Abstract
These experiments were done to learn whether Mycoplasma pulmonis infections of the respiratory tract of rats can potentiate "neurogenic inflammation" and whether this potentiation is amplified by factors that exacerbate the infections. Pathogen-free F344 rats were inoculated intranasally with M. pulmonis or with sterile culture medium and then lived for 4 wk in an ammonia-free atmosphere or in air containing ammonia (100 parts per million). Neurogenic inflammation was evoked by an intravenous injection of capsaicin, and 5 min later the magnitude of the response was quantified by measuring the amount of extravasation of two tracers, Monastral blue pigment and Evans blue dye. We found that vascular permeability in the tracheas of all rats was normal in the absence of capsaicin. However, a 75-micrograms/kg dose of capsaicin, which caused almost no extravasation of Evans blue in the tracheas of pathogen-free controls (17 +/- 3 ng/mg; mean +/- SE), produced extensive extravasation in the infected rats (135 +/- 18 ng/mg; P less than 0.001). Similarly, this dose of capsaicin produced 30 times as much Monastral blue extravasation in the infected rats (area density = 47 +/- 8% of surface area) as it did in the pathogen-free rats (1.6 +/- 0.5%; P less than 0.001), a difference that resulted from increases in the number of Monastral blue-labeled postcapillary venules and in the amount of labeling per venule. Exposure of the infected rats to ammonia exacerbated the infections, further increased the number of Monastral blue-labeled vessels and the amount of labeling per vessel, and made the rats so sensitive to capsaicin that a normally tolerable dose of 150 micrograms/kg i.v. caused fatal apnea. Ammonia did not have these effects in pathogen-free rats. We conclude that M. pulmonis infections of the airway mucosa cause a potent, long-lasting potentiation of neurogenic inflammation, which results in part from an increase in the number and responsiveness of mediator-sensitive postcapillary venules. These changes can be amplified by environmental factors such as ammonia which exacerbate the infections.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker D. G., McDonald D. M., Basbaum C. B., Mitchell R. A. The architecture of nerves and ganglia of the ferret trachea as revealed by acetylcholinesterase histochemistry. J Comp Neurol. 1986 Apr 22;246(4):513–526. doi: 10.1002/cne.902460408. [DOI] [PubMed] [Google Scholar]
- Baljet B., Drukker J. An acetylcholinesterase method for in toto staining of peripheral nerves. Stain Technol. 1975 Jan;50(1):31–36. doi: 10.3109/10520297509117028. [DOI] [PubMed] [Google Scholar]
- Belvisi M. G., Rogers D. F., Barnes P. J. Neurogenic plasma extravasation: inhibition by morphine in guinea pig airways in vivo. J Appl Physiol (1985) 1989 Jan;66(1):268–272. doi: 10.1152/jappl.1989.66.1.268. [DOI] [PubMed] [Google Scholar]
- Borson D. B., Brokaw J. J., Sekizawa K., McDonald D. M., Nadel J. A. Neutral endopeptidase and neurogenic inflammation in rats with respiratory infections. J Appl Physiol (1985) 1989 Jun;66(6):2653–2658. doi: 10.1152/jappl.1989.66.6.2653. [DOI] [PubMed] [Google Scholar]
- Boushey H. A., Holtzman M. J., Sheller J. R., Nadel J. A. Bronchial hyperreactivity. Am Rev Respir Dis. 1980 Feb;121(2):389–413. doi: 10.1164/arrd.1980.121.2.389. [DOI] [PubMed] [Google Scholar]
- Broderson J. R., Lindsey J. R., Crawford J. E. The role of environmental ammonia in respiratory mycoplasmosis of rats. Am J Pathol. 1976 Oct;85(1):115–130. [PMC free article] [PubMed] [Google Scholar]
- Brokaw J. J., McDonald D. M. Neurally mediated increase in vascular permeability in the rat trachea: onset, duration, and tachyphylaxis. Exp Lung Res. 1988;14(6):757–767. doi: 10.3109/01902148809087842. [DOI] [PubMed] [Google Scholar]
- Carthew P., Sparrow S. Sendai virus in nude and germ-free rats. Res Vet Sci. 1980 Nov;29(3):289–292. doi: 10.1016/S0034-5288(18)32629-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castleman W. L. Respiratory tract lesions in weanling outbred rats infected with Sendai virus. Am J Vet Res. 1983 Jun;44(6):1024–1031. [PubMed] [Google Scholar]
- Davidson M. K., Lindsey J. R., Parker R. F., Tully J. G., Cassell G. H. Differences in virulence for mice among strains of Mycoplasma pulmonis. Infect Immun. 1988 Aug;56(8):2156–2162. doi: 10.1128/iai.56.8.2156-2162.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. K., Thorp R. B., Maddox P. A., Brown M. B., Cassell G. H. Murine respiratory mycoplasmosis in F344 and LEW rats: evolution of lesions and lung lymphoid cell populations. Infect Immun. 1982 May;36(2):720–729. doi: 10.1128/iai.36.2.720-729.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusser D. J., Jacoby D. B., Djokic T. D., Rubinstein I., Borson D. B., Nadel J. A. Virus induces airway hyperresponsiveness to tachykinins: role of neutral endopeptidase. J Appl Physiol (1985) 1989 Oct;67(4):1504–1511. doi: 10.1152/jappl.1989.67.4.1504. [DOI] [PubMed] [Google Scholar]
- Empey D. W., Laitinen L. A., Jacobs L., Gold W. M., Nadel J. A. Mechanisms of bronchial hyperreactivity in normal subjects after upper respiratory tract infection. Am Rev Respir Dis. 1976 Feb;113(2):131–139. doi: 10.1164/arrd.1976.113.2.131. [DOI] [PubMed] [Google Scholar]
- Gamse R., Holzer P., Lembeck F. Decrease of substance P in primary afferent neurones and impairment of neurogenic plasma extravasation by capsaicin. Br J Pharmacol. 1980 Feb;68(2):207–213. doi: 10.1111/j.1476-5381.1980.tb10409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgy K. E., Burns G. F., Hayhoe F. G. Discrimination of B, T and null lymphocytes by esterase cytochemistry. Scand J Haematol. 1977 May;18(5):437–448. doi: 10.1111/j.1600-0609.1977.tb02098.x. [DOI] [PubMed] [Google Scholar]
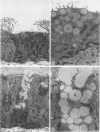
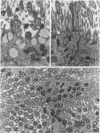
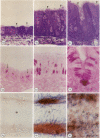
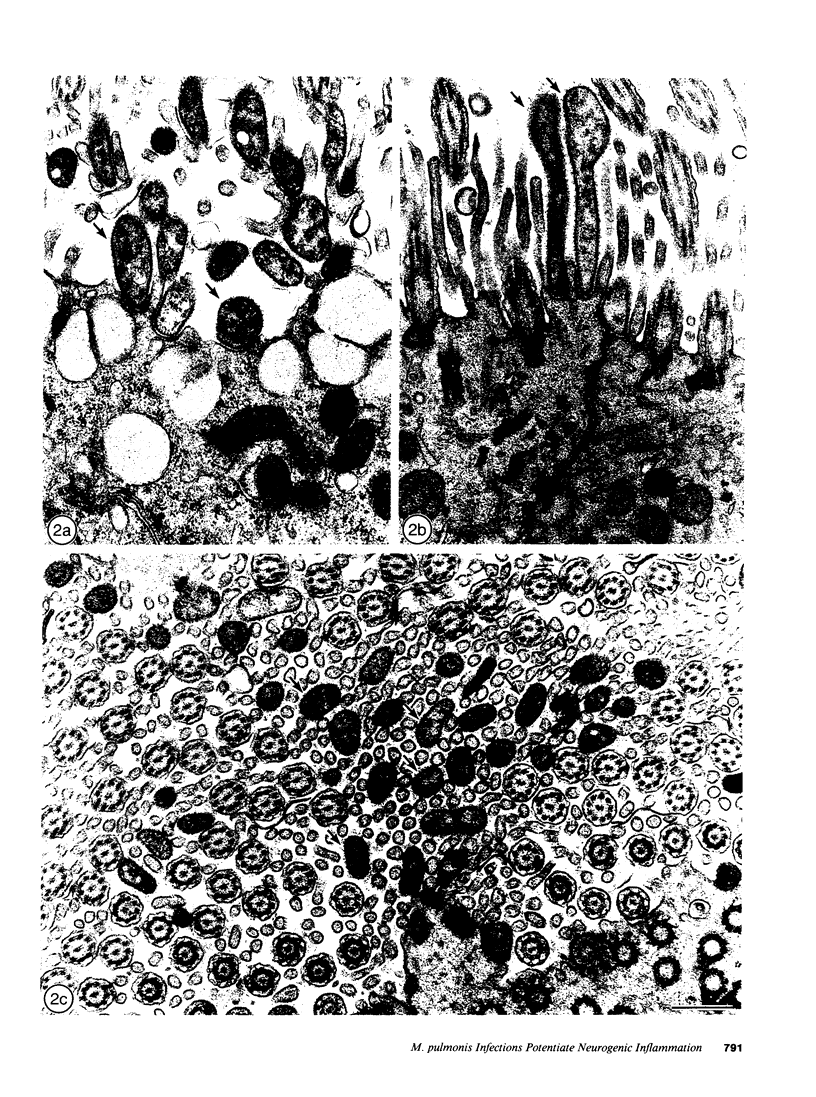
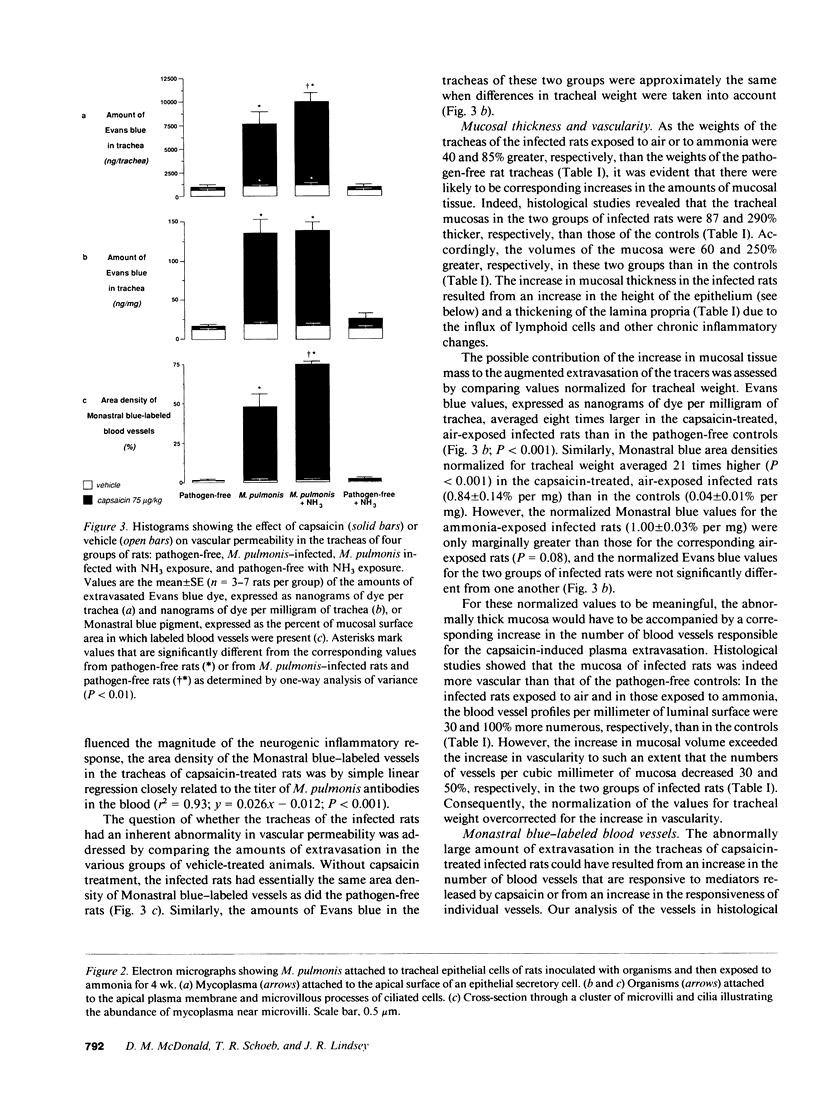
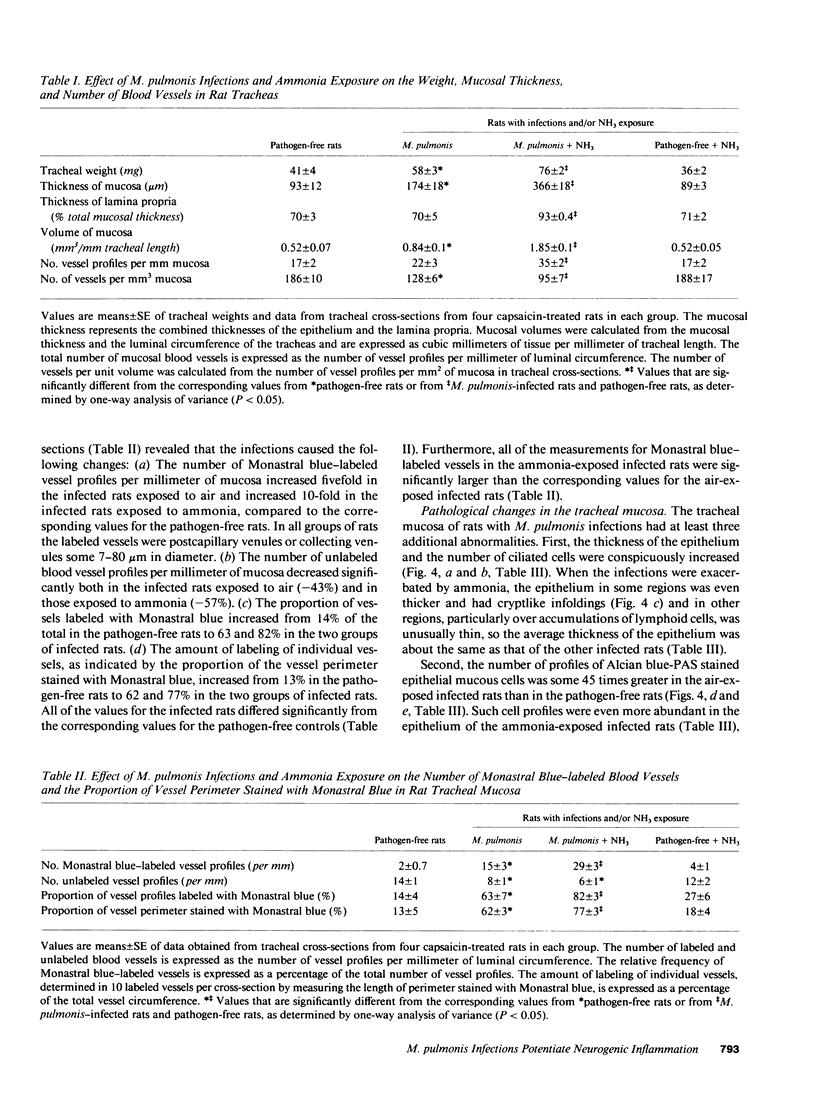
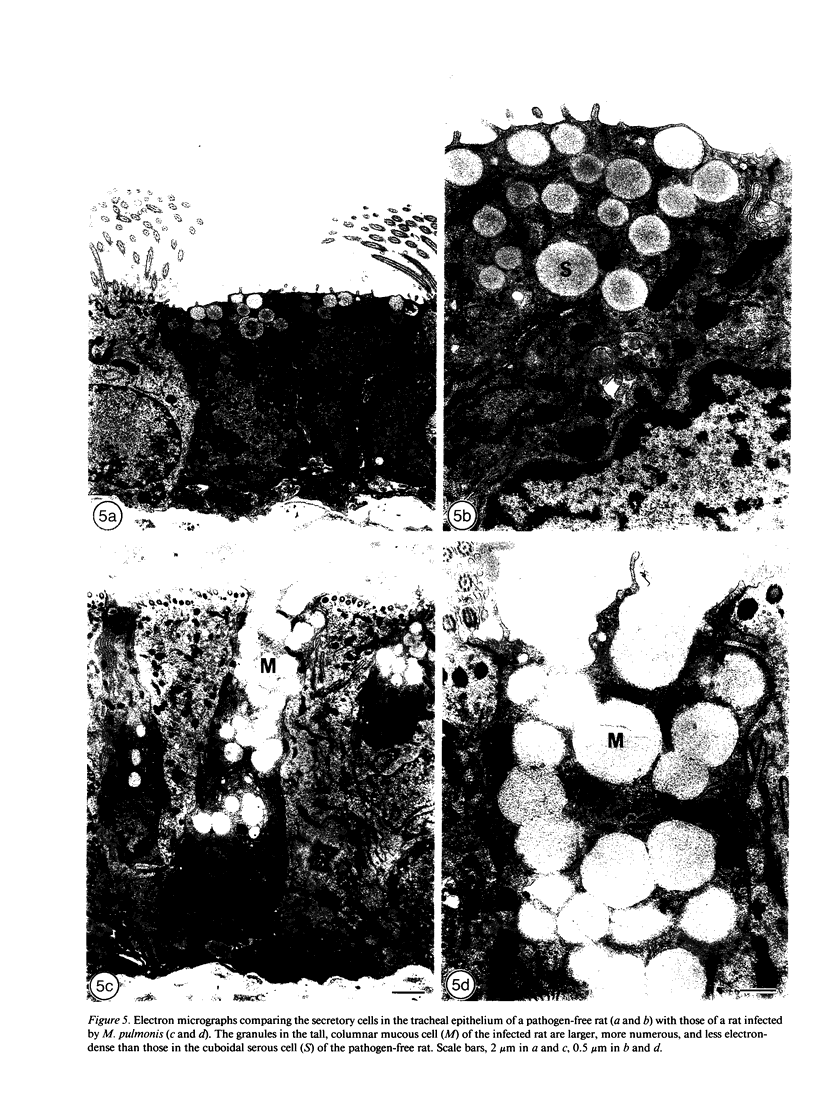
- Huang H. T., Haskell A., McDonald D. M. Changes in epithelial secretory cells and potentiation of neurogenic inflammation in the trachea of rats with respiratory tract infections. Anat Embryol (Berl) 1989;180(4):325–341. doi: 10.1007/BF00311165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jancso-Gabor A., Szolcsanyi J., Jansco N. A simple method for measuring the amount of azovan blue exuded into the skin in response to an inflammatory stimulus. J Pharm Pharmacol. 1967 Jul;19(7):486–487. doi: 10.1111/j.2042-7158.1967.tb08119.x. [DOI] [PubMed] [Google Scholar]
- Jancsó N., Jancsó-Gábor A., Szolcsányi J. Direct evidence for neurogenic inflammation and its prevention by denervation and by pretreatment with capsaicin. Br J Pharmacol Chemother. 1967 Sep;31(1):138–151. doi: 10.1111/j.1476-5381.1967.tb01984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joris I., DeGirolami U., Wortham K., Majno G. Vascular labelling with monastral blue B. Stain Technol. 1982 May;57(3):177–183. doi: 10.3109/10520298209066611. [DOI] [PubMed] [Google Scholar]
- Kowalski M. L., Didier A., Kaliner M. A. Neurogenic inflammation in the airways. I. Neurogenic stimulation induces plasma protein extravasation into the rat airway lumen. Am Rev Respir Dis. 1989 Jul;140(1):101–109. doi: 10.1164/ajrccm/140.1.101. [DOI] [PubMed] [Google Scholar]
- Lindsey J. R., Baker H. J., Overcash R. G., Cassell G. H., Hunt C. E. Murine chronic respiratory disease. Significance as a research complication and experimental production with Mycoplasma pulmonis. Am J Pathol. 1971 Sep;64(3):675–708. [PMC free article] [PubMed] [Google Scholar]
- Lundberg J. M., Brodin E., Hua X., Saria A. Vascular permeability changes and smooth muscle contraction in relation to capsaicin-sensitive substance P afferents in the guinea-pig. Acta Physiol Scand. 1984 Feb;120(2):217–227. doi: 10.1111/j.1748-1716.1984.tb00127.x. [DOI] [PubMed] [Google Scholar]
- Lundberg J. M., Saria A., Brodin E., Rosell S., Folkers K. A substance P antagonist inhibits vagally induced increase in vascular permeability and bronchial smooth muscle contraction in the guinea pig. Proc Natl Acad Sci U S A. 1983 Feb;80(4):1120–1124. doi: 10.1073/pnas.80.4.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg J. M., Saria A. Capsaicin-induced desensitization of airway mucosa to cigarette smoke, mechanical and chemical irritants. Nature. 1983 Mar 17;302(5905):251–253. doi: 10.1038/302251a0. [DOI] [PubMed] [Google Scholar]
- Lundblad L. Protective reflexes and vascular effects in the nasal mucosa elicited by activation of capsaicin-sensitive substance P-immunoreactive trigeminal neurons. Acta Physiol Scand Suppl. 1984;529:1–42. [PubMed] [Google Scholar]
- Makara G. B., György L., Molnár J. Circulatory and respiratory responses to capsaicin, 5-hydroxytryptamine and histamine in rats pretreated with capsaicin. Arch Int Pharmacodyn Ther. 1967 Nov;170(1):39–45. [PubMed] [Google Scholar]
- Martling C. R., Matran R., Alving K., Lacroix J. S., Lundberg J. M. Vagal vasodilatory mechanisms in the pig bronchial circulation preferentially involves sensory nerves. Neurosci Lett. 1989 Jan 30;96(3):306–311. doi: 10.1016/0304-3940(89)90396-0. [DOI] [PubMed] [Google Scholar]
- Matsas R., Kenny A. J., Turner A. J. The metabolism of neuropeptides. The hydrolysis of peptides, including enkephalins, tachykinins and their analogues, by endopeptidase-24.11. Biochem J. 1984 Oct 15;223(2):433–440. doi: 10.1042/bj2230433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald D. M., Mitchell R. A., Gabella G., Haskell A. Neurogenic inflammation in the rat trachea. II. Identity and distribution of nerves mediating the increase in vascular permeability. J Neurocytol. 1988 Oct;17(5):605–628. doi: 10.1007/BF01260989. [DOI] [PubMed] [Google Scholar]
- McDonald D. M. Neurogenic inflammation in the rat trachea. I. Changes in venules, leucocytes and epithelial cells. J Neurocytol. 1988 Oct;17(5):583–603. doi: 10.1007/BF01260988. [DOI] [PubMed] [Google Scholar]
- McDonald D. M. Respiratory tract infections increase susceptibility to neurogenic inflammation in the rat trachea. Am Rev Respir Dis. 1988 Jun;137(6):1432–1440. doi: 10.1164/ajrccm/137.6.1432. [DOI] [PubMed] [Google Scholar]
- McDonald D. M. The ultrastructure and permeability of tracheobronchial blood vessels in health and disease. Eur Respir J Suppl. 1990 Dec;12:572s–585s. [PubMed] [Google Scholar]
- Mumford R. A., Pierzchala P. A., Strauss A. W., Zimmerman M. Purification of a membrane-bound metalloendopeptidase from porcine kidney that degrades peptide hormones. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6623–6627. doi: 10.1073/pnas.78.11.6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naot Y., Davidson S., Lindenbaum E. S. Mitogenicity and pathogenicity of Mycoplasma pulmonis in rats. I. Atypical interstitial pneumonia induced by mitogenic myeoplasmal membranes. J Infect Dis. 1981 Jan;143(1):55–62. doi: 10.1093/infdis/143.1.55. [DOI] [PubMed] [Google Scholar]
- Persson C. G., Erjefält I., Andersson P. Leakage of macromolecules from guinea-pig tracheobronchial microcirculation. Effects of allergen, leukotrienes, tachykinins, and anti-asthma drugs. Acta Physiol Scand. 1986 May;127(1):95–105. doi: 10.1111/j.1748-1716.1986.tb07880.x. [DOI] [PubMed] [Google Scholar]
- Piedimonte G., McDonald D. M., Nadel J. A. Glucocorticoids inhibit neurogenic plasma extravasation and prevent virus-potentiated extravasation in the rat trachea. J Clin Invest. 1990 Nov;86(5):1409–1415. doi: 10.1172/JCI114855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piedimonte G., Nadel J. A., Umeno E., McDonald D. M. Sendai virus infection potentiates neurogenic inflammation in the rat trachea. J Appl Physiol (1985) 1990 Feb;68(2):754–760. doi: 10.1152/jappl.1990.68.2.754. [DOI] [PubMed] [Google Scholar]
- Saban R., Dick E. C., Fishleder R. I., Buckner C. K. Enhancement by parainfluenza 3 infection of contractile responses to substance P and capsaicin in airway smooth muscle from the guinea pig. Am Rev Respir Dis. 1987 Sep;136(3):586–591. doi: 10.1164/ajrccm/136.3.586. [DOI] [PubMed] [Google Scholar]
- Saito M., Nakayama K., Muto T., Nakagawa M. Effects of gaseous ammonia on Mycoplasma pulmonis infection in mice and rats. Jikken Dobutsu. 1982 Jul;31(3):203–206. doi: 10.1538/expanim1978.31.3_203. [DOI] [PubMed] [Google Scholar]
- Saria A., Lundberg J. M. Evans blue fluorescence: quantitative and morphological evaluation of vascular permeability in animal tissues. J Neurosci Methods. 1983 May;8(1):41–49. doi: 10.1016/0165-0270(83)90050-x. [DOI] [PubMed] [Google Scholar]
- Saria A., Lundberg J. M., Skofitsch G., Lembeck F. Vascular protein linkage in various tissue induced by substance P, capsaicin, bradykinin, serotonin, histamine and by antigen challenge. Naunyn Schmiedebergs Arch Pharmacol. 1983 Nov;324(3):212–218. doi: 10.1007/BF00503897. [DOI] [PubMed] [Google Scholar]
- Schoeb T. R., Davidson M. K., Lindsey J. R. Intracage ammonia promotes growth of Mycoplasma pulmonis in the respiratory tract of rats. Infect Immun. 1982 Oct;38(1):212–217. doi: 10.1128/iai.38.1.212-217.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoeb T. R., Kervin K. C., Lindsey J. R. Exacerbation of murine respiratory mycoplasmosis in gnotobiotic F344/N rats by Sendai virus infection. Vet Pathol. 1985 May;22(3):272–282. doi: 10.1177/030098588502200310. [DOI] [PubMed] [Google Scholar]
- Schoeb T. R., Lindsey J. R. Exacerbation of murine respiratory mycoplasmosis by sialodacryoadenitis virus infection in gnotobiotic F344 rats. Vet Pathol. 1987 Sep;24(5):392–399. doi: 10.1177/030098588702400505. [DOI] [PubMed] [Google Scholar]
- Sekizawa K., Tamaoki J., Nadel J. A., Borson D. B. Enkephalinase inhibitor potentiates substance P- and electrically induced contraction in ferret trachea. J Appl Physiol (1985) 1987 Oct;63(4):1401–1405. doi: 10.1152/jappl.1987.63.4.1401. [DOI] [PubMed] [Google Scholar]
- Skidgel R. A., Engelbrecht S., Johnson A. R., Erdös E. G. Hydrolysis of substance p and neurotensin by converting enzyme and neutral endopeptidase. Peptides. 1984 Jul-Aug;5(4):769–776. doi: 10.1016/0196-9781(84)90020-2. [DOI] [PubMed] [Google Scholar]
- Umeno E., Nadel J. A., Huang H. T., McDonald D. M. Inhibition of neutral endopeptidase potentiates neurogenic inflammation in the rat trachea. J Appl Physiol (1985) 1989 Jun;66(6):2647–2652. doi: 10.1152/jappl.1989.66.6.2647. [DOI] [PubMed] [Google Scholar]
- Wei E. T., Kiang J. G. Inhibition of protein exudation from the trachea by corticotropin-releasing factor. Eur J Pharmacol. 1987 Aug 4;140(1):63–67. doi: 10.1016/0014-2999(87)90634-0. [DOI] [PubMed] [Google Scholar]