.2007.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Anjuere F, et al. In vivo adjuvant-induced mobilization and maturation of gut dendritic cells after oral administration of cholera toxin. J Immunol. 2004;173:5103–5111. doi: 10.4049/jimmunol.173.8.5103. [DOI] [PubMed] [Google Scholar]
- 164.Baqar S, et al. Standardization of measurement of immunoglobulin-secreting cells in human peripheral circulation. Clin Diagn Lab Immunol. 1997;4:375–379. doi: 10.1128/cdli.4.3.375-379.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kotloff KL, Sztein MB, Wasserman SS, Losonsky GA, DiLorenzo SC, Walker RI. Safety and immunogenicity of oral inactivated whole-cell Helicobacter pylori vaccine with adjuvant among volunteers with or without subclinical infection. Infect Immun. 2001;69:3581–3590. doi: 10.1128/IAI.69.6.3581-3590.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Losonsky GA, Kotloff KL, Walker RI. B cell responses in gastric antrum and duodenum following oral inactivated Helicobacter pylori whole cell (HWC) vaccine and LT(R192G) in H pylori seronegative individuals. Vaccine. 2003;21:562–565. doi: 10.1016/s0264-410x(02)00259-1. [DOI] [PubMed] [Google Scholar]
- 167.Tribble D, et al. Modified Escherichia coli heat-labile (LT(R192G)) adjuvant dose-range study of an oral, inactivated whole cell Campylobacter (CWC) vaccine in volunteers. 1st International Conference on Modern Vaccines, Adjuvants and Delivery Systems; Dublin, Ireland. 4–6 June 2003. [Google Scholar]
- 168.Lapa JA, et al. Randomized clinical trial assessing the safety and immunogenicity of oral microencapsulated enterotoxigenic Escherichia coli surface antigen 6 with or without heat-labile enterotoxin with mutation R192G. Clin Vaccine Immunol. 2008;15:1222–1228. doi: 10.1128/CVI.00491-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Clements JD. Target tissue responses to antigens and adjuvants. 2nd International Conference on Modern Mucosal Vaccine, Adjuvants and Microbicides; Dublin, Ireland. 28–30 April 2010. [Google Scholar]
- 170.Yuki Y, et al. Oral MucoRice expressing double-mutant cholera toxin A and B subunits induces toxin-specific neutralising immunity. Vaccine. 2009;27:5982–5988. doi: 10.1016/j.vaccine.2009.07.071. [DOI] [PubMed] [Google Scholar]
- 171.Summerton NA, et al. Toward the development of a stable, freeze-dried formulation of Helicobacter pylori killed whole cell vaccine adjuvanted with a novel mutant of Escherichia coli heat-labile toxin. Vaccine. 2010;28:1404–1411. doi: 10.1016/j.vaccine.2009.10.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Brandtzaeg P. Food allergy: separating the science from the mythology. Nat Rev Gastroenterol Hepatol. 2010;7:380–400. doi: 10.1038/nrgastro.2010.80. [DOI] [PubMed] [Google Scholar]
- 173.Levine MM. Use of vaccines for the prevention of typhoid fever. Indian Pediatr. 2003;40:1029–1034. [PubMed] [Google Scholar]
- 174.Tacket CO, Levine MM. CVD 908, CVD 908-htrA, and CVD 909 live oral typhoid vaccines: a logical progression. Clin Infect Dis. 2007;45 (Suppl):S20–S23. doi: 10.1086/518135. [DOI] [PubMed] [Google Scholar]
- 175.Hindle Z, et al. Characterization of Salmonella enterica derivatives harboring defined aroC and Salmonella pathogenicity island 2 type III secretion system (ssaV) mutations by immunization of healthy volunteers. Infect Immun. 2002;70:3457–3467. doi: 10.1128/IAI.70.7.3457-3467.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kirkpatrick BD, Tenney KM, Larsson CJ, et al. The novel oral typhoid vaccine M01ZH09 is well tolerated and highly immunogenic in 2 vaccine presentations. J Infect Dis. 2005;192:360–366. doi: 10.1086/431605. [DOI] [PubMed] [Google Scholar]
- 177.Kirkpatrick BD, et al. Evaluation of Salmonella enterica serovar Typhi (Ty2 aroC-ssaV-) M01ZH09, with a defined mutation in the Salmonella pathogenicity island 2, as a live, oral typhoid vaccine in human volunteers. Vaccine. 2006;24:116–123. doi: 10.1016/j.vaccine.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 178.Hohmann EL, Oletta CA, Killeen KP, Miller SI. PhoP/phoQ-deleted Salmonella typhi (Ty800) is a safe and immunogenic single-dose typhoid fever vaccine in volunteers. J Infect Dis. 1996;173:1408–1414. doi: 10.1093/infdis/173.6.1408. [DOI] [PubMed] [Google Scholar]
- 179.Tacket CO, et al. Safety of live oral Salmonella typhi vaccine strains with deletions in htrA and aroC aroD and immune response in humans. Infect Immun. 1997;65:452–456. doi: 10.1128/iai.65.2.452-456.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Tacket CO, Pasetti MF, Sztein MB, Livio S, Levine MM. Immune responses to an oral typhoid vaccine strain that is modified to constitutively express Vi capsular polysaccharide. J Infect Dis. 2004;190:565–570. doi: 10.1086/421469. [DOI] [PubMed] [Google Scholar]
- 181.Wang JY, et al. Construction, genotypic and phenotypic characterization, and immunogenicity of attenuated ΔguaBA Salmonella enterica serovar Typhi strain CVD 915. Infect Immun. 2001;69:4734–4741. doi: 10.1128/IAI.69.8.4734-4741.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Sztein MB, et al. Cytokine production patterns and lymphoproliferative responses in volunteers orally immunized with attenuated vaccine strains of Salmonella typhi. J Infect Dis. 1994;170:1508–1517. doi: 10.1093/infdis/170.6.1508. [DOI] [PubMed] [Google Scholar]
- 183.Sztein MB, Tanner M, Polotsky Y, Orenstein JM, Levine MM. Cytotoxic T lymphocytes after oral immunization with attenuated vaccine strains of Salmonella typhi in humans. J Immunol. 1995;155:3987–3993. [PubMed] [Google Scholar]
- 184.Wahid R, Salerno-Goncalves R, Tacket CO, Levine MM, Sztein MB. Cell-mediated immune responses in humans after immunization with one or two doses of oral live attenuated typhoid vaccine CVD 909. Vaccine. 2007;25:1416–1425. doi: 10.1016/j.vaccine.2006.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Wyant TL, Tanner MK, Sztein MB. Salmonella typhi flagella are potent inducers of proinflammatory cytokine secretion by human monocytes. Infect Immun. 1999;67:3619–3624. doi: 10.1128/iai.67.7.3619-3624.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wahid R, Salerno-Goncalves R, Tacket CO, Levine MM, Sztein MB. Generation of specific effector and memory T cells with gut- and secondary lymphoid tissue- homing potential by oral attenuated CVD 909 typhoid vaccine in humans. Mucosal Immunol. 2008;1:389–398. doi: 10.1038/mi.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Geginat J, Lanzavecchia A, Sallusto F. Proliferation and differentiation potential of human CD8+ memory T-cell subsets in response to antigen or homeostatic cytokines. Blood. 2003;101:4260–4266. doi: 10.1182/blood-2002-11-3577. [DOI] [PubMed] [Google Scholar]
- 188.Maskey AP, et al. Salmonella enterica serovar Paratyphi A and S. enterica serovar Typhi cause indistinguishable clinical syndromes in Kathmandu, Nepal. Clin Infect Dis. 2006;42:1247–1253. doi: 10.1086/503033. [DOI] [PubMed] [Google Scholar]
- 189.Karkey A, Aryjal A, Basnyat B, Baker S. Kathmandu, Nepal: still an enteric fever capital of the world. J Infect Dev Ctries. 2008;2:461–465. doi: 10.3855/jidc.162. [DOI] [PubMed] [Google Scholar]
- 190.Ochiai RL, Wang X, von SL, et al. Salmonella paratyphi A rates, Asia. Emerg Infect Dis. 2005;11:1764–1766. doi: 10.3201/eid1111.050168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Levine MM, et al. Expanding immunization against human Salmonella infections: vaccines to prevent invasive non-typhoidal Salmonella emerging in Sub-Saharan Africa and Salmonella Paratyphi in Asia. 4th Vaccine and International Society for Vaccines Annual Global Congress; Vienna, Austria. October 3-5, 2010. [Google Scholar]
- 192.Vindurampulle CJ, et al. Construction and pre-clinical evaluation of CVD 1902, a candidate live oral Salmonella Paratyphi vaccine. 2010 Manuscript in preparation. Ref Type: Journal (Full) [Google Scholar]
- 193.Kotloff KL, Pasetti MF, Barry EM, et al. Deletion in the Shigella enterotoxin genes further attenuates Shigella flexneri 2a bearing guanine auxotrophy in a phase 1 trial of CVD 1204 and CVD 1208. J Infect Dis. 2004;190:1745–1754. doi: 10.1086/424680. [DOI] [PubMed] [Google Scholar]
- 194.Cohen D, Green MS, Block C, Slepon R, Ofek I. Prospective study of the association between serum antibodies to lipopolysaccharide O antigen and the attack rate of shigellosis. J Clin Microbiol. 1991;29:386–389. doi: 10.1128/jcm.29.2.386-389.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Black RE, et al. Prevention of shigellosis by a Salmonella typhi-Shigella sonnei bivalent vaccine. J Infect Dis. 1987;155:1260–1265. doi: 10.1093/infdis/155.6.1260. [DOI] [PubMed] [Google Scholar]
- 196.Tacket CO, Binion SB, Bostwick E, Losonsky G, Roy MJ, Edelman R. Efficacy of bovine milk immunoglobulin concentrate in preventing illness after Shigella flexneri challenge. Am J Trop Med Hyg. 1992;47:276–283. doi: 10.4269/ajtmh.1992.47.276. [DOI] [PubMed] [Google Scholar]
- 197.Clemens JD, Stanton B, Stoll B, Shahid NS, Banu H, Chowdhury AK. Breast feeding as a determinant of severity in shigellosis. Evidence for protection throughout the first three years of life in Bangladeshi children. Am J Epidemiol. 1986;123:710–720. doi: 10.1093/oxfordjournals.aje.a114291. [DOI] [PubMed] [Google Scholar]
- 198.Kotloff KL, et al. Evaluation of the safety, immunogenicity, and efficacy in healthy adults of four doses of live oral hybrid Escherichia coli- Shigella flexneri 2a vaccine strain EcSf2a-2. Vaccine. 1995;13:495–502. doi: 10.1016/0264-410x(94)00011-b. [DOI] [PubMed] [Google Scholar]
- 199.Coster TS, et al. Vaccination against shigellosis with attenuated Shigella flexneri 2a strain SC602. Infect Immun. 1999;67:3437–3443. doi: 10.1128/iai.67.7.3437-3443.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Raqib R, Ljungdahl A, Lindberg AA, Andersson U, Andersson J. Local entrapment of interferon gamma in the recovery from Shigella dysenteriae type 1 infection. Gut. 1996;38:328–336. doi: 10.1136/gut.38.3.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Raqib R, Wretlind B, Andersson J, Lindberg AA. Cytokine secretion in acute shigellosis is correlated to disease activity and directed more to stool than to plasma. J Infect Dis. 1995;171:376–384. doi: 10.1093/infdis/171.2.376. [DOI] [PubMed] [Google Scholar]
- 202.Raqib R, Lindberg AA, Wretlind B, Bardhan PK, Andersson U, Andersson J. Persistence of local cytokine production in shigellosis in acute and convalescent stages. Infect Immun. 1995;63:289–296. doi: 10.1128/iai.63.1.289-296.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Samandari T, et al. Production of IFN-gamma and IL-10 to Shigella invasins by mononuclear cells from volunteers orally inoculated with a Shiga toxin-deleted Shigella dysenteriae type 1 strain. J Immunol. 2000;164:2221–2232. doi: 10.4049/jimmunol.164.4.2221. [DOI] [PubMed] [Google Scholar]
- 204.Noriega FR, Liao FM, Maneval DR, Ren S, Formal SB, Levine MM. Strategy for cross-protection among Shigella flexneri serotypes. Infect Immun. 1999;67:782–788. doi: 10.1128/iai.67.2.782-788.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Kotloff KL, et al. Safety and Immunogenicity of CVD 1208S, a Live, Oral ΔaguaBA Δsen Δset Shigella flexneri 2a Vaccine Grown on Animal-Free Media. Hum Vaccin. 2007;3:268–275. doi: 10.4161/hv.4746. [DOI] [PubMed] [Google Scholar]
- 206.Galen JE, Pasetti MF, Tennant S, Ruiz-Olvera P, Sztein MB, Levine MM. Salmonella enterica serovar Typhi live vector vaccines finally come of age. Immunol Cell Biol. 2009;87:400–412. doi: 10.1038/icb.2009.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Curtiss R, III, et al. New technologies in using recombinant attenuated Salmonella vaccine vectors. Crit Rev Immunol. 2010;30:255–270. doi: 10.1615/critrevimmunol.v30.i3.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Galen JE, et al. A new generation of stable, nonantibiotic, low-copy-number plasmids improves immune responses to foreign antigens in Salmonella enterica serovar Typhi live vectors. Infect Immun. 2010;78:337–347. doi: 10.1128/IAI.00916-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Vindurampulle CJ, Cuberos LF, Barry EM, Pasetti MF, Levine MM. Recombinant Salmonella enterica serovar Typhi in a prime-boost strategy. Vaccine. 2004;22:3744–3750. doi: 10.1016/j.vaccine.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 210.Galen JE, et al. Mucosal immunization with attenuated Salmonella enterica serovar Typhi expressing protective antigen of anthrax toxin (PA83) primes monkeys for accelerated serum antibody responses to parenteral PA83 vaccine. J Infect Dis. 2009;199:326–335. doi: 10.1086/596066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Ramirez K, Capozzo AV, Lloyd SA, Sztein MB, Nataro JP, Pasetti MF. Mucosally delivered Salmonella typhi expressing the Yersinia pestis F1 antigen elicits mucosal and systemic immunity early in life and primes the neonatal immune system for a vigorous anamnestic response to parenteral F1 boost. J Immunol. 2009;182:1211–1222. doi: 10.4049/jimmunol.182.2.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Ramirez K, Ditamo Y, Galen JE, Baillie LW, Pasetti MF. Mucosal priming of newborn mice with S. Typhi Ty21a expressing anthrax protective antigen (PA) followed by parenteral PA-boost induces B and T cell-mediated immunity that protects against infection bypassing maternal antibodies. Vaccine. 2010;28:6065–6075. doi: 10.1016/j.vaccine.2010.06.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Woodland DL. Jump-starting the immune system: prime-boosting comes of age. Trends Immunol. 2004;25:98–104. doi: 10.1016/j.it.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 214.Tacket CO, Sztein MB, Losonsky GA, Wasserman SS, Estes MK. Humoral, mucosal, and cellular immune responses to oral Norwalk virus-like particles in volunteers. Clin Immunol. 2003;108:241–247. doi: 10.1016/s1521-6616(03)00120-7. [DOI] [PubMed] [Google Scholar]
- 215.Demberg T, Robert-Guroff M. Mucosal immunity and protection against HIV/SIV infection: strategies and challenges for vaccine design. Int Rev Immunol. 2009;28:20–48. doi: 10.1080/08830180802684331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Brenchley JM, Douek DC. HIV infection and the gastrointestinal immune system. Mucosal Immunol. 2008;1:23–30. doi: 10.1038/mi.2007.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Mercier GT, et al. Oral immunization of rhesus macaques with adenoviral HIV vaccines using enteric-coated capsules. Vaccine. 2007;25:8687–8701. doi: 10.1016/j.vaccine.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Ko SY, et al. Enhanced induction of intestinal cellular immunity by oral priming with enteric adenovirus 41 vectors. J Virol. 2009;83:748–756. doi: 10.1128/JVI.01811-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Lugade AA, Kalathil S, Heald JL, Thanavala Y. Transgenic plant-based oral vaccines. Immunol Invest. 2010;39:468–482. doi: 10.3109/08820131003622643. [DOI] [PubMed] [Google Scholar]
- 220.Tacket CO. Plant-based oral vaccines: results of human trials. Curr Top Microbiol Immunol. 2009;332:103–117. doi: 10.1007/978-3-540-70868-1_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Tacket CO, Mason HS, Losonsky G, Clements JD, Levine MM, Arntzen CJ. Immunogenicity in humans of a recombinant bacterial antigen delivered in a transgenic potato. Nat Med. 1998;4:607–609. doi: 10.1038/nm0598-607. [DOI] [PubMed] [Google Scholar]
- 222.Tacket CO, Mason HS, Losonsky G, Estes MK, Levine MM, Arntzen CJ. Human immune responses to a novel norwalk virus vaccine delivered in transgenic potatoes. J Infect Dis. 2000;182:302–305. doi: 10.1086/315653. [DOI] [PubMed] [Google Scholar]
- 223.Tacket CO, Pasetti MF, Edelman R, Howard JA, Streatfield S. Immunogenicity of recombinant LT-B delivered orally to humans in transgenic corn. Vaccine. 2004;22:4385–4389. doi: 10.1016/j.vaccine.2004.01.073. [DOI] [PubMed] [Google Scholar]
- 224.Chen RT, et al. Measles antibody: reevaluation of protective titers. J Infect Dis. 1990;162:1036–1042. doi: 10.1093/infdis/162.5.1036. [DOI] [PubMed] [Google Scholar]
- 225.Tacket CO, et al. Randomized, double-blind, placebo-controlled, multicentered trial of the efficacy of a single dose of live oral cholera vaccine CVD 103-HgR in preventing cholera following challenge with Vibrio cholerae O1 El tor inaba three months after vaccination. Infect Immun. 1999;67:6341–6345. doi: 10.1128/iai.67.12.6341-6345.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Losonsky GA, et al. Factors influencing secondary vibriocidal immune responses: relevance for understanding immunity to cholera. Infect Immun. 1996;64:10–15. doi: 10.1128/iai.64.1.10-15.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Levine MM, Nalin DR, Hoover DL, Bergquist EJ, Hornick RB, Young CR. Immunity to enterotoxigenic Escherichia coli. Infect Immun. 1979;23:729–736. doi: 10.1128/iai.23.3.729-736.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Levine MM, Black RE, Brinton CC, Jr, et al. Reactogenicity, immunogenicity and efficacy studies of Escherichia coli type 1 somatic pili parenteral vaccine in man. Scand J Infect Dis. 1982;33 (Suppl):83–95. [PubMed] [Google Scholar]
- 229.Tacket CO, et al. Protection by milk immunoglobulin concentrate against oral challenge with enterotoxigenic Escherichia coli. N Engl J Med. 1988;318:1240–1243. doi: 10.1056/NEJM198805123181904. [DOI] [PubMed] [Google Scholar]
- 230.Tribble DR, et al. Assessment of the duration of protection in Campylobacter jejuni experimental infection in humans. Infect Immun. 2010;78:1750–1759. doi: 10.1128/IAI.01021-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Mosley WH, McCormack WM, Ahmed A, Chowdhury AK, Barui RK. Report of the 1966–67 cholera vaccine field trial in rural East Pakistan. 2. Results of the serological surveys in the study population--the relationship of case rate to antibody titre and an estimate of the inapparent infection rate with Vibrio cholerae. Bull World Health Organ. 1969;40:187–197. [PMC free article] [PubMed] [Google Scholar]
- 232.Mosley WH, Woodward WE, Aziz KM, et al. The 1968–1969 cholera-vaccine field trial in rural East Pakistan. Effectiveness of monovalent Ogawa and Inaba vaccines and a purified Inaba antigen, with comparative results of serological and animal protection tests. J Infect Dis. 1970;121 (Suppl):S9. doi: 10.1093/infdis/121.supplement.s1. [DOI] [PubMed] [Google Scholar]
- 233.Mosley WH, Benenson AS, Barui R. A serological survey for cholear antibodies in rural east Pakistan. 1. The distribution of antibody in the control population of a cholera-vaccine field-trial area and the relation of antibody titre to the pattern of endemic cholera. Bull World Health Organ. 1968;38:327–334. [PMC free article] [PubMed] [Google Scholar]
- 234.Cherry JD, Gornbein J, Heininger U, Stehr K. A search for serologic correlates of immunity to Bordetella pertussis cough illnesses. Vaccine. 1998;16:1901–1906. doi: 10.1016/s0264-410x(98)00226-6. [DOI] [PubMed] [Google Scholar]
- 235.Gaucher D, et al. Yellow fever vaccine induces integrated multilineage and polyfunctional immune responses. J Exp Med. 2008;205:3119–3131. doi: 10.1084/jem.20082292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Querec TD, et al. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat Immunol. 2009;10:116–125. doi: 10.1038/ni.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Campbell H, Andrews N, Borrow R, Trotter C, Miller E. Updated postlicensure surveillance of the meningococcal C conjugate vaccine in England and Wales: effectiveness, validation of serological correlates of protection, and modeling predictions of the duration of herd immunity. Clin Vaccine Immunol. 2010;17:840–847. doi: 10.1128/CVI.00529-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.John TJ, Jayabal P. Oral polio vaccination of children in the tropics. I. The poor seroconversion rates and the absence of viral interference. Am J Epidemiol. 1972;96:263–269. doi: 10.1093/oxfordjournals.aje.a121457. [DOI] [PubMed] [Google Scholar]
- 239.Mirzayeva R, Steele AD, Parashar UD, Zaman K, Neuzil KM, Nelson EA. Evaluation of rotavirus vaccines in Asia--are there lessons to be learnt? Vaccine. 2009;27 (Suppl 5):F120–F129. doi: 10.1016/j.vaccine.2009.09.039. [DOI] [PubMed] [Google Scholar]
- 240.Hanlon P, et al. Trial of an attenuated bovine rotavirus vaccine (RIT 4237) in Gambian infants. Lancet. 1987;1:1342–1345. doi: 10.1016/s0140-6736(87)90649-0. [DOI] [PubMed] [Google Scholar]
- 241.Suharyono, Simanjuntak C, Witham N, et al. Safety and immunogenicity of single-dose live oral cholera vaccine CVD 103-HgR in 5–9-year-old Indonesian children. Lancet. 1992;340:689–694. doi: 10.1016/0140-6736(92)92231-4. [DOI] [PubMed] [Google Scholar]
- 242.Gotuzzo E, et al. Safety, immunogenicity, and excretion pattern of single-dose live oral cholera vaccine CVD 103-HgR in Peruvian adults of high and low socioeconomic levels. Infect Immun. 1993;61:3994–3997. doi: 10.1128/iai.61.9.3994-3997.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Su-Arehawaratana P, et al. Safety and immunogenicity of different immunization regimens of CVD 103-HgR live oral cholera vaccine in soldiers and civilians in Thailand. J Infect Dis. 1992;165:1042–1048. doi: 10.1093/infdis/165.6.1042. [DOI] [PubMed] [Google Scholar]
- 244.Hallander HO, et al. Calibrated serological techniques demonstrate significant different serum response rates to an oral killed cholera vaccine between Swedish and Nicaraguan children. Vaccine. 2002;21:138–145. doi: 10.1016/s0264-410x(02)00348-1. [DOI] [PubMed] [Google Scholar]
- 245.Walker A. Breast milk as the gold standard for protective nutrients. J Pediatr. 2010;156:S3–S7. doi: 10.1016/j.jpeds.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 246.Lagos R, Fasano A, Wasserman SS, et al. Effect of small bowel bacterial overgrowth on the immunogenicity of single-dose live oral cholera vaccine CVD 103-HgR. J Infect Dis. 1999;180:1709–1712. doi: 10.1086/315051. [DOI] [PubMed] [Google Scholar]
- 247.Cooper PJ, et al. Albendazole treatment of children with ascariasis enhances the vibriocidal antibody response to the live attenuated oral cholera vaccine CVD 103–HgR. J Infect Dis. 2000;182:1199–1206. doi: 10.1086/315837. [DOI] [PubMed] [Google Scholar]
- 248.Szu SC, Robbins JB, Schneerson R, Lin FY. Polysaccharide-based conjugate vaccines for enteric bacterial infections: typhoid fever, nontyphoidal Salmonellosis, and E. coli O157:H7. In: Levine MM, Dougan G, Good MF, Liu MA, Nabel GJ, Nataro JP, et al., editors. New Generation Vaccine. 4. New York: Informa Healthcare USA; 2010. pp. 489–496. [Google Scholar]