Abstract
Traditionally, perception was considered to be an encapsulated process that was unaffected by top-down processes like affect. Recent work in vision draws this framework into question by showing that changes in the emotional state of the perceiver can impact many different aspects of visual perception. Here, we extend the relationship between affect and perception into another perceptual modality: audition. Participants were induced into a negative or neutral mood by writing about a frightening or neutral experience in their past. They then listened to a series of short, neutral tones (320 and 640 ms) and rated the loudness and duration of the tones. Participants in a negative mood rated the tones as significantly louder, but not longer, than participants in a neutral mood, suggesting that the difference between the groups was perceptual rather than just a response bias. This research shows for the first time that the role of affect in perceptual processes may be more pervasive than previously considered.
Keywords: Auditory perception, negative mood, arousal, negative affect, loudness
The perceptual systems have traditionally been described as encapsulated systems that are unaffected by top-down processes like affect (Pylyshyn, 2003). A growing body of work in visual perception refutes this traditional framework, demonstrating that the affective state of the perceiver can play an important role perception (Alpers & Gerdes, 2007; Becker, 2009; Phelps, Ling, & Carrasco, 2005; Stefanucci & Proffitt, 2009; Stefanucci, Proffitt, Clore, & Parekh, 2008; Stefanucci & Storbeck, 2009). To date, scant research has examined whether the perceptual changes related to mood extend into other perceptual modalities like audition. The research presented herein demonstrates that negative affect can alter at least one type of auditory perception, the perception of loudness.
The hypothesis that affect can act as a top-down influence in audition derives support from an expanding literature in visual perception. When perceivers are briefly exposed to affectively evocative faces or images, they are momentarily more sensitive to changes in low spatial frequency visual information (Bocanegra & Zeelenberg, 2009; Phelps, Ling, & Carrasco, 2006) and have an increase in their field of view (Schmitz, De Rosa, & Anderson, 2009). Negative affect increases visual search efficiency, even when the target of the search is neutral (Becker, 2009). Furthermore, in binocular rivalry (a visual perception task in which two images are presented to each eye and compete for visual dominance) affective faces are perceived first and for longer than neutral objects (Alper & Gerdes, 2007).
Observers in a negative affective state may perceive their physical environment differently than observers in a neutral state. Slants, for example, appear steeper when there is a danger associated with descending the hill (Stefanucci, Proffitt, Clore, & Parekh, 2008). Negative affect, arousal, and trait-level fear have all been shown to affect the perception of heights in the environment. Individuals normatively overestimate vertical distances (Jackson & Cormack, 2007; Stefanucci & Proffitt, 2009) and fear of heights is highly related to this overestimation, such that those with a greater fear of heights estimate the height as taller, especially from the top (Stefanucci & Proffitt, 2009; Teachman, Stefanucci, Clerkin, Cody & Proffitt, 2008). Individuals without a fear of heights who viewed negative, highly arousing images estimated the height of a balcony to be taller than participants who viewed neutral images even when the images were unrelated to heights and ostensibly part of a memory task. Furthermore, when given a strategy to increase their emotional arousal while viewing the images, participants’ overestimations of the height increased further (Stefanucci & Storbeck, 2009).
When combined, the aforementioned research suggests that affect (particularly negative affect) can lead to pervasive changes in visual attention and perception. To date, the role of affect in auditory perception has been underexplored. However, that negative affect, could alter perception in audition seems highly plausible because emotional arousal cues are generally nonspecific and can be easily transferred from one arousing source to another (Stefanucci & Storbeck, 2009; Zillmann, 1971). Furthermore, Wang and colleagues (2009) demonstrate that negative affect can influence auditory processing (in this case, auditory response to speech) in as few as 20 ms, indicating that negative affect can influence auditory function very early in the processing stream. Finally, there is some behavioral evidence to suggest that negative affect and arousal may influence audition. However, the available evidence comes from research on individuals with trait-level anxiety and disorders of arousal, not normative populations (Dess & Edelheit, 1998; Pollack, Carter, Amir, & Marks, 2006).
Two studies, Pollack and colleagues (2006) and Dess and Edelheit (1998), demonstrate a potential relationship between negative affect and changes in auditory perception. Pollack and colleagues (2006) investigating the role of anxiety sensitivity, a trait-level tendency to fear anxiety-related sensations, found that individuals high in anxiety sensitivity showed an elevated false alarm rate and a lower threshold for reporting normal heartbeats in a heartbeat detection task. The authors concluded that fearful cues might affect auditory attention and perceptions in tasks involving threat signals. Dess and Edelheit (1998) also found that stress and temperament could affect auditory perception. Individuals who were high in trait-level arousability rated a tone as louder after exposure to a mild stressor.
This work is the first to examine whether a negative affective state impacts auditory perception in a non-clinical sample. In order to induce negative affect, we asked participants to write about a frightening experience in their past and then rate the loudness and duration of a series of neutral tones of varying pitch. We hypothesized that the induction of negative affect would have the largest influence on ratings of loudness because prior research indicates that patients with anxiety sensitivity perceive stimuli as louder than non-anxious individuals (Dess & Edelheit, 1998). Furthermore, the perception of amplitude (or loudness) occurs early in the auditory processing stream (Plack & Carlyon, 1995) and negative affect has been shown to exert an influence on auditory processing within tens of milliseconds (Wang, et al., 2009).
Method
Participants
Twenty-three (13 female, 10 male, mean age = 21 years, range = 20–22, ethnicity = 96% Caucasian, 4% Hispanic) students from the College of William & Mary received course credit for their participation.
Stimuli and Apparatus
All participants completed a brief survey that was a modified version of the Positive and Negative Affective Schedule (PANAS; Watson, Clark, & Tellegren, 1988). They were given a list of eight adjectives (discouraged, content, frustrated, anxious, happy, nervous, sad, angry) and asked to rate how much they were experiencing those emotions at that moment on a seven-point Likert scale with 1 being not at all and 7 being extremely. At the end of the experiment, participants in both conditions completed another modified PANAS. However, they were given a different list of six adjectives (calm, nervous, anxious, afraid, at ease, and scared) and asked to rate how much they were experiencing those emotions during the writing task on a seven-point Likert scale with 1 being not at all and 7 being extremely.
Mood Induction
In order to induce negative affect, participants in the negative condition were asked: In as much detail as possible, please write about the most frightening experience you’ve had in the last five years. Try to write it with enough detail that a person you’ve never met might begin to feel afraid. Participants in the neutral condition were asked: In as much detail as possible, please write about what you do when you get ready in the morning. Try to write it with enough detail that a person you’ve never met would really understand your process.
Tones
Participants listened to the tones through speakers (Creative, Inspire 290) located in front of them on a desk. The computer controlling the speakers was located behind them. All tones were created using iTunes sound editing software and were presented during the experiment using Windows Media Player. Five of the tones were 320 milliseconds long and consisted of five frequencies (1000Hz, 2000Hz, 3000Hz, 4000Hz, and 5000Hz). The other five tones were 640 milliseconds long and consisted of the same five frequencies (1000Hz, 2000Hz, 3000Hz, 4000Hz, and 5000Hz). Tones were played in two sets: the five 320 millisecond tones were always presented first in random order while the five 640 millisecond tones were presented second, also in random order. The short and long tones were presented in blocks in order to discourage participants from using the previous tone to anchor their estimate of duration. The decibels of the tones were presented at a range of 95–104 decibels throughout the experiment. The inter-stimulus interval was approximately 35s.
Participants rated the tones on a slider. The slider was a modified 12-inch ruler with all of the markings covered. A 0 and 100 were written on the left and right ends of the ruler, respectively, with 9 hash marks written between them, but no numbers corresponding to those marks. The “sliding” portion was a piece of plastic on wheels connected to the ruler which participants slid back and forth to make their ratings of loudness and duration.
Procedure
Participants arrived in the lab and were told that they were going to complete a “writing task.” They were informed that for this task, they would have to wait for 10 minutes after they stopped writing to complete a recall task. The experimenter then explained that instead of waiting, they could take part of in an unrelated pilot study on auditory perception in which they would listen to a series of tones and rate them (the real task of interest). All participants agreed to participate in the pilot study. After consenting to the pilot, participants filled out the current emotions questionnaire and then started the writing task. The instructions for the writing task were given based on the condition to which they were randomly assigned. All participants were told that they would have ten minutes to complete the writing task. In order to maximize the affect induction, participants were interrupted and told to stop writing after they had completed one full page of writing or after 10 minutes elapsed (whichever came first).
Participants then started the auditory perception pilot study. They were told it would involve listening to tones and rating each for loudness and duration on a scale of 0 – 100. Participants listened to two sets of two “anchor” tones, which served as examples of a 0 on the loudness scale, a 100 on the loudness scale, a 0 on the duration scale, and a 100 on the duration scale. The 0 loudness anchor tone was an 800 millisecond tone at 65 decibels and the 100 loudness anchor tone was the same 800 millisecond tone at 121 decibels. After the loudness anchors were presented, the volume on the speakers was set to 50 (~ 95 decibels) for the rest of the experiment. The 0 duration anchor tone was a 50 millisecond tone and the 100 duration anchor was a 4000 millisecond tone. Participants were instructed to use the anchors when making their judgments. Participants were then given the slider and instructed to use it to make their estimates of loudness and duration. Throughout the presentation of the tones, researchers randomized whether participants gave their loudness or duration ratings first.
Participants listened to the anchor tones as many times as they wanted in order to “really get a feel for the scale.” Once participants felt comfortable, presentation of the tones began. After participants listened to each tone, they rated the loudness and duration of the tone. The researcher determined the participants’ ratings by flipping over the slider and recording the number that matched the rating (numbers written on the back of the slider ranged from 0 – 100), out of sight of participants.
Once the researcher had presented all tones to the participant, the participant was asked to complete the manipulation check. Participants were given explicit instructions to report how they felt during the writing task. After the survey, participants reported what they thought the experiment was about. At this point, participants received a thorough debriefing about the true nature of the task and were informed that the two tasks were really part of the same study.
Results
Initial Affect
In order to assess whether there were differences between the negative and neutral groups in their initial affect ratings, we conducted a series of t-tests and found no significant differences between the groups in their ratings of their initial emotions (ps > .27).
Manipulation Check
During the manipulation check, for which participants were asked to rate their affect during the writing task, participants in the negative group reported feeling more scared (negative: M = 2.00, SD = 1.09; neutral: M = 1.00, SD = 0), t (22) = 2.88, p = .01, d = 1.27, more afraid (negative: M = 1.82, SD = 0.98; neutral: M = 1.00, SD = 0), t (22) = 2.63, p = .02, d = 1.16 and more anxious (M = 2.36, SD = 1.12) than neutral participants (M = 1.40, SD = 0.52), t (22) = 2.49, p = .02, d = 1.08. Further, they reported feeling less calm (M = 3.00, SD = 1.0) than neutral participants (M = 4.10, SD = 0.57), t (22) = −3.06, p = .007, d = −1.34, as well as less at ease (M = 2.73, SD = 1.19) than neutral participants (M = 4.00, SD = 0.67), t (39) = −2.98, p = .008, d = −1.30.
Coding of Written Responses
Two independent, but equally trained, research assistants coded each participant’s written response to the manipulation. Research assistants recorded the number of “fear” words used in the document (any verb, noun, or adjective directly describing fear or a frightening experience). They also recorded the total number of “emotion” words (any verb, noun, or adjective directly describing an emotional experience). Finally, they rated the overall emotional tone of the written response on a scale of 1 (not emotional) to 7 (intensely emotional). The raters’ scores were then averaged together and the number of fear words, emotion words, and the tone of the response were combined to produce a “story rating” score for each participant. We conducted a series of independent t-tests in order to assess whether participants’ responses to the writing task were different between groups. In the writing task, participants in the negative group used more fear words in their written responses (M = 2.86, SD = 1.34) than participants in the neutral group (M = 0, SD = 0), t (22) = 6.73, p < .001, d = 2.94. Participants in the negative group also used more general emotion words in their written responses (M = 4.00, SD = 2.90) than did participants in the neutral group (M = .30, SD = .42), t (22) = 3.98, p = .001, d = 1.74. Finally, the tone of the written responses of participants in the negative group (M = 4.54, SD = 1.15) was rated as significantly more negative than the tone in the neutral condition (M = 1.05, SD = .45), t (22) = 9.51, p < .001, d = 3.92.
Loudness Ratings
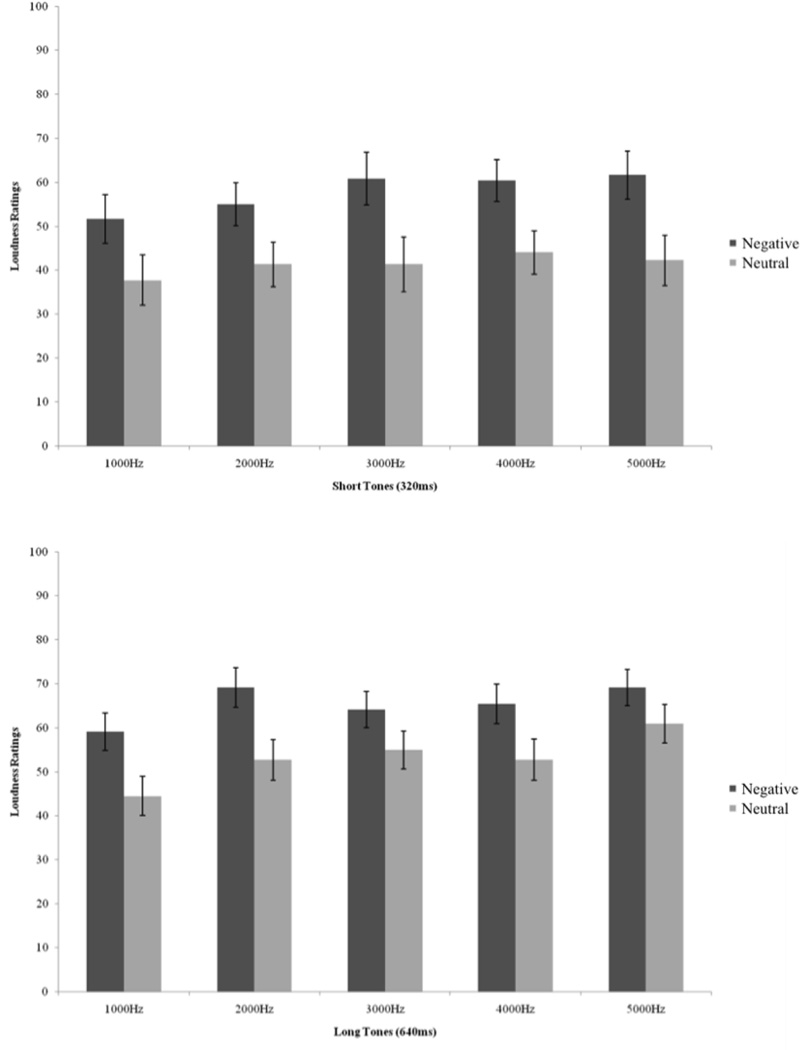
We conducted a 5 (tone frequency: 1000hz, 2000hz, 3000hz, 4000hz, 5000hz) × 2 (tone length: short, long) × 2 (condition: negative, neutral) analysis of variance (ANOVA) to determine if the experimental manipulation affected estimates of loudness. Table 1 presents the mean loudness ratings for each tone by experimental group. There was a significant main effect of condition F (1, 21) = 6.40, p = .01, ηp2 = .23, such that participants in the negative condition estimated the tones to be significantly louder than participants in the neutral condition (See Figure 1). There was also a main effect of tone frequency F(4,18) = 7.07, p < .001, ηp2 = .25, indicating that participants detected a difference in frequency among the tones, as well as a main effect of tone length, F(1, 21) = 21.84, p<.001, ηp2 = .51, indicating that participants’ estimates of loudness increased when the tones got longer.
Table 1.
Mean Loudness and Duration Ratings by Condition
| Loudness M(SE) |
Duration M(SE) |
|||
|---|---|---|---|---|
| Negativea | Neutralb | Negativea | Neutralb | |
| Shortc | ||||
| 1000Hz | 51.67(5.50) | 37.73(5.75) | 20.50(3.62) | 17.82(3.78) |
| 2000Hz | 55.00(4.88) | 41.36(5.09) | 23.75(3.64) | 15.90(3.80) |
| 3000Hz | 60.83(5.98) | 41.36(6.24) | 23.33(2.55) | 14.54(2.66) |
| 4000Hz | 60.42(4.74) | 44.09(4.95) | 26.25(3.71) | 14.18(3.88) |
| 5000Hz | 69.16(5.45) | 42.27(5.69) | 23.75(4.28) | 21.00(4.46) |
| Longd | ||||
| 1000Hz | 59.16(4.23) | 44.54(4.42) | 50.41(5.17) | 46.81(5.40) |
| 2000Hz | 69.16(4.43) | 52.72(4.63) | 53.75(6.22) | 50.91(6.49) |
| 3000Hz | 64.16(4.15) | 55.00(4.33) | 52.08(5.17) | 52.08(5.40) |
| 4000Hz | 65.41(4.49) | 52.72(4.69) | 52.91(5.11) | 47.27(5.34) |
| 5000Hz | 69.16(4.17) | 60.90(4.35) | 57.08(5.68) | 46.82(5.93) |
Note. The short tones were presented first and the long tones were presented last. Within the short and long groups, tone presentation was randomized.
n = 11.
n = 12.
Short = 320 milliseconds.
Long = 640 milliseconds
Figure 1.
Mean loudness ratings (+ 1 SE) of the short (320ms) and long (640ms) tones for the Neutral (n = 11) and Negative (n = 12) groups.
Duration Ratings
We conducted a 5 (tone frequency: 1000hz, 2000hz, 3000hz, 4000hz, 5000hz) × 2 (tone length: short, long) × 2 (condition: negative, neutral) ANOVA, in order to determine if the writing task affected participants’ estimates of the duration of the tones. Table 1 presents the mean duration ratings for each tone by experimental group. There was no main effect of tone frequency, indicating that participants’ ratings of the duration of the tones were not affected by changes in frequency. There was, however, a main effect of tone length, F (1, 21) = 21.00, p < .001, ηp2 = .84, indicating that participants’ ratings of the duration of tones changed as the duration of the tones changed. Finally, there was no main effect of condition F (1, 21) = 1.38, p = .25, ηp2 = .06, suggesting that the writing task did not affect participants’ estimates of the duration of the tones.
Discussion
As predicted, participants in a negative affective state judged the tones as significantly louder than participants in a neutral state. There was no significant difference between the groups in ratings of duration. This indicates that the increased ratings of loudness, in the negative condition, were likely not the result of a response bias which would result in an increase in responses overall. Rather, inducing negative affect had a significant influence only on participants’ perception of loudness.
It is not surprising that the effect on loudness ratings was larger, given previous differences in perceived loudness demonstrated in clinical populations (Dess & Edelheit, 1998) and the relationship between negative affect and early auditory processing (Wang, et, al., 2009). It should be noted however that individuals in the negative group did tend to rate the tones as slightly longer than participants in the neutral state, but the effect did not come close to statistical significance. It is possible that a relationship exists between affective state and the perception of duration but that it could not be adequately detected with this stimulus set. The perception of duration plays an important role in language perception (Gandour, et, al., 2002) and affective state might exert a stronger influence on ratings of duration in linguistic stimuli. Moreover, changing the auditory matching measure to one in which individuals reproduced the tone that they heard (as opposed to passively matching it on a slider) could influence ratings on both measures. However, the perception of loudness, in particular, is highly dependent upon the amplitude of the last sound heard (Plack & Carlyon, 1995). Asking participants to reproduce each tone might lead them to scale their ratings of subsequent tones to the loudness of the tone they just produced (as opposed to the anchors provided to them in the beginning of the task). This could make it difficult to detect the influence of affect on perception of the stimuli.
Participants were asked to write about a frightening experience and this task appears to have changed one aspect of auditory perception. It is impossible to know, however, which component of this task affected auditory perception. Emotional arousal has been reliably associated with changes in visual perception (Stefanucci & Storbeck, 2009) and it seems likely that arousal may have been a factor here. Whether arousal was the driving force behind the differences observed in the loudness ratings is unclear. The writing manipulation used in this experiment was consistent and reliable, but has not been shown to be particularly arousing (Westermann, Spies, Stahl, & Hesse, 1996). One possibility is that the tones themselves served as mild mood induction. Loud noises do induce negative affect and noise bursts are regularly used as aversive stimuli in conditioning studies (LeDoux, 1996). However, given the innocuousness of the tones and the low decibel level at which they were presented, this possibility seems unlikely.
An obvious future direction for this research is to examine arousal and valence separately with regard to their effects on auditory perception. Collecting psychophysiological measures of peripheral arousal like skin conductance or heart rate, for example, might help to tease apart the differential influence of valence and arousal in this finding. An alternate manipulation of affect would also be useful for assessing whether other induction procedures in which valence and arousal can be more carefully controlled produce the observed effects on perceived loudness (see Geuss, Stefanucci, deBenedictis-Kessner, & Stevens, in press and Stefanucci & Storbeck, 2009 for manipulations of arousal only).
The neural mechanisms that underlie our finding are not well understood. However, there are potential neural substrates that may be important for understanding the relationship between affective state and auditory perception. The amygdala, for example, projects to multiple auditory areas in the temporal lobe (Schafe & Ledoux, 2004). Research in visual perception suggests that amygdala activity enhances visual awareness, fundamentally changing low-level visual perception (Duncan & Barrett, 2007). Affect may enhance or change aspects of auditory perception in a similar way. Additionally, and perhaps more importantly, the orbitofrontal cortex (OFC) is integral for synthesizing sensory information and affect. The OFC has direct projections to primary auditory areas (Kringelbach, 2005) as well as cortical and sub-cortical affect processing areas (Barrett & Barr, 2009). The relationship between affective and auditory processing occurring in these areas is intriguing. More research is necessary to understand the functional significance of these neural pathways.
The final question is why a negative affective state would change auditory perception. Mineka and Ohman (2002) suggest that low-level negative affects are selective, automatic, impenetrable to conscious cognitive control, and evolved in order to help us form quick associations when threatened. In this context, affect may influence auditory perception so that we respond more quickly when threatened. If a bear is chasing a listener in the woods, perceiving the bear to be closer than he really is (because his roar sounds louder) could motivate the listener to move more quickly out of his way and to safety. Regardless of underlying processes or their origins, the present study suggests that a negative affective state may qualitatively change the way that we hear loudness in our environment. Moreover, it extends previous research showing that affect influences visual processes to another perceptual modality, suggesting that affect may crucially impact the way we experience and interact with the world.
Acknowledgments
The authors would like to thank Kelsey Mihaloew for her help in collecting data and constructing the slider. This research was supported in part by NIH RO1MH075781-01A2 grant, for which the second author serves as a consultant.
References
- Alpers GW, Gerdes ABM. Here’s looking at you: Emotional faces predominate in binocular rivalry. Emotion. 2007;7:495–506. doi: 10.1037/1528-3542.7.3.495. [DOI] [PubMed] [Google Scholar]
- Barrett LF, Bar M. See it with feeling: Affective predictions in the human brain. Royal Society Phil Trans B. 2009;364:1325–1334. doi: 10.1098/rstb.2008.0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker MW. Panic Search: Fear produces efficient visual searching for nonthreatening objects. Psychological Science. 2009;20:435–437. doi: 10.1111/j.1467-9280.2009.02303.x. [DOI] [PubMed] [Google Scholar]
- Bocanegra BR, Zeelenberg R. Emotion improves and impairs early vision. Psychological Science. 2009;20:707–713. doi: 10.1111/j.1467-9280.2009.02354.x. [DOI] [PubMed] [Google Scholar]
- Dess NK, Edelheit D. The bitter with the sweet: The taste/stress/temperament nexus. Biological Psychology. 1998;48:103–119. doi: 10.1016/s0301-0511(98)00014-3. [DOI] [PubMed] [Google Scholar]
- Duncan SL, Barrett LF. The amygdala in visual awareness. Trends in Cognitive Sciences. 2007;11:190–192. doi: 10.1016/j.tics.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandour J, Wong D, Lowe M, Dzemidzic M, Satthamnuwong N, Long Y, Luritoc J. Neural circuitry underlying perception of duration depends on language experience. Brain & Language. 2002;83:268–290. doi: 10.1016/s0093-934x(02)00033-0. [DOI] [PubMed] [Google Scholar]
- Gasper K, Clore GL. Attending to the big picture: Mood and global verses local processing of visual information. Psychological Science. 2002;13:34–40. doi: 10.1111/1467-9280.00406. [DOI] [PubMed] [Google Scholar]
- Geuss M, Stefanucci JK, de Benedictis-Kessner J, Stevens NR. A balancing act: Physical balance influences the perception of spatial layout. Attention, Perception, & Psychophysics. doi: 10.3758/APP.72.7.1890. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML. Linking reward to hedonic experience. Nat. Rev. Neurosci. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- LeDoux J. The Emotional Brain. New York: Simon and Schuster; 1996. A few degrees of separation; pp. 138–178. [Google Scholar]
- Mineka S, Ohman A. Phobias and preparedness: The selective, automatic, and encapsulated nature of fear. Biological Psychiatry. 2002;52:927–937. doi: 10.1016/s0006-3223(02)01669-4. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Ling S, Carrasco M. Emotion facilitates perception and potentiates the perceptual benefits of attention. Psychological Science. 2006;17:292–299. doi: 10.1111/j.1467-9280.2006.01701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plack CJ, Carlyon RP. Loudness perception and intensity coding. In: More B, editor. Hearing. 2nd ed. San Diego, CA: Academic Press; 1995. pp. 123–156. [Google Scholar]
- Pollock RA, Carter AS, Amir N, Marks LE. Anxiety sensitivity and auditory perception of heartbeat. Behavior Research and Therapy. 2006;44:1739–1756. doi: 10.1016/j.brat.2005.12.013. [DOI] [PubMed] [Google Scholar]
- Pylyshyn ZW. Explaining mental imagery: Are there really pictures in the brain? Trends in Cognitive Sciences. 2003;7:113–118. doi: 10.1016/s1364-6613(03)00004-4. [DOI] [PubMed] [Google Scholar]
- Schafe GE, Ledoux JE. The neural basis of fear. In: Gazzaniga M, editor. The Cognitive Neurosciences. 3rd ed. Cambridge, MA: MIT Press; 2004. pp. 987–1003. [Google Scholar]
- Schmitz TW, De Rosa E, Anderson AK. Opposing influences of affective state valence on visual cortical encoding. Journal of Neuroscience. 2009;29(22):7199–7207. doi: 10.1523/JNEUROSCI.5387-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanucci JK, Proffitt DR. The roles of altitude and fear in the perception of height. Journal of Experimental Psychology: Human Perception & Performance. 2009 doi: 10.1037/a0013894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanucci JK, Storbeck J. Don’t look down: Emotional arousal elevates height perception. Journal of Experimental Psychology: General. 2009;138:131–145. doi: 10.1037/a0014797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanucci JK, Proffitt DR, Clore G, Parekh Skating down a steeper slope: Fear influences the perception of geographical slant. Perception. 2008;37:321–323. doi: 10.1068/p5796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teachman BA, Stefanucci JK, Clerkin EM, Cody MW, Proffitt DR. A new mode of fear expression: Perceptual bias in height fear. Emotion. 2008;8:296–301. doi: 10.1037/1528-3542.8.2.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Nicol T, Skoe E, Sams M, Krous N. Emotion modulates early auditory response to speech. Journal of Cognitive Neuroscience. 2009;21(11):2121–2128. doi: 10.1162/jocn.2008.21147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Westermann R, Spies K, Stahl G, Hesse FW. The relative effectiveness and validity of mood induction procedures: A meta-analysis. The European Journal of Social Psychology. 1996;26:557–580. [Google Scholar]