Abstract
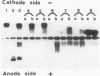
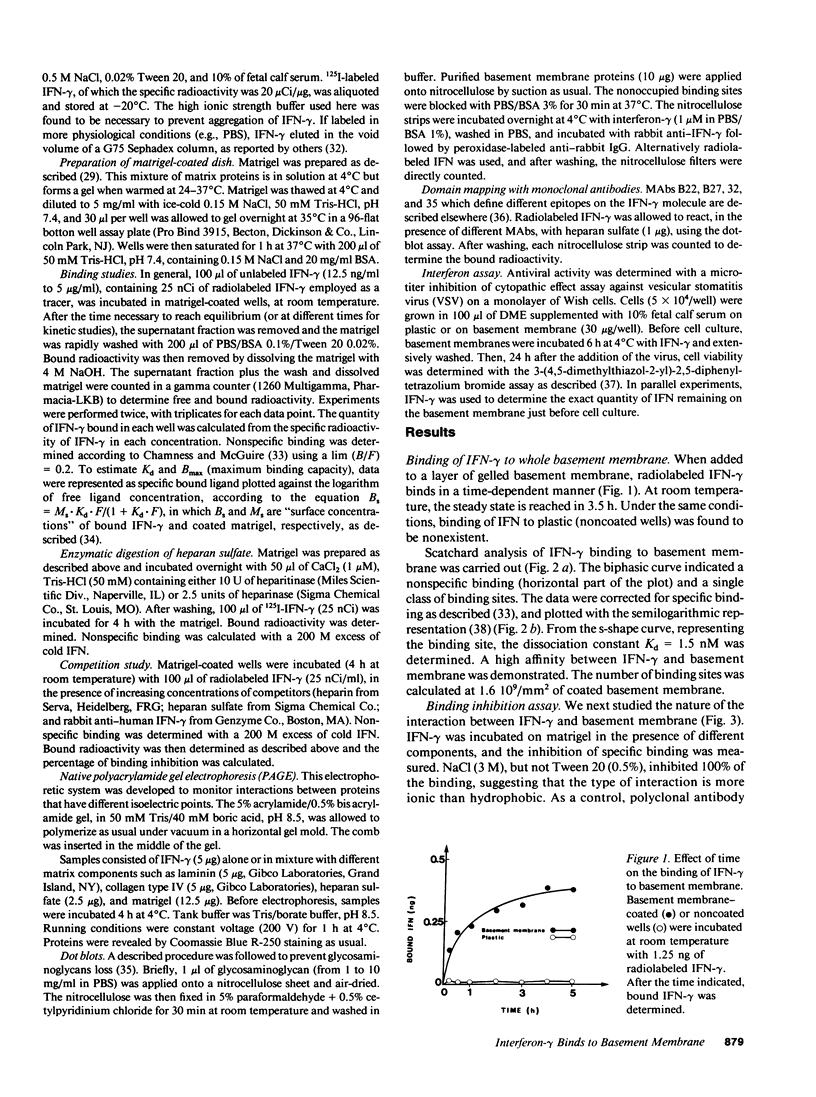
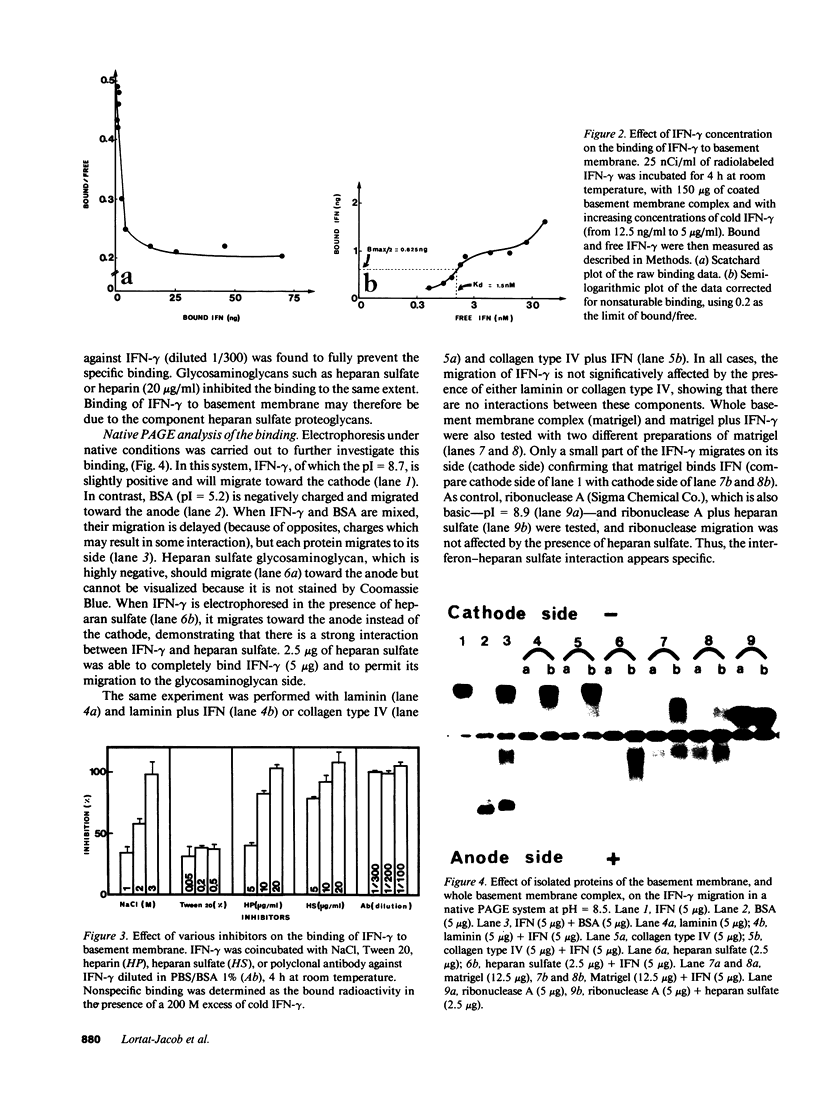
Recently it was demonstrated that growth factors are bound to the extracellular matrix, and can regulate cell behavior. Using three different types of binding assays, we have examined the interaction of interferon-gamma with a basement membrane produced by the Engelbreth-Holm-Swarm tumor. Basement membrane was found to bind interferon-gamma in both a time- and concentration-dependent manner. Equilibrium binding analysis revealed a high-affinity site with a dissociation constant of 1.5 10(-9) M and a maximum binding capacity of 1.6 10(9) sites/mm2 of basement membrane. Competition studies show that the binding is inhibited by heparan sulfate, suggesting that basement membrane-heparan sulfate proteoglycan could be the binding site. This interaction was clearly confirmed by native polyacrylamide gel electrophoresis and dot-blot analysis with purified basement membrane molecules. Furthermore, the carboxy-terminal part of the interferon-gamma molecule contains an amino acid cluster, very closely related to a consensus sequence, present in more than 20 proteins known to bind sulfated glycosaminoglycans such as heparin. These data demonstrate a possible role of extracellular matrix components in storing cytokines and in modulating the cellular response to such factors.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baird A., Ling N. Fibroblast growth factors are present in the extracellular matrix produced by endothelial cells in vitro: implications for a role of heparinase-like enzymes in the neovascular response. Biochem Biophys Res Commun. 1987 Jan 30;142(2):428–435. doi: 10.1016/0006-291x(87)90292-0. [DOI] [PubMed] [Google Scholar]
- Balkwill F. R., Burke F. The cytokine network. Immunol Today. 1989 Sep;10(9):299–304. doi: 10.1016/0167-5699(89)90085-6. [DOI] [PubMed] [Google Scholar]
- Bashkin P., Doctrow S., Klagsbrun M., Svahn C. M., Folkman J., Vlodavsky I. Basic fibroblast growth factor binds to subendothelial extracellular matrix and is released by heparitinase and heparin-like molecules. Biochemistry. 1989 Feb 21;28(4):1737–1743. doi: 10.1021/bi00430a047. [DOI] [PubMed] [Google Scholar]
- Berg K., Hansen M. B., Nielsen S. E. A new sensitive bioassay for precise quantification of interferon activity as measured via the mitochondrial dehydrogenase function in cells (MTT-method). APMIS. 1990 Feb;98(2):156–162. doi: 10.1111/j.1699-0463.1990.tb01016.x. [DOI] [PubMed] [Google Scholar]
- Bissell M. J., Barcellos-Hoff M. H. The influence of extracellular matrix on gene expression: is structure the message? J Cell Sci Suppl. 1987;8:327–343. doi: 10.1242/jcs.1987.supplement_8.18. [DOI] [PubMed] [Google Scholar]
- Burgess A. W. Cell surface heparan sulphate implicated in haemopoietic growth factor signalling. Immunol Today. 1988 Sep;9(9):267–268. doi: 10.1016/0167-5699(88)91307-2. [DOI] [PubMed] [Google Scholar]
- Cardin A. D., Weintraub H. J. Molecular modeling of protein-glycosaminoglycan interactions. Arteriosclerosis. 1989 Jan-Feb;9(1):21–32. doi: 10.1161/01.atv.9.1.21. [DOI] [PubMed] [Google Scholar]
- Chakravarti S., Tam M. F., Chung A. E. The basement membrane glycoprotein entactin promotes cell attachment and binds calcium ions. J Biol Chem. 1990 Jun 25;265(18):10597–10603. [PubMed] [Google Scholar]
- Chamness G. C., McGuire W. L. Scatchard plots: common errors in correction and interpretation. Steroids. 1975 Oct;26(4):538–542. doi: 10.1016/0039-128x(75)90073-2. [DOI] [PubMed] [Google Scholar]
- Cidadão A. J. Interactions between fibronectin, glycosaminoglycans and native collagen fibrils: an EM study in artificial three-dimensional extracellular matrices. Eur J Cell Biol. 1989 Apr;48(2):303–312. [PubMed] [Google Scholar]
- Clemens M. J., McNurlan M. A. Regulation of cell proliferation and differentiation by interferons. Biochem J. 1985 Mar 1;226(2):345–360. doi: 10.1042/bj2260345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clément B., Segui-Real B., Hassell J. R., Martin G. R., Yamada Y. Identification of a cell surface-binding protein for the core protein of the basement membrane proteoglycan. J Biol Chem. 1989 Jul 25;264(21):12467–12471. [PubMed] [Google Scholar]
- Faltynek C. R., Princler G. L., Ortaldo J. R. Expression of IFN-alpha and IFN-gamma receptors on normal human small resting T lymphocytes and large granular lymphocytes. J Immunol. 1986 Jun 1;136(11):4134–4139. [PubMed] [Google Scholar]
- Favre C., Wijdenes J., Cabrillat H., Djossou O., Banchereau J., de Vries J. E. Epitope mapping of recombinant human gamma interferon using monoclonal antibodies. Mol Immunol. 1989 Jan;26(1):17–25. doi: 10.1016/0161-5890(89)90015-1. [DOI] [PubMed] [Google Scholar]
- Gallagher J. T. The extended family of proteoglycans: social residents of the pericellular zone. Curr Opin Cell Biol. 1989 Dec;1(6):1201–1218. doi: 10.1016/s0955-0674(89)80072-9. [DOI] [PubMed] [Google Scholar]
- Grant D. S., Kleinman H. K., Leblond C. P., Inoue S., Chung A. E., Martin G. R. The basement-membrane-like matrix of the mouse EHS tumor: II. Immunohistochemical quantitation of six of its components. Am J Anat. 1985 Dec;174(4):387–398. doi: 10.1002/aja.1001740403. [DOI] [PubMed] [Google Scholar]
- Gray P. W., Leung D. W., Pennica D., Yelverton E., Najarian R., Simonsen C. C., Derynck R., Sherwood P. J., Wallace D. M., Berger S. L. Expression of human immune interferon cDNA in E. coli and monkey cells. Nature. 1982 Feb 11;295(5849):503–508. doi: 10.1038/295503a0. [DOI] [PubMed] [Google Scholar]
- Hay E. D. Extracellular matrix. J Cell Biol. 1981 Dec;91(3 Pt 2):205s–223s. doi: 10.1083/jcb.91.3.205s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst T. J., McCarthy J. B., Tsilibary E. C., Furcht L. T. Differential effects of laminin, intact type IV collagen, and specific domains of type IV collagen on endothelial cell adhesion and migration. J Cell Biol. 1988 Apr;106(4):1365–1373. doi: 10.1083/jcb.106.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano R. L. Membrane receptors for extracellular matrix macromolecules: relationship to cell adhesion and tumor metastasis. Biochim Biophys Acta. 1987 Nov 25;907(3):261–278. doi: 10.1016/0304-419x(87)90009-6. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., Cannon F. B., Laurie G. W., Hassell J. R., Aumailley M., Terranova V. P., Martin G. R., DuBois-Dalcq M. Biological activities of laminin. J Cell Biochem. 1985;27(4):317–325. doi: 10.1002/jcb.240270402. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., Graf J., Iwamoto Y., Sasaki M., Schasteen C. S., Yamada Y., Martin G. R., Robey F. A. Identification of a second active site in laminin for promotion of cell adhesion and migration and inhibition of in vivo melanoma lung colonization. Arch Biochem Biophys. 1989 Jul;272(1):39–45. doi: 10.1016/0003-9861(89)90192-6. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., Klebe R. J., Martin G. R. Role of collagenous matrices in the adhesion and growth of cells. J Cell Biol. 1981 Mar;88(3):473–485. doi: 10.1083/jcb.88.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman H. K., McGarvey M. L., Hassell J. R., Star V. L., Cannon F. B., Laurie G. W., Martin G. R. Basement membrane complexes with biological activity. Biochemistry. 1986 Jan 28;25(2):312–318. doi: 10.1021/bi00350a005. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., Ogle R. C., Cannon F. B., Little C. D., Sweeney T. M., Luckenbill-Edds L. Laminin receptors for neurite formation. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1282–1286. doi: 10.1073/pnas.85.4.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz I. M. Numbers of receptor sites from Scatchard graphs: facts and fantasies. Science. 1982 Sep 24;217(4566):1247–1249. doi: 10.1126/science.6287580. [DOI] [PubMed] [Google Scholar]
- Lander A. D., Fujii D. K., Gospodarowicz D., Reichardt L. F. Characterization of a factor that promotes neurite outgrowth: evidence linking activity to a heparan sulfate proteoglycan. J Cell Biol. 1982 Sep;94(3):574–585. doi: 10.1083/jcb.94.3.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer J. A., Pestka S. Interferon receptors. Immunol Today. 1988 Dec;9(12):393–400. doi: 10.1016/0167-5699(88)91241-8. [DOI] [PubMed] [Google Scholar]
- Leblond C. P., Inoue S. Structure, composition, and assembly of basement membrane. Am J Anat. 1989 Aug;185(4):367–390. doi: 10.1002/aja.1001850403. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Rao C. N., Wewer U. M. Biochemical interactions of tumor cells with the basement membrane. Annu Rev Biochem. 1986;55:1037–1057. doi: 10.1146/annurev.bi.55.070186.005133. [DOI] [PubMed] [Google Scholar]
- Liotta L. A. Tumor invasion and metastases--role of the extracellular matrix: Rhoads Memorial Award lecture. Cancer Res. 1986 Jan;46(1):1–7. [PubMed] [Google Scholar]
- Morrison-Graham K., Weston J. A. Mouse mutants provide new insights into the role of extracellular matrix in cell migration and differentiation. Trends Genet. 1989 Apr;5(4):116–121. doi: 10.1016/0168-9525(89)90042-5. [DOI] [PubMed] [Google Scholar]
- Noonan D. M., Horigan E. A., Ledbetter S. R., Vogeli G., Sasaki M., Yamada Y., Hassell J. R. Identification of cDNA clones encoding different domains of the basement membrane heparan sulfate proteoglycan. J Biol Chem. 1988 Nov 5;263(31):16379–16387. [PubMed] [Google Scholar]
- Pauli B. U., Knudson W. Tumor invasion: a consequence of destructive and compositional matrix alterations. Hum Pathol. 1988 Jun;19(6):628–639. doi: 10.1016/s0046-8177(88)80168-0. [DOI] [PubMed] [Google Scholar]
- Roberts R., Gallagher J., Spooncer E., Allen T. D., Bloomfield F., Dexter T. M. Heparan sulphate bound growth factors: a mechanism for stromal cell mediated haemopoiesis. Nature. 1988 Mar 24;332(6162):376–378. doi: 10.1038/332376a0. [DOI] [PubMed] [Google Scholar]
- Romeo G., Fiorucci G., Rossi G. B. Interferons in cell growth and development. Trends Genet. 1989 Jan;5(1):19–24. doi: 10.1016/0168-9525(89)90007-3. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Collins M. Molecular aspects of growth factor action: receptors and intracellular signals. J Pathol. 1983 Nov;141(3):309–331. doi: 10.1002/path.1711410310. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Pierschbacher M. D. New perspectives in cell adhesion: RGD and integrins. Science. 1987 Oct 23;238(4826):491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- Shearer M., Taylor-Papadimitriou J. Regulation of cell growth by interferon. Cancer Metastasis Rev. 1987;6(3):199–221. doi: 10.1007/BF00144264. [DOI] [PubMed] [Google Scholar]
- Stenberg M., Nygren H. Kinetics of antigen-antibody reactions at solid-liquid interfaces. J Immunol Methods. 1988 Oct 4;113(1):3–15. doi: 10.1016/0022-1759(88)90376-6. [DOI] [PubMed] [Google Scholar]
- Timpl R. Structure and biological activity of basement membrane proteins. Eur J Biochem. 1989 Apr 1;180(3):487–502. doi: 10.1111/j.1432-1033.1989.tb14673.x. [DOI] [PubMed] [Google Scholar]