Abstract
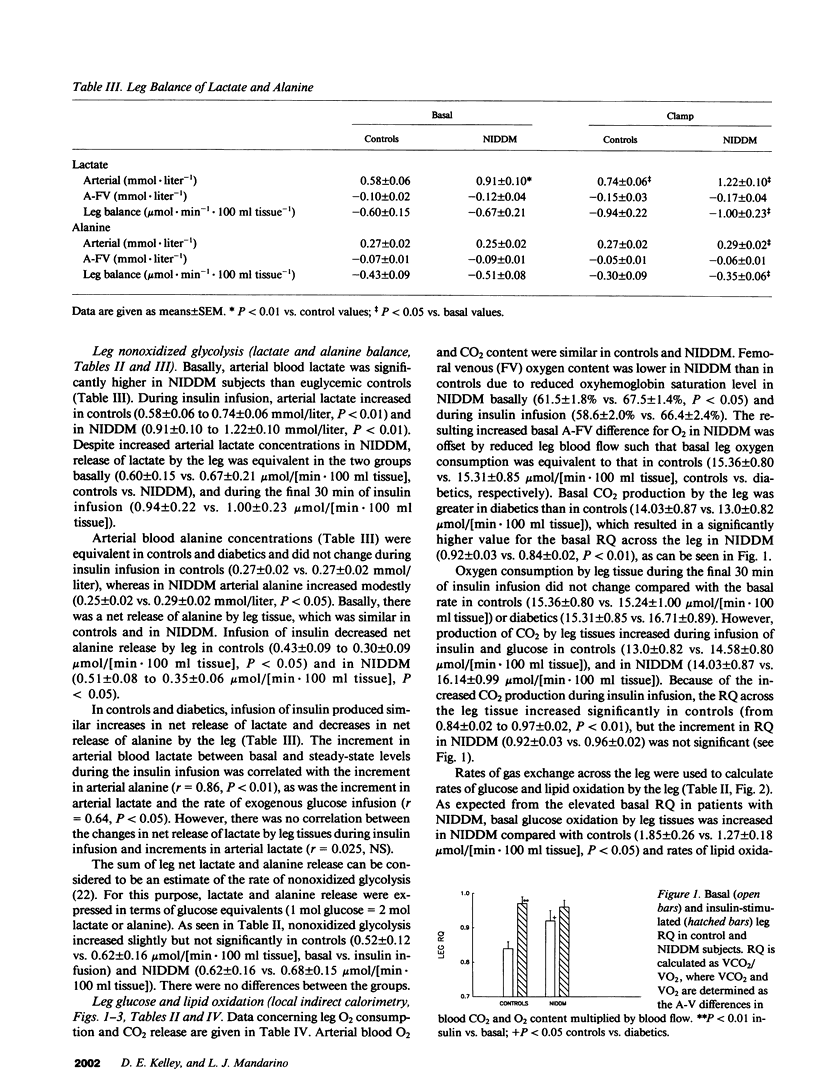
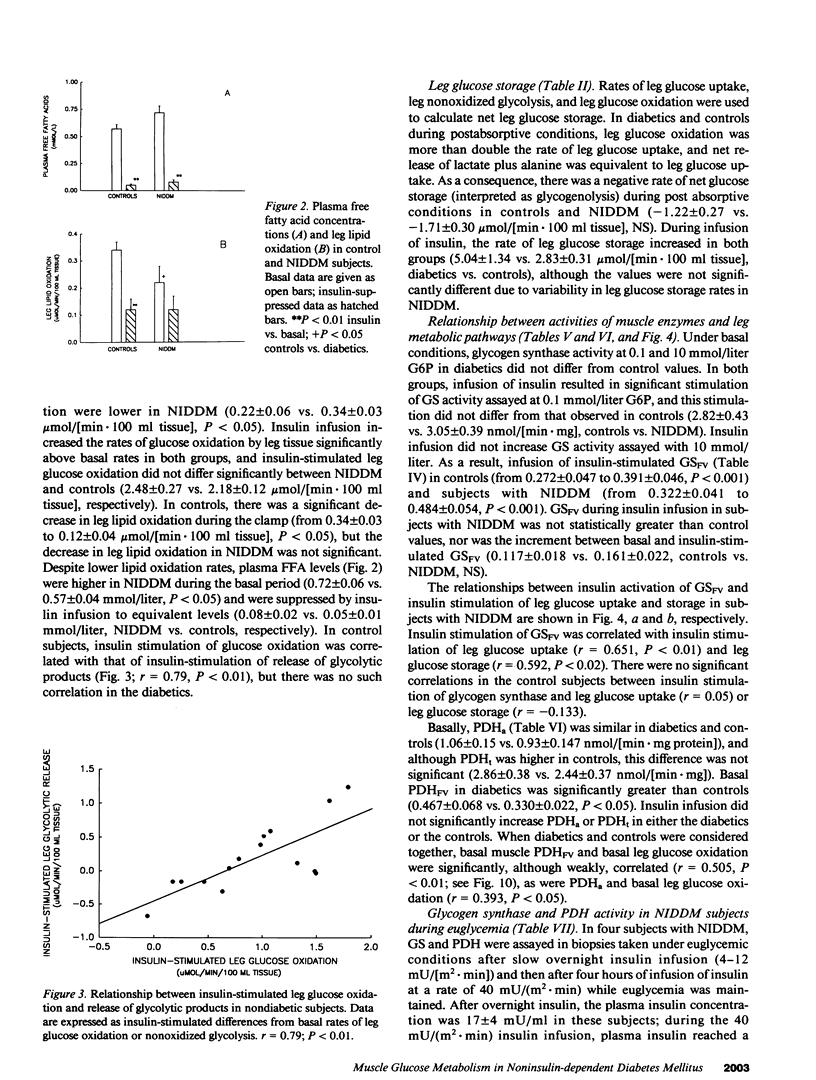
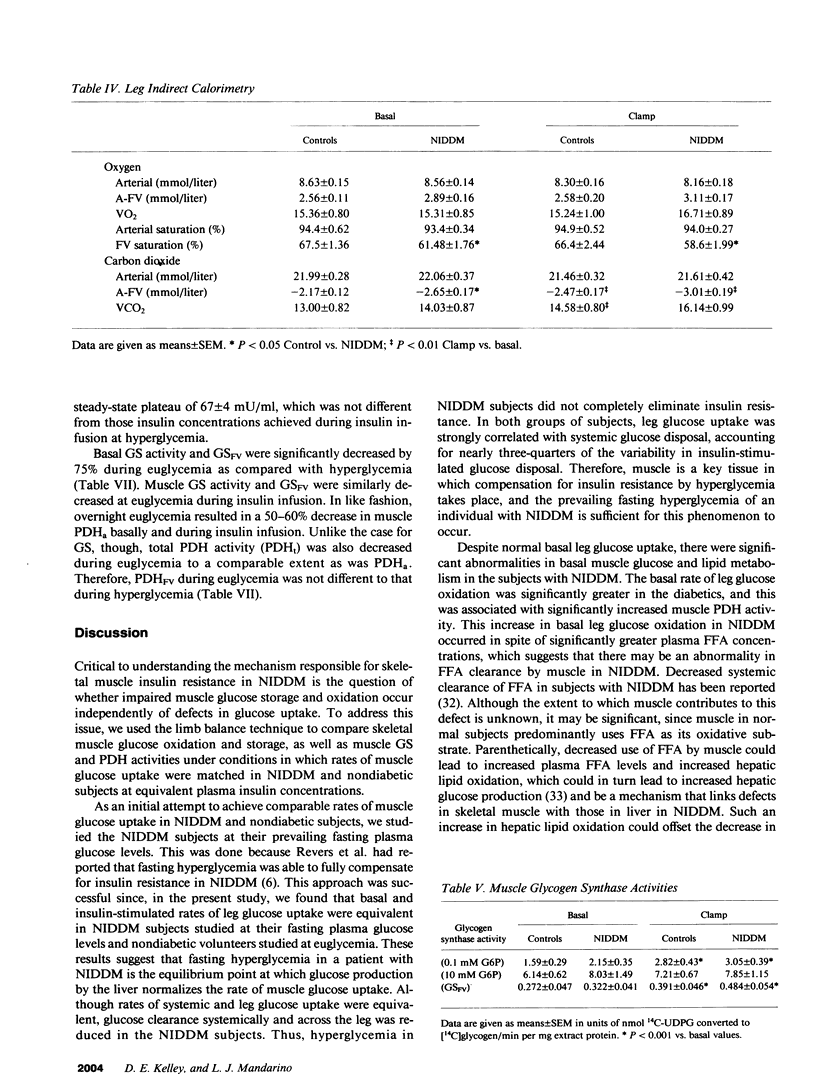
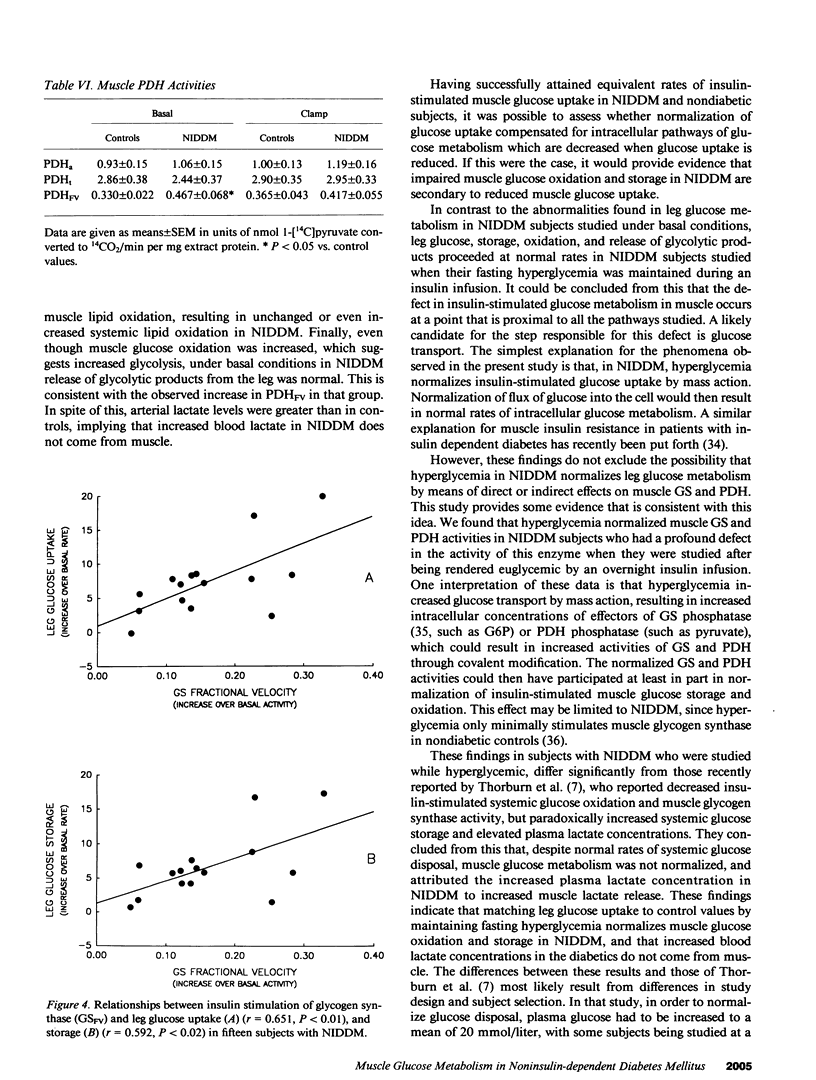
The diminished ability of insulin to promote glucose disposal and storage in muscle has been ascribed to impaired activation of glycogen synthase (GS). It is possible that decreased glucose storage could occur as a consequence of decreased glucose uptake, and that GS is impaired secondarily. Muscle glucose uptake in 15 diabetic subjects was matched to 15 nondiabetic subjects by maintaining fasting hyperglycemia during infusion of insulin. Leg muscle glucose uptake, glucose oxidation (local indirect calorimetry), release of glycolytic products, and muscle glucose storage, as well as muscle GS and pyruvate dehydrogenase (PDH) were determined before and during insulin infusion. Basal leg glucose oxidation and PDH were increased in the diabetics. Insulin-stimulated leg glucose uptake in the diabetics (8.05 +/- 1.41 mumol/[min.100 ml leg tissue]) did not differ from controls (5.64 +/- 0.37). Insulin-stimulated leg glucose oxidation, nonoxidized glycolysis, and glucose storage (2.48 +/- 0.27, 0.68 +/- 0.15, and 5.04 +/- 1.34 mumol/[min.100 ml], respectively) were not different from controls (2.18 +/- 0.12, 0.62 +/- 0.16, and 2.83 +/- 0.31). PDH and GS in noninsulin-dependent diabetes mellitus (NIDDM) were also normal during insulin infusion. When diabetics were restudied after being rendered euglycemic by overnight insulin infusion, GS and PDH were reduced compared with hyperglycemia. Thus, fasting hyperglycemia is sufficient to normalize insulin-stimulated muscle glucose uptake in NIDDM, and glucose is distributed normally to glycogenesis and glucose oxidation, possibly by normalization of GS and PDH.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDRES R., CADER G., ZIERLER K. L. The quantitatively minor role of carbohydrate in oxidative metabolism by skeletal muscle in intact man in the basal state; measurements of oxygen and glucose uptake and carbon dioxide and lactate production in the forearm. J Clin Invest. 1956 Jun;35(6):671–682. doi: 10.1172/JCI103324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adkins-Marshall B. A., Myers S. R., Hendrick G. K., Williams P. E., Triebwasser K., Floyd B., Cherrington A. D. Interaction between insulin and glucose-delivery route in regulation of net hepatic glucose uptake in conscious dogs. Diabetes. 1990 Jan;39(1):87–95. doi: 10.2337/diacare.39.1.87. [DOI] [PubMed] [Google Scholar]
- Barrett E. J., Ferrannini E., Gusberg R., Bevilacqua S., DeFronzo R. A. Hepatic and extrahepatic splanchnic glucose metabolism in the postabsorptive and glucose fed dog. Metabolism. 1985 May;34(5):410–420. doi: 10.1016/0026-0495(85)90205-7. [DOI] [PubMed] [Google Scholar]
- Bergman R. N., Beir J. R., Hourigan P. M. Intraportal glucose infusion matched to oral glucose absorption. Lack of evidence for "gut factor" involvement in hepatic glucose storage. Diabetes. 1982 Jan;31(1):27–35. doi: 10.2337/diab.31.1.27. [DOI] [PubMed] [Google Scholar]
- Bogardus C., Lillioja S., Howard B. V., Reaven G., Mott D. Relationships between insulin secretion, insulin action, and fasting plasma glucose concentration in nondiabetic and noninsulin-dependent diabetic subjects. J Clin Invest. 1984 Oct;74(4):1238–1246. doi: 10.1172/JCI111533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogardus C., Lillioja S., Stone K., Mott D. Correlation between muscle glycogen synthase activity and in vivo insulin action in man. J Clin Invest. 1984 Apr;73(4):1185–1190. doi: 10.1172/JCI111304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherrington A. D., Williams P. E., Abou-Mourad N., Lacy W. W., Steiner K. E., Liljenquist J. E. Insulin as a mediator of hepatic glucose uptake in the conscious dog. Am J Physiol. 1982 Feb;242(2):E97–101. doi: 10.1152/ajpendo.1982.242.2.E97. [DOI] [PubMed] [Google Scholar]
- Davis M. A., Williams P. E., Cherrington A. D. Effect of a mixed meal on hepatic lactate and gluconeogenic precursor metabolism in dogs. Am J Physiol. 1984 Sep;247(3 Pt 1):E362–E369. doi: 10.1152/ajpendo.1984.247.3.E362. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Ferrannini E., Hendler R., Felig P., Wahren J. Regulation of splanchnic and peripheral glucose uptake by insulin and hyperglycemia in man. Diabetes. 1983 Jan;32(1):35–45. doi: 10.2337/diab.32.1.35. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Ferrannini E., Hendler R., Wahren J., Felig P. Influence of hyperinsulinemia, hyperglycemia, and the route of glucose administration on splanchnic glucose exchange. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5173–5177. doi: 10.1073/pnas.75.10.5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo R. A., Jacot E., Jequier E., Maeder E., Wahren J., Felber J. P. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes. 1981 Dec;30(12):1000–1007. doi: 10.2337/diab.30.12.1000. [DOI] [PubMed] [Google Scholar]
- DeFronzo R., Deibert D., Hendler R., Felig P., Soman V. Insulin sensitivity and insulin binding to monocytes in maturity-onset diabetes. J Clin Invest. 1979 May;63(5):939–946. doi: 10.1172/JCI109394. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Douglas A. R., Jones N. L., Reed J. W. Calculation of whole blood CO2 content. J Appl Physiol (1985) 1988 Jul;65(1):473–477. doi: 10.1152/jappl.1988.65.1.473. [DOI] [PubMed] [Google Scholar]
- Ferrannini E., Barrett E. J., Bevilacqua S., DeFronzo R. A. Effect of fatty acids on glucose production and utilization in man. J Clin Invest. 1983 Nov;72(5):1737–1747. doi: 10.1172/JCI111133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frayn K. N. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol Respir Environ Exerc Physiol. 1983 Aug;55(2):628–634. doi: 10.1152/jappl.1983.55.2.628. [DOI] [PubMed] [Google Scholar]
- Gelfand R. A., Barrett E. J. Effect of physiologic hyperinsulinemia on skeletal muscle protein synthesis and breakdown in man. J Clin Invest. 1987 Jul;80(1):1–6. doi: 10.1172/JCI113033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert V., Lau K. S., Gottlieb C. W., Bleicher S. J. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965 Oct;25(10):1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- Ishida T., Chap Z., Chou J., Lewis R., Hartley C., Entman M., Field J. B. Differential effects of oral, peripheral intravenous, and intraportal glucose on hepatic glucose uptake and insulin and glucagon extraction in conscious dogs. J Clin Invest. 1983 Aug;72(2):590–601. doi: 10.1172/JCI111007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R. A., Roshania R. D., Hawa M. I., Sim B. M., DiSilvio L. Impact of glucose ingestion on hepatic and peripheral glucose metabolism in man: an analysis based on simultaneous use of the forearm and double isotope techniques. J Clin Endocrinol Metab. 1986 Sep;63(3):541–549. doi: 10.1210/jcem-63-3-541. [DOI] [PubMed] [Google Scholar]
- Karl I. E., Pagliara A. S., Kipnis D. M. A microfluorometric enzymatic assay for the determination of alanine and pyruvate in plasma and tissues. J Lab Clin Med. 1972 Sep;80(3):434–441. [PubMed] [Google Scholar]
- Kelley D. E., Reilly J. P., Veneman T., Mandarino L. J. Effects of insulin on skeletal muscle glucose storage, oxidation, and glycolysis in humans. Am J Physiol. 1990 Jun;258(6 Pt 1):E923–E929. doi: 10.1152/ajpendo.1990.258.6.E923. [DOI] [PubMed] [Google Scholar]
- Kida Y., Esposito-Del Puente A., Bogardus C., Mott D. M. Insulin resistance is associated with reduced fasting and insulin-stimulated glycogen synthase phosphatase activity in human skeletal muscle. J Clin Invest. 1990 Feb;85(2):476–481. doi: 10.1172/JCI114462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolterman O. G., Gray R. S., Griffin J., Burstein P., Insel J., Scarlett J. A., Olefsky J. M. Receptor and postreceptor defects contribute to the insulin resistance in noninsulin-dependent diabetes mellitus. J Clin Invest. 1981 Oct;68(4):957–969. doi: 10.1172/JCI110350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandarino L. J., Wright K. S., Verity L. S., Nichols J., Bell J. M., Kolterman O. G., Beck-Nielsen H. Effects of insulin infusion on human skeletal muscle pyruvate dehydrogenase, phosphofructokinase, and glycogen synthase. Evidence for their role in oxidative and nonoxidative glucose metabolism. J Clin Invest. 1987 Sep;80(3):655–663. doi: 10.1172/JCI113118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyboer J., Murray P., Sedensky J. A. Blood-flow indices in amputee and control limbs by mutual electrical impedance plethysmography. Am Heart J. 1974 Jun;87(6):704–710. doi: 10.1016/0002-8703(74)90414-1. [DOI] [PubMed] [Google Scholar]
- Revers R. R., Fink R., Griffin J., Olefsky J. M., Kolterman O. G. Influence of hyperglycemia on insulin's in vivo effects in type II diabetes. J Clin Invest. 1984 Mar;73(3):664–672. doi: 10.1172/JCI111258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizza R. A., Mandarino L. J., Gerich J. E. Mechanism and significance of insulin resistance in non-insulin-dependent diabetes mellitus. Diabetes. 1981 Dec;30(12):990–995. doi: 10.2337/diab.30.12.990. [DOI] [PubMed] [Google Scholar]
- Saccà L., Cicala M., Trimarco B., Ungaro B., Vigorito C. Differential effects of insulin on splanchnic and peripheral glucose disposal after an intravenous glucose load in man. J Clin Invest. 1982 Jul;70(1):117–126. doi: 10.1172/JCI110583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman G. I., Rothman D. L., Jue T., Stein P., DeFronzo R. A., Shulman R. G. Quantitation of muscle glycogen synthesis in normal subjects and subjects with non-insulin-dependent diabetes by 13C nuclear magnetic resonance spectroscopy. N Engl J Med. 1990 Jan 25;322(4):223–228. doi: 10.1056/NEJM199001253220403. [DOI] [PubMed] [Google Scholar]
- Taskinen M. R., Bogardus C., Kennedy A., Howard B. V. Multiple disturbances of free fatty acid metabolism in noninsulin-dependent diabetes. Effect of oral hypoglycemic therapy. J Clin Invest. 1985 Aug;76(2):637–644. doi: 10.1172/JCI112016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorburn A. W., Gumbiner B., Bulacan F., Wallace P., Henry R. R. Intracellular glucose oxidation and glycogen synthase activity are reduced in non-insulin-dependent (type II) diabetes independent of impaired glucose uptake. J Clin Invest. 1990 Feb;85(2):522–529. doi: 10.1172/JCI114468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yki-Järvinen H., Mott D., Young A. A., Stone K., Bogardus C. Regulation of glycogen synthase and phosphorylase activities by glucose and insulin in human skeletal muscle. J Clin Invest. 1987 Jul;80(1):95–100. doi: 10.1172/JCI113069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yki-Järvinen H., Sahlin K., Ren J. M., Koivisto V. A. Localization of rate-limiting defect for glucose disposal in skeletal muscle of insulin-resistant type I diabetic patients. Diabetes. 1990 Feb;39(2):157–167. doi: 10.2337/diab.39.2.157. [DOI] [PubMed] [Google Scholar]
- Yki-Järvinen H., Young A. A., Lamkin C., Foley J. E. Kinetics of glucose disposal in whole body and across the forearm in man. J Clin Invest. 1987 Jun;79(6):1713–1719. doi: 10.1172/JCI113011. [DOI] [PMC free article] [PubMed] [Google Scholar]


