Abstract
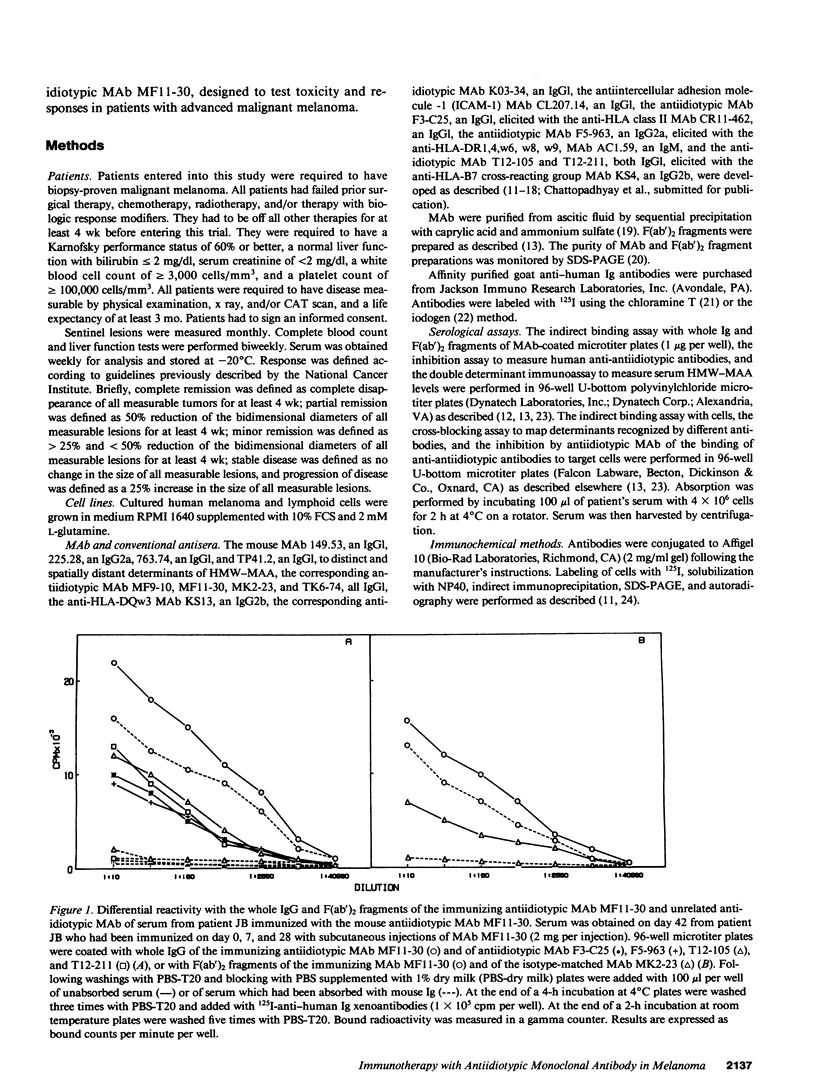
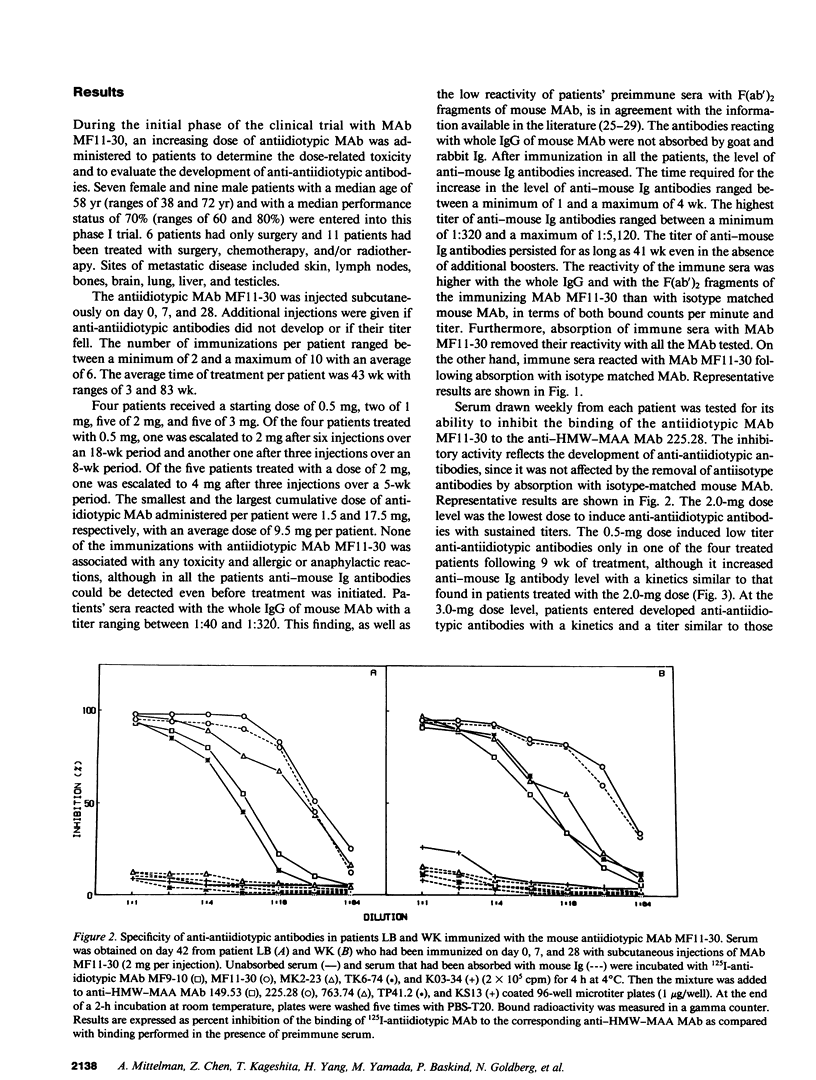
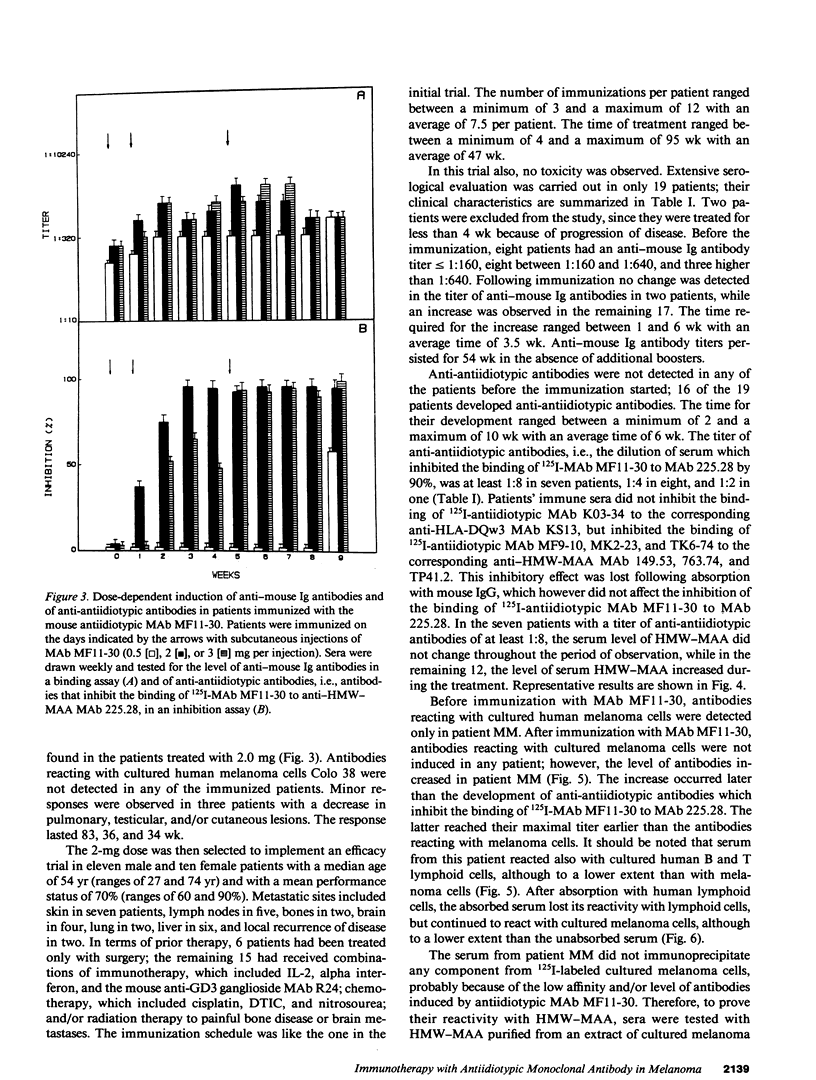
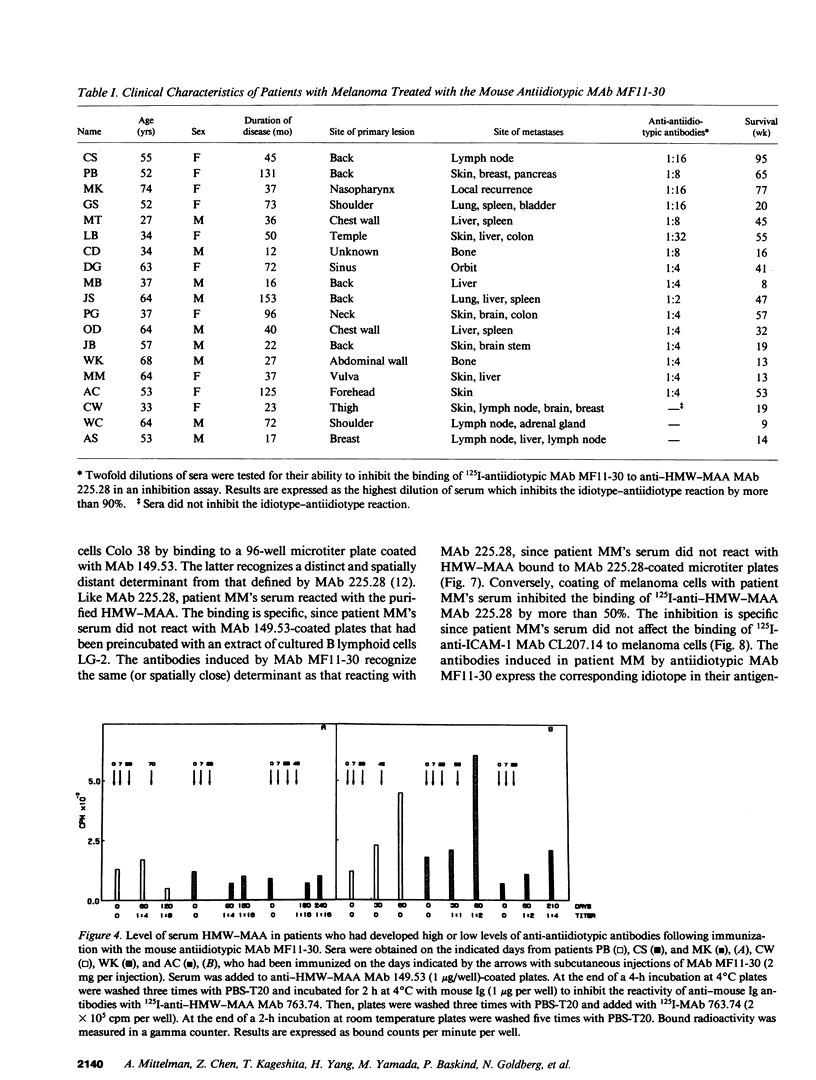
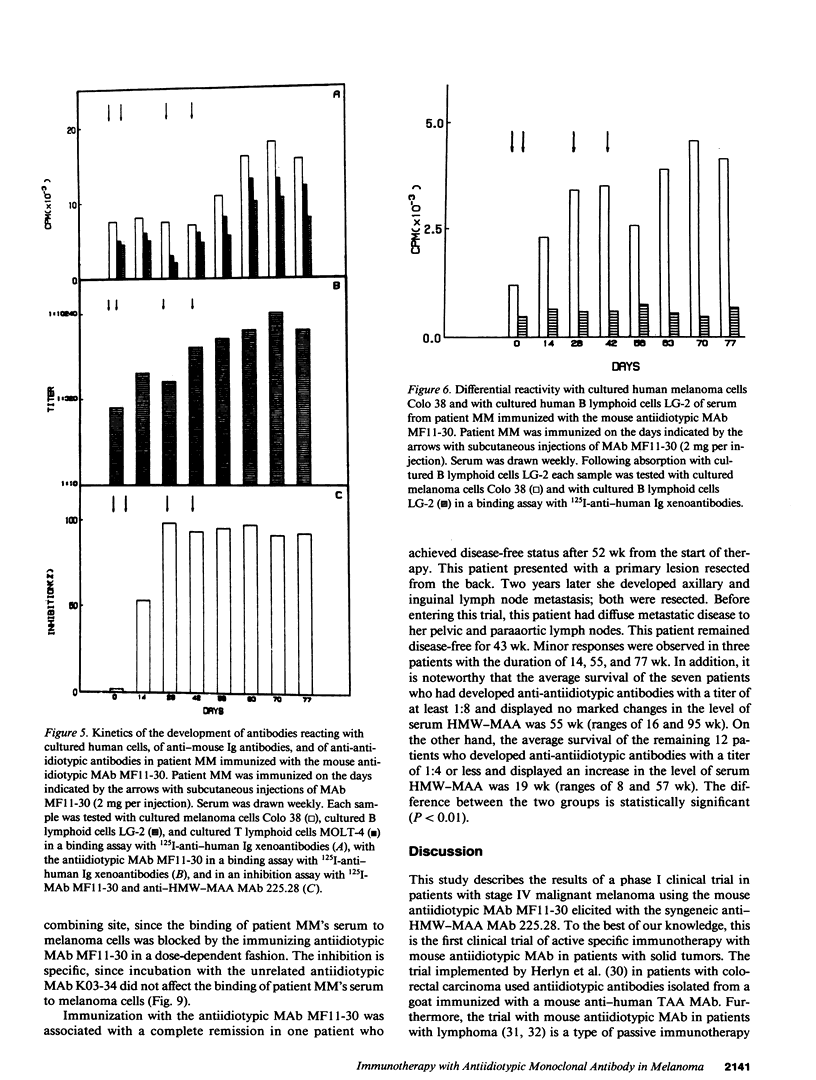
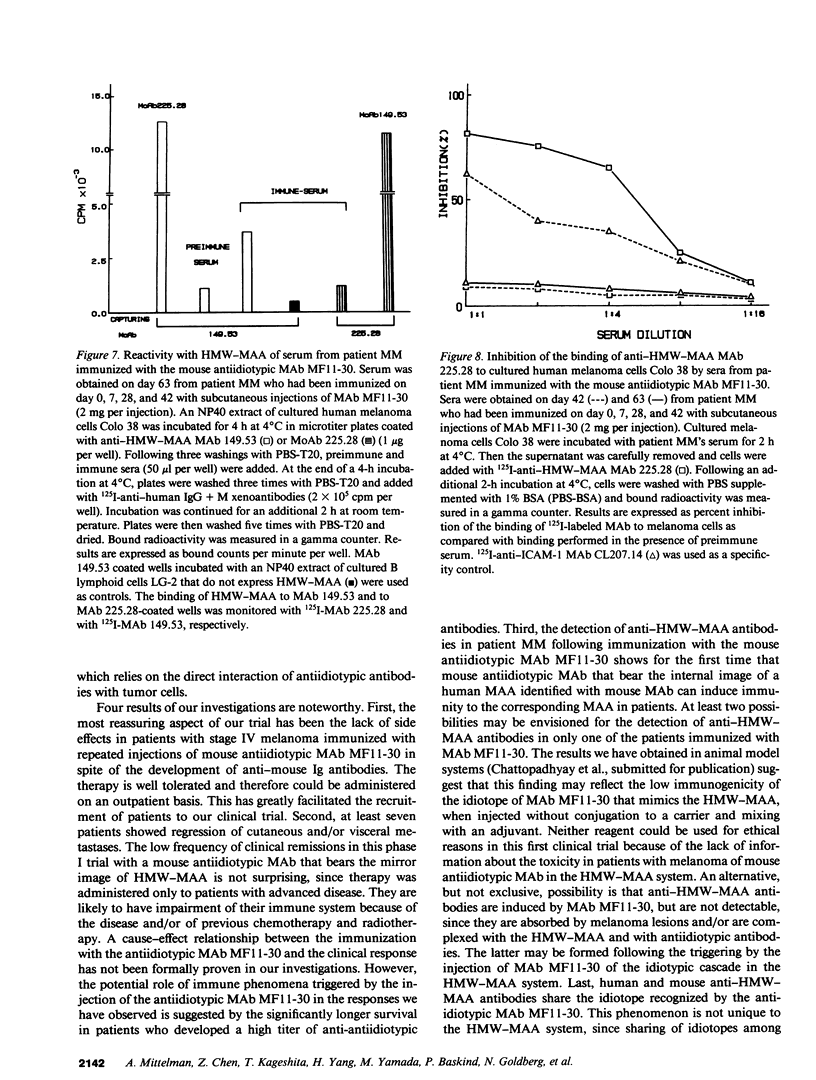
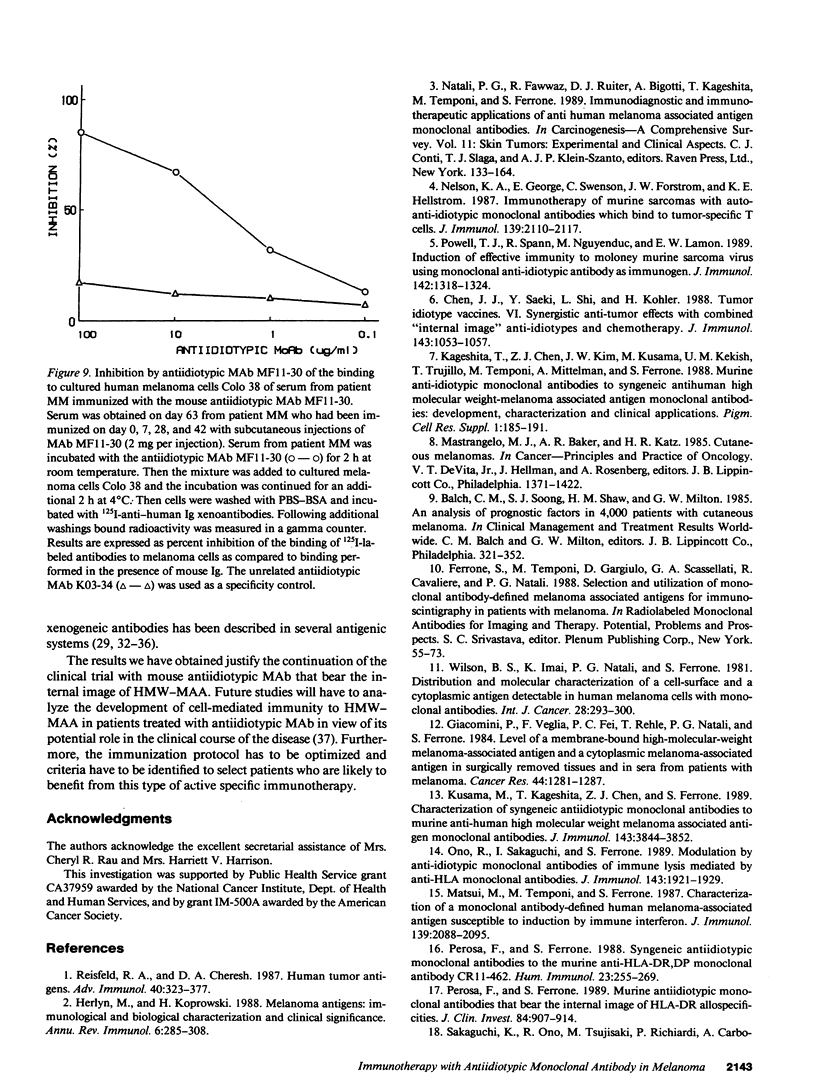
In two clinical trials the mouse antiidiotypic monoclonal antibody (MAb) MF11-30, which bears the internal image of human high-molecular-weight-melanoma-associated antigen (HMW-MAA) was administered by subcutaneous route without adjuvants to patients with stage IV malignant melanoma on day 0, 7, and 28. Additional injections were administered if anti-antiidiotypic antibodies were not found or their titer decreased. In the first phase I trial with 16 patients the initial dose was 0.5 mg per injection and escalated to 4 mg per injection. Neither toxicity nor allergic reactions were observed despite the development of anti-mouse Ig antibodies. Minor responses were observed in three patients. In a second clinical trial MAb MF11-30 was administered to 21 patients at a dose of 2 mg per injection, since this dose had been shown in the initial study to be effective in inducing anti-antiidiotypic antibodies. Two patients were inevaluable; in the remaining 19 patients, the average duration of treatment was 34 wk. In this trial as well, neither toxicity nor allergic reactions were observed. 17 of the 19 immunized patients increased the levels of anti-mouse Ig antibodies and 16 developed antibodies that inhibit the binding of antiidiotypic MAb MF11-30 to the immunizing anti-HMW-MAA MAb 225.28. One patient increased the level of anti-HMW-MAA antibodies. One patient achieved a complete remission with disappearance of multiple abdominal lymph nodes for a duration of 95 wk. Minor responses were observed in three patients. These results suggest that mouse antiidiotypic MAb that bear the internal image of HMW-MAA may be useful reagents to implement active specific immunotherapy in patients with melanoma.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen J. J., Saeki Y., Shi L. F., Köhler H. Tumor idiotype vaccines. VI. Synergistic anti-tumor effects with combined "internal image" anti-idiotypes and chemotherapy. J Immunol. 1989 Aug 1;143(3):1053–1057. [PubMed] [Google Scholar]
- Claflin J. L., Davie J. M. Clonal nature of the immune response to phosphorylcholine. IV. Idiotypic uniformity of binding site-associated antigenic determinants among mouse antiphosphorylcholine antibodies. J Exp Med. 1974 Sep 1;140(3):673–686. doi: 10.1084/jem.140.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtenay-Luck N. S., Epenetos A. A., Moore R., Larche M., Pectasides D., Dhokia B., Ritter M. A. Development of primary and secondary immune responses to mouse monoclonal antibodies used in the diagnosis and therapy of malignant neoplasms. Cancer Res. 1986 Dec;46(12 Pt 1):6489–6493. [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacomini P., Veglia F., Cordiali Fei P., Rehle T., Natali P. G., Ferrone S. Level of a membrane-bound high-molecular-weight melanoma-associated antigen and a cytoplasmic melanoma-associated antigen in surgically removed tissues and in sera from patients with melanoma. Cancer Res. 1984 Mar;44(3):1281–1287. [PubMed] [Google Scholar]
- Hellström K. E., Hellström I. Cellular immunity against tumor antigens. Adv Cancer Res. 1969;12:167–223. doi: 10.1016/s0065-230x(08)60331-0. [DOI] [PubMed] [Google Scholar]
- Herlyn D., Wettendorff M., Schmoll E., Iliopoulos D., Schedel I., Dreikhausen U., Raab R., Ross A. H., Jaksche H., Scriba M. Anti-idiotype immunization of cancer patients: modulation of the immune response. Proc Natl Acad Sci U S A. 1987 Nov;84(22):8055–8059. doi: 10.1073/pnas.84.22.8055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlyn M., Koprowski H. Melanoma antigens: immunological and biological characterization and clinical significance. Annu Rev Immunol. 1988;6:283–308. doi: 10.1146/annurev.iy.06.040188.001435. [DOI] [PubMed] [Google Scholar]
- Ju S. T., Benacerraf B., Dorf M. E. Idiotypic analysis of antibodies to poly(Glu60Ala30Tyr10): interstrain and interspecies idiotypic crossreactions. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6192–6196. doi: 10.1073/pnas.75.12.6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju S. T., Cramer D. V., Dorf M. E. Idiotypic analysis of anti-GAT antibodies. V. Distribution of an interspecies cross-reactive idiotype. J Immunol. 1979 Aug;123(2):877–883. [PubMed] [Google Scholar]
- Karol R. A., Reichlin M., Noble R. W. Evolution of an idiotypic determinant: anti-Val. J Exp Med. 1977 Aug 1;146(2):435–444. doi: 10.1084/jem.146.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy R. C., Ionescu-Matiu I., Sanchez Y., Dreesman G. R. Detection of interspecies idiotypic cross-reactions associated with antibodies to hepatitis B surface antigen. Eur J Immunol. 1983 Mar;13(3):232–235. doi: 10.1002/eji.1830130310. [DOI] [PubMed] [Google Scholar]
- Kusama M., Kageshita T., Chen Z. J., Ferrone S. Characterization of syngeneic antiidiotypic monoclonal antibodies to murine anti-human high molecular weight melanoma-associated antigen monoclonal antibodies. J Immunol. 1989 Dec 1;143(11):3844–3852. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matsui M., Temponi M., Ferrone S. Characterization of a monoclonal antibody-defined human melanoma-associated antigen susceptible to induction by immune interferon. J Immunol. 1987 Sep 15;139(6):2088–2095. [PubMed] [Google Scholar]
- Meeker T., Lowder J., Cleary M. L., Stewart S., Warnke R., Sklar J., Levy R. Emergence of idiotype variants during treatment of B-cell lymphoma with anti-idiotype antibodies. N Engl J Med. 1985 Jun 27;312(26):1658–1665. doi: 10.1056/NEJM198506273122602. [DOI] [PubMed] [Google Scholar]
- Miller R. A., Maloney D. G., Warnke R., Levy R. Treatment of B-cell lymphoma with monoclonal anti-idiotype antibody. N Engl J Med. 1982 Mar 4;306(9):517–522. doi: 10.1056/NEJM198203043060906. [DOI] [PubMed] [Google Scholar]
- Nelson K. A., George E., Swenson C., Forstrom J. W., Hellström K. E. Immunotherapy of murine sarcomas with auto-anti-idiotypic monoclonal antibodies which bind to tumor-specific T cells. J Immunol. 1987 Sep 15;139(6):2110–2117. [PubMed] [Google Scholar]
- Ono R., Sakaguchi K., Ferrone S. Modulation by antiidiotypic monoclonal antibodies of immune lysis mediated by anti-HLA monoclonal antibodies. J Immunol. 1989 Sep 15;143(6):1921–1929. [PubMed] [Google Scholar]
- Perosa F., Ferrone S. Murine antiidiotypic monoclonal antibodies that bear the internal image of HLA-DR allospecificities. J Clin Invest. 1989 Sep;84(3):907–914. doi: 10.1172/JCI114252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perosa F., Ferrone S. Syngeneic antiidiotypic monoclonal antibodies to the murine anti-HLA-DR,DP monoclonal antibody CR11-462. Hum Immunol. 1988 Dec;23(4):255–269. doi: 10.1016/0198-8859(88)90061-4. [DOI] [PubMed] [Google Scholar]
- Perosa F., Kageshita T., Ono R., Ferrone S. Serological methods to detect anti-idiotypic antibodies. Methods Enzymol. 1989;178:74–90. doi: 10.1016/0076-6879(89)78007-1. [DOI] [PubMed] [Google Scholar]
- Powell T. J., Spann R., Nguyenduc M., Lamon E. W. Induction of effective immunity to Moloney murine sarcoma virus using monoclonal anti-idiotypic antibody as immunogen. J Immunol. 1989 Feb 15;142(4):1318–1324. [PubMed] [Google Scholar]
- Reisfeld R. A., Cheresh D. A. Human tumor antigens. Adv Immunol. 1987;40:323–377. doi: 10.1016/s0065-2776(08)60242-4. [DOI] [PubMed] [Google Scholar]
- Sakaguchi K., Ono R., Tsujisaki M., Richiardi P., Carbonara A., Park M. S., Tonai R., Terasaki P. I., Ferrone S. Anti-HLA-B7,B27,Bw42,Bw54,Bw55,Bw56, Bw67,Bw73 monoclonal antibodies: specificity, idiotypes, and application for a double determinant immunoassay. Hum Immunol. 1988 Mar;21(3):193–207. doi: 10.1016/0198-8859(88)90071-7. [DOI] [PubMed] [Google Scholar]
- Schroff R. W., Foon K. A., Beatty S. M., Oldham R. K., Morgan A. C., Jr Human anti-murine immunoglobulin responses in patients receiving monoclonal antibody therapy. Cancer Res. 1985 Feb;45(2):879–885. [PubMed] [Google Scholar]
- Shawler D. L., Bartholomew R. M., Smith L. M., Dillman R. O. Human immune response to multiple injections of murine monoclonal IgG. J Immunol. 1985 Aug;135(2):1530–1535. [PubMed] [Google Scholar]
- Temponi M., Kageshita T., Perosa F., Ono R., Okada H., Ferrone S. Purification of murine IgG monoclonal antibodies by precipitation with caprylic acid: comparison with other methods of purification. Hybridoma. 1989 Feb;8(1):85–95. doi: 10.1089/hyb.1989.8.85. [DOI] [PubMed] [Google Scholar]
- Thompson R. J., Jackson A. P., Langlois N. Circulating antibodies to mouse monoclonal immunoglobulins in normal subjects--incidence, species specificity, and effects on a two-site assay for creatine kinase-MB isoenzyme. Clin Chem. 1986 Mar;32(3):476–481. [PubMed] [Google Scholar]
- Wilson B. S., Imai K., Natali P. G., Ferrone S. Distribution and molecular characterization of a cell-surface and a cytoplasmic antigen detectable in human melanoma cells with monoclonal antibodies. Int J Cancer. 1981 Sep 15;28(3):293–300. doi: 10.1002/ijc.2910280307. [DOI] [PubMed] [Google Scholar]
- Zweig S. E., Shevach E. M. Production and properties of monoclonal antibodies to guinea pig Ia antigens. Methods Enzymol. 1983;92:66–85. doi: 10.1016/0076-6879(83)92010-4. [DOI] [PubMed] [Google Scholar]


