Abstract
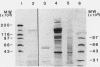
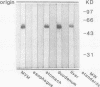
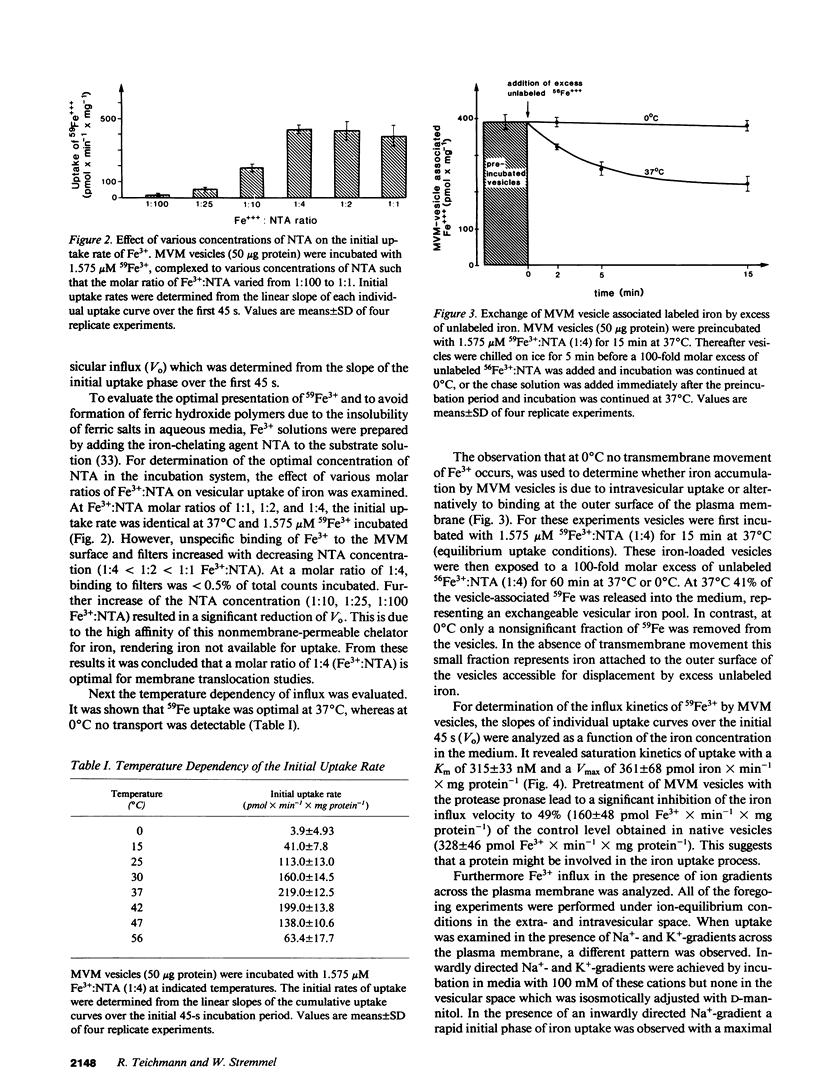
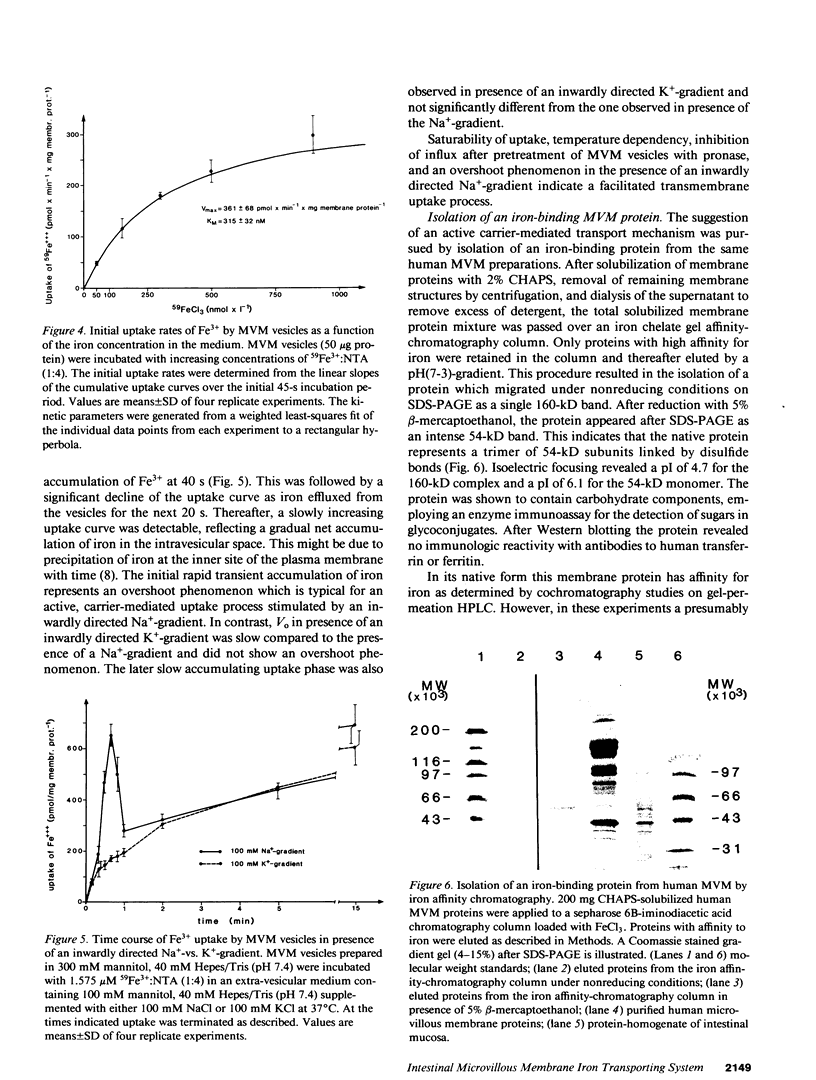
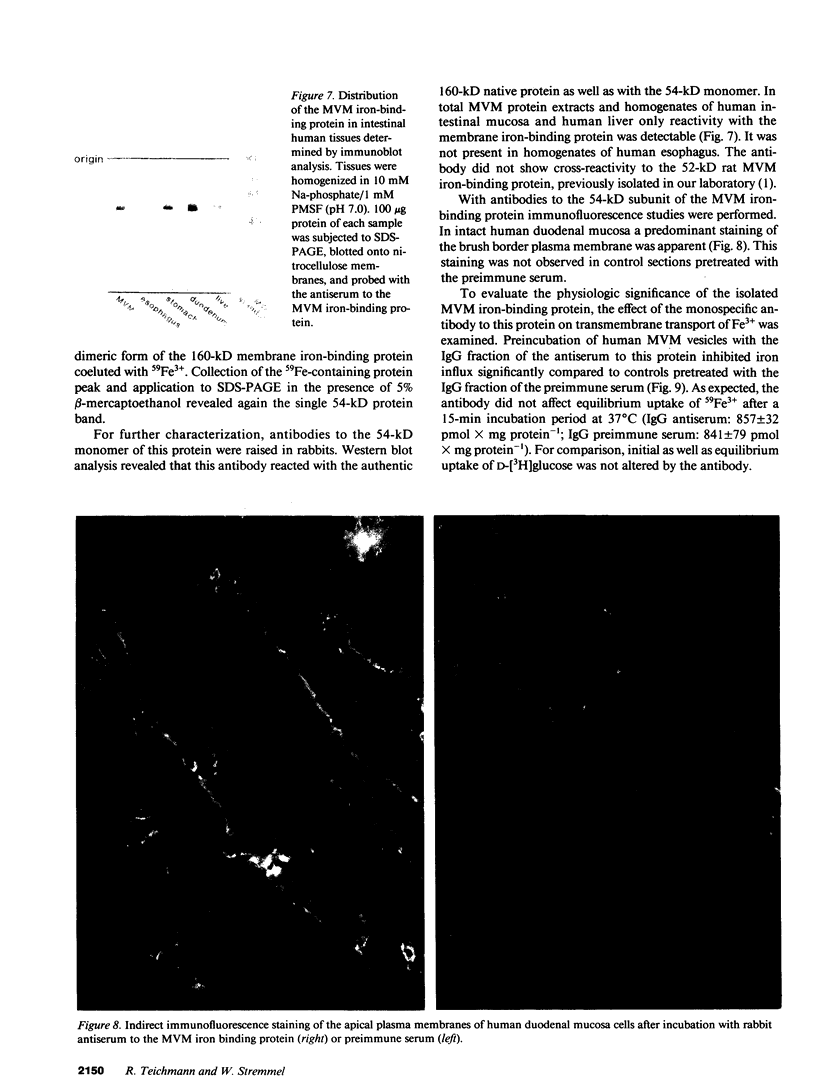
To investigate the hypothesis that iron absorption in man involves a carrier-mediated cellular uptake mechanism, influx velocity (Vo) of 59Fe3+ by isolated human microvillous membrane (MVM) vesicles of the upper small intestine was examined. Vo revealed saturation kinetics (Km = 315 nM; Vmax = 361 pmol Fe3+ x min-1 x mg protein-1) was temperature dependent and inhibited by pronase pretreatment of MVM. In the presence of an inwardly directed Na(+)-gradient a typical overshoot phenomenon with maximal uptake at 30-40 s was observed. The suggestion of an active, carrier-mediated uptake mechanism for iron was pursued by isolation of a 160-kD iron-binding protein from solubilized human MVM proteins. This glycoprotein was assembled as a trimer composed of 54-kD monomers. A monospecific antibody against the 54-kD subunit inhibited vesicular influx of Fe3+ into MVM by greater than 50%. Immunofluorescence and immunoblot analysis confirmed the localization of the protein in brush border plasma membranes. It was detectable in human intestinal mucosa and liver, but not in esophagus. These data indicate that the translocation of Fe3+ across human MVM represents a facilitated transport mechanism which is, at least in part, mediated by a membrane iron-binding protein.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bates G. W., Wernicke J. The kinetics and mechanism of iron(3) exchange between chelates and transferrin. IV. The reaction of transferrin with iron(3) nitrilotriacetate. J Biol Chem. 1971 Jun 10;246(11):3679–3685. [PubMed] [Google Scholar]
- Bates G. W., Wernicke J. The kinetics and mechanism of iron(3) exchange between chelates and transferrin. IV. The reaction of transferrin with iron(3) nitrilotriacetate. J Biol Chem. 1971 Jun 10;246(11):3679–3685. [PubMed] [Google Scholar]
- Bluett M. K., Abumrad N. N., Arab N., Ghishan F. K. Aboral changes in D-glucose transport by human intestinal brush-border membrane vesicles. Biochem J. 1986 Jul 1;237(1):229–234. doi: 10.1042/bj2370229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cleland W. W. Statistical analysis of enzyme kinetic data. Methods Enzymol. 1979;63:103–138. doi: 10.1016/0076-6879(79)63008-2. [DOI] [PubMed] [Google Scholar]
- Conrad M. E., Umbreit J. N., Moore E. G., Peterson R. D., Jones M. B. A newly identified iron binding protein in duodenal mucosa of rats. Purification and characterization of mobilferrin. J Biol Chem. 1990 Mar 25;265(9):5273–5279. [PubMed] [Google Scholar]
- Cox T. M., Mazurier J., Spik G., Montreuil J., Peters T. J. Iron binding proteins and influx of iron across the duodenal brush border. Evidence for specific lactotransferrin receptors in the human intestine. Biochim Biophys Acta. 1979 Nov 15;588(1):120–128. doi: 10.1016/0304-4165(79)90377-5. [DOI] [PubMed] [Google Scholar]
- Cox T. M., O'Donnell M. W. Studies on the binding of iron by rabbit intestinal microvillus membranes. Biochem J. 1981 Mar 15;194(3):753–759. doi: 10.1042/bj1940753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox T. M., Peters T. J. The kinetics of iron uptake in vitro by human duodenal mucosa: studies in normal subjects. J Physiol. 1979 Apr;289:469–478. doi: 10.1113/jphysiol.1979.sp012747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox T. M., Peters T. J. Uptake of iron by duodenal biopsy specimens from patients with iron-deficiency anaemia and primary haemochromatosis. Lancet. 1978 Jan 21;1(8056):123–124. doi: 10.1016/s0140-6736(78)90420-8. [DOI] [PubMed] [Google Scholar]
- Eastham E. J., Bell J. I., Douglas A. P. Iron-transport characteristics of vesicles of brush-border and basolateral plasma membrane from the rat enterocyte. Biochem J. 1977 May 15;164(2):289–294. doi: 10.1042/bj1640289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forth W., Rummel W. Iron absorption. Physiol Rev. 1973 Jul;53(3):724–792. doi: 10.1152/physrev.1973.53.3.724. [DOI] [PubMed] [Google Scholar]
- Forth W., Schäfer S. G. Absorption of di- and trivalent iron. Experimental evidence. Arzneimittelforschung. 1987 Jan;37(1A):96–100. [PubMed] [Google Scholar]
- Hjelm H., Hjelm K., Sjöquist J. Protein A from Staphylococcus aureus. Its isolation by affinity chromatography and its use as an immunosorbent for isolation of immunoglobulins. FEBS Lett. 1972 Nov 15;28(1):73–76. doi: 10.1016/0014-5793(72)80680-x. [DOI] [PubMed] [Google Scholar]
- Hurn B. A., Chantler S. M. Production of reagent antibodies. Methods Enzymol. 1980;70(A):104–142. doi: 10.1016/s0076-6879(80)70044-7. [DOI] [PubMed] [Google Scholar]
- Kessler M., Acuto O., Storelli C., Murer H., Müller M., Semenza G. A modified procedure for the rapid preparation of efficiently transporting vesicles from small intestinal brush border membranes. Their use in investigating some properties of D-glucose and choline transport systems. Biochim Biophys Acta. 1978 Jan 4;506(1):136–154. doi: 10.1016/0005-2736(78)90440-6. [DOI] [PubMed] [Google Scholar]
- Kimber C. L., Mukherjee T., Deller D. J. In vitro iron attachment to the intestinal brush border. Effect of iron stores and other environmental factors. Am J Dig Dis. 1973 Sep;18(9):781–791. doi: 10.1007/BF01070848. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marx J. J., Aisen P. Iron uptake by rabbit intestinal mucosal membrane vesicles. Biochim Biophys Acta. 1981 Dec 7;649(2):297–304. doi: 10.1016/0005-2736(81)90418-1. [DOI] [PubMed] [Google Scholar]
- Nathanson M. H., Muir A., McLaren G. D. Iron absorption in normal and iron-deficient beagle dogs: mucosal iron kinetics. Am J Physiol. 1985 Oct;249(4 Pt 1):G439–G448. doi: 10.1152/ajpgi.1985.249.4.G439. [DOI] [PubMed] [Google Scholar]
- PLOTKIN G. R., ISSELBACHER K. J. SECONDARY DISACCHARIDASE DEFICIENCY IN ADULT CELIAC DISEASE (NONTROPICAL SPRUE) AND OTHER MALABSORPTION STATES. N Engl J Med. 1964 Nov 12;271:1033–1037. doi: 10.1056/NEJM196411122712003. [DOI] [PubMed] [Google Scholar]
- Quarterman J. Metal absorption and the intestinal mucus layer. Digestion. 1987;37(1):1–9. doi: 10.1159/000199480. [DOI] [PubMed] [Google Scholar]
- Raja K. B., Bjarnason I., Simpson R. J., Peters T. J. In vitro measurement and adaptive response of Fe3+ uptake by mouse intestine. Cell Biochem Funct. 1987 Jan;5(1):69–76. doi: 10.1002/cbf.290050109. [DOI] [PubMed] [Google Scholar]
- Raja K. B., Simpson R. J., Peters T. J. Membrane potential dependence of Fe(III) uptake by mouse duodenum. Biochim Biophys Acta. 1989 Sep 18;984(3):262–266. doi: 10.1016/0005-2736(89)90291-5. [DOI] [PubMed] [Google Scholar]
- Savin M. A., Cook J. D. Iron transport by isolated rat intestinal mucosal cells. Gastroenterology. 1978 Oct;75(4):688–694. [PubMed] [Google Scholar]
- Simpson R. J., Peters T. J. Iron-binding lipids of rabbit duodenal brush-border membrane. Biochim Biophys Acta. 1987 Apr 9;898(2):181–186. doi: 10.1016/0005-2736(87)90036-8. [DOI] [PubMed] [Google Scholar]
- Simpson R. J., Peters T. J. Studies of Fe3+ transport across isolated intestinal brush-border membrane of the mouse. Biochim Biophys Acta. 1984 May 16;772(2):220–226. doi: 10.1016/0005-2736(84)90047-6. [DOI] [PubMed] [Google Scholar]
- Simpson R. J., Peters T. J. Transport of Fe2+ across lipid bilayers: possible role of free fatty acids. Biochim Biophys Acta. 1987 Apr 9;898(2):187–195. doi: 10.1016/0005-2736(87)90037-x. [DOI] [PubMed] [Google Scholar]
- Stremmel W., Lotz G., Niederau C., Teschke R., Strohmeyer G. Iron uptake by rat duodenal microvillous membrane vesicles: evidence for a carrier mediated transport system. Eur J Clin Invest. 1987 Apr;17(2):136–145. doi: 10.1111/j.1365-2362.1987.tb02393.x. [DOI] [PubMed] [Google Scholar]
- Stremmel W., Lotz G., Strohmeyer G., Berk P. D. Identification, isolation, and partial characterization of a fatty acid binding protein from rat jejunal microvillous membranes. J Clin Invest. 1985 Mar;75(3):1068–1076. doi: 10.1172/JCI111769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturrock A., Alexander J., Lamb J., Craven C. M., Kaplan J. Characterization of a transferrin-independent uptake system for iron in HeLa cells. J Biol Chem. 1990 Feb 25;265(6):3139–3145. [PubMed] [Google Scholar]
- Thomson A. B., Valberg L. S. Intestinal uptake of iron, cobalt, and manganese in the iron-deficient rat. Am J Physiol. 1972 Dec;223(6):1327–1329. doi: 10.1152/ajplegacy.1972.223.6.1327. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright T. L., Fitz J. G., Weisiger R. A. Non-transferrin-bound iron uptake by rat liver. Role of membrane potential difference. J Biol Chem. 1988 Feb 5;263(4):1842–1847. [PubMed] [Google Scholar]